Abstract
A total of 227 clinical Mycobacterium avium complex isolates from Thailand were differentiated into species and types by using PCR-restriction enzyme analysis of hsp65. The distribution of types showed the predominance of M. avium I (77%) in blood specimens, whereas M. intracellulare I was more commonly found in pulmonary specimens (44.2%). In addition, infections with M. avium were more likely to be found in younger adults (20 to 39 years old), while infections with M. intracellulare were more likely to be found in older adults (≥60 years old). Our results provide the useful epidemiological information that some particular types have more invasive and virulent characters than others.
The Mycobacterium avium complex (MAC) consists of two closely related species, M. avium and M. intracellulare, which are found widely in the environment, both in soil and water, and cause diseases in humans and animals (7). Both are capable of infecting diverse species, including birds, pigs, and humans, with consequences ranging from asymptomatic infection to clinically significant and even fatal disease. MAC is clinically important, as it is a frequent cause of disseminated disease and death in AIDS patients (5, 8). Clinically as well as genetically significant differences between M. avium and M. intracellulare have been shown. Mycobacterium avium is the most common MAC species isolated from AIDS patients and is also a pathogenic bacterium isolated from animals, whereas M. intracellulare is more frequently isolated from immunocompetent patients, especially from individuals with pulmonary illnesses (4, 9).
Based on the gene encoding the 65-kDa heat shock protein (hsp65), MAC can be identified and differentiated into species and types using PCR-restriction enzyme analysis (REA) (1, 2, 14). The present study demonstrates the usefulness of this method for rapidly differentiating MAC into M. avium types I, II, and III and M. intracellulare types I, II, III, and IV, providing useful epidemiological data.
Two mycobacterial reference strains, M. avium ATCC 25291 and M. intracellulare ATCC 13950, and 227 clinical isolates of M. avium complex bacteria from different patients, identified by the biochemical method, were submitted for molecular identification by using hsp65 PCR-REA in the Molecular Mycology and Mycobacteriology Laboratory, Department of Microbiology, Faculty of Medicine, Siriraj Hospital, Mahidol University, during 2003 and 2004. A total of 189 patients provided complete information on their sexes and ages; this group of patients consisted of 106 males and 83 females, with a mean age of 42.2 (range, 4 to 87) years. Clinical data about the human immunodeficiency virus (HIV) status of patients in this study were not completely available; only 67 (29.5%; 37 male and 30 female) of 227 patients confirmed that they were HIV+ patients. Genomic DNA was extracted by a boiling technique and used as a template for the hsp65 PCR-REA as previously described (1).
Mycobacterium avium ATCC 25291 and M. intracellulare ATCC 13950 were identified as M. avium I and M. intracellulare I, respectively. Of 227 MAC isolates, 146 isolates were identified as M. avium (64.3%), whereas 81 were M. intracellulare (35.7%). Interestingly, the 227 clinical isolates showed three distinct digestion patterns for M. avium and two for M. intracellulare (Fig. 1). According to PCR-REA patterns, 146 isolates of M. avium could be differentiated into type I (130 isolates [89%]), type II (15 isolates [10.3%]), and type III (1 isolate [0.7%]). Likewise, 81 isolates of M. intracellulare were differentiated into type I (62 isolates [76.5%]) and type IV (19 isolates [23.5%]).
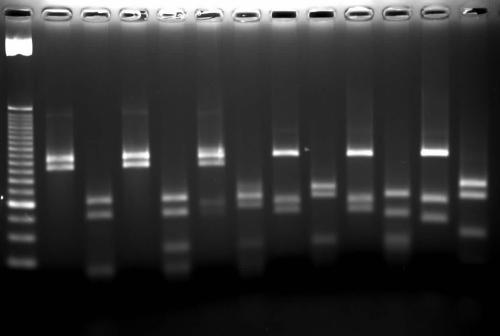
FIG. 1.
Restriction patterns of hsp65 amplicons after digestion with BstEII (lanes 1, 3, 5, 7, 9, and 11) and HaeIII (lanes 2, 4, 6, 8, 10, and 12). The digested bands of <60 bp were not used for interpretation. Lane M, 25-bp DNA ladder; lanes 1 and 2, M. avium I (BstEII 245/220 [values here and below are sizes of digested bands]; HaeIII 140/105); lanes 3 and 4, M. avium II (BstEII 245/220; HaeIII 140/105/60); lanes 5 and 6, M. avium III (BstEII 245/220; HaeIII 140/115); lanes 7 and 8, M. intracellulare I (BstEII 245/120/100; HaeIII 155/140/60); lanes 9 and 10, M. intracellulare IV (BstEII 245/120/100; HaeIII 140/90/60); lanes 11 and 12, an internal marker, M. tuberculosis (BstEII 245/120/80; HaeIII 160/140/70).
