Abstract
The phage surface display technique was used to identify Borrelia burgdorferi antigens. By affinity selection with immunoglobulin G from pooled sera of six Lyme borreliosis (LB) patients, the ribosomal protein L25 was identified. The diagnostic value of L25 was investigated by an enzyme-linked immunosorbent assay, using sera from 80 LB patients and 75 controls, and the use of the protein resulted in a specificity of 99% and a 23% sensitivity, which qualify L25 as a useful antigen when combined with others.
Lyme borreliosis (LB) is increasingly recognized to cause chronic manifestations, such as arthritis, neurological disorders, skin manifestations, and arrhythmia (25, 26). Commonly, LB diagnosis is based on clinical signs and confirmed by serological findings. For serodiagnosis, a two-step process, including an enzyme-linked immunosorbent assay (ELISA) followed by Western immunoblot analysis, is recommended in Europe and the United States. Serological tests are widely used despite several shortcomings, such as the heterogeneity of antigen preparations and a lack of standardization causing interlaboratory variation (3, 23). Furthermore, serological tests show insensitivity in the early stages of LB (1, 13). It is well established that Borrelia species express different surface components depending on the temperature (21, 27), pH (4, 21) and cell density (30). Through differential gene expression, the pathogen is able to adapt to different hosts and to evade immune responses (14, 15). Therefore, assays using antigens derived from Borrelia cultures might not represent antigens which are selectively expressed in the human host. Recent studies with the recombinant proteins BBK32 and VlsE as well as two synthetic peptides have proven to be superior to assays currently used for LB serodiagnosis (1, 13, 24).
In the present study, we used the pJuFo phage surface display method (6) to identify Borrelia antigens with affinity for immunoglobulin G (IgG) from Borrelia-infected patients. This technology has previously been applied successfully to identify allergens from cDNA libraries of Aspergillus fumigatus (5), Cladosporium herbarum (29), peanuts (12), and mites (8) by employing IgE antibodies from patients. Genomic phage surface display libraries of three different Borrelia strains and one mixed library consisting of all three strains were constructed by helper phage superinfection according to a published protocol (2). The strains (Borrelia burgdorferi N40, Borrelia afzelii VS461, and Borrelia garinii PSTH), which were kindly provided by T. Kamradt (Berlin, Germany), were grown in BSK-H medium (Sigma-Aldrich, Deisendorf, Germany) as described previously (7), and the genomic DNAs were purified (QiaAmp tissue kit; QIAGEN, Hilden, Germany). The DNAs were partially digested with MboI, ligated into the BglII-restricted pJuFo vector (6), and electrotransformed into Escherichia coli XL1-Blue. Recombinant phage were enriched by five cycles of affinity selection with a pool of sera from six LB patients (Table 1). Microtiter plates were coated with anti-human IgG monoclonal antibodies (Zymed Laboratories Inc., San Francisco, Calif.), blocked with 3% nonfat dry milk, and incubated with LB patient sera (20 μl/well in 80 μl Tris-buffered saline) overnight at 4°C. After washing of the plates, 1.8 × 1011 to 1.6 × 1012 CFU of each library was separately added and incubated for 2 h at 37°C. After 10 (cycles one to three) or 20 (cycles four and five) consecutive washing steps, adherent phage were eluted by a pH shift (100 mM glycine-HCl, pH 2.2), and E. coli was reinfected for further cycles of affinity enrichment. Phage enrichment was monitored by titration of ampicillin-resistant CFU.
TABLE 1.
