Abstract
Micafungin is an echinocandin antifungal agent that has recently been approved for the prevention of invasive fungal infection and the treatment of esophageal candidiasis. Prospective sentinel surveillance for the emergence of in vitro resistance to micafungin among invasive Candida sp. isolates is indicated. We determined the in vitro activity of micafungin against 2,656 invasive (bloodstream or sterile site) unique patient isolates of Candida spp. collected from 60 medical centers worldwide in 2004 and 2005. We performed antifungal susceptibility testing according to the Clinical and Laboratory Standards Institute (CLSI) M27-A2 method and used a 24-hour prominent inhibition endpoint for determination of the MIC. Caspofungin was tested in parallel against all isolates. Of 2,656 invasive Candida sp. isolates, species distribution was 55.6% Candida albicans, 14.4% Candida parapsilosis, 13.4% Candida glabrata, 10.1% Candida tropicalis, 2.4% Candida krusei, 1.7% Candida guilliermondii, 0.9% Candida lusitaniae, 0.6% Candida kefyr, and 0.9% other Candida species. Overall, micafungin was very active against Candida (MIC50/MIC at which 90% of the isolates tested are inhibited [MIC90], 0.015/1 μg/ml; 96% inhibited at a MIC of ≤1 μg/ml, 100% inhibited at a MIC of ≤2 μg/ml) and comparable to caspofungin (MIC50/MIC90, 0.03/0.25 μg/ml; 99% inhibited at a MIC of ≤2 μg/ml). Results by species, expressed as MIC50/MIC90 (micrograms per milliliter), were as follows: C. albicans, 0.015/0.03; C. glabrata, 0.015/0.015; C. tropicalis, 0.03/0.06; C. krusei, 0.06/0.12; C. kefyr, 0.06/0.06; C. parapsilosis, 1/2; C. guilliermondii, 0.5/1; C. lusitaniae, 0.12/0.25; other Candida spp., 0.25/1. Although the species distribution varied considerably among the different geographic regions, there was no difference in micafungin activity across the regions. Micafungin has excellent in vitro activity against invasive clinical isolates of Candida from centers worldwide.
Three echinocandin antifungal agents (caspofungin, micafungin, and anidulafungin) are now available for the prevention and/or treatment of invasive fungal infection (1, 2, 5, 11, 18). Micafungin has been licensed by the U.S. Food and Drug Administration for prophylaxis against invasive fungal infection in neutropenia and for the treatment of esophageal candidiasis (2, 18). Although the results from a randomized clinical trial for the treatment of candidemia are pending, micafungin has been shown to be safe and efficacious in the treatment of candidemia in a recently published open-label clinical trial (1, 11).
Through a consensus process the Clinical and Laboratory Standards Institute (CLSI, formerly the National Committee for Clinical Laboratory Standards [NCCLS]) has developed a standardized method for broth microdilution (BMD) testing of echinocandins (i.e., caspofungin, micafungin, and anidulafungin) against Candida species (9, 14). BMD testing using RPMI 1640 broth, incubation for no longer than 24 h, and a MIC endpoint criterion of prominent reduction in growth (MIC-2 or ≥50% inhibition) relative to control growth provides both excellent reproducibility of results within and between laboratories and differentiation of isolates with “normal” or “wild-type” susceptibilities from glucan synthesis mutant strains with decreased susceptibilities to echinocandins (9, 14).
Although the above conditions have been applied to the testing of caspofungin (14, 16) and anidulafungin (15) versus Candida spp., there are limited in vitro data available for micafungin and Candida using these optimized methods. Previously, Ostrosky-Zeichner et al. (10) reported micafungin MICs for 2,000 Candida bloodstream infection (BSI) isolates determined by BMD using RPMI 1640 and a prominent inhibition (MIC-2) endpoint. However, the MICs were read after 48 h of incubation rather than 24 h. Recently, we have tested a smaller collection of 315 fluconazole-resistant isolates of Candida spp. using the CLSI consensus conditions and found excellent activity for both micafungin and caspofungin (6). Although the echinocandins appear to have excellent activity against Candida spp., recent reports describing the development of resistance to caspofungin and micafungin during treatment of endocarditis (7) and to caspofungin during treatment of esophagitis (4) raise the specter of the emergence of echinocandin-resistant Candida species. Thus, surveillance of the activity of the echinocandins is important as they are used more broadly worldwide (10, 15, 16).
