Abstract
We assessed the performance of the Genotype MTBDR line probe assay that offers the simultaneous identification of Mycobacterium tuberculosis and its resistance to rifampin (RIF) and isoniazid (INH) by detecting the most commonly found mutations in the rpoB and katG genes. One hundred thirteen M. tuberculosis isolates were tested. The nucleotide sequences of the katG and inhA genes and the mabA-inhA promoter region were also determined. The MTBDR assay detected 100% and 67% (n = 64) of the strains resistant to RIF and INH, respectively. Among the latter, 62 strains carried a Ser315Thr mutation in katG, 59 of them displaying a high level of resistance to INH. Two strains with a low level of INH resistance had a Ser315Asn mutation. No mutation was found by the MTBDR assay for 31 INH-resistant strains (33%), of which 24 showed a low level of resistance. By DNA sequencing, we found among them various mutations in the KatG protein for 7 strains, a C→T mutation in position −15 of the mabA-inhA promoter in 17 strains, and a Ser94Ala mutation in InhA for 7 strains. In conclusion, the MTBDR assay, which fits easily in the workflow of a routine laboratory, enabled the detection of 100% of the RIF-resistant strains and 89% of the INH-resistant strains with a high level of resistance but only 17% of the strains characterized by a low level of INH resistance, indicating that the test can be used as a rapid method to detect in the same experiment the rifampin-resistant and the high-level isoniazid-resistant strains of M. tuberculosis.
The rapid identification of antibiotic resistance in Mycobacterium tuberculosis is an important challenge to ensure a rapid and adequate therapy of tuberculosis and to limit the dissemination of multiresistant strains. Rifampin (RIF) and isoniazid (INH) are two of the four first-line drugs used for the treatment of tuberculosis. RIF inhibits the RNA polymerase at the level of the beta subunit encoded by the rpoB gene. Resistance to RIF in mycobacteria results from point mutations predominantly located in the 511 to 533 region of the RpoB polypeptide (4, 5, 17). The most common mutations found in RIF-resistant M. tuberculosis isolates are Ser531Leu, His526Asp or Tyr, and Asp516Val (24). INH inhibits InhA, the enoyl-ACP-reductase, which is a key enzyme for the biosynthesis of mycolic acids found in the cell wall (2). As INH is a prodrug, this antibiotic needs to be converted to an active form by the catalase-peroxidase KatG encoded by the katG gene (8). Resistance to INH can therefore arise from a wide variety of mutations affecting either the binding of INH to the target InhA (such as Ile21Thr or Val, Ser94Ala, and Ile194Thr), the activation of INH by KatG (the most frequent mutation being Ser315Thr), or finally, the level of expression of the target InhA (by the C-to-T nucleotide substitution at −15 affecting the promoter region of the mabA-inhA operon) (7, 10, 12, 15, 17). Resistance to INH is predominantly associated with the amino acid substitution Ser315Thr in KatG (in roughly 70% of INH-resistant strains) and the −15 C-to-T mutation in the inhA promoter (in 15 to 35% of INH-resistant strains) (7, 10, 17, 18). Other mutations in various genes, including kasA, furA, iniA, iniB, iniC, and the oxyR-ahpC intergenic region, were also reported from INH-resistant clinical isolates, but these mutations are found with much lower frequencies and their contribution to resistance to INH remains to be elucidated (19, 26).
In the last few years, several PCR-based molecular methods have been developed to detect resistance to RIF and INH (1, 4, 5, 7, 20, 21, 23). Among the recent molecular diagnostic methods, two commercial DNA strip assays have been developed, the INNO-LIPA Rif TB (Innogenetics, Ghent, Belgium) and the Genotype MTBDR (Hain Life-Science, Nehren, Germany). Both tests are based on reverse hybridization of amplicons (rpoB in the INNO-LIPA Rif TB and rpoB plus katG in the Genotype MTBDR) to immobilized membrane-bound probes, allowing the detection of mutations at the level of the most frequently mutated codons. The presence of a mutation is revealed by the absence of hybridization at the level of the wild-type probes (rpoB WT1 to WT5 and katG WT), with a possible positive hybridization signal at the level of the mutant probes (rpoB MUT D516V, MUT H526Y, MUT H526D, MUT S531L, and katG MUT S315T). These two DNA line probe assays have been recently evaluated in a comparative study for their abilities to detect RIF and INH resistance in M. tuberculosis isolates (9, 13). For RIF resistance, both assays showed very good performances, with 98% of the test results concordant with the drug susceptibility testing results. For INH resistance, the sensitivity of the MTBDR assay, compared to conventional drug susceptibility testing, was found to be 90% (9, 13). In these previous studies, the isoniazid resistance molecular mechanism was established by partial sequencing of the key regions involved in the development of resistance, and the levels of resistance to INH were not taken into account in the determination of the performances of the strip assays.
