Abstract
The ability to readily and accurately diagnose Kaposi's sarcoma-associated herpesvirus (KSHV, or human herpesvirus 8) infection in individuals remains a demanding task. Among the available diagnostic methods, sensitivities and specificities range widely, and many are inadequate for large-scale screening studies. We examined a serological algorithm for detecting KSHV in human sera having high sensitivity and specificity. This method uses previously described open reading frame (ORF) K8.1 and ORF65 peptide-based enzyme-linked immunosorbent assays and a novel purified recombinant full-length LANA1 protein. We generated two multiantigen algorithms: one that maximized sensitivity and one that maximized specificity. These serological algorithms were then used to evaluate seroprevalence rates among populations of clinical and epidemiological importance. The serological algorithms yielded sensitivities of 96% and 93% and specificities of 94% and 98% for the more sensitive and specific algorithms, respectively. Among kidney donors, seroprevalence was low, 4.0% (2/50), and similar to that of blood donors (P = 0.46; odds ratio [OR], 1.4; confidence interval [CI], 0.14 to 7.9) using the highly specific algorithm. Using the sensitive algorithm, 8.0% (4/50) were infected compared to 6.4% (16/250) observed among blood donors (OR, 1.3; CI, 0.41 to 4.0; P = 0.43). Among subjects requiring bone marrow transplantation, seroprevalence rates were not elevated compared to those of blood donors (OR, 2.0; 95% CI, 0.10 to 122.9; P = 0.50). Because the need for high-quality KSHV detection methods are warranted and because questions remain about the optimal methods for assessing KSHV infection in individuals, we propose a systematic approach to standardize and optimize the assessment of KSHV infection rates using a combination of established and novel serological assays and methods.
Kaposi's sarcoma-associated herpesvirus (KSHV) (7), also known as human herpesvirus 8, causes several neoplastic diseases in the immunocompromised host, including Kaposi's sarcoma (KS), multicentric Castleman's disease, and primary effusion lymphoma (36). Among immunocompromised individuals who are KSHV infected, the lifetime risk of developing KS is 23 to 28% (6, 14, 41, 46). With a prevalence of 3 to 10% (5, 45) among iatrogenically immunosuppressed patients and 20 to 40% among human immunodeficiency virus (HIV)-infected individuals (11, 16, 17, 20, 42), KSHV is clearly of significant public health concern in the United States.
KSHV can be transmitted during transplantation, and both de novo infection and reactivated infection are associated with significant morbidity and mortality among transplant patients (10, 19, 26, 28, 29, 33, 36, 43). In one study of liver transplant recipients, all four KSHV-negative recipients who received a KSHV-infected allograft seroconverted within 6 months posttransplantation, and two patients developed KS and multiorgan failure and died within 1 year posttransplantation (30). Among kidney and heart transplant recipients seropositive for KSHV prior to transplantation, 23% to 28% develop KS (6, 12, 14). Organ recipients can also acquire KSHV from an infected allograft (2, 27, 30, 35, 40, 43, 46). In addition, transplant recipients who are infected with KSHV are at increased risk of bone marrow failure (28). Serodetection of KSHV infection may also have public health importance in securing the transfusion blood supply. Although blood-borne transmission is estimated to be uncommon (0.1% per transfused component) (9), transfusion and intravenous-drug user status have been described as risk factors for KSHV infection (5, 32). Given the large numbers of blood transfusions that are given each year in the United States (approximately 5 million transfusions) (1), up to 5,000 cases of transfusion-associated KSHV infection might occur every year in the United States.
Though the existence of KSHV has been recognized for over a decade (7), screening for KSHV infection remains challenging. Some of the factors which make diagnosis difficult include a large proportion of KSHV-infected individuals who do not have detectable levels of KSHV DNA in peripheral blood (39, 50), disconcordance between serological assays targeting different antigens (13, 18, 47), and difficulties in assembling gold standard positive and negative reference populations for antigen testing (21, 31).
Serologic tests for KSHV have been developed for both latent and lytic viral antigens (15, 16, 20, 24, 38, 49). Latency-associated nuclear antigen (LANA)-based assays are highly specific for KSHV infection but are negative in up to 20% of confirmed KSHV-infected persons. LANA antibodies are measured by immunoblotting and indirect immunofluorescence assays; enzyme-linked immunosorbent assays (ELISA) detection of LANA antibodies has been disappointing due to poor retention of the antigen on plates, resulting in low test sensitivity (8). KSHV lytic antigen ELISAs against open reading frame 65 (ORF65) and K8.1 antigens overcome some of the deficiencies that occur in LANA assays and are more sensitive but have higher rates of false positivity (8). At present, no single recombinant antigen assay has sufficient sensitivity/specificity characteristics for use in routine clinical screening. Optimal detection of KSHV seropositivity appears to require use of both latent and lytic antigens which can be independently screened and scored for seroreactivity (3, 23).
Because of the need for improved KSHV serodiagnostic methods, we used a systematic approach to standardize and optimize the assessment of KSHV infection rates using established and novel serological assays and methods. To achieve this, we have modified LANA ELISAs to increase antigen retention on plates. After validating our assay algorithm, we examined seroprevalence rates among high-risk populations of clinical interest, including transplant donors and patients with autoimmune disease.
MATERIALS AND METHODS
Subjects.
The initial assay evaluation was performed on 90 case patient sera obtained from persons with biopsy-confirmed KS. The sera were drawn within 12 months after KS diagnosis, and all case subjects were HIV-infected males with a diagnosis of AIDS. These sera were made available from participating centers of the National Cancer Institute AIDS and Cancer Specimen Resource. Control sera (100) were obtained from New York City Blood Bank (NYCBB) donors, all of whom had previously been screened and found to be LANA1 immunofluorescence assay (IFA) seronegative as well as negative for HIV, hepatitis C virus, hepatitis B virus, syphilis, and human T-cell leukemia virus type 1. A second independent set of case and control sera were tested for purposes of validating our assay algorithm. Case sera for the independent test set, a total of 87, were obtained from two large longitudinal studies of HIV-infected men (23, 48) and defined as sera drawn from subjects after a clinical diagnosis of KS. Control sera for the test set were an additional 100 blood donors from the NYCBB.
