Abstract
Sapoviruses (SVs) are an important cause of acute pediatric gastroenteritis. Due to the lack of appropriate diagnostic methods, the epidemiology of SV-associated illness remains poorly understood. Baculovirus and Escherichia coli expression systems were evaluated for the development of antibody detection enzyme immunoassays (EIA). Age-related antibody prevalence in children was studied using the new EIA. Because of the low yield of the baculovirus system, the E. coli-expressed SV capsid proteins were used to develop the EIA. The antigenic specificities of the E. coli-expressed SV capsid proteins were demonstrated using hyperimmune antisera raised in animals and sera collected from patients. A high prevalence (>90%) of antibodies to both SV (strain Mex340) and norovirus (strain VA387) was observed in children involved in a birth cohort at 20 to 24 months of age; however, at 1 to 3 months of age, <25% of the children possessed anti-SV antibodies versus >90% with anti-NV antibodies. The E. coli-derived SV proteins are an excellent source of antigens for the EIA. SV infection is common in the first 2 years of life. The low prevalence of maternal antibodies detected in Mexican children against SVs in this study is unique and needs to be addressed in future studies.
The family Caliciviridae consists of four genera, Norovirus (NV), Sapovirus (SV), Lagovirus, and Vesivirus, from which NV and SV cause disease mainly in humans and therefore are also referred to as human caliciviruses (HuCVs) (9). NVs are the leading cause of epidemic gastroenteritis, often causing large water- or food-borne outbreaks in all ages, while SVs are implicated mainly in pediatric gastroenteritis. In a study among Mexican children, we found that 19% of the sporadic acute gastroenteritis cases were associated with HuCVs and over a third of these were caused by SVs (8). Pang et al. reported 20% and 9% detection rates for NVs and SVs, respectively, in stool specimens of children with acute gastroenteritis in Finland (31, 32). It is generally accepted that SV gastroenteritis is less severe than that of rotavirus or NV; however, SVs have been detected in 1 to 3% of stool specimens collected from children hospitalized with gastroenteritis, indicating that SVs can cause severe illness (34, 38, 47). Also, with the increasing awareness of SV gastroenteritis and improved diagnostic methods, SVs were recently implicated in several gastroenteritis outbreaks in adult populations (22, 43).
Because of the lack of a suitable animal model and tissue culture system for the propagation of HuCVs (5), the study of these pathogens has advanced slowly since the description of the prototype Norwalk and Sapporo viruses in the 1970s (3, 23). The cloning and genomic characterization of the Norwalk virus (14) and subsequently other HuCVs opened the way for the development of diagnostic techniques, such as reverse transcription-PCR (16) and enzyme immunoassays (EIA) utilizing recombinant virus-like particles (VLPs) (15, 17). These techniques proved to be superior to previously used techniques that relied mainly on clinical materials as reagents (1). The tremendous genetic and possibly antigenic diversity of HuCVs further complicates their diagnosis. With the accumulation of HuCV genomic sequence information, the Norovirus genus is further classified into five genogroups and more than 25 NV genetic clusters (42), and similarly, five genogroups and at least 9 genetic clusters within Sapovirus have been reported (7).
The development of EIA systems using recombinant VLPs greatly increased our knowledge on the seroepidemiology of NVs (4, 6, 21, 33). Baculovirus expression and VLP formation of SV capsid proteins have also been reported (10, 12, 19, 30); however, the yield of SV VLPs obtained is typically low. Since there are very limited reports on the seroprevalence of SVs (26, 28, 29, 36), in this article we describe the bacterial expression of SV capsid fusion proteins, the development of an EIA for measuring anti-SV antibodies, and its application in a study of SV seroprevalence in children.
MATERIALS AND METHODS
Virus strains.
The virus strains used in this study are listed in Table 1 and were described in our previous studies (7, 20). cDNA clones covering the C-terminal part of the viral genomes (2.3 to 3.2 kb) were stored at −70°C and used as templates for PCR amplification of virus-specific sequences in this study, using high-fidelity Pfu DNA polymerase (Promega, Madison, WI).
TABLE 1.
Virus strains used in this studya
Clinical samples.
A total of 567 achieved serum samples collected between November 1987 and June 1990 from 102 Mexican children between 0 and 24 months of age were tested for the prevalence of anti-Mex340- and anti-VA387-specific antibodies. Ninety-nine serum samples were collected between 0 and 3 months, 91 between 4 and 7 months, 97 between 8 and 11 months, 91 between 12 and 15 months, 95 between 16 and 19 months, and 94 between 20 and 24 months of age. Serum samples were stored at −70°C. The original study was approved by the Institutional Review Board of the Institute of Medical Services and Nutrition, and informed consent was obtained from the parents of the children.
