Abstract
We investigated the relationship between serum ribavirin concentrations and clearance, as well as therapeutic efficacy and adverse reactions, in 97 Japanese patients with chronic hepatitis C virus infections treated with a 6-month course of high-dose alpha2b interferon (6 million units/day) plus ribavirin (600 to 800 mg/day) combination therapy. This randomized trial showed that the saturation of ribavirin uptake after taking ribavirin capsules does not occur within a dose range of 600 to 800 mg/day, which is a standard dosage used clinically in Japan. Serum ribavirin concentrations and clearance did not correlate with sustained virological response rates. Fourteen patients discontinued therapy because of adverse reactions, and sustained virological response rates were significantly reduced by discontinuation of therapy, while dose reduction of ribavirin did not alter the therapeutic effects. Ribavirin concentrations after 1 week and ribavirin clearance were significantly correlated with discontinuation of ribavirin; however, a multiple-regression analysis revealed that only hemoglobin concentration, but not ribavirin clearance, was a significant factor for discontinuation of therapy (odds ratio, 0.514; 95% confidence interval, 0.311 to 0.85; P = 0.0095). It appears that peripheral erythrocytes may act as a reservoir for ribavirin and regulate serum ribavirin levels in the very early phase of treatment.
It is estimated that 170 million people are infected with hepatitis C virus (HCV) worldwide, and the prevalence is 1% to 2% in developed countries (12, 17). The natural history of hepatitis C involves the gradual progression to liver cirrhosis, with hepatocellular carcinoma as a final complication (44). The goal of therapy is to halt or decrease the progression of liver fibrosis (38), and interferon (IFN) therapy has been used for this purpose for more than a decade (33, 34). However, IFN-α and -β monotherapy is effective in a limited number of patients, with only 10% to 30% of patients experiencing a sustained virological response (SVR) to monotherapy (1, 10, 36, 37). Many investigators, including our group, have tried alternative approaches to enhance SVR rates (5, 9), and based on these investigations, oral ribavirin (RBV) combination therapy is now a first-line treatment (2, 42). Recently, the pegylated form of IFN (PEG-IFN) was demonstrated to be significantly more effective in achieving SVRs than IFN combination therapy (23), and as a result, PEG-IFN and RBV combination therapy has been recently recommended as a standard therapy for HCV infection.
Several types of adverse reactions have been reported in patients undergoing antiviral therapy for chronic hepatitis C (35). In recipients of combination therapy, consisting of either standard IFN or PEG-IFN with RBV, hemolytic anemia (hemoglobin concentration of <100 g/liter) occurred frequently and led to dosage reduction or discontinuation of RBV treatment in 1% to 15% of patients (21, 43). Most patients treated with IFN plus RBV combination therapy experience a decrease in hemoglobin levels from baseline of at least 20 to 30 g/liter (21). In a study of 832 treatment-naive patients with chronic hepatitis C virus infection, there was a mean maximum decrease of hemoglobin from baseline of 29 g/liter after 24 weeks of therapy. The decrease in hemoglobin concentrations began after 1 week and stabilized after 4 weeks of therapy, and reticulocyte counts increased, reaching a peak at 4 weeks (32). Following cessation of therapy, hemoglobin concentrations and reticulocyte counts returned to pretreatment levels after 3 to 10 weeks. Several other adverse events have been reported with IFN and RBV combination therapy, and approximately 25% of patients require dosage modification of one or both agents during treatment (41). Several laboratory parameters should be carefully monitored at various time points before and during treatment. Additionally, it would also be valuable to identify factors that can be monitored to predict treatment efficacy and adverse effects.
There are several proposed antiviral mechanisms of RBV, including immunomodulating and direct antiviral effects. RBV is a water-soluble synthetic guanosine analog, and it exerts direct antiviral activity after intracellular phosphorylation to its mono-, di-, and triphosphate forms, which are pharmacologically active in vivo. The uptake rate of RBV by erythrocytes is species and concentration dependent (47). Human erythrocytes pretreated in vitro with RBV retain 45% of the drug, and the erythrocyte concentration of RBV greatly exceeds that in plasma. This is probably caused by the absence of the enzymes, 5′ nucleotidases, and alkaline phosphatase, which hydrolyze RBV phosphates (29), and the inability of RBV phosphates to cross the erythrocyte membrane. As a consequence, RBV is accumulated within the erythrocytes as a mixture of phosphorylated derivatives, which causes a marked decrease in ATP levels in erythrocytes, inducing oxidative stress and, finally, membrane damage of the erythrocyte (4). Therefore, the serum concentration of RBV may be one of the factors that cause hemolytic anemia. In addition to hemolytic anemia, various other side effects have been reported with IFN and RBV combination therapy, such as gastrointestinal, respiratory tract, and dermatologic symptoms and emotional disturbance, and these symptoms sometimes cause discontinuation of RBV therapy (3, 22, 25, 31). It is thought that some of these symptoms are also correlated with serum RBV concentrations. Furthermore, several investigators have reported that the RBV concentrations at weeks 4 and 8 are associated with SVR. Kamar et al. (16) recently demonstrated that RBV apparent clearance (CL/F) is a good marker for determining RBV clearance in HCV-positive transplant patients with normal or impaired renal function. Toyota et al. (45) reported that discontinuation of RBV and decrease in hemoglobin concentration depended on CL/F levels.
Thus, the serum RBV concentration and its clearance seem to have clinical significance for predicting treatment efficacy and the occurrence of side effects. We measured RBV levels at 1, 2, 4, and 8 weeks after the start of treatment and analyzed the correlation of serum drug levels with therapeutic effects and severe side effects that led to discontinuation of the treatment. We also assessed the effect of RBV dosing frequency (2 or 3 times per day) because intestinal absorption of this drug also affects serum concentrations.
MATERIALS AND METHODS
Patients, treatment, and parameters.