Sera from patients with severe aplastic anemia and myelodysplastic syndrome were obtained from the National Marrow Donor Program and matched to each other with respect to age, gender, and history of blood transfusion. Screened cadaveric renal transplant donor sera were obtained from the Center for Organ Recovery and Education, Pittsburgh, Pa.
Sera from patients with systemic lupus erythematosus (SLE) (5 male, 45 female) were obtained from the University of Pittsburgh Division of Rheumatology and Clinical Immunology, and 50 blood donor controls were obtained from the NYCBB. Prior to KSHV testing, case and control specimens, as well as 5 positive laboratory controls from the University of Pittsburgh Cancer Institute were blinded. Informed consent from all study participants and Institutional Review Board approval were obtained in accordance with the guidelines for human experimentation of the University of Pittsburgh.
Serological testing.
Sera were tested by using serological ELISAs against recombinant-baculovirus expressed LANA1 and against peptide-based ELISAs for ORF65 and K8.1 antigens. In addition, sera from patients with SLE were tested using a LANA1 IFA. For the ELISAs, serum at a dilution of 1:100 was added to 4 wells: 2 wells coated with peptide (5 μg/μl) and the remaining 2 wells without peptide. Optical density (OD) values were read on a Dynatech Laboratories (Chantilly, VA) MRX 1CXA0716 plate reader at a 405-nm wavelength after reaction with rabbit anti-human immunoglobulin G horseradish peroxidase (1:6,000; DAKO, Carpinteria, CA) and development in 3,3′,5,5′-tetramethylbenizidine (Bio-Rad, Hercules, CA).
Quality control testing.
All peptides and proteins were generated from single batches that were aliquoted and used throughout the study. For each antigen, test plates were generated that measured peptide deposition using well-characterized KSHV-reactive sera and, in the case of LANA1 protein, using a mouse monoclonal antibody. These tests demonstrated uniform and reproducible antigen deposition on plates. For testing of human sera, each plate included three test sera (KSHV high- and low-titered sera and a KSHV-negative serum), which were plotted over time. These reference standard sera were aliquoted and used throughout the study. If results from one of the three test sera deviated by greater than 2 standard deviations from the global mean optical density, then the results from that plate were discarded and the test repeated. This allowed us to monitor quality control throughout the study period and to discover any systematic changes in test sensitivity or specificity.
All sera were tested in duplicate, and average ODs for a given sample were calculated as the mean OD of the wells containing peptide minus the mean OD of the wells containing no peptide (Fig. 1). Duplicate tests were performed independently, and all serological testing was done in a blinded fashion.
FIG. 1.
ELISA plating scheme. Each sample tested has two wells coated with antigen (indicated by gray shading in columns 1, 2, 5, 6, 9, and 10) and two wells that are not coated with antigen (unshaded circles of columns 3, 4, 7, 8, 11, and 12). The average of the noncoated wells, used as a background correction, is subtracted from the average of the peptide-coated wells to give the adjusted optical density.
K8.l and ORF65 peptide ELISAs.
Antibody testing was performed using ELISAs based on peptides from the ORF65 and K8.1 with sequences ASDILTTLSSTTETAAPAVADARKPPSGKKK and RSHLGFWQEGWSGQVYQDWLGRMNCSYENMT, respectively, as previously described (44, 49) with the modifications noted above.
Recombinant LANA1-GST ELISA.
ORF73 recombinant baculovirus was produced via transfection with a pDEST20 plasmid vector (Invitrogen, Carlsbad, CA) containing glutathione S-transferase (GST)-fused full-length LANA1 protein. Sf9 cells were grown in BacPak-supplemented medium (Clontech BD-Biosciences, Palo Alto, California) on a shaker at 135 rpm in a 27°C incubator. At the time of infection, the cell concentration was adjusted to 1 × 106 cells/ml, spun down, and resuspended in 1/10 total volume with unsupplemented medium. Baculovirus was used to infect the cells at a multiplicity of infection equal to 1 for 45 min at room temperature. Infected cells were brought back up to full volume and incubated for 3 days at 27°C prior to harvest. Cells were pelleted, frozen in an ethanol-dry ice bath, and thawed in a solution of 10 mM Tris-HCl, pH 7.5, 130 mM NaCl, 10 mM NaF, 10 mM sodium phosphate, 10 mM sodium pyrophosphate, and 1% Triton X-100, with protease inhibitors, 1 μg/ml (each) of aprotinin (Roche, Indianapolis, IN), leupeptin (Roche, Indianapolis, IN), pepstatin (Roche, Indianapolis, IN), and 0.25 mM phenylmethylsulfonyl fluoride (Sigma-Aldrich, St. Louis, MO) added immediately prior to use. Lysed cells were spun down, and the supernatant was collected and purified using 1 ml glutathione-Sepharose beads (Amersham, Piscataway, NJ) per 5 ml of cell lysate. Purified GST-LANA1 was eluted with a solution of 20 mM glutathione (Sigma-Aldrich, St. Louis, MO) in 50 mM Tris, pH 8.0.
Reacti-bind glutathione plates were used according to the manufacturer-provided protocol (Pierce, Rockford, IL). Plates were washed 3 times (200 μl/well) with 1× phosphate-buffered saline (PBS)-0.05% Tween 20. Purified GST-LANA1 protein was diluted in wash buffer (0.018 μg/μl) and added to the wells at a volume of 110 μl. Plates were covered, left to incubate at room temperature for 1 h, and washed 3 times with 200 μl wash buffer per well.
To measure anti-LANA1 antibodies, serum was added at a 1:100 dilution in a 5% nonfat milk wash buffer solution, covered, and incubated for 1 h at room temperature. Rabbit anti-human immunoglobulin G horseradish peroxidase-conjugated antibody (DAKO, Carpinteria, CA) was diluted 1:6,000 in milk buffer, and 100 μl/well was added and incubated for 1 h at room temperature. A colorimetric detection was performed as described for the peptide ELISAs.
LANA1 IFA.