Baculovirus expression.
Baculovirus expression of VA387 has been described previously (20). For baculovirus expression of Mex340 and Mex14917 capsid proteins, virus-specific sequences from the capsid protein initiation codon to the poly(A) tail were cloned into pFastBac1 vector (Invitrogen, Carlsbad, CA), utilizing restriction enzyme sites (BamHI and NotI) added to the PCR primers.
Recombinant baculoviruses were constructed by a Bac-to-Bac baculovirus expression system (Invitrogen, Carlsbad, CA), according to the manufacturer's instructions. For large-scale production of recombinant SV capsid proteins, insect cell cultures were inoculated with a multiplicity of infection of 5 to 10 of recombinant baculoviruses. Cultures were harvested at 5 days postinoculation, and recombinant SV VLPs were purified on 10 to 50% sucrose gradients as previously described for NV VLPs (20). Sucrose gradient fractions were analyzed on 10% polyacrylamide gels containing sodium dodecyl sulfate (SDS) and/or by Western blotting, using hyperimmune sera generated against SV capsid fusion proteins or human sera collected during previous gastroenteritis outbreak investigations.
Electron microscopy (EM).
Drops of sucrose gradient-purified VLPs were adsorbed onto Formvar-carbon 200-mesh copper grids (Electron Microscopy Sciences, Fort Washington, PA), stained with 1% ammonium molybdate (pH 5), and examined using a Zeiss EM 10 transmission electron microscope.
Bacterial fusion protein expression and purification.
For bacterial fusion protein expression, the capsid protein genes of individual SV strains were amplified from cDNA clones with strain-specific primers containing restriction enzyme sites for in-frame cloning. PCR amplicons were gel purified, digested with the appropriate restriction enzyme(s), and in frame cloned into XmnI (blunt end)- and XbaI-digested pMAL-c2 vector (New England BioLabs, Beverly, MA) or BamHI- and NotI-digested (in the case of Hou7-1181, EcoRI and NotI) pGEX-4T-1 vector (Amersham, Piscataway, NJ). Escherichia coli JM109 cells were used for amplifying and screening the recombinant plasmids, and protease-deficient BL21 E. coli cells were used for protein expression. Selected clones were confirmed by sequencing.
Glutathione S-transferase (GST)-capsid fusion protein clones were grown in LB (10 g/liter tryptone, 5 g/liter yeast extract, 10 g/liter NaCl, 100 μg/ml ampicillin) and induced by 0.3 mM IPTG (isopropyl-β-d-thiogalactopyranoside) overnight at room temperature. Maltose binding protein (MBP)-capsid fusion protein clones were grown and induced under the same conditions, except rich medium (10 g/liter tryptone, 5 g/liter yeast extract, 5 g/liter NaCl, 2 g/liter glucose, 100 μg/ml ampicillin) was used. Affinity purification of fusion proteins was performed according to the manufacturer's protocols. Briefly, soluble fractions of sonicated cell lysates in phosphate-buffered saline (PBS) (pH 7.4) were bound to corresponding affinity resins (glutathione-Sepharose 4B for GST and amylose resin for MBP) at room temperature for 45 min. Affinity resins were packed into Flex Columns (Kimble/Kontes, Vineland, NJ) and intensively washed by PBS. Copurifying GroEL was removed from the affinity column-bound fusion proteins by subsequent washes with bacterial protein extraction reagent (B-PER; Pierce Biotechnology Inc., Rockford, IL). After the columns were reequilibrated with PBS, the fusion proteins were eluted with the appropriate elution buffer (10 mM maltose or 10 mM glutathione in PBS). All wash and elution steps were monitored visually by adding 10 μl eluate to 50 μl of 1:4 diluted Bio-Rad protein assay dye reagent (Bio-Rad Laboratories, Hercules, CA). Affinity resin-purified MBP fusion proteins with a C-terminal six-His tag were rebound to Talon metal affinity resin (Clontech, Palo Alto, CA), washed with PBS and B-PER, and eluted in PBS containing 150 mM imidazole. Protein concentrations were determined by an optical density reading at 280 nm (OD280) and/or visually by loading aliquots on SDS-polyacrylamide gel electrophoresis (PAGE) along with bovine serum albumin standards. Purified proteins were concentrated or buffer exchanged by centrifugation in Amicon Ultra-15 centrifugal filter units (Millipore Co., Billerica, MA). Fusion proteins were stored at −70°C in PBS with or without 20% glycerol at 0.5- to 1-mg/ml concentrations.