The aim of the present study was to assess the performance of the Genotype MTBDR assay for the detection of INH-resistant clinical isolates of M. tuberculosis and compare the results (i) to those obtained by conventional sequencing of the entire rpoB, katG, and inhA genes and the mabA-inhA regulatory region and (ii) to the phenotypic drug susceptibility testing results analyzed according to the INH resistance levels.
MATERIALS AND METHODS
Strains.
The Pitié-Salpêtrière Laboratory of Bacteriology serves as the National Reference Center for Mycobacteria in France. A total of 113 Mycobacterium tuberculosis complex isolates were included in the study. They corresponded to 94 clinical isolates collected by the National Reference Center for Mycobacteria during the study period (2003 to 2004) and 19 strains sent by the WHO for evaluation purposes. The latter were included in the study because they were suspected to carry rarely encountered mutations in rpoB. Drug susceptibility testing for RIF and INH was performed on Lowenstein-Jensen medium with the standard proportions method, as previously described (3), using concentrations of 40 mg/liter for RIF and 0.1, 0.2, 1, and 10 mg/liter for INH. A low level of resistance to INH was defined by resistance to 0.1 to 0.2 mg/liter of INH, while a high level of resistance was defined by resistance to INH concentrations of ≥1 mg/liter. Ninety-five strains were INH-resistant isolates, of which 72 were RIF resistant and 23 were RIF susceptible. Among these 95 strains, 66 isolates displayed a high level of resistance to INH, while 29 isolates were resistant to INH at a low level. Among the INH-susceptible strains, 4 were resistant to RIF, while 14 were fully susceptible to both RIF and INH.
DNA sequencing of genes associated with drug resistance.
Genomic DNA was isolated from bacteria grown on Lowenstein-Jensen medium. A loop of culture was suspended in water (500 μl) and killed at 95°C over the course of 15 min. The DNA used for amplification by PCR was obtained by heat shock extraction (1 min at 95°C, 1 min in ice, repeated 5 times). Five microliters was submitted to PCR amplification using the oligonucleotide primers described in Table 1. For RIF resistance, a 69-bp region of rpoB was amplified and sequenced using primers rifip 1 and rifip 2. The amplification protocol consisted of an initial denaturation step of 5 min at 95°C, followed by 35 cycles of 1 min at 95°C, 1 min at 63°C, and 1 min at 72°C, ending with a final extension step of 7 min at 72°C. For INH resistance, katG was amplified and sequenced using 3 pairs of primers (katA and katCas, kat2 and kat4as, and katC and katEas). The inhA promoter region and the inhA gene were amplified using primers Pro1 and Pro2, FabG1 and FabG2, INH1 and INH2, and INH3 and INHD (Table 1). After amplification, unincorporated nucleotides and primers were removed by filtration with Microcon 100 microconcentrators (Amicon Inc., Beverly, Mass.), and the gene targets were sequenced using the Big Dye Terminator cycle sequencing ready reaction kit (Applied Biosystems Inc., Foster City, Calif.) in an ABI Prism 310 DNA sequencer (Applied Biosystems). The sequences obtained were compared with the available sequences for the rpoB and katG genes (GenBank accession numbers U12205 and X68081, respectively) and the mabA-inhA operon (GenBank accession numbers U66801 and U02492).
TABLE 1.