The LANA1 IFA was performed as previously described (16, 22). Two independent readers scored each sample. Two discordants occurred and were resolved by a third reader.
RESULTS
Recombinant LANA1-GST ELISA development and optimization.
We hypothesized that the low sensitivity of LANA1 ELISAs may be in part due to poor retention of antigen on plates due to the unusual charge structure of the LANA1 protein. To address this possibility, we generated a baculovirus-expressed recombinant LANA1-GST (rLANA-G) fusion protein which was coated to glutathione-linked 96-well plates. Using a mouse monoclonal antibody to LANA1, GST-LANA1 retention on glutathione plates was found to be approximately 20 to 30% greater than on standard ELISA plates (data not shown). Increased seroreactivity was seen when patient sera were examined.
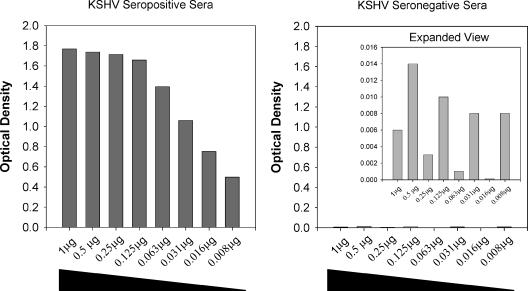
In all but one KS sera, OD values are higher on the glutathione plates, while no significant increase in reactivity is observed among the control sera. Using dilutions of rLANA-G among seroreactive and nonreactive serum samples, 1.0 μg was found to give the maximum seroreactivity among the case sera, with no appreciable reactivity observed among the control sera (Fig. 2).
FIG. 2.
Effect of antigen concentration on rLANA-G reactivity for case (left) and a control (right) sera. Increasing amounts of rLANA-G antigen increase case serum reactivity until a plateau is reached at about 0.125 μg per plate, without increasing nonspecific reactivity for the control serum. Note that the control serum reactivities are shown in a rescaled graph to allow comparison.
KSHV multiantigen algorithm.
The rLANA-G ELISA was evaluated together with the previously developed ORF65 and K8.1 ELISAs (22, 44, 49). In this analysis, 90 KS patient sera were compared to 100 blood donor sera. As seen in Fig. 3, case and control sera show significant overlap in optical densities for each of the three individual antigens. The rLANA-G ELISA shows the highest specificity at very low OD values, while the K8.1 assay is the best discriminator overall when assessing the assays independently.
FIG. 3.
Distribution of optical density values among blood donors (closed circles) and subjects with Kaposi's sarcoma (open circles) for K8.1 (A), ORF65 (B), and LANA1 (C) ELISAs.
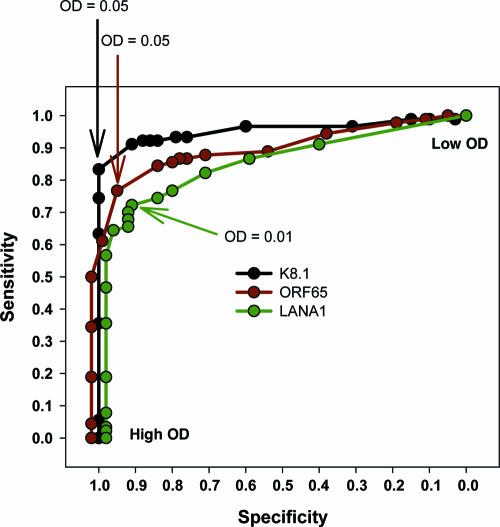
To determine the optimal discriminator cutoff value for each assay alone, the data were plotted in a receiver operator characteristic curve (Fig. 4). Based on the results of individual assays, we performed multiple iterations of OD cutoff values to obtain two algorithm models: one that maximizes sensitivity and one that maximizes specificity, while not allowing either value to drop below 90%.
FIG. 4.
Receiver operator characteristic curve of individual assay performance. Symbols indicate sensitivity and specificity of assays at differing optical density values.
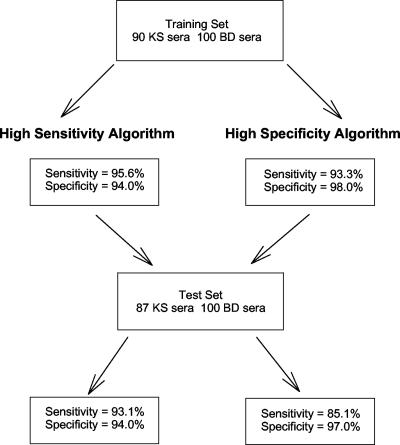
Based upon these calculations, a specimen was classified as positive if either the K8.1 assay OD was greater than 0.045, the ORF65 was greater than 0.0573, or the LANA1 OD was greater than 0.041. This algorithm yielded a sensitivity of 95.6% and a specificity of 94.0% (Table 1). For the high-specificity algorithm, a model was chosen where specimens were classified as positive if either the K8.1 assay OD was greater than 0.045, the ORF65 was greater than 0.155, or the LANA1 OD was greater than 0.048. This algorithm yielded a sensitivity of 93.3% and a specificity of 98.0% (Table 1).
TABLE 1.
Training set contingency table and multiassay sensitivity and specificity
| Algorithm and KSHV test result | No. of samples with true statusa:
|
Total | |
|---|---|---|---|
| KS+ | BD | ||
| High sensitivityb | |||
| Positive | 86 | 6 | 92 |
| Negative | 4 | 94 | 98 |
| Total | 90 | 100 | 190 |
| High specificityc | |||
| Positive | 84 | 2 | 86 |
| Negative | 6 | 98 | 104 |
| Total | 90 | 100 | 190 |
BD, blood donor.
High-sensitivity algorithm values are based on a serological algorithm where a specimen is classified as positive if the adjusted optical density is >0.045 for the K8.1 assay, >0.0573 for the ORF65 assay, or >0.041 for the LANA1 assay. Sensitivity, 86/90 = 0.956; specificity, 94/100 = 0.94.