Clinical data for six panning pool sera from LB patients
| Patient no.a | Age (yr) | Tick bite recall | Presence of erythema migrans | ELISA titer | IgG Western blot reactivity (bands [kDa]) | LB symptom(s) |
|---|---|---|---|---|---|---|
| 1 | 61 | + | + | 1:320 | 39, 41, 58, 66, 75, 100 | Lyme arthritis (knee), myocarditis |
| 2 | 35 | − | − | 1:80 | 30, 41, 58, 100 | Lyme arthritis (knee, elbow), arthralgia |
| 3 | 58 | − | − | 1:640 | 17, 30, 35, 41, 58, 75, 100 | Lyme arthritis |
| 4 | 35 | − | − | 1:40 | 58 | Lyme arthritis, ACA |
| 5 | 59 | + | − | 1:160 | 30, 58, 75, 100 | Lyme arthritis (knee, fingers), neuropathy |
| 6 | 28 | − | − | 1:80 | 25, 30, 39, 41, 58, 66, 75, 100 | Lyme arthritis (knee), palpitation |
Patients were selected by an experienced general practitioner.
After five cycles of selection, the phagemid DNAs from 124 randomly picked E. coli clones were analyzed by restriction with PstI and sequencing. Fifty clones contained Borrelia sequences with the incorrect orientation in the pJuFo vector, and three clones could not be correlated with any published Borrelia sequence. Among the remaining clones with Borrelia-specific sequences, 10, 14, 4, and 43 were derived from the B. burgdorferi, B. garinii, B. afzelii, and mixed libraries, respectively. These clones carried inserts of 16 different sizes, which could be matched with nine genomic sequences of B. burgdorferi B31, namely, BB0182, BB0272, BB0335, BB0371, BB0713, BB0786, BBA26, BBK32, and BBL38. One sequence encoded the fibronectin binding protein BBK32, a well-established immunogenic protein (9) with high potential as a target for serodiagnosis of human LB (11, 13). Another sequence identified from the B. burgdorferi and mixed libraries encoded the ribosomal protein L25. This protein is part of the 50S ribosomal subunit that binds to a specific portion of the 5S rRNA called loop E (28). Although L25 has conserved regions involved in RNA binding (28), the amino acid residues interacting with the 5S rRNA show no conservation among different eubacterial species (17). Among Borrelia species, the deduced amino acid sequence of L25 from B. burgdorferi N40 is 98% and 90% identical with those from B. burgdorferi B31 and B. garinii PBi, respectively.
In order to investigate the diagnostic value of L25, the protein was expressed as a six-histidine-tagged recombinant protein in E. coli M15 (QIAGEN). As a control, the antigenic protein OspC was expressed in E. coli BL21 (Stratagene, La Jolla, Calif.). The full-length coding sequence of L25 was amplified from the genomic DNA of B. burgdorferi N40 by PCR with primers including a BamHI and a KpnI site (forward primer, CGGGATCCGGACGTCGACAAGTGGTAAG; reverse primer, GGGGTACCAAATCACTTTATAATAACAACTTCC) and then ligated into the pQE30 expression plasmid (QIAGEN). The sequence of OspC was amplified with primers including an EcoRI and an XhoI site (CCGGAATTCATGAAAAAGAATACATTAAGTGC and CCGCTCGAGCTTATAATATTGATCTTAATTAAGG) and then ligated into the pTYB12 plasmid (New England Biolabs, Ipswich, Conn.). Protein expression of L25 and OspC was induced for 20 h at 16°C. Recombinant L25 (rL25) was purified by Ni2+-chelate affinity chromatography in the presence of 8 M urea, and rOspC was purified according to the manufacturer's instructions (NEB) with an elution buffer containing 8 M urea. For serological analysis, 80 serum samples from patients with late-stage LB, i.e., Lyme arthritis, neuroborreliosis, or acrodermatis chronica atrophicans (ACA), were collected in the southwest of Germany by an experienced physician. The diagnosis of LB was based on characteristic clinical findings, a history of exposure, and an antibody response, which was further confirmed by IgG ELISA based on a C6 peptide antigen of the VlsE protein (16). A peptide with the sequence CMKKDDQIAAAMVLRGMAKDGQFALK was synthesized in the Department of Analytical Chemistry (M. Przybyslki, University of Konstanz, Germany). Control serum samples from 75 healthy donors without any signs of LB and no serum reactivity against the C6 peptide were collected in the same area of Germany.