Primer sequences
| Primer target(s) | Primer designation | Primer sequence (5′-3′) | PCR product size (bp) | Hybridization temp (°C) |
|---|---|---|---|---|
| rpoB | rifip1 | GGT CGG CAT GTC GCG GAT GG | 257 | 63 |
| rifip2 | GCA CGT CGC GGA CCT CCA GC | |||
| katG | katA | CCC GAT AAC ACC TCC TG | 817 | 59 |
| katCas | GTT TCG ACG TCG TTC ATG GC | |||
| kat2 | CTC GGC GAT GAG CGT TAC AG | 1,318 | 59 | |
| kat4as | CCA GCG GTA AGC GCT TGT AG | |||
| katC | CCG AGT ACA TGC TGC TCG AC | 609 | 59 | |
| katEas | GGT GAT CGC ACA TCC AGC AC | |||
| Operon inhA-mabA and | Pro1 | TCA ATA CAG CCG CAG CCA | 493 | 53 |
| its promoter | Pro2 | GTC ATC CGC ATG AGG AAT | ||
| FabG1 | TCA CGG CGG TAG AAG AGC | 553 | 53 | |
| FabG2 | CAT GTG CGT CCT TGT GTT | |||
| INHA1 | AGG ACG CAC ATG ACA AGC | 412 | 53 | |
| INHA2 | TCA TGA TCG GCA GCA GCG | |||
| INHA3 | CCA CAT CTC GGC GTA TTC | 601 | 53 | |
| INHAD | CGA AAT GCA GGT AGT GCT C |
Genotype MTBDR assay.
The Genotype MTBDR assay (Hain Lifescience, Nehren, Germany) was performed as recommended by the manufacturer. The amplification mixture contained 35 μl of primer-nucleotide mix provided in the kit, 5 μl of 10× Taq polymerase incubation buffer containing 2 mM of MgCl2, 1 to 2 unit(s) of thermostable Taq DNA polymerase, and 5 μl of extracted chromosomal DNA solution in a final volume of 50 μl. The following amplification parameters were used: 5 min of denaturation at 95°C, followed by 10 cycles of 30 s at 95°C and 2 min at 58°C, followed by 20 additional cycles of 25 s at 95°C, 40 s at 53°C, and 40 s at 70°C, ending with a final extension step of 8 min at 70°C. Hybridization and detection were performed with a TwinCubator semiautomated washing and shaking device according to the manufacturer's instructions and using the reagents provided with the kit. Briefly, 20 μl of denaturation solution was mixed to 20 μl of amplified sample and incubated at room temperature for 5 min. One milliliter of prewarmed hybridization buffer was added before the membrane strips were placed and shaken in the hybridization solution for 30 min at 45°C. After two washing steps, a colorimetric detection of the hybridized amplicons was obtained by the addition of the streptavidin alkaline phosphatase conjugate.
RESULTS AND DISCUSSION
Among the 113 M. tuberculosis clinical isolates included in this study, 76 were resistant to RIF. Comparison of the results obtained by the MTBDR DNA strip assay and by automated DNA sequencing showed that the sensitivity and specificity of the Genotype MTBDR test in detecting RIF resistance was 100%. Indeed, no mutation was found by both methods in the 37 RIF-susceptible clinical isolates, while the 76 RIF-resistant isolates were all identified by DNA sequencing of the amplified rpoB region and by the Genotype MTBDR assay. Fifty-four of the 76 RIF-resistant strains (71%) gave positive hybridization results with the mutation-specific probes MUT:D516V, MUT:H526Y, MUT:H526D, and MUT:S531L, which were the mutations the most frequently found in this study (in 5, 8, 10, and 47% of the RIF-resistant clinical isolates, respectively) (Table 2). These data are in accordance with the mutation frequencies previously reported in other studies (24). The amino acid modifications detected in the other RIF-resistant strains (n = 22 or 29%) corresponded to mutations that were not targeted by an oligonucleotide probe on the strip. They were therefore detected by a negative hybridization result with one of the five wild-type-specific probes WT1 to WT5 (Table 2). Overall, they affected codon 526 in 7 strains (9%), codons 531 and 533 in a total of 6 strains (8%), codon 516 in 5 strains (6.5%), and codon 513 and 522 in a total of 4 strains (5%) (Table 2). It must be pointed out here that 3 RIF-resistant isolates were found to carry multiple substitutions in the rpoB hot spot region by sequencing analysis. These strains were control strains sent by the WHO for evaluation purposes. One strain gave negative hybridization results with the WT1 and WT5 wild-type probes because of the presence of 3 mutations (F505L and L511P in the WT1 region and S531C in the WT5 region), and the two other strains displayed two mutations (M515I and D516Y), both located in the WT2 region. These results indicate that the MTBDR DNA strip assay is also capable of revealing the presence of rarely found mutations that can be encountered with a higher prevalence in certain countries (24).
TABLE 2.