The clinical protocol was approved by the ethical committees of the Keio University, School of Medicine, and the University Hospital. Ninety-seven patients with chronic hepatitis C who visited the Keio University Hospital and affiliated hospitals from April 2002 to March 2003 were enrolled in this study. Written informed consent was obtained from all patients. Patients were excluded if they had hepatitis B virus or human immunodeficiency virus infection, daily alcohol consumption greater than 60 g, or other forms of liver disease. Patients were randomly assigned to two groups, receiving RBV twice a day (group I) or three times a day (group II). Patients were cautioned to take RBV capsules after a meal to facilitate absorption and were reminded at each physician visit. Patients received a liver needle biopsy before having treatment and histological assessment was performed, after processing using standard techniques, according to the new Inuyama classification (13), which is a standard diagnostic criteria in Japan. HCV RNA levels and genotypes were examined using an AmpliCor GT-HCV monitor version 2.0 (Roche Molecular Systems, Pleasanton, CA) according to the method described by Okamoto et al. (28). Serum transaminase levels (aspartate amino transferase [AST] and alanine aminotransferase [ALT]), blood cell counts, and serum levels of creatinine were sequentially measured. To assess liver fibrosis, hyaluronic acid, type IV collagen, type IV collagen 7S, matrix metalloproteinase 1 (MMP-1), MMP-2, MMP-3, tissue inhibitor of MMP 1 (TIMP-1), and TIMP-2 were also measured. Adverse effects were monitored clinically by careful interview and medical examination throughout the study. Compliance with treatment was examined using a questionnaire and medical records. Renal function was assessed by serum creatinine (Cr) concentration (mg/dl) and Cr clearance (CrCl; ml/min). RBV apparent clearance was obtained from the formula CL/F (liter/h) = 32.3 × (body weight [kg]) × (1 − 0.0094 × age) × (1 − 0.42 × gender [M = 0, F = 1])/Cr (μmol/liter), according to previous reports (16, 45).
Treatment regimen.
Patients received 6 million units (MU) of intramuscular IFN-α2b (IntronA; Schering-Plough, Kenilworth, NJ) daily for 2 weeks and three times a week for 22 weeks. The RBV (Schering-Plough) dosage was determined according to body weight (600 mg/day for those weighing ≤60 kg and 800 mg/day for those weighing more than 60 kg) and administered for 24 weeks concurrently with IFN treatment. SVR was defined as the absence of serum HCV RNA after the end of treatment until 6 months later. The dose of RBV was reduced when hemoglobin concentrations reached less than 100 g/liter, and RBV was discontinued when the hemoglobin concentration reached less than 85 g/liter and restored to the initial dose upon recovery to 100 g/liter. This is a standard protocol recommended by the Ministry of Labor and Welfare of Japan.
Measurement of serum RBV levels.
Serum RBV concentrations were measured by high-performance liquid chromatography (HPLC, LC-10A; Shimadzu Corporation, Kyoto, Japan) at 28°C and optical density at 215 nm. This measuring system was already established in Japan (8) and was shown to be suitable for clinical use (48). Briefly, serum samples (200 μl) were pretreated with 20 μl of 25% perchloric acid to exclude protein and then centrifuged at 12,000 × g at 10°C for 10 min. The supernatant (120 μl) was added with 20 μl of 17% potassium phosphate tribasic solution and centrifuged at 10°C and 12,000 × g for 5 min. The supernatant (100 μl) was mixed with 140 μl of distilled water, and this solution was used as the HPLC sample. Forty microliters of the HPLC sample was loaded onto the HPLC columns (precolumn [PREGASIL ODS; Senshu Scientific Co. Ltd., Tokyo, Japan], 0.1% phosphate solution-methanol = 1,000/1 [vol/vol], 0.8 ml/min for 2 min; main column [Inertisil ODS-3; GL Sciences, Tokyo, Japan], 0.01% Na2 EDTA phosphate buffer [pH 6.7]-acetonitrile = 1,000/2.4 [vol/vol], 0.8 ml/min for 4 min).
Statistical analysis.
Treatment outcomes were analyzed on an intent-to-treat basis. Correlation between RBV concentrations and SVR rates or hemoglobin concentrations was analyzed by Fisher's exact test, Mann-Whitney U test, or Pearson's test. SVR rates of two different treatment groups were analyzed in relation to viral genotypes by the Mantel-Haenszel test. Multivariate analysis was performed using a logistic regression analysis. The criterion for statistical significance was a P value of <0.05. All statistical calculations were performed using SAS software version 8.2 (SAS Institute, Cary, NC).
RESULTS
Background of the patients.
Of the 97 patients enrolled in this study, 60 (61.9%) were male, 37 (38.1%) were over 60 years of age (median age, 57 years; range, 20 to 70 years), and 63.9% of the patients were treatment naive. HCV genotype 1b was found in 67.0% (65/97) of the patients, HCV RNA levels less than 100 kIU/ml were found in 7 of 97 the patients (7.2%), and 54 (55.7%) had level greater than 500 kIU/ml.
Fourteen patients discontinued IFN and RBV combination therapy because of anemia (n = 14), anxiety and insomnia (n = 3), itchy eruption (n = 4), fatigue (n = 3), severe headache (n = 1), or retinopathy (n = 1). One patient stopped therapy because of discovery of lung tuberculosis (n = 1). All side effects disappeared after discontinuation of the combination therapy with adequate treatment. A patient with lung tuberculosis was adequately treated with antituberculosis drugs.
The overall SVR rate in this study was 38.1% (37/97). The SVR rate of patients depended on HCV genotypes and HCV RNA levels. The SVR rate was 20.0% (13/65) in patients with genotype 1b and 75.0% (24/32) in patients with genotypes 2a/2b. In patients with genotype 1b, the SVR rate was 50.0% (2/4) in patients with low viral load (<100 kIU/ml), 36.0% (9/25) in those with moderate viral loads (100 to 500 kIU/ml), and 5.6% (2/36) in those with high viral loads (≥500 kIU/ml). These responses were compatible with other studies previously reported (22).
Effect of dosing frequency on serum RBV concentrations.