When we considered the correlation of M. avium and M. intracellulare infections with sex and age (Table 1), it was noted that M. avium was found to infect mostly younger adults (age, 20 to 39 years; P < 0.001, χ2 test) and infected males more significantly than females (P < 0.05, χ2 test). These results may be due to the prevalence of HIV infection in younger males. In contrast, frequencies of infections with M. intracellulare were not different between males and females, but such infections were more likely to be found in older adults (age, ≥60 years; P < 0.001, χ2 test). Eight M. intracellulare isolates were obtained from pulmonary specimens from patients with pulmonary illnesses, five with chronic obstructive pulmonary diseases, two with chronic bronchiectasis, and one with relapsed pulmonary tuberculosis. M. intracellulare I was isolated from all five patients (age, >60 years) with chronic obstructive pulmonary diseases and one (age, 21 years) with chronic bronchiectasis, whereas two M. intracellulare IV isolates were obtained from one patient (age, 39 years) with chronic bronchiectasis and one (age, 65 years) with relapsed pulmonary tuberculosis. These results were in agreement with those of a previous study showing that infections with M. intracellulare were more frequent among non-AIDS patients and more likely to be found in older patients (age, ≥50 years) (6).
TABLE 1.
Effect of age and sex on the distribution of MAC types
| Infection type | No. of patients by age (yr) and sexa
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤19
|
20-39
|
40-59
|
≥60
|
|||||||||
| M | F | Both | M | F | Both | M | F | Both | M | F | Both | |
| M. avium I | 2 | 5 | 7 | 43 | 34 | 77 | 19 | 4 | 23 | 2 | 6 | 8 |
| M. avium II | 1 | 0 | 1 | 6 | 5 | 11 | 0 | 1 | 1 | 0 | 0 | 0 |
| M. avium III | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Total M. avium | 3 | 5 | 8 | 49 | 39 | 88 | 19 | 5 | 24 | 3 | 6 | 9 |
| M. intracellulare I | 1 | 0 | 1 | 4 | 1 | 5 | 3 | 5 | 8 | 18 | 14 | 32 |
| M. intracellulare IV | 0 | 0 | 0 | 3 | 4 | 7 | 2 | 2 | 4 | 1 | 2 | 3 |
| Total M. intracellulare | 1 | 0 | 1 | 7 | 5 | 12 | 5 | 7 | 12 | 19 | 16 | 35 |
F, female; M, male.
The distributions of M. avium and M. intracellulare PCR-REA types varied among isolates from different sources (Table 2). Of the blood isolates, 77% were M. avium I, whereas 10.3, 9.2, and 3.5% were M. avium II, M. intracellulare IV, and M. intracellulare I, respectively. In contrast, M. intracellulare was the most common species, accounting for 51.9% of the isolates from the pulmonary specimens. The difference in the percentages of M. avium and M. intracellulare isolates from the pulmonary site was not statistically significant. Concerning the distribution of particular types, M. avium I and M. intracellulare I were found almost equally in 43.4 versus 44.2% of isolates, respectively. Furthermore, it was noted that M. intracellulare I was isolated from the pulmonary specimens (44.2%) much more often than from the blood specimens (3.6%) (P < 0.01, χ2 test). In contrast, M. intracellulare IV was isolated from blood significantly more often than was M. intracellulare I (P < 0.01, χ2 test). For other specimens, M. avium I was still the most common type (Table 2). Among 67 isolates from HIV+ patients, 54 (80.6%) were identified as M. avium I, whereas the remaining isolates were M. avium II (11.9%) and M. intracellulare IV (7.5%). Most of the specimens (44 of 67, 65.7%) were blood isolates.
TABLE 2.