Comparison of MTBDR assay and sequencing results for the detection of mutations conferring resistance to rifampin for 76 RIF-resistant M. tuberculosis clinical isolates
| rpoB region | MTBDR result with:
|
Sequencing data (amino acid modification[s] in RpoB) | No. (%) of strains | |
|---|---|---|---|---|
| MUT WTa | MUTb | |||
| 509-514 | MUT WT1 | Q513K | 1 | |
| MUT WT1 | Q513P | 1 | ||
| 515-520 | MUT WT2 | MUT:D516V | D516V | 4 (5) |
| MUT WT2 | D516Y | 3 | ||
| MUT WT2 | M515I + D516Y | 2 | ||
| 521-525 | MUT WT3 | S522L | 2 | |
| 525-529 | MUT WT4 | MUT:H526D | H526D | 8 (10) |
| MUT WT4 | MUT:H526Y | H526Y | 6 (8) | |
| MUT WT4 | H526A | 1 | ||
| MUT WT4 | H526L | 3 | ||
| MUT WT4 | H526R | 3 | ||
| 530-534 | MUT WT5 | MUT:S531L | S531L | 36 (47) |
| MUT WT5 | S531W | 2 | ||
| MUT WT5 | S531T | 1 | ||
| MUT WT5 | L533P | 2 | ||
| MUT WT5+WT1 | S531C + L511P + F505L | 1 | ||
Absence of hybridization signal with the wild-type probes (WT1 to WT5).
Positive hybridization result with the specified mutant probe (MUT D516V, MUT H526D, MUT H526Y, or MUT S531L).
In this study, 95 INH-resistant and 18 INH-susceptible M. tuberculosis strains were assessed by the MTBDR DNA strip assay. We first sequenced the genes katG and inhA, including the inhA promoter region, in the 18 INH-susceptible control strains. No mutation was found in 11 strains, while one mutation, R463L, was detected in katG from 6 susceptible strains, indicating that R463L is not related to resistance to INH (Table 3). Accordingly, several reports have previously shown that this mutation can be found in INH-susceptible isolates (22, 24). Finally, one mutation, G279A, was found in the remaining INH-susceptible strain. This mutation has never been described before and likely represents a phenotypically silent mutation.
TABLE 3.
Results of the MTBDR assay, sequencing data, and drug resistance phenotypes for 18 susceptible and 95 isoniazid-resistant M. tuberculosis isolates
| Type of mutation | MTBDR result for katG-315 | Sequencing data (amino acid substitution[s] for InhA and KatG)
|
No. of strains exhibiting INH resistance phenotype:
|
||||
|---|---|---|---|---|---|---|---|
| InhA | InhA promoter region | KatG | Susceptible | Resistant
|
|||
| Low | High | ||||||
| No mutation | wt | wt | wt | wt | 11 | ||
| wt | wt | wt | R463L | 6 | |||
| wt | wt | wt | G279A | 1 | |||
| KatG-315 | S315T1 | NDb | ND | S315T | 3 | 59 | |
| MUTa | wt | wt | S315N + R463L | 2 | |||
| InhA promoter region | wt | wt | −15C→T | wt | 3 | ||
| wt | wt | −15C→T | R463L | 2 | 1 | ||
| wt | wt | −15C→T | A110V | 6 | 1 | ||
| InhA | wt | S94A | −15C→T | wt | 4 | ||
| wt | S94A | wt | R463L | 2 | |||
| wt | S94A | wt | D419H | 1 | |||
| Other mutations in KatG | wt | wt | wt | W341G + R463L | 1 | ||
| wt | wt | wt | G494D + R463L | 2 | |||
| wt | wt | wt | R595→STOP | 1 | |||
| wt | wt | wt | L141F + R463L | 1 | |||
| wt | wt | wt | E553K | 1 | |||
| wt | wt | wt | F658V | 1 | |||
| Other | wt | wt | −102G→A | R463L | 1 | ||
| wt | wt | wt | R463L | 2 | |||
| wt | wt | wt | wt | 1 | |||
MUT, absence of hybridization signal with the wild-type 315 probe.
ND, not determined.