In the present study we have employed the optimal testing conditions described previously (6, 9, 14), to examine geographic trends in the activity of micafungin against an international collection of 2,656 BSI isolates of Candida spp. obtained from 60 different medical centers in 2004 and 2005. We have used caspofungin, tested in parallel with micafungin, as an echinocandin comparator.
MATERIALS AND METHODS
Organisms.
A total of 2,656 clinical isolates obtained from 60 different medical centers internationally in 2004 and 2005 were tested. The collection included 1,476 strains of Candida albicans, 383 of Candida parapsilosis, 356 of Candida glabrata, 269 of Candida tropicalis, 63 of Candida krusei, 45 of Candida guilliermondii, 24 of Candida lusitaniae, 17 of Candida kefyr, 10 of Candida famata, 4 of Candida dubliniensis, 4 of Candida lipolytica, 3 of Candida pelliculosa, and 1 each of Candida rugosa and Candida zeylanoides. All isolates were obtained from blood or other normally sterile sites and represented individual infectious episodes. The isolates were collected at the individual study sites and were sent to the University of Iowa (Iowa City) for identification and susceptibility testing as described previously (6, 13-16). The isolates were identified by standard methods (3) and stored as water suspensions until used in the study. Prior to testing, each isolate was passaged at least twice onto potato dextrose agar (Remel) and CHROMagar Candida (Hardy Diagnostics, Santa Maria, Calif.) to ensure purity and viability.
Antifungal agents.
Reference powders of micafungin and caspofungin were obtained from their respective manufacturers. Stock solutions were prepared in water, and serial twofold dilutions were made in RPMI 1640 medium (Sigma, St. Louis, Mo.) buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid (MOPS) buffer (Sigma).
Antifungal susceptibility testing.
BMD testing was performed in accordance with the guidelines in CLSI document M27-A2 (8) using RPMI 1640 medium, an inoculum of 0.5 × 103 to 2.5 × 103 cells/ml, and incubation at 35°C. MICs were determined visually after 24 h of incubation as the lowest concentration of drug that caused a significant diminution (MIC-2 or ≥50%) of growth below control levels (6, 14-16).
Quality control.
Quality control was performed by testing CLSI-recommended strains C. krusei ATCC 6258 and C. parapsilosis ATCC 22019 (8).
RESULTS AND DISCUSSION
Table 1 demonstrates the species distribution of Candida BSI isolates according to the geographic region of origin. A total of 2,656 isolates were obtained from 60 different medical centers in the Asia-Pacific region (12 sites), Latin America (13 sites), Europe (18 sites), Canada (3 sites), and the United States (14 sites). As was seen previously for the years 1992 to 2001 (13) and 2001 to 2004 (16), the distribution of Candida species isolated from blood and other sterile sites varied considerably across the different regions. Whereas C. albicans accounted for ≥60% of all isolates in Europe, Canada, and the Asia-Pacific regions, <50% of isolates from Latin America and the United States were C. albicans. Likewise, C. parapsilosis and C. tropicalis were prominent in the Asia-Pacific and Latin American regions but less so in Europe, Canada, and the United States. Although C. glabrata accounted for more than 25% of Candida isolates in North America (21.8% in Canada and 27.4% in the United States), this species was distinctly less common in the other regions and especially so (4.2%) in Latin America. Finally, although very uncommon (<1%) in the rest of the world, C. guilliermondii may be emerging as an important species of Candida in Latin America (6.6%), where it has surpassed both C. glabrata (4.2%) and C. krusei (1.8%) as a percentage of all invasive (BSI and other sterile site) isolates of Candida submitted to our surveillance program.
TABLE 1.