Patients randomized into group I received RBV twice a day (n = 49), and those randomized to group II received RBV three times a day (n = 48). Serum RBV concentrations after oral administration of RBV reached a plateau at up to 8 weeks. The mean serum RBV concentrations of group I and II were as follows: at 1 week, 1,159.9 and 1,244.3 ng/ml; at 2 weeks, 1,626.6 and 1,671.5 ng/ml; at 4 weeks, 2,145.1 and 2,258.4 ng/ml; at 8 weeks, 2,314.6 and 2,538.9 ng/ml; at 24 weeks, 2,243.8 and 2,148.8 ng/ml, respectively. No significant difference in RBV concentrations was found in relation to daily dosing frequency.
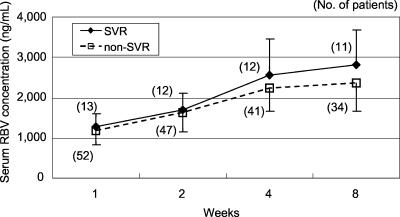
The SVR rates in groups I and II were 42.9% (21/49) and 33.3% (16/48), respectively, and there was no significant difference between the groups (P = 0.366, Mantel-Haenszel test). Serum RBV concentrations were compared between SVR patients and non-SVR patients independent of HCV genotype, and there was no significant difference between them. This result was also found when the concentration was compared within groups of patients infected with HCV genotype 1b (Fig. 1) or genotype 2a/2b.
FIG. 1.
Serum levels of RBV 1, 2, 4, and 8 weeks after the start of therapy in patients with genotype 1b HCV infection. Serum RBV concentrations were compared between patients with SVR and those who did not achieve SVR. No significant difference was found between serum RBV levels in SVR and non-SVR patients at any time point. The number of patients is indicated at each point.
SVR rates and discontinuation of RBV administration.
The backgrounds of patients who did or did not achieve an SVR were compared, and earlier stage of fibrotic change in the liver, HCV genotype (non-1b), lower basal HCV RNA levels, higher platelet counts, and lower basal MMP-2 levels were significant factors for contributing to SVR. A logistic regression analysis using the stepwise method showed that HCV genotype and HCV RNA levels were significant contributing factors for obtaining SVR among the significant factors in the univariate analysis (genotype non-1b/1b, odds ratio = 21.2, 95% confidence interval [CI] = 6.21 to 72.47, P < 0.0001; HCV RNA levels, odds ratio = 0.997, 95% CI = 0.995 to 0.999, P = 0.0066).
To exclude genotype differences, patients with genotype 1b (n = 65) were analyzed (Table 1). Contributing factors for obtaining SVR (univariate analysis) were fibrosis staging, HCV RNA levels, and MMP-2 levels. Logistic regression analysis between these factors revealed that the HCV RNA levels were a significant factor in determining the SVR of patients with genotype 1b (odds ratio = 0.995, 95% CI = 0.992 to 0.998, P = 0.0014).
TABLE 1.
Background of patients with genotype 1b hepatitis C virus infection (n = 65) according to response to therapy
| Parameter (unit) | Result for therapy outcome:
|
P valuea | |
|---|---|---|---|
| SVR | Non-SVR | ||
| Total no. of patients | 13 | 52 | |
| Administration (n) | |||
| Group I | 6 | 26 | 1.000 |
| Group II | 7 | 26 | |
| Gender (n) | |||
| Male | 8 | 35 | 0.749 |
| Female | 5 | 17 | |
| Mean age (yr) | 57 | 59 | 0.483 |
| Histology | |||
| A factor | 0.605 | ||
| F factor | 0.033* | ||
| HCV RNA (kIU/ml) | 180 | 806 | 0.00027* |
| ALT (IU/liter) | 76 | 72 | 0.476 |
| AST (IU/liter) | 78 | 73 | 0.365 |
| Creatinine (mg/dl) | 0.7 | 0.7 | 1.000 |
| Cr clearance (ml/min) | 96.5 | 109.2 | 0.498 |
| CL/F (liters/h) | 11.9 | 12.6 | 0.702 |
| White blood cell count (/μl) | 4,900 | 5,000 | 0.577 |
| Hemoglobin (g/liter) | 139 | 146 | 0.131 |
| Platelet count (104/μl) | 15.0 | 14.8 | 0.151 |
| Hyaluronate | 64.5 | 69.0 | 0.715 |
| RBV concn (ng/ml) at wk: | |||
| 1 | 1,222 | 1,149 | 0.517 |
| 2 | 1,753 | 1,535 | 0.770 |
| 4 | 2,250 | 2,239 | 0.438 |
| 8 | 2,919 | 2,366 | 0.104 |
| IV collagen (ng/ml) | 130 | 150 | 0.444 |
| IV collagen 7S (ng/ml) | 4.6 | 5.2 | 0.127 |
| MMP-1 (ng/ml) | 7.2 | 7.0 | 0.974 |
| MMP-2 (ng/ml) | 757 | 925 | 0.035* |
| MMP-3 (ng/ml) | 37.6 | 37.4 | 0.664 |
| TIMP-1 (ng/ml) | 211 | 199 | 0.470 |
| TIMP-2 (ng/ml) | 48 | 54 | 0.571 |
*, statistically significant. A logistic regression analysis using the stepwise method showed that only HCV RNA level was a significant contributing factor for obtaining sustained viral response among the factors significant in the univariate analysis (odds ratio = 0.995; 95% CI = 0.992 to 0.998; P < 0.0014).
The SVR rates of patients who stopped taking RBV or who stopped both IFN and RBV were significantly reduced. There were no patients who stopped only IFN. SVR rates in the different groups of patients are summarized in Table 2. The SVR rate of patients who completed combination therapy was 47.5% (29/61), and the SVR rate of those who needed a dose reduction of RBV was 36.4% (8/22). No significant difference was found between these groups. The SVR rate of patients who stopped RBV treatment was 0% (0/14). SVR rates for patients with genotype 1b who completed combination therapy and who had a reduced dosage were 26.3% (10/38) and 18.8% (3/16), respectively. Those of non-1b patients were 82.6% (19/23) and 83.3% (5/6), respectively. SVR rates of genotype 1b and non-1b patients who stopped RBV were both 0% (0/11 and 0/3, respectively). Thus, SVR was not obtained in patients who discontinued RBV, but the SVR rate was not affected by reduction of dosage.