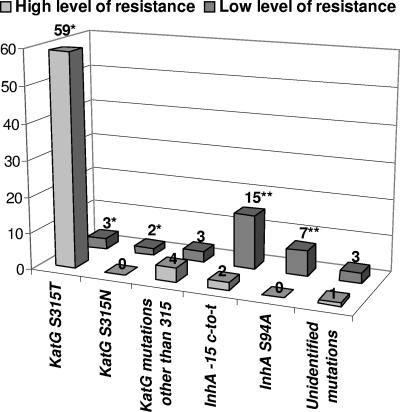
Sixty-two of the 95 INH-resistant strains (65%) were found to have an S315T substitution by DNA sequencing. They were all identified by the MTBDR assay (Table 3). It must be pointed out here that this amino acid modification can result from two different nucleotide substitutions in the wild-type codon 315 (AGC). In our study, all of the S315T amino acid substitutions found were due to the so-called S315T1 mutation (or katG MUT1) corresponding to the AGC→ACC modification at codon 315. In contrast, we found no strain with the S315T2 (or katG MUT2) mutation corresponding to AGC→ACA, which is also tested on the MTDBR strip. Interestingly, we observed that 59 of the 62 strains with an S315T mutation were characterized by a high level of resistance to INH, with the 3 remaining showing a low level of INH resistance (Fig. 1). These results confirm previous reports indicating that the replacement of Ser315 by Thr is the most frequent substitution encountered in INH-resistant strains of M. tuberculosis (1, 6, 10, 19, 24, 26).
FIG. 1.
Distribution of the mutations found in katG, inhA, and the inhA promoter region according to the level of resistance to INH (high level in light gray; low level in dark gray). The y axis indicates the number of strains. *, mutations detected by the MTBDR assay (at position 315); **, 4 of the low-level INH-resistant strains with a mutation affecting inhA and/or its promoter cumulated a −15 C-to-T substitution plus a S94A mutation.
Among the INH-resistant strains characterized by a mutation at codon 315, 2 had KatG substitutions that were different from S315T, which were revealed by a negative hybridization signal at the level of the corresponding wild-type-specific probe. The mutations, identified by DNA sequencing, corresponded to an S315N mutation found in association with the R463L mutation identified in several susceptible control strains (Table 3). These two isolates with S315N displayed a low level of resistance to INH. Accordingly, the mutation was previously shown to result in significantly reduced KatG activities (25).
Thirty-one INH-resistant strains (33%) were found to have no mutation at codon 315 in katG and were therefore undetectable by the MTBDR DNA strip. In addition to being associated with katG gene mutations, resistance to isoniazid has also been associated with mutations in the inhA gene and its promoter found upstream from the mabA-inhA locus (10, 12, 15). These three loci were therefore entirely sequenced in the 31 INH-resistant strains that had neither S315T nor S315N. Seventeen of them showed a −15 C-to-T substitution in the inhA promoter region (Table 3). This mutation was found to be associated with a low level of INH resistance in 15 of the 17 strains, a result that confirms previous reports showing that the −15 C-to-T substitution is generally associated with an intermediate level of resistance to INH (10, 15). It must be noted here that, in addition to the −15 C-to-T substitution found in the promoter region, these strains also showed various amino acid modifications in InhA (S94A in 4 strains, which are discussed in the next paragraph) and in KatG, including R463L in 3 strains and A110V in 7 strains (Table 3). Previous reports have shown that the mutation A110V is not associated with resistance to INH, since it has no significant impact on the catalase activity of KatG (25). Its presence in a significant proportion (41%) of the INH-resistant strains with a −15 C-to-T mutation in the inhA promoter remains to be explained.
Strikingly, seven strains showing a low level of INH resistance had an S94A substitution in InhA (Table 3). As mentioned above, 4 of them also had a −15 C-to-T substitution in the promoter region, indicating that the association of the two mutations, −15 C-to-T plus S94A, do not confer a high level of INH resistance. Three other strains with S94A were also found to have additional mutations in KatG, R463L in 2 strains and D419H in 1 strain, with the latter having not been described before (Table 3). Even if the InhA protein is generally recognized as being the primary target of INH, there has been much debate about whether the InhA S94A mutation is involved in INH resistance in M. tuberculosis. Indeed, this mutation was initially identified in INH-resistant laboratory mutants of Mycobacterium smegmatis and Mycobacterium bovis (11) and was very recently confirmed by structural and biochemical studies to reduce the NADH binding affinity of the M. tuberculosis InhA protein, thus protecting the enzyme from INH inactivation (16). However, despite the numerous genetic studies carried out in the last 10 years on the molecular mechanisms of INH resistance in M. tuberculosis, there has been only one report describing the S94A amino acid substitution from clinical isolates (15). In this previous study, the investigators identified the S94A mutation in 3 (7%) clinical isolates showing an intermediate level of resistance to INH. Interestingly, the mutation was the only alteration found in 1 of the 3 strains, while a −15 C-to-T mutation and a missense mutation in KatG (E195K) were also found in the two others, respectively (15). In our study, S94A was detected in 24% of the low-level INH-resistant strains and was found to be the only mutation identified in 2 of them. These findings, taken together with the previous report of Morlock et al. (15) and the recent data published by Oliveira et al. (16), provide substantial new evidence confirming the role of S94A in low-level INH resistance in M. tuberculosis.