Species distribution of Candida isolates by geographic region
| Candida species | % of isolatesa
|
|||||
|---|---|---|---|---|---|---|
| APAC (518) | LAM (548) | EU (847) | Canada (156) | U.S. (587) | Total (2,656) | |
| C. albicans | 60.2 | 48.9 | 63.5 | 64.1 | 44.0 | 55.6 |
| C. parapsilosis | 16.2 | 19.7 | 10.6 | 9.0 | 14.8 | 14.4 |
| C. glabrata | 7.3 | 4.2 | 11.8 | 21.8 | 27.4 | 13.4 |
| C. tropicalis | 12.5 | 16.4 | 7.6 | 2.6 | 7.8 | 10.1 |
| C. krusei | 0.8 | 1.8 | 4.1 | 1.3 | 2.0 | 2.4 |
| C. guilliermondii | 0.8 | 6.6 | 0.2 | 0.5 | 1.7 | |
| C. lusitaniae | 1.0 | 0.5 | 0.4 | 0.6 | 2.0 | 0.9 |
| C. kefyr | 0.2 | 0.4 | 1.3 | 0.5 | 0.6 | |
| Candida spp.b | 1.0 | 1.5 | 0.5 | 0.6 | 1.0 | 0.8 |
Regions: APAC, Asia-Pacific (12 study sites); LAM, Latin America (13 study sites); EU, Europe (18 study sites); Canada (3 study sites); U.S., United States (14 study sites). For each region, the number of isolates is given in parentheses.
Includes C. famata (10 isolates), C. dubliniensis (4 isolates), C. lipolytica (4 isolates), C. pelliculosa (3 isolates), C. rugosa (1 isolate), and C. zeylanoides (1 isolate).
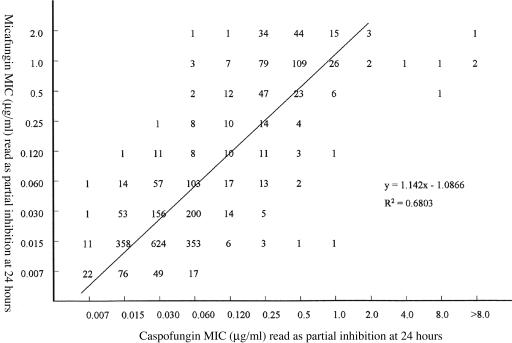
Table 2 summarizes the in vitro susceptibilities of 2,656 isolates of Candida spp. to micafungin and caspofungin when tested in RPMI 1640 medium with 24 h of incubation and the prominent reduction endpoint criteria. The MICs for caspofungin obtained with this recent (2004 to 2005) collection of BSI isolates are comparable to those reported previously for isolates collected between 1992 and 2000 (14) and between 2001 and 2004 (16) using the same test methods and MIC endpoint (14, 16). As reported previously for micafungin (6, 10), isolates with elevated MICs (i.e., >2 μg/ml) were not identified and the in vitro activity of this agent against virtually all species of Candida was comparable to that of caspofungin. Indeed, a scatterplot of micafungin and caspofungin MICs shows a high level of correlation (R2 = 0.68) with 97% of all MICs for the two agents within ±2 log2 dilutions of one another (Fig. 1). The six isolates with caspofungin MICs of >2 μg/ml included three of C. guilliermondii and one each of C. parapsilosis, C. glabrata, and C. tropicalis.
TABLE 2.