TABLE 2.
Sustained virological response rates according to adherence of ribavirina
| RBV dose modification | No. of patients with SVR/total no. of patients (% SVR rate) |
P value:
|
|
|---|---|---|---|
| Between groups | Overall | ||
| None | 29/61 (47.5) | 0.456 | |
| Dose reduction of RBV | 8/22 (36.4) | 0.00053* | |
| Discontinuation of RBV or both drugs | 0/14 (0.0) | 0.013* | |
Dose reduction of RBV during the combination therapy did not affect SVR rates. *, statistical significance.
Correlation between RBV concentration and hemoglobin concentration.
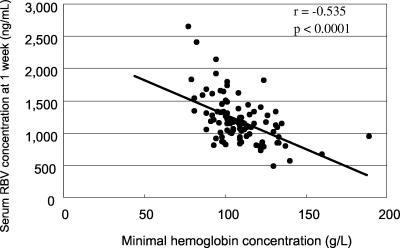
Hemoglobin levels gradually decreased after the start of therapy and reached a plateau at week 4. The correlation between RBV concentration and minimal hemoglobin level is shown in Table 3. The RBV concentrations on days 7, 14, and 28 were significantly correlated with the minimal level and maximal decrease of hemoglobin, but the RBV concentration on day 56 (week 8) was not correlated with those levels. The correlation coefficient was highest at 1 week and gradually decreased according to the time course from 1 week. The serum RBV concentration at 1 week, when hemoglobin levels did not reach minimal levels, was significantly correlated with the minimal hemoglobin concentration (Fig. 2).
TABLE 3.
Correlation between ribavirin concentration and minimal hemoglobin concentration
| Hemoglobin concn | Correlation vs:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hemoglobin concn at baseline
|
RBV concn
|
|||||||||
| Wk 1
|
Wk 2
|
Wk 4
|
Wk 8
|
|||||||
| r | P value | r | P value | r | P value | r | P value | r | P value | |
| Baseline | −0.392 | <0.0001 | −0.279 | 0.0094 | −0.142 | 0.217 | −0.119 | 0.334 | ||
| Maximal λ | −0.405 | <0.0001 | −0.230 | 0.027 | −0.239 | 0.027 | −0.217 | 0.058 | −0.100 | 0.416 |
| Minimal | 0.499 | <0.0001 | −0.535 | <0.0001 | −0.464 | <0.0001 | −0.325 | 0.0035 | −0.190 | 0.116 |
FIG. 2.
Correlation between serum ribavirin concentrations (ng/ml) and minimum hemoglobin concentration (g/liter). These two parameters were significantly correlated.
Analysis of factors affecting discontinuation of RBV.
We further investigated the factors responsible for discontinuation of RBV therapy because the suspension of RBV administration causes a significant reduction in SVR rates (Table 4). Positive factors contributing to RBV discontinuation were female gender, lower hemoglobin concentration, lower CrCl, lower CL/F, and higher serum RBV concentration at 1 week. Of the significant factors contributing to discontinuation of RBV identified in the univariate analysis (gender, hemoglobin, CrCl, CL/F, and RBV concentration at 1 week), none were significant according to multivariate analysis with logistic regression. If the factor CL/F was excluded from these factors (among gender, hemoglobin, CrCl, RBV concentration), hemoglobin concentration was identified as a significant factor (odds ratio = 0.514; 95% CI = 0.31 to 0.85; P = 0.0095).
TABLE 4.
Factors affecting discontinuation of ribavirin therapy
| Parameter | RBV therapy status
|
P valuea | |
|---|---|---|---|
| Continued | Discontinued | ||
| Treatment response (n) | |||
| SVR | 37 | 0 | 0.00075* |
| Non-SVR | 46 | 14 | |
| Gender (n) | |||
| Male | 55 | 5 | 0.039* |
| Female | 28 | 9 | |
| Age (yr) | 56 | 59 | 0.111 |
| Previous IFN therapy (n) | |||
| Naive | 51 | 11 | 0.452 |
| Relapser | 14 | 2 | |
| Null | 10 | 0 | |
| HCV genotype (n) | |||
| 1b | 54 | 11 | 0.376 |
| 2a, 2b | 29 | 3 | |
| HCV RNA (kIU/ml) | 555 | >850 | 0.060 |
| ALT (IU/liter) | 76 | 53 | 0.431 |
| AST (IU/liter) | 79 | 63 | 0.151 |
| Cr (mg/dl) | 0.7 | 0.7 | 0.528 |
| CrCl (ml/min) | 114 | 97 | 0.034* |
| CL/F (liters/h) | 13.6 | 11.0 | 0.012* |
| White blood cell count (/μl) | 4,900 | 4,600 | 0.111 |
| Hemoglobin (g/liter) | 144 | 134 | 0.018* |
| Platelet count (104/μl) | 14.8 | 16.6 | 0.611 |
| Type IV collagen (ng/ml) | 140 | 170 | 0.106 |
| Type IC collagen 7S (ng/ml) | 4.7 | 5.9 | 0.070 |
| MMP-1 (ng/ml) | 7.0 | 7.9 | 0.558 |
| MMP-2 (ng/ml) | 872 | 912 | 0.196 |
| MMP-3 (ng/ml) | 34.8 | 32.8 | 0.488 |
| TIMP-1 (ng/ml) | 203 | 206 | 0.589 |
| TIPM-2 (ng/ml) | 49 | 59 | 0.053 |
| RBV concn (ng/ml) at wk: | |||
| 1 | 1,149 | 1,315 | 0.021* |
| 2 | 1,558 | 1,599 | 0.280 |
| 4 | 2,170 | 2,170 | 0.968 |
| 8 | 2,453 | 2,131 | 0.075 |
*, statistically significant.