Among the 11 remaining INH-resistant strains having wild-type sequences for the katG codon 315, the fabG1-inhA regulatory region, and the inhA structural gene, 7 displayed various amino acid modifications located in KatG (Table 3). The 4 strains with a high level of resistance were found to have the following mutations: W341G plus R463L (1 strain), G494D plus R463L (2 strains), and R595STOP (1 strain) (Table 3). Among these mutations, only the presence of a STOP codon in the KatG protein has been previously demonstrated to be associated with resistance to INH in clinical strains (14, 19, 26). The other amino acid substitutions, W341G and G494D, have not been previously reported in the literature, but it is likely, as suggested by the data obtained in this study, that they are related to a high level of resistance to INH, a point that will require demonstration by further molecular studies. Three other strains with a low level of INH resistance also harbored mutations in KatG that were found to be widely scattered along the polypeptide sequence (L141F, E553K, and F658V) (Table 3). These mutations represent novel mutations that may play a role in the low-level INH resistance observed in the corresponding clinical isolates, but this hypothesis will also require molecular confirmation.
Finally, 4 strains were found to have no significant mutation in KatG (if one excludes the R463L substitution which is not involved in INH resistance) (Table 3). Among them, one strain with a high level of resistance had a −102 G-to-A mutation upstream from the inhA promoter. Several nucleotide substitutions in the inhA regulatory region have been previously reported from INH resistant strains, including positions −15 (C→T), −8 (T→C), −17 (G→T), and −39 (C→T) (1, 6, 7, 26) but not the −102 G-to-A mutation found in the present study. The previously described mutations lead to the increased expression of the InhA protein, producing INH resistance via a titration mechanism (10). It is possible that the −102 G-to-A mutation acts by a similar mechanism, although the association between this mutation and resistance to INH has yet to be proven. For the 3 remaining strains, it is very likely that the low level of INH resistance observed is caused by mutations affecting genes other than katG and inhA, as previously suggested (12, 19, 26).
In conclusion, the MTBDR assay can identify the most frequent mutations involved in resistance to RIF and INH and can reveal the presence of additional mutations by negative hybridization results with the wild-type probes. Regarding RIF resistance, the MTBDR assay identified 100% of the phenotypically resistant strains. For INH resistance, the MTBDR test results correlated with the results of susceptibility testing for 59 of the 66 (89%) INH-resistant strains showing a high level of resistance (Fig. 1). By contrast, only 5 of the 29 (17%) INH-resistant strains with a low level of resistance were detected by the MTBDR assay, since most of these strains are characterized by mutations in either InhA (S94A) or the promoter of the inhA gene (−15 C-to-T), or by various KatG mutations different from S315T/N (Fig. 1). Because of the genetic diversity of the mutations found in the low-level INH-resistant clinical isolates, the Genotype MTBDR assay can be reliably applied only for detecting a high level of resistance to INH in M. tuberculosis. In contrast, complementary tests are necessary for detection of the M. tuberculosis strains with a low level of resistance, and it must be stressed here that a genotypic strip assay including the fabG1-inhA −15 regulatory region and the inhA-Ser94 mutation, in complement to katG-Ser315, may cover 88% of INH-resistant isolates. Nevertheless, despite the fact that the MTBDR assay cannot identify, in its actual form, all of the INH-resistant strains, the test is able to detect 89% of the high-level-resistant strains, i.e., the strains that have the strongest impact on INH therapy. Therefore, the Genotype MTBDR assay can be included as a valuable molecular tool in a diagnostic strategy aimed at the rapid and specific detection of the most frequent mutations leading to high-level INH resistance in M. tuberculosis.