In vitro susceptibilities of 2,656 clinical isolates of Candida spp. to micafungin and caspofungin
| Organism (no. of isolates tested) and antifungal agent | Cumulative % susceptible at a MIC (μg/ml) ofa:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.007 | 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | |
| C. albicans (1,476) | |||||||||||
| Micafungin | 10 | 76 | 95 | >99 | >99 | 100 | |||||
| Caspofungin | 2 | 28 | 68 | 98 | >99 | >99 | 100 | ||||
| C. glabrata (356) | |||||||||||
| Micafungin | 4 | 91 | 96 | 98 | 99 | >99 | >99 | 100 | |||
| Caspofungin | 9 | 57 | 96 | 98 | 99 | 99 | >99 | >99 | >99 | 100 | |
| C. tropicalis (269) | |||||||||||
| Micafungin | 2 | 28 | 70 | 94 | 99 | 99 | >99 | 100 | |||
| Caspofungin | 1 | 28 | 73 | 97 | 98 | 99 | 99 | >99 | >99 | >99 | >99 |
| C. krusei (63) | |||||||||||
| Micafungin | 3 | 16 | 86 | 98 | 100 | ||||||
| Caspofungin | 2 | 2 | 51 | 71 | 92 | 98 | 100 | ||||
| C. kefyr (17) | |||||||||||
| Micafungin | 41 | 100 | |||||||||
| Caspofungin | 12 | 94 | 100 | ||||||||
| C. parapsilosis (383) | |||||||||||
| Micafungin | 1 | 1 | 1 | 1 | 5 | 21 | 76 | 100 | |||
| Caspofungin | 1 | 1 | 4 | 8 | 47 | 88 | 98 | >99 | 100 | ||
| C. guilliermondii (45) | |||||||||||
| Micafungin | 2 | 9 | 18 | 58 | 98 | 100 | |||||
| Caspofungin | 2 | 5 | 14 | 41 | 81 | 93 | 93 | 93 | 95 | ||
| C. lusitaniae (24) | |||||||||||
| Micafungin | 8 | 67 | 100 | ||||||||
| Caspofungin | 4 | 4 | 58 | 92 | 100 | ||||||
| Candida spp. (23) | |||||||||||
| Micafungin | 4 | 26 | 35 | 35 | 52 | 78 | 91 | 100 | |||
| Caspofungin | 13 | 22 | 39 | 48 | 83 | 96 | 100 | ||||
| Total (2,656) | |||||||||||
| Micafungin | 6 | 57 | 74 | 81 | 83 | 84 | 88 | 96 | 100 | ||
| Caspofungin | 1 | 20 | 54 | 80 | 83 | 91 | 98 | >99 | >99 | >99 | >99 |
Broth microdilution testing was performed according to CLSI M27-A2 (8), using 24-h incubation and a MIC-2 endpoint.
FIG. 1.
Scatterplot of micafungin MICs versus caspofungin MICs for 2,656 isolates of Candida spp. MICs were determined for each drug using RPMI 1640 medium, 24-h incubation, and a partial inhibition (MIC-2) endpoint.
Although no MIC breakpoints for echinocandins have been established, a caspofungin MIC of ≤2 μg/ml encompasses >99% of all clinical isolates of Candida spp. without bisecting any species group and represents a concentration that is easily maintained throughout the dosing interval (17). Available clinical, pharmacokinetic, and pharmacodynamic data also support the contention that infections due to Candida spp. in this MIC range are likely respond to therapy (data reviewed in reference 17).
As seen with both caspofungin (14, 16) and anidulafungin (15), the micafungin MIC distribution defined two broad groups among the eight major species tested (Table 2). C. albicans, C. glabrata, C. tropicalis, and C. kefyr were all highly susceptible to both micafungin and caspofungin (MIC at which 90% of isolates tested are inhibited [MIC90], 0.015 to 0.06 μg/ml), whereas C. parapsilosis (MIC90, 1 to 2 μg/ml), C. guilliermondii (MIC90, 1 μg/ml), and C. lusitaniae (MIC90, 0.25 μg/ml) were significantly less susceptible to both agents. Similarly to anidulafungin (15), micafungin was also quite active against C. krusei (MIC90, 0.12 μg/ml). Thus, micafungin exhibits broad-spectrum in vitro potency against virtually all Candida species encountered clinically. Given the mechanism of action shared among the echinocandins, it is not surprising that a strong correlation was demonstrated between micafungin and caspofungin MICs (Fig. 1). Likewise, one might expect that if resistance emerges to one of these agents it will likely encompass the other as well (7, 12).
The micafungin susceptibilities of isolates stratified by geographic region and by species are shown in Table 3. Despite the differences in species distribution noted previously, the overall activity of micafungin was similar for all regions: 94 to 97% of isolates were inhibited by ≤1 μg/ml and 100% by 2 μg/ml.
TABLE 3.