Distribution of MAC isolates from different sources
| Source | No. of isolates by typeb
|
Total | ||||
|---|---|---|---|---|---|---|
| Mav I | Mav II | Mav III | Min I | Min IV | ||
| Blood | 67 | 9 | 0 | 3 | 8 | 87 |
| Pulmonary specimensa | 56 | 5 | 1 | 57 | 10 | 129 |
| Stool specimens | 4 | 0 | 0 | 0 | 0 | 4 |
| Tissue biopsy specimens | 1 | 1 | 0 | 0 | 1 | 3 |
| Cerebrospinal fluid | 2 | 0 | 0 | 0 | 0 | 2 |
| Joint fluid | 0 | 0 | 0 | 2 | 0 | 2 |
| Total | 130 | 15 | 1 | 62 | 19 | 227 |
Pulmonary specimens include sputum, bronchial wash, and gastric wash specimens.
Mav, M. avium; Min, M. intracellulare.
In this study, MAC isolates were specifically selected from both pulmonary and disseminated infections. The PCR-REA could differentiate M. avium and M. intracellulare into three and two types, respectively. Expectedly, most clinical isolates of MAC bacteria from patients with disseminated infections were M. avium I (P < 0.01, χ2 test), as reported in previous studies (10, 13). In those studies, M. avium I was the predominant pathogen found in pigs and was the most common species isolated from human blood, whereas M. avium II was more frequently isolated from pulmonary specimens. Interestingly, M. avium II was shown to be the most predominant type among environmental isolates, and even M. avium III was more common than M. avium I (13). Our results were consistent with those studies in that M. avium I was the predominant variant among the blood isolates, but the majority of M. avium isolates from the pulmonary specimens were still M. avium I. These differences could result from the geographic variations between the isolates from the earlier study in the United States (13) and those from our study in Thailand. However, the distribution of MAC variants in our environment should be investigated in order to clarify the predominant variant in the environment.
Mycobacterium intracellulare was differentiated into two types, which have already been described in previous studies (3, 14); M. intracellulare I was a single type originally described by Telenti et al. (14), whereas M. intracellulare IV was an environmental isolate from India recently identified by Devallois et al. (3). Mycobacterium intracellulare II and III, the types of clinical isolates described at PRASITE (http://app.chuv.ch/prasite/index.html), were not found in this study. Notably, among the M. intracellulare types, M. intracellulare IV was shown to be more invasive than M. intracellulare I, as 42.1% of the M. intracellulare IV isolates were blood isolates, relative to 4.8% of the M. intracellulare I isolates. The genetic difference between these two types would be valuable for further investigation. However, most infections caused by M. intracellulare were limited to the pulmonary area (51.9%) instead of invading the bloodstream (12.6%). In contrast, M. avium was the species predominantly isolated from the blood specimens (87.4%), whereas it was isolated less frequently from the pulmonary specimens (48.1%). This novel observation suggests that M. avium is likely more virulent than M. intracellulare, and among the M. avium isolates, M. avium I has a greater propensity for causing invasive, disseminated infections than other types do. These conclusions are in agreement with previous studies in which virulence-related determinants like the macrophage-induced gene (mig), the insertion sequence IS1245, and the hemolysin of MAC have been found mostly in M. avium (11, 12). However, the presence of those reported virulence determinants among the M. avium types should be further investigated, together with other virulence factors (currently undefined) that make some types of M. avium more invasive, more virulent, or longer survivors in the hosts. The characterization of the precise genetic changes that separate these variants will certainly provide the epidemiological connections and will define, if not all, some factors involved in virulent, pathogenic mechanisms and even routes of transmission.
Earlier studies demonstrated that, among AIDS patients, 98% of MAC infections caused by M. avium (5) and M. intracellulare were found in 13% of respiratory isolates but only 1.3% were found in blood isolates (15). Our results confirm those studies that showed that 92.5% of MAC infections among HIV+ patients were due to M. avium (M. avium I, 80.6%, versus M. avium II, 11.9%), whereas 7.5% were due to M. intracellulare. It was noted that the M. intracellulare isolated from hemocultures of HIV+ patients was M. intracellulare IV, emphasizing the particular genetic determinants responsible for an invasive characteristic of this type.
Acknowledgments
We thank Nattawut Kongkarath, Numpung Makhao, and Piyaporn Sakulmaiwatana for routine laboratory work and Chertsak Dhirabutra, the former head of the Department of Microbiology, for his enthusiastic support of this study. Kanogkan Leerojanaprapa is acknowledged for recommending statistical analysis.
This study was partly supported by a Siriraj Research and Development Grant, Faculty of Medicine, Siriraj Hospital, Mahidol University.
Footnotes
Published ahead of print on 23 August 2006.