For the determination of anti-rL25 and anti-rOspC antibodies, specific ELISAs were established and optimized. In brief, the wells of a microtiter plate (Nunc) were coated with 0.5 μg recombinant protein overnight at 4°C. After being washed with PBST (10 mM sodium phosphate, 140 mM NaCl, 0.1% Tween 20), the wells were blocked with 5% nonfat dry milk for 2 h. Human serum samples were diluted 1:200 in blocking solution, and 100 μl was added to the wells and incubated for 3 h. After four washing steps, the wells were incubated with 100 μl horseradish peroxidase-conjugated rabbit anti-human IgG antibody (Dako, Denmark) diluted 1:5,000 in blocking solution for 45 min. The substrate (3,3′,5,5′-tetramethylbenzidine; Sigma-Aldrich) was added after eight washing steps. The reaction was stopped with 50 μl 1 M H2SO4, and the optical density (OD) was measured at 450 nm. To determine the specific reactivities of rL25 and rOspC, each OD value for serum samples added to wells coated with the negative control (chromatography elution fractions of lysates from E. coli M15 or BL21 transformed with pQE30 or pTYP12) was subtracted from the OD values for serum samples added to wells coated with the respective protein of interest. The cutoff value for each protein evaluated by ELISA was defined as the mean OD plus 3 standard deviations for all control sera.
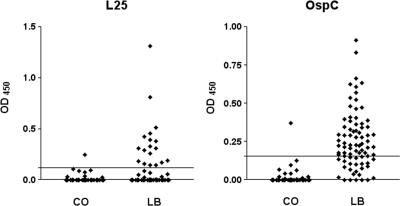
As shown in Fig. 1, rL25 specifically bound IgG antibodies in serum samples from LB patients. For the LB patients, 18 of 80 serum samples were positive when rL25 was used as the antigen and 54 of 80 were positive for rOspC, resulting in sensitivities of 23% and 68%, respectively. Of the 75 control serum samples, only one reacted with rL25 and another reacted with rOspC, corresponding to a specificity of 99% for both antigens. Even though a substantial number of patient sera showed reactivity only with the more sensitive rOspC antigen, 7 of 80 samples reacted exclusively with rL25, which might be of potential value for the serodiagnosis of late-stage LB. The observed sensitivity of 68% for rOspC in IgG serology was higher than those reported for other European seroepidemiological studies (10, 20) but was in agreement with results from North American studies (18, 19), where an OspC protein with a sequence 99% identical to ours was used. To our knowledge, the diagnostic value of L25 has not been recognized so far. It can be assumed that our patients acquired LB in Europe. For the southwest region of Germany, we have recently shown that the predominant genospecies in ticks are B. burgdorferi (11%), B. garinii (18%), and B. afzelii (53%), while mixed infections (18%) also occur (22). Although the available sequence data indicate that L25 is rather conserved among species, we cannot exclude the possibility that the use of B. garinii or B. afzelii L25 would lead to increased sensitivity. However, the low sensitivity of L25 limits its ability as a stand-alone diagnostic antigen, while its high specificity qualifies the protein as a useful antigen for LB serodiagnosis when combined with other antigens.
FIG. 1.
IgG ELISA OD values for reactivities of rL25 (BB0786) and rOspC from B. burgdorferi N40 with serum samples from 80 patients with different phases of LB and 75 control (CO) serum samples from healthy blood donors. The cutoff value (mean for control serum samples plus 3 standard deviations) is indicated by a horizontal line.
Nucleotide sequence accession number.
The nucleotide sequence of L25 from B. burgdorferi N40 has been submitted to GenBank database with the accession number DQ400710.
Acknowledgments
We thank Claudio Rhyner and Sabine Flückiger for helpful discussions and laboratory/technical support. We thank Sonja von Aulock for a critical reading of the manuscript.
Work at SIAF was supported by Swiss National Science Foundation grant 3100.063381.00.