High-specificity algorithm values are based on a serological algorithm where a specimen is classified as positive if the adjusted optical density is >0.045 for the K8.1 assay, >0.155 for the ORF65 assay, or >0.048 for the LANA1 assay. Sensitivity, 84/90 = 0.933; specificity, 98/100 = 0.98.
To validate the multiassay algorithms, an independent test set of case and control sera were evaluated (Fig. 5). Among the 100 new blood donor sera, we observed specificities of 94% and 97% with the sensitive and specific algorithms, respectively. Among the 87 KS patient sera, the sensitivity dropped to 85.1% using the highly specific algorithm but remained at 93.1% with the more sensitive model.
FIG. 5.
Selection method for determination of high-sensitivity and high-specificity assay algorithms.
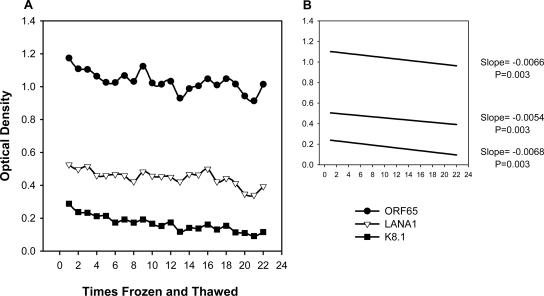
To evaluate the rLANA-G, ORF65, and K8.1 ELISAs, and because specimens were received from multiple repositories, we examined the integrity of the sera subjected to multiple freeze-thaws (Fig. 6). All assays showed a statistically significant linear decrease in OD over 22 freeze-thaws (P < 0.01). The total percentage decrease in signal intensity from the first measurement to the last was 13.6% for the ORF65 assay, 25.2% for LANA1, and 60.0% for K8.1, suggesting that handling can affect KSHV assay performance, but under most conditions, with proper handling, freeze-thaw cycles are unlikely to account for significant loss of seroreactivity.
FIG. 6.
The effects of multiple freeze-thaws on a human seroreactive serum sample. (A) One patient serum seroreactive for ORF65, LANA1, and K8.1 was frozen and thawed a total of 22 times and tested in each assay. (B) Linear regression of samples from panel A.
Serosurvey of cadaveric renal transplant donors.
Because transmission of KSHV can occur from an infected kidney donor to an uninfected recipient, the optimized assay algorithms were used to test 50 screened sera from US renal transplant donors. Using the highly specific algorithm, overall seroprevalence was low at 4.0% (2/50), similar to the 2.8% (7/250) observed among US blood donors tested in this study (P = 0.46) (Table 2). The odds ratio (OR) associated with this comparison is 1.4, and the 95% confidence interval (CI) ranges from 0.14 to 7.9. The more specific algorithm identified 8.0% (4/50) of the kidney donors as KSHV infected, though when compared to the 6.4% (16/250) found among blood donors with this method, no significant difference was observed between the groups (P = 0.43; OR = 1.3; CI = 0.41 to 4.0).
TABLE 2.
Comparison of seroprevalence of KSHV in different populations with a high-sensitivity algorithm versus a high-specificity algorithm
| Population | Sample size | Result for algorithm:
|
|||
|---|---|---|---|---|---|
| High sensitivity
|
High specificity
|
||||
| Seroprevalence (%) | P valuea | Seroprevalence (%) | P value | ||
| Blood donors | |||||
| Training set | 100 | 6.0 | 2.0 | ||
| Test set | 100 | 6.0 | 0.62 | 3.0 | 0.50 |
| SLE controls | 50 | 8.0 | 0.43 | 4.0 | 0.41 |
| Cadaveric renal transplant donors | 50 | 8.0 | 0.43 | 4.0 | 0.41 |
| Severe aplastic anemia patients | 50 | 10.0 | 0.27 | 4.0 | 0.41 |
| Myelodysplastic syndrome patients | 50 | 10.0 | 0.27 | 2.0 | 0.71 |
| SLE patients | 50 | 24.0 | 0.002 | 20.0 | 0.0003 |
| KS patients | |||||
| Training set | 90 | 95.6 | 93.3 | ||
| Test set | 87 | 93.1 | 0.35 | 85.1 | 0.06 |
P values were computed with Fisher's exact test, using U.S. blood donors in the training set as the referent group for low-risk populations and subjects with KS in the training set as the referent group for comparisons in the high-risk populations.
KSHV case control study of patients with myelodysplastic syndrome and severe aplastic anemia.
Among subjects requiring bone marrow transplantation, the overall seroprevalence was 3.0% for the more specific model and 10.0% for the sensitive model and did not significantly differ from that observed among blood donors (P = 0.4) (Table 2). In addition, KSHV seroprevalence did not significantly differ between patients with severe aplastic anemia (4%) and patients with myelodysplastic syndrome (2%) using the more specific method (OR = 2.0; 95% CI = 0.10 to 122.9; P = 0.50) or with the more sensitive method, where both groups had an equivalent KSHV seroprevalence of 10%.
Case control study of patients with SLE and blood donor controls.
In using a case control study design among 50 patients with SLE and 50 blood donors, our study objectives were twofold; first, to rigorously test the utility of our assay system using a patient population known to produce false-positive viral ELISA results, and second, to accurately identify the rate of KSHV infection among patients at increased risk of being on a course of immunosuppressive therapy, an important risk factor for KS. Our a priori null hypothesis was that KSHV seroprevalence among subjects with SLE and blood donor controls would be equivalent. However, using our ELISAs, we observed a significantly higher KSHV seroprevalence among the SLE patients using the more specific algorithm (OR = 6.0; 95% CI, 1.2 to 29.0) and the more sensitive algorithm (OR = 3.6; 95% CI, 1.1 to 12.2).
Median OD values among SLE patients were significantly elevated compared to blood donors for the LANA1 assay (P = 0.02) and the K8.1 assay (P = 0.01). This trend was observed for ORF65 but did not reach a level of statistical significance (P = 0.18). Based upon our more specific ELISA serological algorithm, of the 10 positive SLE samples, none were ORF65 positive, four were K8.1 positive only, six were LANA1 positive only, and one specimen was classified positive by reactivity to both K8.1 and LANA1. To further explore the apparent KSHV seroreactivity among patients with SLE, the LANA1 IFA was utilized.