Acknowledgments
This work was supported by the Ministère de la Recherche (grant UPRES EA 1541).
REFERENCES
- 1.Baker, L. V., T. J. Brown, O. Maxwell, A. L. Gibson, Z. Fang, M. D. Yates, and F. A. Drobniewski. 2005. Molecular analysis of isoniazid-resistant Mycobacterium tuberculosis isolates from England and Wales reveals the phylogenetic significance of the ahpC −46A polymorphism. Antimicrob. Agents Chemother. 49:1455-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banerjee, A., E. Dubnau, A. Quemard, V. Balasubramanian, K. S. Um, T. Wilson, D. Collins, G. de Lisle, and W. R. Jacobs, Jr. 1994. inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science 263:227-230. [DOI] [PubMed] [Google Scholar]
- 3.Canetti, G., N. Rist, and J. Grosset. 1963. Measurement of sensitivity of the tuberculous bacillus to antibacillary drugs by the method of proportions. Methodology, resistance criteria, results and interpretation. Rev. Tuberc. Pneumol. (Paris) 27:217-272. [PubMed] [Google Scholar]
- 4.Edwards, K. J., L. A. Metherell, M. Yates, and N. A. Saunders. 2001. Detection of rpoB mutations in Mycobacterium tuberculosis by biprobe analysis. J. Clin. Microbiol. 39:3350-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Hajj, H. H., S. A. Marras, S. Tyagi, F. R. Kramer, and D. Alland. 2001. Detection of rifampin resistance in Mycobacterium tuberculosis in a single tube with molecular beacons. J. Clin. Microbiol. 39:4131-4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gali, N., J. Dominguez, S. Blanco, C. Prat, F. Alcaide, P. Coll, and V. Ausina. 2006. Use of a mycobacteriophage-based assay for rapid assessment of susceptibilities of Mycobacterium tuberculosis isolates to isoniazid and influence of resistance level on assay performance. J. Clin. Microbiol. 44:201-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herrera-Leon, L., T. Molina, P. Saiz, J. A. Saez-Nieto, and M. S. Jimenez. 2005. New multiplex PCR for rapid detection of isoniazid-resistant Mycobacterium tuberculosis clinical isolates. Antimicrob. Agents Chemother. 49:144-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heym, B., Y. Zhang, S. Poulet, D. Young, and S. T. Cole. 1993. Characterization of the katG gene encoding a catalase-peroxidase required for the isoniazid susceptibility of Mycobacterium tuberculosis. J. Bacteriol. 175:4255-4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hillemann, D., M. Weizenegger, T. Kubica, E. Richter, and S. Niemann. 2005. Use of the genotype MTBDR assay for rapid detection of rifampin and isoniazid resistance in Mycobacterium tuberculosis complex isolates. J. Clin. Microbiol. 43:3699-3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lavender, C., M. Globan, A. Sievers, H. Billman-Jacobe, and J. Fyfe. 2005. Molecular characterization of isoniazid-resistant Mycobacterium tuberculosis isolates collected in Australia. Antimicrob. Agents Chemother. 49:4068-4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lei, B., C. J. Wei, and S. C. Tu. 2000. Action mechanism of antitubercular isoniazid. Activation by Mycobacterium tuberculosis KatG, isolation, and characterization of inha inhibitor. J. Biol. Chem. 275:2520-2526. [DOI] [PubMed] [Google Scholar]
- 12.Leung, E. T., P. L. Ho, K. Y. Yuen, W. L. Woo, T. H. Lam, R. Y. Kao, W. H. Seto, and W. C. Yam. 2006. Molecular characterization of isoniazid resistance in Mycobacterium tuberculosis: identification of a novel mutation in inhA. Antimicrob. Agents Chemother. 50:1075-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makinen, J., H. J. Marttila, M. Marjamaki, M. K. Viljanen, and H. Soini. 2006. Comparison of two commercially available DNA line probe assays for detection of multidrug-resistant Mycobacterium tuberculosis. J. Clin. Microbiol. 44:350-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marttila, H. J., H. Soini, P. Huovinen, and M. K. Viljanen. 1996. katG mutations in isoniazid-resistant Mycobacterium tuberculosis isolates recovered from Finnish patients. Antimicrob. Agents Chemother. 