DISCUSSION
The combination therapy of IFN and RBV is associated with higher rates of SVR than IFN monotherapy (1, 3, 25, 32, 40). SVR rates, especially those for patients with HCV genotype 1b and high viral load (≥100 kIU/ml, difficult-to-treat patients), were very low with IFN monotherapy, and the SVR rate for difficult-to-treat patients on IFN monotherapy was below 5% in our series (36, 39). The combination of RBV and IFN increased this rate to 18.0% (11/61) in the present study. The SVR rate of genotype 1b patients with viral loads of 100 to 500 KIU/ml was 36.0%, and that of patients with a viral load of more than 500 KIU/ml was 5.6% in this study. Multivariate analysis showed that the significant contributing factors for achieving SVR were genotype and HCV RNA levels within genotype. These results are compatible with previous reports. PEG-IFN plus RBV combination therapy has overcome this therapeutic limitation caused by HCV RNA levels.
We measured markers of fibrosis in relation to therapeutic responses, and MMP-2 was identified as a significant factor correlating with SVR, although this was not significant in a multivariate analysis. The response was correlated with histological staging (F factor), suggesting a correlation between fibrosis markers and histological staging. Kasahara et al. (18) previously reported a relationship between MMP-2 and the response to interferon monotherapy, and Ninomiya et al. (27) suggested a role of MMPs in the IFN-induced inhibition of hepatic fibrosis. It is interesting that MMP-2 was the only marker that was identified as a significant factor; however, the relevance of this finding is unclear. Marinosci et al. (24) recently reported a role for MMP-9 in response to PEG-IFN and RBV treatment.
It has been reported that a higher serum RBV concentration was associated with a higher likelihood of SVR (46); the serum RBV concentration at week 4 and 8 seemed to be significantly associated with SVR (15), although one study showed that RBV concentrations in SVR patients at week 12 and 24 were significantly higher than those of nonresponders (19). However, in the present study, no significant correlation between serum RBV concentrations at weeks 1, 2, 4, and 8 and SVR rates were found. Tsubota et al. (47) described the pharmacokinetic profiles of single and multiple dosing regimens of RBV and noted that only patients with sustained serum concentrations of 3,000 ng/ml or more exhibited an SVR. Jen et al. (15) also found a relationship between serum RBV concentration and treatment outcome, but they found that RBV-induced HCV clearance was affected by body weight, gender, age, and serum Cr. The present study did not include obese patients, and there was no difference between SVR patients and non-SVR patients with respect to gender, age, serum Cr levels, and serum Cr clearance.
It is unclear why serum RBV level was not correlated with SVR in our study, but in addition to the antiviral and “error catastrophic” effects of RBV, it also has immunoregulatory activity; RBV induces the Th1 cytokine phenotype while suppressing the Th2 cytokine response. Immunologic shift to the Th1 response is thought to be important for viral clearance and recovery. The immunoregulatory effect of RBV has been demonstrated in several animal models and a clinical study. In the present study, serum RBV concentration did not correlate with viral clearance, and reduction of the total dose of RBV did not affect therapeutic outcome. If RBV exerts its therapeutic effect by stimulating the production of antiviral substances and the induction of error-prone replication of HCV, its effect would be expected to be dose dependent. However, we demonstrated that the effect was not dose dependent, since dose reduction did not alter RBV efficacy. Therefore, our results suggest that the immunological effect of RBV could play a significant role in the antiviral response to IFN plus RBV combination treatment.
It has been reported that hemolytic anemia induced by RBV depends primarily on the serum concentration of RBV but does not depend on the dose per kilogram of body weight (20), since RBV is mainly eliminated by the kidneys. Other important factors affecting serum RBV concentrations are absorption, uptake of RBV from the intestine, metabolism, and excretion. Following administration of a single capsule of RBV, the mean bioavailability of RBV is reported to be up to 40%, and saturable absorption of the drug has been suggested by the fact that the peak plasma concentration does not increase proportionally with dosage (6, 30). This saturable absorption of RBV has been explained by saturation of its transport by the N1 Na+ nucleoside transporter located in the brush border membrane in the human jejunum and intestine (30). Other nucleoside transporters have been implicated in RBV uptake by human erythrocytes (14). If nucleoside transporter saturation affects absorption of RBV, and if the total dose remains constant, the serum RBV concentration might be affected by each additional dose. Serum RBV levels, however, were not affected by whether RBV was taken twice or three times per day, suggesting that saturable absorption does not occur if the dose of RBV is between 600 and 800 mg/day, which is the standard dosage used in Japan. Further studies at higher RBV dosages are necessary to clarify this issue.
The SVR rate was not affected by the reduction of the RBV dosage, but RBV discontinuation had a considerable effect. This result is compatible with another report using PEG-IFN (7), although the 80% compliance with RBV therapy has also been reported to achieve a good outcome (26). Therefore, it is important to identify predictive factors responsible for the discontinuance of RBV administration. We compared various factors that contributed to discontinuance of RBV; these included gender, hemoglobin levels, CrCl, CL/F, and RBV concentration at 1 week. Hemoglobin concentration was the only significant factor for RBV discontinuation identified by logistic regression analysis with CL/F excluded from the factor analysis. These results suggest that both hemoglobin concentration and RBV clearance may contribute to RBV discontinuation, although hemoglobin levels appear to be more important than RBV clearance for determining RBV effects. Thus, erythrocytes seem to play an important role in modulating the effects of RBV.