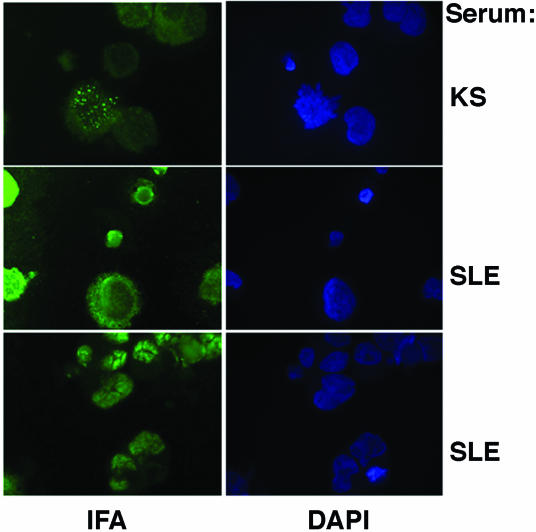
Figure 7 shows representative IFA examples from a KSHV-infected individual with KS and from two SLE patients. An increased level of diffuse background positivity was seen among SLE patients. Specific speckled nuclear staining, indicative of KSHV positivity, was absent in contrast to the positive control sample.
FIG. 7.
Representative IFA examples from a KSHV-infected subject with KS and from two SLE patients with seroreactive ELISA results. Note that the characteristic speckled nuclear staining pattern seen in the KS patient serum is absent from the SLE patient sera, suggesting nonspecific cross-reactivity.
DISCUSSION
Past reports have suggested that KSHV testing using multiple serological assays or seroassays to target multiple epitopes are needed to achieve maximal sensitivity (3, 23, 31). We have demonstrated the potential to increase KSHV detection accuracy rates beyond what has been previously reported through the combined use of established and novel assays.
We demonstrate in our study that recombinant LANA antigen can be reliably used in an ELISA format if the antigen is modified to specifically bind to the test plate. We utilized a GST tag and glutathione ELISA plates, which allowed for the attachment of our recombinant LANA1 protein to the plates with high efficiency. As an independent assay, the sensitivity and specificity of the rLANA-G ELISA is similar to that observed for the LANA1 IFA but with significantly higher throughput. In addition, as one component of a multiassay algorithm, the assay provides unique information which increases sensitivity beyond what is observed using an independent lytic-based ELISA.
By making minor adjustments in the OD cutoff values in the multiassay algorithm, we are able to present our results in comparative models that favor sensitivity or specificity. We have demonstrated that the relations in the case control studies remain relatively constant even with minor changes in assay sensitivity and specificity (based upon P values and odds ratios). Though the more sensitive algorithm definition consistently identified more seropositives, no significant difference in the ratio of positives to negatives was observed between the two different systems for any study population.
The assays used in our study are not commercially available for routine clinical testing. The results of this study, however, are sufficiently encouraging to suggest that minor technical improvements can be made so that routine KSHV serodiagnosis is reliable and robust and has sufficient sensitivity and specificity for clinical screening. One clinical testing strategy is directly derived from the results of our study. Serum samples can be first screened using high-sensitivity cutoff values to maximize detection of KSHV antibodies in serum. This small group of suspect sera can then be reexamined using the high-specificity cutoff algorithm without retesting. Sera that are positive by both algorithms would be considered positive for KSHV antibodies, while sera that are negative on the highly specific screen should be considered “indeterminant.” Evidence for KSHV infection can then be sought on indeteriminant sera using repeated blood samples and other test technologies, such as immunoblotting and IFA.
For screening efforts, high sensitivity is always desired; however, for an assay to provide clinical utility, high specificity must also be achieved. The need for clinical testing of KSHV has been addressed in the past (34, 37), and the KSHV algorithms proposed here achieve this high level of specificity (94% to 98%). Among all US blood donors in the study, 2.8% were observed to be KSHV infected with the algorithm designed for higher specificity and 6.0% when evaluated with the algorithm designed for higher sensitivity. It should be noted that an absolute evaluation of assay specificity is not possible when using US blood donors as a gold standard negative population. US blood donor prevalence has been estimated to be 2 to 4% (45), therefore it can be reasonably expected that some proportion of the 2 to 6% of seropositive blood donors observed in this study are actually infected with KSHV, which could increase the overall specificity of both algorithms.
Epidemiologically, it is implausible to suppose that KSHV infection is three to six times greater among patients with SLE than among blood donors. We have provided evidence here of the potential that exists for false-positive KSHV results among SLE patients. As an alternative to the ELISAs, we utilized a LANA1 IFA which visibly revealed the increase in nonspecific antibody reactivity. Though the LANA1 IFA may be impractical for large-scale population screening, it can be reliably expected to produce accurate results among patient groups at increased risk of producing erroneous results among standard KSHV ELISAs. Therefore, medical screening which assesses factors such as whether a patient has autoantibodies can be used as one component in determining the type of KSHV testing algorithm that would best increase KSHV diagnostic specificity.
In conclusion, we describe a novel serological approach for defining KSHV seropositivity which has high sensitivity and specificity and can be performed in a high-throughput format with relative ease. Additionally, we present KSHV seroprevalence estimates of populations of particular clinical importance. Substantial evidence exists to suggest that KSHV can be transmitted to low-risk populations through blood transfusion (4, 9) and organ transplantation (25), where a proportion will go on to develop KSHV-related morbidity and mortality. KSHV screening of transplant donors and recipients could potentially prevent cases of KS from developing, aid in earlier diagnoses of KS that do develop, reduce non-KS morbidities associated with KSHV, and potentially block one of the viral transmission pathways of KSHV. The serological methods presented here may provide a mechanism to address these issues in the future.
Acknowledgments
We thank Jodi Black and members of the National Cancer Institute's AIDS and Cancer Specimen Resource for providing some of the specimens used in this analysis. We also thank Charles Rinaldo for help in obtaining Multicenter AIDS Cohort Study specimens. We claim no conflicts of interest.