Variation in micafungin MIC profiles by geographic region
| Region(s) and species | No. of isolates tested | No. of isolates for which MIC (μg/ml) was:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.007 | 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | ||
| Asia-Pacific | ||||||||||||
| C. albicans | 312 | 23 | 199 | 69 | 21 | |||||||
| C. glabrata | 38 | 3 | 32 | 1 | 2 | |||||||
| C. tropicalis | 65 | 8 | 40 | 11 | 5 | 1 | ||||||
| C. krusei | 4 | 2 | 2 | |||||||||
| C. kefyr | 1 | 1 | ||||||||||
| C. parapsilosis | 84 | 1 | 16 | 51 | 16 | |||||||
| C. guilliermondii | 4 | 1 | 1 | 1 | 1 | |||||||
| C. lusitaniae | 5 | 1 | 3 | 1 | ||||||||
| Candida spp. | 5 | 1 | 1 | 1 | 2 | |||||||
| Total | 518 | 26 | 240 | 110 | 38 | 12 | 4 | 17 | 53 | 18 | ||
| Latin America | ||||||||||||
| C. albicans | 268 | 32 | 159 | 56 | 21 | |||||||
| C. glabrata | 23 | 3 | 20 | |||||||||
| C. tropicalis | 90 | 2 | 27 | 35 | 22 | 3 | 1 | |||||
| C. krusei | 10 | 1 | 9 | |||||||||
| C. kefyr | 2 | 2 | ||||||||||
| C. parapsilosis | 108 | 1 | 6 | 23 | 65 | 13 | ||||||
| C. guilliermondii | 36 | 3 | 2 | 14 | 16 | 1 | ||||||
| C. lusitaniae | 3 | 2 | 1 | |||||||||
| Candida spp. | 8 | 1 | 2 | 3 | 2 | |||||||
| Total | 548 | 37 | 206 | 95 | 52 | 9 | 12 | 40 | 83 | 14 | ||
| Europe | ||||||||||||
| C. albicans | 538 | 52 | 387 | 73 | 22 | 2 | 2 | |||||
| C. glabrata | 100 | 2 | 89 | 7 | 1 | 1 | ||||||
| C. tropicalis | 64 | 2 | 21 | 25 | 15 | 1 | ||||||
| C. krusei | 35 | 2 | 6 | 23 | 4 | |||||||
| C. kefyr | 11 | 3 | 8 | |||||||||
| C. parapsilosis | 90 | 1 | 4 | 11 | 47 | 27 | ||||||
| C. guilliermondii | 2 | 2 | ||||||||||
| C. lusitaniae | 3 | 1 | 2 | |||||||||
| Candida spp. | 4 | 1 | 1 | 1 | 1 | |||||||
| Total | 847 | 56 | 500 | 115 | 69 | 10 | 9 | 14 | 47 | 27 | ||
| Canada | ||||||||||||
| C. albicans | 100 | 3 | 59 | 35 | 3 | |||||||
| C. glabrata | 34 | 1 | 30 | 2 | 1 | |||||||
| C. tropicalis | 4 | 1 | 1 | 2 | ||||||||
| C. krusei | 2 | 1 | 1 | |||||||||
| C. kefyr | ||||||||||||
| C. parapsilosis | 14 | 1 | 9 | 4 | ||||||||
| C. guilliermondii | ||||||||||||
| C. lusitaniae | 1 | 1 | ||||||||||
| Candida spp. | 1 | 1 | ||||||||||
| Total | 156 | 4 | 90 | 38 | 8 | 2 | 1 | 9 | 4 | |||
| United States | ||||||||||||
| C. albicans | 258 | 33 | 173 | 42 | 10 | |||||||
| C. glabrata | 161 | 6 | 137 | 10 | 4 | 1 | 2 | 1 | ||||
| C. tropicalis | 46 | 2 | 12 | 13 | 15 | 2 | 1 | 1 | ||||
| C. krusei | 12 | 1 | 9 | 1 | 1 | |||||||
| C. kefyr | 3 | 2 | 1 | |||||||||
| C. parapsilosis | 87 | 5 | 13 | 35 | 34 | |||||||
| C. guilliermondii | 3 | 1 | 1 | 1 | ||||||||
| C. lusitaniae | 12 | 1 | 7 | 4 | ||||||||
| Candida spp. | 5 | 3 | 2 | |||||||||
| Total | 587 | 41 | 322 | 71 | 40 | 11 | 13 | 17 | 38 | 35 | ||
| All | ||||||||||||
| C. albicans | 1,476 | 143 | 977 | 275 | 77 | 3 | 1 | |||||