RBV concentration after 2 weeks was not a significant factor for discontinuation of RBV administration. It has been suggested that the transmembrane transfer of RBV is much faster in erythrocytes than in other kinds of cells, so the early-phase serum RBV levels might be decreased mainly due to absorption by erythrocytes, while in subsequent phases, serum RBV levels might be affected by absorption by other organ cells (14). There was a negative correlation between pretreatment hemoglobin levels and serum RBV concentrations at 1 week. If excess RBV not absorbed by erythrocytes during the first week gradually distributes to other organs, several side effects might occur according to the RBV levels in cells other than erythrocytes. Homma et al. (11) clearly demonstrated that marked elevation of erythrocyte RBV, including its phosphorylated metabolites, was associated with hemoglobin reduction, although hemoglobin concentration during the first 7 days was not decreased. This result indicated that almost all absorbed RBV was stored in erythrocytes during the first week from the start of therapy. Therefore, erythrocytes may serve as a reservoir for RBV during the first week of treatment, and if the serum RBV remaining at 1 week is high, then the RBV distributes much more to other organs, and side effects might easily occur. This hypothesis suggests that patients with low pretreatment hemoglobin levels may be more susceptible to higher serum RBV levels and more-frequent side effects. This speculation is compatible with the result of a univariate analysis and a logistic regression analysis of factors affecting discontinuation of RBV administration, in which gender, hemoglobin levels, RBV levels, CrCl, and CL/F were identified in the univariate analysis but only hemoglobin level was significant when CL/F was excluded. It has been recently reported that one gram or more of RBV per day increases the risk of developing anemia (43), and therefore, early measurement of RBV concentration might be more beneficial for patients receiving higher doses of RBV than those used in our study.
Acknowledgments
We thank Kanji Wakabayashi (TEPCO Hospital), Hideo Yoshida (Eiju General Hospital), Nobuyuki Ohkubo, Takeshi Yoshida (Saitama Social Insurance Hospital), Shunsuke Kohmoto (Tokyo Metropolitan Ohtsuka Hospital), Toshiyuki Tahara (Tochigi Saisei-kai Utsunomiya Hospital), Masaharu Miyazawa (Tchikawa Hospital), Yoshikazu Yonei, Yasutaka Inagaki (Nihon Kohkan Hospital), Naoki Kumagai (Kitasato Institute Hospital), Kotaro Kaneko (Kasumigaura Hospital), Hidehiko Matsukawa (Tachikawa Houspital), Kazuhiro Atsukawa, Masao Arai (Hiratsuka Civil Hospital), and Nobuhiro Nakamoto (Keio University Hospital), who also participated in this study.
No financial disclosure or other relationship was noted by us or by those included above.
REFERENCES
- 1.Ahmed, A., and E. B. Keeffe. 1999. Treatment strategies for chronic hepatitis C: update since the 1997 National Institutes of Health Consensus Development Conference. J. Gastroenterol. Hepatol. 14(Suppl.):S12-S18. [DOI] [PubMed] [Google Scholar]
- 2.Alberti, A., and L. Benvegnu. 2003. Management of hepatitis C. J. Hepatol. 38(Suppl. 1):S104-S118. [DOI] [PubMed] [Google Scholar]
- 3.Davis, G. L., R. Esteban-Mur, V. Rustgi, J. Hoefs, S. C. Gordon, C. Trepo, M. L. Shiffman, S. Zeuzem, A. Craxi, M. H. Ling, J. Albrecht, et al. 1998. Interferon alfa-2b alone or in combination with ribavirin for the treatment of relapse of chronic hepatitis C. N. Engl. J. Med. 339:1493-1499. [DOI] [PubMed] [Google Scholar]
- 4.De Franceschi, L., G. Fattovich, F. Turrini, K. Ayi, C. Brugnara, F. Manzato, F. Noventa, A. M. Stanzial, P. Solero, and R. Corrocher. 2000. Hemolytic anemia induced by ribavirin therapy in patients with chronic hepatitis C virus infection: role of membrane oxidative damage. Hepatology 31:997-1004. [DOI] [PubMed] [Google Scholar]
- 5.Ebinuma, H., H. Saito, S. Tada, T. Masuda, T. Kamiya, J. Nishida, M. Yoshioka, and H. Ishii. 2004. Additive therapeutic effects of the liver extract preparation mixture adelavin-9 on interferon-beta treatment for chronic hepatitis C. Hepatogastroenterology 51:1109-1114. [PubMed] [Google Scholar]
- 6.Fernandez, H., G. Banks, and R. Smith. 1986. Ribavirin: a clinical overview. Eur. J. Epidemiol. 2:1-14. [DOI] [PubMed] [Google Scholar]
- 7.Fried, M. W., M. L. Shiffman, K. R. Reddy, C. Smith, G. Marinos, F. L. Goncales, Jr., D. Haussinger, M. Diago, G. Carosi, D. Dhumeaux, A. Craxi, A. Lin, J. Hoffman, and J. Yu. 2002. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 347:975-982. [DOI] [PubMed] [Google Scholar]
- 8.Goudou, K., S. Enoki, A. Tsubota, and H. Kumada. 2002. Establishment of the assay for ribavirin in human serum by high-performance liquid chromatography with column-switching. Igaku Yakugaku 47:317-320. [Google Scholar]
- 9.Helbling, B., I. Stamenic, F. Viani, J. J. Gonvers, J. F. Dufour, J. Reichen, G. Cathomas, M. Steuerwald, J. Borovicka, M. Sagmeister, and E. L. Renner. 2002. Interferon and amantadine in naive chronic hepatitis C: a double-blind, randomized, placebo-controlled trial. Hepatology 35:447-454. [DOI] [PubMed] [Google Scholar]