Gel filtration chromatography.
A Superdex 200 gel filtration column (separation range, 10 to 600 kDa) powered by an ÄKTA FPLC system (model 920; Amersham Biosciences, Piscataway, NJ) was used for further separation of the affinity chromatography-purified proteins. Several buffer systems containing nonionic detergents (0.1% Triton X-100, 0.1 to 0.4% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}, 1% octyl glucoside), reducing agent (0.2 to 1 mM dithiothreitol), 5 to 10% glycerol, 10 to 15% acetonitrile, high salt (500 to 700 mM NaCl), and low or high pH (pH 4.5, pH 8.9, or pH 11) have been tested with a flow rate of 1 ml/min.
SDS-PAGE and Western blot analysis.
Proteins separated by SDS-PAGE were either stained by Coomassie brilliant blue or electroblotted onto nitrocellulose membranes (Osmonics Nitro Bind; GE Osmonics Labstore, Minnetonka, MN). Membranes were blocked with 5% dried milk (Blotto)-PBS containing 0.5% Tween 20 (PBS-T) overnight at 4°C and stained with first and horseradish peroxidase-labeled secondary antibodies diluted in 2.5% Blotto-PBS-T. Signals were detected with the ECL detection system (Amersham Biosciences Corp., Piscataway, NJ), according to the manufacturer's instructions. All wash steps were done with PBS-T.
Production of hyperimmune sera against SV capsid fusion proteins.
Hyperimmune sera were raised in rabbits (150 μg protein/immunization) and guinea pigs (50 μg protein/immunization). Animals were immunized subcutaneously three times in 2-week intervals. Complete Freund's adjuvant was used in the primary immunization, and incomplete Freund's adjuvant was used in the following immunizations. Preblood samples, blood samples drawn during the immunization schedule, and the final blood samples were titrated in antibody detection EIA. Serum samples were stored in aliquots at −70°C. The animal protocol was approved by the Institutional Animal Care and Use Committee of Cincinnati Children's Hospital Medical Center.
Antibody detection EIA.
To measure specific antibodies in serum samples, antigens diluted in PBS were coated onto 96-well microtiter plates (Dynex Immulon; Dynatech) at 30 ng/well for VA387 VLPs and 150 ng/well for MBP2* (New England BioLabs, Beverly, MA) and MBP-Mex340-6xHis overnight at 4°C. The optimal antigen concentration for coating was determined by testing serial dilutions of the antigens with positive and negative patients' sera and plotting saturation curves (data not shown). Plates were blocked with 5% Blotto-PBS for 1 h at 37°C. Serum samples diluted to 1:100 in 1% Blotto-PBS were added, and the plates were incubated for 1 h at 37°C. Horseradish peroxidase-conjugated goat anti-human immunoglobulin G (Cappel Organon Teknika, Durham, N.C.) was added at a 1:5,000 dilution in 1% Blotto-PBS, and the plates were incubated for 1 h at 37°C. Between each step, the plates were washed five times with PBS-0.5% Tween 20 in an automated plate washer (ELx 405 auto plate washer; Bio-Tek Instruments, Inc., Winooski, VT). Finally, tetramethylbenzidine substrate (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, MD) was added, the plates were incubated for 10 min at room temperature, and the color development was stopped by adding 100 μl of a 1 M H3PO4 solution per well. Results were read by a Tecan Spectra II microtiter plate reader (Tecan AG, Switzerland) at OD450. The cutoff point of the VA387 EIA was determined by analyzing a group of samples collected during gastroenteritis outbreak investigations (P. W. Huang, unpublished data). The cutoff point of the Mex340 EIA was determined as the mean OD450 ± 2 standard deviations of the 567 serum samples on the MBP2*-coated wells.
RESULTS
Expression of SV capsid proteins in baculovirus.