| C. glabrata | 356 | 15 | 308 | 20 | 6 | 4 | 2 | 1 | ||||
| C. tropicalis | 269 | 6 | 69 | 114 | 65 | 11 | 2 | 1 | 1 | |||
| C. krusei | 63 | 2 | 8 | 44 | 8 | 1 | ||||||
| C. kefyr | 17 | 7 | 10 | |||||||||
| C. parapsilosis | 383 | 1 | 2 | 15 | 64 | 207 | 94 | |||||
| C. guilliermondii | 45 | 1 | 3 | 4 | 18 | 18 | 1 | |||||
| C. lusitaniae | 24 | 2 | 14 | 8 | ||||||||
| Candida spp. | 23 | 1 | 5 | 2 | 4 | 6 | 3 | 2 | ||||
| Total | 2,656 | 164 | 1,358 | 429 | 207 | 45 | 37 | 89 | 230 | 97 | ||
The data in Table 3 underscore the fact that species-specific differences in echinocandin MICs noted for the aggregate population are true for each of the individual regions as well. Thus, the modal MIC for the common species C. albicans, C. glabrata, and C. tropicalis was 0.015 to 0.03 μg/ml, in all five regions. The species-specific differences in the potency of the echinocandins must be kept in mind, and these differences emphasize the need for any surveillance program to include accurate species identification of the monitored isolates. Whether these major differences in drug potency will impact dosing and patient management remains to be seen. The data we present can serve as a baseline for comparison in future studies of these regions.
In summary, we document the in vitro potency and spectrum of micafungin against Candida spp. We provide evidence for comparability between micafungin and caspofungin MICs and suggest that this may be important when resistance profiles for either agent are determined. We have shown that, similarly to caspofungin (16), the activity of micafungin remains consistent over broad geographic regions and that species-specific differences in micafungin activity against Candida are apparent worldwide. These MIC distributions, all determined by a single optimized test method, should provide a useful baseline for subsequent studies of this agent.
Acknowledgments
We thank Linda Elliott for excellent secretarial assistance in the preparation of the manuscript.
This study was supported in part by Astellas Pharmaceuticals.
REFERENCES
- 1.Andes, D., and N. Safdar. 2005. Efficacy of micafungin for the treatment of candidemia. Eur. J. Clin. Microbiol. Infect. Dis. 24:662-664. [DOI] [PubMed] [Google Scholar]
- 2.De Wet, N., A. Llanos-Cuentas, J. Suleiman, E. Baraldi, E. F. Krantz, M. Negra, and H. Diekmann-Berndt. 2004. A randomized double-blind, parallel-group, dose-response study of micafungin compared with fluconazole for the treatment of esophageal candidiasis in HIV-positive patients. Clin. Infect. Dis. 39:842-849. [DOI] [PubMed] [Google Scholar]
- 3.Hazen, K. C., and S. A. Howell. 2003. Candida, Cryptococcus, and other yeasts of medical importance, p. 1693-1711. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. J. Yolken (ed.), Manual of clinical microbiology, 8th ed. ASM Press, Washington, D.C.