REFERENCES
- 1.Cheunoy, W., T. Prammananan, A. Chaiprasert, and S. Foongladda. 2005. Comparative evaluation of PCR-restriction enzyme analysis: two amplified targets, hsp65 and rpoB, for identification of cultured mycobacteria. Diagn. Microbiol. Infect. Dis. 51:165-171. [DOI] [PubMed] [Google Scholar]
- 2.Devallois, A., K. S. Goh, and N. Rastogi. 1997. Rapid identification of mycobacteria to species level by PCR-restriction fragment length polymorphism analysis of hsp65 gene and proposition of an algorithm to differentiate 34 mycobacterial species. J. Clin. Microbiol. 35:2969-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devallois, A., M. Picardeau, C. N. Paramasivan, V. Vincent, and N. Rastogi. 1997. Molecular characterization of Mycobacterium avium complex isolates giving discordant results in AccuProbe tests by PCR-restriction enzyme analysis, 16S rRNA gene sequencing, and DT1-DT6 PCR. J. Clin. Microbiol. 35:2767-2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falkinham, J. O., III. 1994. Epidemiology of Mycobacterium avium infection in the pre- and post-HIV era. Res. Microbiol. 145:169-172. [DOI] [PubMed] [Google Scholar]
- 5.Guthertz, L. S., B. Damsker, E. J. Bottone, E. G. Ford, T. F. Midura, and J. M. Janda. 1989. Mycobacterium avium and Mycobacterium intracellulare infections in patients with and without AIDS. J. Infect. Dis. 160:1037-1041. [DOI] [PubMed] [Google Scholar]
- 6.Han, X. Y., J. J. Tarrand, R. Infante, K. L. Jacobson, and M. Truong. 2005. Clinical significance and epidemiologic analyses of Mycobacterium avium and Mycobacterium intracellulare among patients without AIDS. J. Clin. Microbiol. 43:4407-4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inderlied, C. B., C. A. Kemper, and L. E. M. Bermudez. 1993. The Mycobacterium avium complex. Clin. Microbiol. Rev. 6:266-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobson, M. A., P. C. Hopewell, D. M. Yajko, W. K. Hadley, E. Lazarus, P. K. Mohanty, G. W. Feigal, P. S. Cusick, and M. A. Sande. 1991. Natural history of disseminated Mycobacterium avium complex infection in AIDS. J. Infect. Dis. 164:994-998. [DOI] [PubMed] [Google Scholar]
- 9.Kyriakopoulos, A. M., P. T. Tassios, P. Matsiota-Bernard, E. Marinis, S. Tsaousidou, and N. J. Legakis. 1997. Characterization to species level of Mycobacterium avium complex strains from human immunodeficiency virus-positive and -negative patients. J. Clin. Microbiol. 35:3001-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leão, S. C., M. R. S. Briones, M. P. Sircili, S. C. Balian, N. Mores, and J. S. Ferreira-Neto. 1999. Identification of two novel Mycobacterium avium allelic variants in pig and human isolates from Brazil by PCR-restriction enzyme analysis. J. Clin. Microbiol. 37:2592-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maslow, J. N., D. Dawson, E. A. Carlin, and S. M. Holland. 1999. Hemolysin as a virulence factor for systemic infection with isolates of Mycobacterium avium complex. J. Clin. Microbiol. 37:445-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer, M., P. W. R. von Grünberg, T. Knoop, P. Hartmann, and G. Plum. 1998. The macrophage-induced gene mig as a marker for clinical pathogenicity and in vitro virulence of Mycobacterium avium complex strains. Infect. Immun. 66:4549-4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smole, S. C., F. McAleese, J. Ngampasutadol, C. F. von Reyn, and R. D. Arbeit. 2002. Clinical and epidemiological correlates of genotypes within the Mycobacterium avium complex defined by restriction and sequence of hsp65. J. Clin. Microbiol. 40:3374-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Telenti, A., F. Marchesi, M. Balz, F. Bally, E. C. Böttger, and T. Bodmer. 1993. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction analysis. J. Clin. Microbiol. 31:175-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yakrus, M. A., and R. C. Good. 1990. Geographic distribution, frequency, and specimen source of Mycobacterium avium complex serotypes isolated from patients with acquired immunodeficiency syndrome. J. Clin. Microbiol. 28:926-929. [DOI] [PMC free article] [PubMed] [Google Scholar]