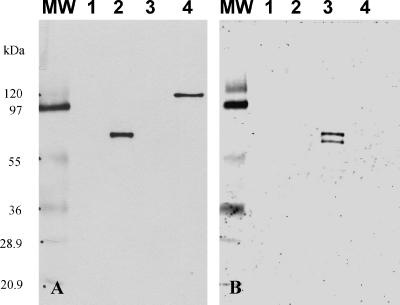
The expression levels of two SV capsid proteins, Mex340 (GII/2) and Mex14917 (GI/3), were evaluated in the baculovirus system in this study. As a positive control for expression, a previously made construct expressing an NV capsid protein (SW918) with high VLP yield was used. The proteins of neither of the SV strains could be detected by SDS-PAGE analysis; however, a typical 60-kDa protein band was visible in Western blots of sucrose gradient-purified fractions of Mex340, immunostained with human sera of patients who were subclinically infected with a genetically related strain (Cruise ship virus; GII/3). The sucrose gradient analysis also indicated that Mex340 capsid proteins formed VLPs, and EM examination of positive fractions revealed scattered virus-like particles (data not shown). Furthermore, hyperimmune sera produced against E. coli-expressed GST-Mex340 and GST-Mex14917 fusion proteins reacted specifically with the homologous baculovirus-expressed antigens in both Western blots (Fig. 1) and EIA. We were unable to increase the yield of the SV capsid proteins by using different constructs of the expression vectors, including an insertion of a lobster tropomyosin leader sequence upstream to the start codon, which was reported to enhance protein expression in insect cells (37), or by changing the host cells (Sf21 or H5).
FIG. 1.
Hyperimmune sera raised against SV bacterial fusion proteins recognized baculovirus-expressed SV capsid proteins in a type-specific manner. Western blot immunostained with (A) guinea pig anti-GST-Mex340 hyperimmune serum (1:5,000) and (B) guinea pig anti-GST-Mex14917 hyperimmune serum (1:5,000). Lane 1, wild-type baculovirus; lane 2, baculovirus-expressed recombinant Mex340; lane 3, baculovirus-expressed recombinant Mex14917; lane 4, MBP-Mex340 fusion protein. MW, molecular weight marker (molecular weights in thousands). For lanes 1 to 3, sucrose gradient fraction 6-containing recombinant VLPs were used.
Expression of SV capsid proteins in bacteria.
To overcome the low yield of the baculovirus expression system, SV capsid proteins were expressed as GST fusion proteins in E. coli. After affinity column purification, high yields (15 to 20 mg/liter culture) of soluble fusion proteins were obtained; however, a ∼60-kDa bacterial protein that was recognized by an anti-GroEL antibody (Sigma-Aldrich Co., St. Louis, MO) copurified with the SV fusion proteins (Fig. 2). Copurification of the GroEL chaperonin remained an issue even when SV capsid proteins were expressed with the much larger MBP as the fusion tag. Removal of GroEL is necessary to develop an antibody detection EIA because anti-GroEL antibodies have frequently been found in human sera (2, 11, 25). In fact, when a set of randomly selected human sera was tested in Western blot analyses, several of them reacted with GroEL.
FIG. 2.
SV fusion protein purification. (A) SDS-PAGE. (B) Western blot immunostained with anti-GST-Mex340 hyperimmune serum (1:10,000). (C) Western blot immunostained with anti-GroEL hyperimmune serum (1:10,000; Sigma). Lane 1, amylose affinity resin-purified MBP-Mex340-6xHis; lane 2, GroEL (B-PER wash); lane 3, amylose and Talon affinity resin-purified full-length MBP-Mex340-6xHis; lane 4, MBP2. MW, molecular weight marker (molecular weights in thousands).
Fast protein liquid chromatography analysis on a Superdex 200 16/60 column of affinity column-purified SV fusion proteins indicated that the GST- or MBP-fused capsid proteins formed microaggregates with GroEL as they eluted in the void. The addition of commonly used nonionic detergents (0.1% Triton X-100, 0.1 to 0.4% CHAPS, 1% OG), reducing agent (0.2 to 1 mM dithiothreitol), 5 to 10% glycerol, 10 to 15% acetonitrile to the buffer or high salt (500 to 700 mM NaCl) or low or high pH buffers (pH 4.5, pH 8.9, or pH 11) did not result in separation. The methods described in the literature for the removal of GroEL from GST fusion proteins (35, 41) also failed in our case. However, we identified a commercial product, B-PER (Pierce Biotechnology Inc., Rockford, IL), to be highly effective in releasing GroEL from the affinity column-bound SV fusion proteins (Fig. 2). Since protein degradation was still noticeable, MBP-SV fusion proteins with a C-terminal His tag were expressed for the selective purification of full-length MBP-SV-6xHis proteins by double-affinity resin purification and B-PER washes (Fig. 2). Removal of the MBP or GST tag was unsuccessful because factor Xa or thrombin cleavage, respectively, resulted in the degradation of the SV capsid proteins. For this reason, uncleaved fusion proteins were used for the development of antibody detection EIA.