- 10.Hepatology. 1997. National Institutes of Health Consensus Development Conference Panel statement: management of hepatitis C. Hepatology 26(3 Suppl. 1):2S-10S. [DOI] [PubMed] [Google Scholar]
- 11.Homma, M., Y. Matsuzaki, Y. Inoue, M. Shibata, K. Mitamura, N. Tanaka, and Y. Kohda. 2004. Marked elevation of erythrocyte ribavirin levels in interferon and ribavirin-induced anemia. Clin. Gastroenterol. Hepatol. 2:337-339. [DOI] [PubMed] [Google Scholar]
- 12.Hoofnagle, J. H. 1997. Hepatitis C: the clinical spectrum of disease. Hepatology 26:15S-20S. [DOI] [PubMed] [Google Scholar]
- 13.Ichida, F., T. Tsuji, M. Omata, T. Ichida, K. Inoue, T. Kamimura, G. Yamada, K. Hino, O. Yokosuka, and H. Suzuki. 1996. New Inuyama classification new criteria for histological assessment of chronic hepatitis. Int. Hepatol. Commun. 6:112-119. [Google Scholar]
- 14.Jarvis, S. M., J. A. Thorn, and P. Glue. 1998. Ribavirin uptake by human erythrocytes and the involvement of nitrobenzylthioinosine-sensitive (es)-nucleoside transporters. Br J. Pharmacol. 123:1587-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jen, J. F., P. Glue, S. Gupta, D. Zambas, and G. Hajian. 2000. Population pharmacokinetic and pharmacodynamic analysis of ribavirin in patients with chronic hepatitis C. Ther. Drug Monit. 22:555-565. [DOI] [PubMed] [Google Scholar]
- 16.Kamar, N., E. Chatelut, E. Manolis, T. Lafont, J. Izopet, and L. Rostaing. 2004. Ribavirin pharmacokinetics in renal and liver transplant patients: evidence that it depends on renal function. Am. J. Kidney Dis. 43:140-146. [DOI] [PubMed] [Google Scholar]
- 17.Kasahara, A. 2000. Treatment strategies for chronic hepatitis C virus infection. J. Gastroenterol. 35:411-423. [DOI] [PubMed] [Google Scholar]
- 18.Kasahara, A., N. Hayashi, K. Mochizuki, M. Oshita, K. Katayama, M. Kato, M. Masuzawa, H. Yoshihara, M. Naito, T. Miyamoto, A. Inoue, A. Asai, T. Hijioka, H. Fusamoto, and T. Kamada. 1997. Circulating matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-1 as serum markers of fibrosis in patients with chronic hepatitis C. Relationship to interferon response. J. Hepatol. 26:574-583. [DOI] [PubMed] [Google Scholar]
- 19.Larrat, S., F. Stanke-Labesque, A. Plages, J. P. Zarski, G. Bessard, and C. Souvignet. 2003. Ribavirin quantification in combination treatment of chronic hepatitis C. Antimicrob. Agents Chemother. 47:124-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindahl, K., R. Schvarcz, A. Bruchfeld, and L. Stahle. 2004. Evidence that plasma concentration rather than dose per kilogram body weight predicts ribavirin-induced anaemia. J. Viral Hepat. 11:84-87. [DOI] [PubMed] [Google Scholar]
- 21.Maddrey, W. C. 1999. Safety of combination interferon alfa-2b/ribavirin therapy in chronic hepatitis C-relapsed and treatment-naive patients. Semin. Liver Dis. 19(Suppl. 1):67-75. [PubMed] [Google Scholar]
- 22.Mangia, A., R. Santoro, M. Piattelli, G. Leandro, N. Minerva, M. Annese, D. Bacca, F. Spirito, V. Carretta, F. Ventrella, M. Cela, and A. Andriulli. 2002. High doses of interferon in combination with ribavirin are more effective than the standard regimen in patients with HCV genotype 1 chronic hepatitis. J. Hepatol. 37:109-116. [DOI] [PubMed] [Google Scholar]
- 23.Manns, M. P., J. G. McHutchison, S. C. Gordon, V. K. Rustgi, M. Shiffman, R. Reindollar, Z. D. Goodman, K. Koury, M. Ling, and J. K. Albrecht. 2001. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 358:958-965. [DOI] [PubMed] [Google Scholar]
- 24.Marinosci, F., C. Bergamini, E. Fransvea, N. Napoli, P. Maurel, P. Dentico, S. Antonaci, and G. Giannelli. 2005. Clinical role of serum and tissue matrix metalloprotease-9 expression in chronic HCV patients treated with pegylated IFN-alpha2b and ribavirin. J. Interferon Cytokine Res. 25:453-458. [DOI] [PubMed] [Google Scholar]
- 25.McHutchison, J. G., S. C. Gordon, E. R. Schiff, M. L. Shiffman, W. M. Lee, V. K. Rustgi, Z. D. Goodman, M. H. Ling, S. Cort, J. K. Albrecht, et al. 1998. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N. Engl. J. Med. 339:1485-1492. [DOI] [PubMed] [Google Scholar]
- 26.McHutchison, J. G., M. Manns, K. Patel, T. Poynard, K. L. Lindsay, C. Trepo, J. Dienstag, W. M. Lee, C. Mak, J.-J. Garaud, J. K. Albrecht, and the International Hepatitis Interventional Therapy Group. 2002. Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology 123:1061-1069. [DOI] [PubMed] [Google Scholar]
- 27.Ninomiya, T., S. Yoon, H. Nagano, Y. Kumon, Y. Seo, M. Kasuga, Y. Yano, M. Nakaji, and Y. Hayashi. 2001. Significance of serum matrix metalloproteinases and their inhibitors on the antifibrogenetic effect of interferon-alfa in chronic hepatitis C patients. Intervirology 44:227-231. [DOI] [PubMed] [Google Scholar]
- 28.Okamoto, H., K. Kurai, S. Okada, K. Yamamoto, H. Lizuka, T. Tanaka, S. Fukuda, F. Tsuda, and S. Mishiro. 1992. Full-length sequence of a hepatitis C virus genome having poor homology to reported isolates: comparative study of four distinct genotypes. Virology 188:331-341. [DOI] [PubMed] [Google Scholar]
- 29.Page, T., and J. D. Connor. 1990. The metabolism of ribavirin in erythrocytes and nucleated cells. Int. J. Biochem. 22:379-383. [DOI] [PubMed] [Google Scholar]