REFERENCES
- 1.Bacon, R. M., B. J. Biggerstaff, M. E. Schriefer, R. D. Gilmore, Jr., M. T. Philipp, A. C. Steere, G. P. Wormser, A. R. Marques, and B. J. Johnson. 2003. Serodiagnosis of Lyme disease by kinetic enzyme-linked immunosorbent assay using recombinant VlsE1 or peptide antigens of Borrelia burgdorferi compared with 2-tiered testing using whole-cell lysates. J. Infect. Dis. 187:1187-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbas, C. F., III, A. S. Kang, R. A. Lerner, and S. J. Benkovic. 1991. Assembly of combinatorial antibody libraries on phage surfaces: the gene III site. Proc. Natl. Acad. Sci. USA 88:7978-7982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown, S. L., S. L. Hansen, and J. J. Langone. 1999. Role of serology in the diagnosis of Lyme disease. JAMA 282:62-66. [DOI] [PubMed] [Google Scholar]
- 4.Carroll, J. A., C. F. Garon, and T. G. Schwan. 1999. Effects of environmental pH on membrane proteins in Borrelia burgdorferi. Infect. Immun. 67:3181-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crameri, R., and K. Blaser. 1996. Cloning Aspergillus fumigatus allergens by the pJuFo filamentous phage display system. Int. Arch. Allergy Immunol. 110:41-45. [DOI] [PubMed] [Google Scholar]
- 6.Crameri, R., and M. Suter. 1993. Display of biologically active proteins on the surface of filamentous phages: a cDNA cloning system for selection of functional gene products linked to the genetic information responsible for their production. Gene 137:69-75. [DOI] [PubMed] [Google Scholar]
- 7.Diterich, I., L. Härter, D. Hassler, A. Wendel, and T. Hartung. 2001. Modulation of cytokine release in ex vivo stimulated blood from borreliosis patients. Infect. Immun. 69:687-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eriksson, T. L., O. Rasool, S. Huecas, P. Whitley, R. Crameri, U. Appenzeller, G. Gafvelin, and M. van Hage-Hamsten. 2001. Cloning of three new allergens from the dust mite Lepidoglyphus destructor using phage surface display technology. Eur. J. Biochem. 268:287-294. [DOI] [PubMed] [Google Scholar]
- 9.Fikrig, E., S. W. Barthold, W. Sun, W. Feng, S. R. Telford III, and R. A. Flavell. 1997. Borrelia burgdorferi P35 and P37 proteins, expressed in vivo, elicit protective immunity. Immunity 6:531-539. [DOI] [PubMed] [Google Scholar]
- 10.Hauser, U., G. Lehnert, and B. Wilske. 1998. Diagnostic value of proteins of three Borrelia species (Borrelia burgdorferi sensu lato) and implications for development and use of recombinant antigens for serodiagnosis of Lyme borreliosis in Europe. Clin. Diagn. Lab. Immunol. 5:456-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heikkila, T., I. Seppala, H. Saxen, J. Panelius, M. Peltomaa, T. Julin, S. A. Carlsson, and P. Lahdenne. 2002. Recombinant BBK32 protein in serodiagnosis of early and late Lyme borreliosis. J. Clin. Microbiol. 40:1174-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleber-Janke, T., R. Crameri, U. Appenzeller, M. Schlaak, and W. M. Becker. 1999. Selective cloning of peanut allergens, including profilin and 2S albumins, by phage display technology. Int. Arch. Allergy Immunol. 119:265-274. [DOI] [PubMed] [Google Scholar]
- 13.Lahdenne, P., J. Panelius, H. Saxen, T. Heikkila, H. Sillanpaa, M. Peltomaa, M. Arnez, H. I. Huppertz, and I. J. Seppala. 2003. Improved serodiagnosis of erythema migrans using novel recombinant borrelial BBK32 antigens. J. Med. Microbiol. 52:563-567. [DOI] [PubMed] [Google Scholar]