Supported by grant RO1 CA67391 and U01-AI35041 from the National Cancer Institute/National Institutes of Health, Bethesda, Maryland.
REFERENCES
- 1.American Association of Blood Banks. 2005. Recieving a blood transfusion: what every patient should know. [Online.] http://www.aabb.org/Documents/Programs_and_Services/NBDRC/faqs.htm.
- 2.Barozzi, P., M. Luppi, F. Facchetti, C. Mecucci, M. Alu, R. Sarid, V. Rasini, L. Ravazzini, E. Rossi, S. Festa, B. Crescenzi, D. G. Wolf, T. F. Schulz, and G. Torelli. 2003. Post-transplant Kaposi sarcoma originates from the seeding of donor-derived progenitors. Nat. Med. 9:554-561. [DOI] [PubMed] [Google Scholar]
- 3.Biggar, R. J., E. A. Engels, D. Whitby, D. H. Kedes, and J. J. Goedert. 2003. Antibody reactivity to latent and lytic antigens to human herpesvirus-8 in longitudinally followed homosexual men. J. Infect. Dis. 187:12-18. [DOI] [PubMed] [Google Scholar]
- 4.Blackbourn, D. J., J. Ambroziak, E. Lennette, M. Adams, B. Ramachandran, and J. A. Levy. 1997. Infectious human herpesvirus 8 in a healthy North American blood donor. Lancet 349:609-611. [DOI] [PubMed] [Google Scholar]
- 5.Cannon, M. J., S. C. Dollard, D. K. Smith, R. S. Klein, P. Schuman, J. D. Rich, D. Vlahov, and P. E. Pellett. 2001. Blood-borne and sexual transmission of human herpesvirus 8 in women with or at risk for human immunodeficiency virus infection. N. Engl. J. Med. 344:637-643. [DOI] [PubMed] [Google Scholar]
- 6.Cattani, P., M. Capuano, R. Graffeo, R. Ricci, F. Cerimele, D. Cerimele, G. Nanni, and G. Fadda. 2001. Kaposi's sarcoma associated with previous human herpesvirus 8 infection in kidney transplant recipients. J. Clin. Microbiol. 39:506-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 8.Corchero, J. L., E. C. Mar, T. J. Spira, P. E. Pellett, and N. Inoue. 2001. Comparison of serologic assays for detection of antibodies against human herpesvirus 8. Clin. Diagn. Lab. Immunol. 8:913-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dollard, S. C., K. E. Nelson, P. M. Ness, V. Stambolis, M. J. Kuehnert, P. E. Pellett, and M. J. Cannon. 2005. Possible transmission of human herpesvirus-8 by blood transfusion in a historical United States cohort. Transfusion 45:500-503. [DOI] [PubMed] [Google Scholar]
- 10.Dotti, G., R. Fiocchi, T. Motta, B. Facchinetti, B. Chiodini, G. M. Borleri, G. Gavazzeni, T. Barbui, and A. Rambaldi. 1999. Primary effusion lymphoma after heart transplantation: a new entity associated with human herpesvirus-8. Leukemia 13:664-670. [DOI] [PubMed] [Google Scholar]
- 11.Dukers, N. H., N. Renwick, M. Prins, R. B. Geskus, T. F. Schulz, G. J. Weverling, R. A. Coutinho, and J. Goudsmit. 2000. Risk factors for human herpesvirus 8 seropositivity and seroconversion in a cohort of homosexual men. Am. J. Epidemiol. 151:213-224. [DOI] [PubMed] [Google Scholar]
- 12.Emond, J. P., A. G. Marcelin, R. Dorent, C. Milliancourt, N. Dupin, C. Frances, H. Agut, I. Gandjbakhch, and V. Calvez. 2002. Kaposi's sarcoma associated with previous human herpesvirus 8 infection in heart transplant recipients. J. Clin. Microbiol. 40:2217-2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engels, E. A., D. Whitby, P. B. Goebel, A. Stossel, D. Waters, A. Pintus, L. Contu, R. J. Biggar, and J. J. Goedert. 2000. Identifying human herpesvirus 8 infection: performance characteristics of serologic assays. J. Acquir. Immune Defic. Syndr. 23:346-354. [DOI] [PubMed] [Google Scholar]
- 14.Frances, C., C. Mouquet, A. G. Marcelin, S. Barete, R. Agher, D. Charron, H. Benalia, N. Dupin, J. C. Piette, M. O. Bitker, and V. Calvez. 2000. Outcome of kidney transplant recipients with previous human herpesvirus-8 infection. Transplantation 69:1776-1779. [DOI] [PubMed] [Google Scholar]
- 15.Gao, S. J., L. Kingsley, D. R. Hoover, T. J. Spira, C. R. Rinaldo, A. Saah, J. Phair, R. Detels, P. Parry, Y. Chang, and P. S. Moore. 1996. Seroconversion to antibodies against Kaposi's sarcoma-associated herpesvirus-related latent nuclear antigens before the development of Kaposi's sarcoma. N. Engl. J. Med. 335:233-241. [DOI] [PubMed] [Google Scholar]
- 16.Gao, S. J., L. Kingsley, M. Li, W. Zheng, C. Parravicini, J. Ziegler, R. Newton, C. R. Rinaldo, A. Saah, J. Phair, R. Detels, Y. Chang, and P. S. Moore. 1996. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi's sarcoma. Nat. Med. 2:925-928. [DOI] [PubMed] [Google Scholar]
- 17.Gnann, J. W., Jr., P. E. Pellett, and H. W. Jaffe. 2000. Human herpesvirus 8 and Kaposi's sarcoma in persons infected with human immunodeficiency virus. Clin. Infect. Dis. 30(Suppl. 1):S72-S76. [DOI] [PubMed] [Google Scholar]
- 18.Inoue, N., T. Spira, L. Lam, J. L. Corchero, and W. Luo. 2004. Comparison of serologic responses between Kaposi's sarcoma-positive and -negative men who were seropositive for both human herpesvirus 8 and human immunodeficiency virus. J. Med. Virol. 74:202-206. [DOI] [PubMed] [Google Scholar]