- 30.Patil, S. D., L. Y. Ngo, P. Glue, and J. D. Unadkat. 1998. Intestinal absorption of ribavirin is preferentially mediated by the Na+-nucleoside purine (N1) transporter. Pharm. Res. 15:950-952. [DOI] [PubMed] [Google Scholar]
- 31.Pol, S., P. Couzigou, M. Bourliere, A. Abergel, J. M. Combis, D. Larrey, A. Tran, J. Moussalli, R. Poupon, P. Berthelot, and C. Brechot. 1999. A randomized trial of ribavirin and interferon-alpha vs. interferon-alpha alone in patients with chronic hepatitis C who were non-responders to a previous treatment. Multicenter Study Group under the coordination of the Necker Hospital, Paris, France. J. Hepatol. 31:1-7. [DOI] [PubMed] [Google Scholar]
- 32.Poynard, T., P. Marcellin, S. S. Lee, C. Niederau, G. S. Minuk, G. Ideo, V. Bain, J. Heathcote, S. Zeuzem, C. Trepo, J. Albrecht, et al. 1998. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. Lancet 352:1426-1432. [DOI] [PubMed] [Google Scholar]
- 33.Poynard, T., J. McHutchison, G. L. Davis, R. Esteban-Mur, Z. Goodman, P. Bedossa, and J. Albrecht. 2000. Impact of interferon alfa-2b and ribavirin on progression of liver fibrosis in patients with chronic hepatitis C. Hepatology 32:1131-1137. [DOI] [PubMed] [Google Scholar]
- 34.Poynard, T., J. McHutchison, M. Manns, C. Trepo, K. Lindsay, Z. Goodman, M. H. Ling, and J. Albrecht. 2002. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology 122:1303-1313. [DOI] [PubMed] [Google Scholar]
- 35.Saito, H., H. Ebinuma, H. Nagata, Y. Inagaki, Y. Saito, K. Wakabayashi, T. Takagi, M. Nakamura, H. Katsura, Y. Oguchi, and H. Ishii. 2001. Interferon-associated retinopathy in a uniform regimen of natural interferon-alpha therapy for chronic hepatitis C. Liver 21:192-197. [DOI] [PubMed] [Google Scholar]
- 36.Saito, H., H. Ebinuma, M. Oda, H. Ishii, et al. 1997. Results of the multicenter natural interferon-alpha treatment for chronic hepatitis C: correlation between total dose, administration periods and efficacy of therapy. Keio J. Med. 46:177-183. [DOI] [PubMed] [Google Scholar]
- 37.Saito, H., H. Ebinuma, I. Satoh, S. Miyaguchi, S. Tada, N. Iwabuchi, N. Kumagai, K. Tsuchimoto, T. Morizane, and H. Ishii. 2000. Immunological and virological predictors of outcome during interferon-alpha therapy of chronic hepatitis C. J. Viral Hepat. 7:64-74. [DOI] [PubMed] [Google Scholar]
- 38.Saito, H., S. Tada, N. Nakamoto, K. Kitamura, H. Horikawa, S. Kurita, Y. Saito, H. Iwai, and H. Ishii. 2004. Efficacy of non-invasive elastometry on staging of hepatic fibrosis. Hepatol. Res. 29:97-103. [DOI] [PubMed] [Google Scholar]
- 39.Saito, H., K. Tsuchimoto, S. Miyaguchi, S. Tada, K. Sawaguchi, K. Komatsu, K. Kaneko, T. Watanabe, T. Morizane, H. Ishii, et al. 1996. Keio multicenter trial in high-dose interferon-alpha 2b treatment for chronic hepatitis C. Keio J. Med. 45:161-167. [DOI] [PubMed] [Google Scholar]
- 40.Schalm, S. W., O. Weiland, B. E. Hansen, M. Milella, M. Y. Lai, A. Hollander, P. P. Michielsen, A. Bellobuono, L. Chemello, G. Pastore, D. S. Chen, J. T. Brouwer, et al. 1999. Interferon-ribavirin for chronic hepatitis C with and without cirrhosis: analysis of individual patient data of six controlled trials. Gastroenterology 117:408-413. [DOI] [PubMed] [Google Scholar]
- 41.Scott, L. J., and C. M. Perry. 2002. Interferon-alpha-2b plus ribavirin: a review of its use in the management of chronic hepatitis C. Drugs 62:507-556. [DOI] [PubMed] [Google Scholar]
- 42.Strader, D. B., T. Wright, D. L. Thomas, and L. B. Seeff. 2004. Diagnosis, management, and treatment of hepatitis C. Hepatology 39:1147-1171. [DOI] [PubMed] [Google Scholar]
- 43.Sulkowski, M. S., R. Wasserman, L. Brooks, L. Ball, and R. Gish. 2004. Changes in haemoglobin during interferon alpha-2b plus ribavirin combination therapy for chronic hepatitis C virus infection. J. Viral Hepat. 11:243-250. [DOI] [PubMed] [Google Scholar]
- 44.Tong, M. J., N. S. el-Farra, A. R. Reikes, and R. L. Co. 1995. Clinical outcomes after transfusion-associated hepatitis C. N. Engl. J. Med. 332:1463-1466. [DOI] [PubMed] [Google Scholar]
- 45.Toyota, J., Y. Karino, J. Akaike, T. Ohmura, T. Sato, K. Yamazaki, Y. Kuwata, and S. Iino. 2005. Total clearance (CL/F) of ribavirin is the factor most influencing incidence of hemolytic anemia in interferon a plus ribavirin combination therapy. Kanzo 46:107-118. (In Japanese.) [Google Scholar]
- 46.Tsubota, A., N. Akuta, F. Suzuki, Y. Suzuki, T. Someya, M. Kobayashi, Y. Arase, S. Saitoh, K. Ikeda, and H. Kumada. 2002. Viral dynamics and pharmacokinetics in combined interferon alfa-2b and ribavirin therapy for patients infected with hepatitis C virus of genotype 1b and high pretreatment viral load. Intervirology 45:33-42. [DOI] [PubMed] [Google Scholar]
- 47.Tsubota, A., Y. Hirose, N. Izumi, and H. Kumada. 2003. Pharmacokinetics of ribavirin in combined interferon-alpha 2b and ribavirin therapy for chronic hepatitis C virus infection. Br. J. Clin. Pharmacol. 55:360-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsubota, A., F. Suzuki, K. Arase, M. Matsuda, M. Kobayashi, J. Sato, K. Takagi, S. Saito, Y. Suzuki, T. Someya, N. Kaida, M. Kobayashi, K. Ikeda, and M. Kumada. 2002. Efficacy of a newly developed measuring system for serum ribavirin concentration (HPLC assay). Kanzo 43:212-213. (In Japanese.) [Google Scholar]