40:2187-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morlock, G. P., B. Metchock, D. Sikes, J. T. Crawford, and R. C. Cooksey. 2003. ethA, inhA, and katG loci of ethionamide-resistant clinical Mycobacterium tuberculosis isolates. Antimicrob. Agents Chemother. 47:3799-3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oliveira, J. S., J. H. Pereira, F. Canduri, N. C. Rodrigues, O. N. de Souza, W. F. de Azevedo, Jr., L. A. Basso, and D. S. Santos. 2006. Crystallographic and pre-steady-state kinetics studies on binding of NADH to wild-type and isoniazid-resistant enoyl-ACP(CoA) reductase enzymes from Mycobacterium tuberculosis. J. Mol. Biol. 359:646-666. [DOI] [PubMed] [Google Scholar]
- 17.Park, H., E. J. Song, E. S. Song, E. Y. Lee, C. M. Kim, S. H. Jeong, J. H. Shin, J. Jeong, S. Kim, Y. K. Park, G. H. Bai, and C. L. Chang. 2006. Comparison of a conventional antimicrobial susceptibility assay to an oligonucleotide chip system for detection of drug resistance in Mycobacterium tuberculosis isolates. J. Clin. Microbiol. 44:1619-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piatek, A. S., A. Telenti, M. R. Murray, H. El-Hajj, W. R. Jacobs, Jr., F. R. Kramer, and D. Alland. 2000. Genotypic analysis of Mycobacterium tuberculosis in two distinct populations using molecular beacons: implications for rapid susceptibility testing. Antimicrob. Agents Chemother. 44:103-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramaswamy, S. V., R. Reich, S. J. Dou, L. Jasperse, X. Pan, A. Wanger, T. Quitugua, and E. A. Graviss. 2003. Single nucleotide polymorphisms in genes associated with isoniazid resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 47:1241-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sajduda, A., A. Brzostek, M. Poplawska, E. Augustynowicz-Kopec, Z. Zwolska, S. Niemann, J. Dziadek, and D. Hillemann. 2004. Molecular characterization of rifampin- and isoniazid-resistant Mycobacterium tuberculosis strains isolated in Poland. J. Clin. Microbiol. 42:2425-2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Doorn, H. R., E. C. Claas, K. E. Templeton, A. G. van der Zanden, A. te Koppele Vije, M. D. de Jong, J. Dankert, and E. J. Kuijper. 2003. Detection of a point mutation associated with high-level isoniazid resistance in Mycobacterium tuberculosis by using real-time PCR technology with 3′-minor groove binder-DNA probes. J. Clin. Microbiol. 41:4630-4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Doorn, H. R., E. J. Kuijper, A. van der Ende, A. G. Welten, D. van Soolingen, P. E. de Haas, and J. Dankert. 2001. The susceptibility of Mycobacterium tuberculosis to isoniazid and the Arg->Leu mutation at codon 463 of katG are not associated. J. Clin. Microbiol. 39:1591-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veziris, N., A. Aubry, W. Sougakoff, C. Truffot-Pernot, and V. Jarlier. 2004. Utility of molecular tools in diagnosis, treatment, and epidemiology of mycobacterial infections. Med. Trop. (Marseilles) 64:243-249. [PubMed] [Google Scholar]
- 24.Viader-Salvado, J. M., C. M. Luna-Aguirre, J. M. Reyes-Ruiz, R. Valdez-Leal, L. del Bosque-Moncayo Mde, R. Tijerina-Menchaca, and M. Guerrero-Olazaran. 2003. Frequency of mutations in rpoB and codons 315 and 463 of katG in rifampin- and/or isoniazid-resistant Mycobacterium tuberculosis isolates from northeast Mexico. Microb. Drug Resist. 9:33-38. [DOI] [PubMed] [Google Scholar]
- 25.Wei, C. J., B. Lei, J. M. Musser, and S. C. Tu. 2003. Isoniazid activation defects in recombinant Mycobacterium tuberculosis catalase-peroxidase (KatG) mutants evident in InhA inhibitor production. Antimicrob. Agents Chemother. 47:670-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang, M., J. Yue, Y. P. Yang, H. M. Zhang, J. Q. Lei, R. L. Jin, X. L. Zhang, and H. H. Wang. 2005. Detection of mutations associated with isoniazid resistance in Mycobacterium tuberculosis isolates from China. J. Clin. Microbiol. 43:5477-5482. [DOI] [PMC free article] [PubMed] [Google Scholar]