- 19.Kapelushnik, J., S. Ariad, D. Benharroch, D. Landau, A. Moser, G. Delsol, and P. Brousset. 2001. Post renal transplantation human herpesvirus 8-associated lymphoproliferative disorder and Kaposi's sarcoma. Br J. Haematol. 113:425-428. [DOI] [PubMed] [Google Scholar]
- 20.Kedes, D. H., E. Operskalski, M. Busch, R. Kohn, J. Flood, and D. Ganem. 1996. The seroepidemiology of human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus): distribution of infection in KS risk groups and evidence for sexual transmission. Nat. Med. 2:918-924. [DOI] [PubMed] [Google Scholar]
- 21.LaDuca, J. R., J. L. Love, L. Z. Abbott, S. Dube, A. E. Freidman-Kien, and B. J. Poiesz. 1998. Detection of human herpesvirus 8 DNA sequences in tissues and bodily fluids. J. Infect. Dis. 178:1610-1615. [DOI] [PubMed] [Google Scholar]
- 22.Laney, A. S., T. De Marco, J. S. Peters, M. Malloy, C. Teehankee, P. S. Moore, and Y. Chang. 2005. Kaposi sarcoma-associated herpesvirus and primary and secondary pulmonary hypertension. Chest 127:762-767. [DOI] [PubMed] [Google Scholar]
- 23.Laney, A. S., S. C. Dollard, H. W. Jaffe, M. K. Offermann, T. J. Spira, C. J. Gunthel, P. E. Pellett, and M. J. Cannon. 2004. Repeated measures study of human herpesvirus 8 (HHV-8) DNA and antibodies in men seropositive for both HHV-8 and HIV. AIDS 18:1819-1826. [DOI] [PubMed] [Google Scholar]
- 24.Lennette, E. T., D. J. Blackbourn, and J. A. Levy. 1996. Antibodies to human herpesvirus type 8 in the general population and in Kaposi's sarcoma patients. Lancet 348:858-861. [DOI] [PubMed] [Google Scholar]
- 25.Luppi, M., P. Barozzi, G. Guaraldi, L. Ravazzini, V. Rasini, C. Spano, G. Riva, D. Vallerini, A. D. Pinna, and G. Torelli. 2003. Human herpesvirus 8-associated diseases in solid-organ transplantation: importance of viral transmission from the donor. Clin. Infect. Dis. 37:606-607. [DOI] [PubMed] [Google Scholar]
- 26.Luppi, M., P. Barozzi, V. Rasini, and G. Torelli. 2002. HHV-8 infection in the transplantation setting: a concern only for solid organ transplant patients? Leuk. Lymphoma 43:517-522. [DOI] [PubMed] [Google Scholar]
- 27.Luppi, M., P. Barozzi, G. Santagostino, R. Trovato, T. F. Schulz, R. Marasca, D. Bottalico, L. Bignardi, and G. Torelli. 2000. Molecular evidence of organ-related transmission of Kaposi sarcoma-associated herpesvirus or human herpesvirus-8 in transplant patients. Blood 96:3279-3281. [PubMed] [Google Scholar]
- 28.Luppi, M., P. Barozzi, T. F. Schulz, G. Setti, K. Staskus, R. Trovato, F. Narni, A. Donelli, A. Maiorana, R. Marasca, S. Sandrini, and G. Torelli. 2000. Bone marrow failure associated with human herpesvirus 8 infection after transplantation. N. Engl. J. Med. 343:1378-1385. [DOI] [PubMed] [Google Scholar]
- 29.Luppi, M., P. Barozzi, T. F. Schulz, R. Trovato, A. Donelli, F. Narni, J. Sheldon, R. Marasca, and G. Torelli. 2000. Nonmalignant disease associated with human herpesvirus 8 reactivation in patients who have undergone autologous peripheral blood stem cell transplantation. Blood 96:2355-2357. [PubMed] [Google Scholar]
- 30.Marcelin, A. G., A. M. Roque-Afonso, M. Hurtova, N. Dupin, M. Tulliez, M. Sebagh, Z. A. Arkoub, C. Guettier, D. Samuel, V. Calvez, and E. Dussaix. 2004. Fatal disseminated Kaposi's sarcoma following human herpesvirus 8 primary infections in liver-transplant recipients. Liver Transpl. 10:295-300. [DOI] [PubMed] [Google Scholar]
- 31.Martin, J. N., Z. Amad, C. Cossen, P. K. Lam, D. H. Kedes, K. A. Page-Shafer, D. H. Osmond, and B. Forghani. 2000. Use of epidemiologically well-defined subjects and existing immunofluorescence assays to calibrate a new enzyme immunoassay for human herpesvirus 8 antibodies. J. Clin. Microbiol. 38:696-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin, J. N., D. E. Ganem, D. H. Osmond, K. A. Page-Shafer, D. Macrae, and D. H. Kedes. 1998. Sexual transmission and the natural history of human herpesvirus 8 infection. N. Engl. J. Med. 338:948-954. [DOI] [PubMed] [Google Scholar]
- 33.Matsushima, A. Y., J. A. Strauchen, G. Lee, E. Scigliano, E. E. Hale, M. T. Weisse, D. Burstein, O. Kamel, P. S. Moore, and Y. Chang. 1999. Posttransplantation plasmacytic proliferations related to Kaposi's sarcoma-associated herpesvirus. Am. J. Surg. Pathol. 23:1393-1400. [DOI] [PubMed] [Google Scholar]
- 34.Michaels, M. G., and F. J. Jenkins. 2003. Human herpesvirus 8: is it time for routine surveillance in pediatric solid organ transplant recipients to prevent the development of Kaposi's sarcoma? Pediatr. Transplant. 7:1-3. [DOI] [PubMed] [Google Scholar]
- 35.Milliancourt, C., S. Barete, A. G. Marcelin, C. Mouquet, N. Dupin, C. Frances, H. Agut, M. O. Bitker, and V. Calvez. 2001. Human herpesvirus-8 seroconversions after renal transplantation. Transplantation 72:1319-1320. [DOI] [PubMed] [Google Scholar]