- 14.Liang, F. T., M. B. Jacobs, L. C. Bowers, and M. T. Philipp. 2002. An immune evasion mechanism for spirochetal persistence in Lyme borreliosis. J. Exp. Med. 195:415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang, F. T., F. K. Nelson, and E. Fikrig. 2002. Molecular adaptation of Borrelia burgdorferi in the murine host. J. Exp. Med. 196:275-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang, F. T., A. C. Steere, A. R. Marques, B. J. Johnson, J. N. Miller, and M. T. Philipp. 1999. Sensitive and specific serodiagnosis of Lyme disease by enzyme-linked immunosorbent assay with a peptide based on an immunodominant conserved region of Borrelia burgdorferi VlsE. J. Clin. Microbiol. 37:3990-3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu, M., and T. A. Steitz. 2000. Structure of Escherichia coli ribosomal protein L25 complexed with a 5S rRNA fragment at 1.8-A resolution. Proc. Natl. Acad. Sci. USA 97:2023-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magnarelli, L. A., J. W. Ijdo, S. J. Padula, R. A. Flavell, and E. Fikrig. 2000. Serologic diagnosis of Lyme borreliosis by using enzyme-linked immunosorbent assays with recombinant antigens. J. Clin. Microbiol. 38:1735-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magnarelli, L. A., M. Lawrenz, S. J. Norris, and E. Fikrig. 2002. Comparative reactivity of human sera to recombinant VlsE and other Borrelia burgdorferi antigens in class-specific enzyme-linked immunosorbent assays for Lyme borreliosis. J. Med. Microbiol. 51:649-655. [DOI] [PubMed] [Google Scholar]
- 20.Panelius, J., P. Lahdenne, T. Heikkila, M. Peltomaa, J. Oksi, and I. Seppala. 2002. Recombinant OspC from Borrelia burgdorferi sensu stricto, B. afzelii and B. garinii in the serodiagnosis of Lyme borreliosis. J. Med. Microbiol. 51:731-739. [DOI] [PubMed] [Google Scholar]
- 21.Ramamoorthy, R., and D. Scholl-Meeker. 2001. Borrelia burgdorferi proteins whose expression is similarly affected by culture temperature and pH. Infect. Immun. 69:2739-2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rauter, C., R. Oehme, I. Diterich, M. Engele, and T. Hartung. 2002. Distribution of clinically relevant Borrelia genospecies in ticks assessed by a novel, single-run, real-time PCR. J. Clin. Microbiol. 40:36-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reed, K. D. 2002. Laboratory testing for Lyme disease: possibilities and practicalities. J. Clin. Microbiol. 40:319-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schulte-Spechtel, U., G. Lehnert, G. Liegl, V. Fingerle, C. Heimerl, B. J. Johnson, and B. Wilske. 2003. Significant improvement of the recombinant Borrelia-specific immunoglobulin G immunoblot test by addition of VlsE and a DbpA homologue derived from Borrelia garinii for diagnosis of early neuroborreliosis. J. Clin. Microbiol. 41:1299-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steere, A. C. 2001. Lyme disease. N. Engl. J. Med. 345:115-125. [DOI] [PubMed] [Google Scholar]
- 26.Steere, A. C., J. Coburn, and L. Glickstein. 2004. The emergence of Lyme disease. J. Clin. Investig. 113:1093-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevenson, B., T. G. Schwan, and P. A. Rosa. 1995. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect. Immun. 63:4535-4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stoldt, M., J. Wohnert, M. Gorlach, and L. R. Brown. 1998. The NMR structure of Escherichia coli ribosomal protein L25 shows homology to general stress proteins and glutaminyl-tRNA synthetases. EMBO J. 17:6377-6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weichel, M., P. Schmid-Grendelmeier, C. Rhyner, G. Achatz, K. Blaser, and R. Crameri. 2003. Immunoglobulin E-binding and skin test reactivity to hydrophobin HCh-1 from Cladosporium herbarum, the first allergenic cell wall component of fungi. Clin. Exp. Allergy 33:72-77. [DOI] [PubMed] [Google Scholar]
- 30.Yang, X. F., A. Hubner, T. G. Popova, K. E. Hagman, and M. V. Norgard. 2003. Regulation of expression of the paralogous Mlp family in Borrelia burgdorferi. Infect. Immun. 71:5012-5020. [DOI] [PMC free article] [PubMed] [Google Scholar]