- 4.Hernandez, S., J. L. Lopez-Ribot, L. K. Najvar, D. I. McCarthy, R. Bocanegra, and J. R. Graybill. 2004. Caspofungin resistance in Candida albicans: correlating clinical outcome with laboratory susceptibility testing of three isogenic isolates serially obtained from a patient with progressive Candida esophagitis. Antimicrob. Agents Chemother. 48:1382-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarvis, B., D. P. Figgitt, and L. J. Scott. 2004. Micafungin. Drugs 64:969-982. [DOI] [PubMed] [Google Scholar]
- 6.Messer, S. A., D. J. Diekema, L. Boyken, S. Tendolkar, R. J. Hollis, and M. A. Pfaller. 2006. Activities of micafungin against 315 invasive clinical isolates of fluconazole-resistant Candida spp. J. Clin. Microbiol. 44:324-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moudgal, V., T. Little, D. Boikov, and J. A. Vazquez. 2005. Multiechinocandin- and multiazole-resistant Candida parapsilosis isolates serially obtained during therapy for prosthetic valve endocarditis. Antimicrob. Agents Chemother 49:767-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard—second edition M27-A2. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 9.Odds, F. C., M. Motyl, R. Andrade, J. Bille, E. Canton, M. Cuenca-Estrella, A. Davison, C. Durussel, D. Ellis, E. Foraker, A. W. Fothergill, M. A. Ghannoum, R. A. Giacobbe, M. Gobernado, R. Handke, M. Lavediere, W. Lee-Yang, W. G. Merz, L. Ostrosky-Zeichner, J. Peman, S. Perea, J. R. Perfect, M. A. Pfaller, L. Prioa, J. H. Rex, M. G. Rinaldi, J. L. Rodriguez-Tudela, W. A. Schell, C. Shields, D. A. Sutton, P. E. Verweij, and D. W. Warnock. 2004. Interlaboratory comparison of results of susceptibility testing with caspofungin against Candida and Aspergillus species. J. Clin. Microbiol. 42:3475-3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ostrosky-Zeichner, L., J. H. Rex, P. G. Pappas, R. J. Hamill, R. A. Larsen, H. W. Horowitz, W. G. Powderly, N. Hyslop, C. A. Kauffman, J. Cleary, J. E. Mangino, and J. Lee. 2003. Antifungal susceptibility survey of 2,000 bloodstream Candida isolates in the United States. Antimicrob. Agents Chemother 47:3149-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ostrosky-Zeichner, L., D. Kontoyiannis, J. Raffalli, K. M. Mullane, J. Vazquez, E. J. Anaissie, J. Lipton, P. Jacobs, J. H. Jansen van Rensburg, J. H. Rex, W. Lau, D. Facklam, and D. N. Buell. 2005. International, open-label, noncomparative, clinical trial of micafungin alone and in combination for treatment of newly diagnosed and refractory candidemia. Eur. J. Clin. Microbiol. Infect. Dis. 24:654-661. [DOI] [PubMed] [Google Scholar]
- 12.Park, S., R. Kelly, J. Nielsen Kahn, J. Robles, M. J. Hsu, E. Register, W. Li, V. Vyas, H. Fan, G. Abruzzo, A. Flattery, C. Gill, G. Chrebet, S. A. Parent, M. Kurtz, H. Teppler, C. M. Douglas, and D. S. Perlin. 2005. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob. Agents Chemother. 49:3264-3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfaller, M. A., and D. J. Diekema. 2004. Twelve years of fluconazole in clinical practice: global trends in species distribution and fluconazole susceptibility of bloodstream isolates of Candida. Clin. Microbiol. Infect. 10(Suppl. 1):11-23. [DOI] [PubMed] [Google Scholar]
- 14.Pfaller, M. A., S. A. Messer, L. Boyken, C. Rice, S. Tendolkar, R. J. Hollis, and D. J. Diekema. 2004. Further standardization of broth microdilution methodology for in vitro susceptibility testing of caspofungin against Candida species by use of an international collection of more than 3,000 clinical isolates. J. Clin. Microbiol. 42:3117-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfaller, M. A., L. Boyken, R. J. Hollis, S. A. Messer, S. Tendolkar, and D. J. Diekema. 2005. In vitro activities of anidulafungin against more than 2,500 clinical isolates of Candida spp., including 315 clinical isolates resistant to fluconazole. J. Clin. Microbiol. 43:5425-5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfaller, M. A., L. Boyken, R. J. Hollis, S. A. Messer, S. Tendolkar, and D. J. Diekema. 2006. In vitro susceptibility of Candida spp. to caspofungin: four years of global surveillance. J. Clin. Microbiol. 44:760-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfaller, M. A., and D. J. Diekema. The epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev., in press. [DOI] [PMC free article] [PubMed]
- 18.van Burik, J. A., V. Ratanathorn, D. E. Stepan, C. B. Miller, J. H. Lipton, D. H. Vesole, N. Burin, D. A. Wall, J. W. Hiemenz, Y. Satoi, J. M. Lee, and T. J. Walsh for the National Institute of Allergy and Infectious Diseases Mycoses Study Group. 2004. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin. Infect. Dis. 39:1407-1416. [DOI] [PubMed] [Google Scholar]