- 36.Moore, P., and Y. Chang. 2001. Kaposi's sarcoma-associated herpesvirus, p. 2803-2833. In D. Knipe, P. Howley, D. Griffin, R. Lamb, M. Martin, and S. Straus (ed.), Field's virology, 4th ed., vol. 2. Lippincott, Williams, and Wilkins, Philadelphia, Pa. [Google Scholar]
- 37.Moore, P. S. 2003. Transplanting cancer: donor-cell transmission of Kaposi sarcoma. Nat. Med. 9:506-508. [DOI] [PubMed] [Google Scholar]
- 38.Moore, P. S., S. J. Gao, G. Dominguez, E. Cesarman, O. Lungu, D. M. Knowles, R. Garber, P. E. Pellett, D. J. McGeoch, and Y. Chang. 1996. Primary characterization of a herpesvirus agent associated with Kaposi's sarcomae. J. Virol. 70:549-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moore, P. S., L. A. Kingsley, S. D. Holmberg, T. Spira, P. Gupta, D. R. Hoover, J. P. Parry, L. J. Conley, H. W. Jaffe, and Y. Chang. 1996. Kaposi's sarcoma-associated herpesvirus infection prior to onset of Kaposi's sarcoma. AIDS 10:175-180. [DOI] [PubMed] [Google Scholar]
- 40.Nocera, A., M. Corbellino, U. Valente, S. Barocci, F. Torre, R. De Palma, A. Sementa, G. B. Traverso, A. Icardi, I. Fontana, V. Arcuri, F. Poli, P. Cagetti, P. Moore, and C. Parravicini. 1998. Posttransplant human herpes virus 8 infection and seroconversion in a Kaposi's sarcoma affected kidney recipient transplanted from a human herpes virus 8 positive living related donor. Transplant. Proc. 30:2095-2096. [DOI] [PubMed] [Google Scholar]
- 41.O'Brien, T. R., D. Kedes, D. Ganem, D. R. Macrae, P. S. Rosenberg, J. Molden, and J. J. Goedert. 1999. Evidence for concurrent epidemics of human herpesvirus 8 and human immunodeficiency virus type 1 in US homosexual men: rates, risk factors, and relationship to Kaposi's sarcoma. J. Infect. Dis. 180:1010-1017. [DOI] [PubMed] [Google Scholar]
- 42.Parisi, S. G., L. Sarmati, M. Pappagallo, R. Mazzi, G. Carolo, F. Farchi, E. Nicastri, E. Concia, G. Rezza, and M. Andreoni. 2002. Prevalence trend and correlates of HHV-8 infection in HIV-infected patients. J. Acquir. Immune Defic. Syndr. 29:295-299. [DOI] [PubMed] [Google Scholar]
- 43.Parravicini, C., S. J. Olsen, M. Capra, F. Poli, G. Sirchia, S. J. Gao, E. Berti, A. Nocera, E. Rossi, G. Bestetti, M. Pizzuto, M. Galli, M. Moroni, P. S. Moore, and M. Corbellino. 1997. Risk of Kaposi's sarcoma-associated herpes virus transmission from donor allografts among Italian posttransplant Kaposi's sarcoma patients. Blood 90:2826-2829. [PubMed] [Google Scholar]
- 44.Pau, C. P., L. L. Lam, T. J. Spira, J. B. Black, J. A. Stewart, P. E. Pellett, and R. A. Respess. 1998. Mapping and serodiagnostic application of a dominant epitope within the human herpesvirus 8 ORF 65-encoded protein. J. Clin. Microbiol. 36:1574-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pellett, P. E., D. J. Wright, E. A. Engels, D. V. Ablashi, S. C. Dollard, B. Forghani, S. A. Glynn, J. J. Goedert, F. J. Jenkins, T. H. Lee, F. Neipel, D. S. Todd, D. Whitby, G. J. Nemo, and M. P. Busch. 2003. Multicenter comparison of serologic assays and estimation of human herpesvirus 8 seroprevalence among US blood donors. Transfusion 43:1260-1268. [DOI] [PubMed] [Google Scholar]
- 46.Regamey, N., M. Tamm, M. Wernli, A. Witschi, G. Thiel, G. Cathomas, and P. Erb. 1998. Transmission of human herpesvirus 8 infection from renal-transplant donors to recipients. N. Engl. J. Med. 339:1358-1363. [DOI] [PubMed] [Google Scholar]
- 47.Schatz, O., P. Monini, R. Bugarini, F. Neipel, T. F. Schulz, M. Andreoni, P. Erb, M. Eggers, J. Haas, S. Butto, M. Lukwiya, J. R. Bogner, S. Yaguboglu, J. Sheldon, L. Sarmati, F. D. Goebel, R. Hintermaier, G. Enders, N. Regamey, M. Wernli, M. Sturzl, G. Rezza, and B. Ensoli. 2001. Kaposi's sarcoma-associated herpesvirus serology in Europe and Uganda: multicentre study with multiple and novel assays. J. Med. Virol. 65:123-132. [PubMed] [Google Scholar]
- 48.Seaberg, E. C., A. Munoz, M. Lu, R. Detels, J. B. Margolick, S. A. Riddler, C. M. Williams, and J. P. Phair. 2005. Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. AIDS 19:953-960. [DOI] [PubMed] [Google Scholar]
- 49.Spira, T. J., L. Lam, S. C. Dollard, Y. X. Meng, C. P. Pau, J. B. Black, D. Burns, B. Cooper, M. Hamid, J. Huong, K. Kite-Powell, and P. E. Pellett. 2000. Comparison of serologic assays and PCR for diagnosis of human herpesvirus 8 infection. J. Clin. Microbiol. 38:2174-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whitby, D., M. R. Howard, M. Tenant-Flowers, N. S. Brink, A. Copas, C. Boshoff, T. Hatzioannou, F. E. Suggett, D. M. Aldam, A. S. Denton, et al. 1995. Detection of Kaposi sarcoma-associated herpesvirus in peripheral blood of HIV-infected individuals and progression to Kaposi's sarcoma. Lancet 346:799-802. [DOI] [PubMed] [Google Scholar]