Development of an antibody detection EIA for SVs.
Since patient sera detected both baculovirus- and bacterium-expressed SV capsid proteins in a type-specific manner and rabbit or guinea pig hyperimmune sera raised against E. coli-expressed SV fusion proteins similarly detected the corresponding baculovirus-expressed proteins (Fig. 1), we concluded that the bacterium-expressed SV capsid fusion proteins exhibit the major antigenic epitopes of SV virions and can be used for measuring seroresponses to SV infection. To study the antigenic relationship between Mex340 (GII/2) and other SVs, we analyzed the reactivity of the anti-MBP-Mex340-6xHis hyperimmune rabbit sera with GST and a panel of GST-SV fusion proteins representing different genetic types. The anti-MBP-Mex340-6xHis serum specifically reacted with GST-Mex340 fusion protein at a 1:102,400 dilution (homologous titer). It also recognized GST-Cruise ship (GII/3) but with an eightfold-lower titer (1:12,800). SV fusion proteins representing heterologous genogroups showed only minor cross-reactivities (1:800 to 1:1,600) (Fig. 3). These results indicated that an antibody detection EIA based on the Mex340 capsid fusion protein is likely to detect antibodies not only against the homologous strain but also against heterologous strains within the genogroup. To evaluate the application of this assay for measuring SV-specific serum antibodies in patients, full-length GroEL-free MBP-Mex340-6xHis was used. As a negative control, each serum sample was also tested for antibody against MBP.
FIG. 3.
Titration of rabbit anti-MBP-Mex340-6xHis hyperimmune serum against GST-SV fusion proteins. Rabbit anti-MBP-Mex340-6xHis hyperimmune serum was titrated in twofold dilutions starting from 1:100 against GST and GST-SV fusion proteins (100 ng/well) in an EIA. End titers were read as the highest dilution for a given antigen with an OD450 of ≥0.2.
Prevalence of anti-SV antibodies in Mexican children.
The Mex340 EIA was used to study the age-related prevalence of anti-SV antibodies in children involved in a birth cohort in Mexico. For a comparison, serum samples were also tested for antibodies against an NV (VA387; GII/4). Among the 567 serum samples collected from 102 children in the cohort between birth and 2 years of age, 92 (93%) of the 99 samples collected between 0 and 3 months of age contained antibodies against VA387 but only 23 (23%) of them were positive for antibodies against Mex340. The prevalence of antibodies against VA387 showed a slight decrease between 4 and 7 months to 85% and then increased and reached its maximum (98%) at 16 to 19 months of age. The prevalence of antibody to Mex340 linearly increased by age, without the drop at 4 to 7 months seen for VA387, and reached its plateau (91%) at 20 to 24 months of age (Fig. 4).
FIG. 4.
Age-related prevalence (%) of antibodies to VA387 and Mex340 in Mexican children of ≤2 years of age.
DISCUSSION
In this study, E. coli-expressed SV capsid proteins were used for the development of an antibody detection EIA because of the insufficient yields of SV capsid protein expression in the baculovirus system. Several previous studies also reported low yields of SV VLP expressions in baculovirus (10, 12, 19). The reason(s) behind the low yield is under investigation in our laboratory.
Yoda et al. reported the expression of capsid fusion proteins of NVs in E. coli and found that hyperimmune sera produced against a GII MBP-NV capsid fusion protein detected several GII and even a GI NV capsid fusion protein in Western blot analysis and EIA (45). In addition, this antiserum also detected authentic GII NVs derived from stool samples of patients in Western blot analysis. The usefulness of bacterium-expressed recombinant proteins was also demonstrated in our previous studies in which the E. coli-expressed NV capsid proteins or the capsid P domains retained strain-specific receptor binding characteristics (13, 39, 40). Thus, the bacterial expression system can be an alternative of the baculovirus expression system in immunological assay development for HuCVs.
The biggest challenge in our study was the copurification of GroEL, a problem frequently encountered during fusion protein production in E. coli. This problem was also encountered in our studies of NV capsid fusion protein expression in E. coli, although it was not reported in the study by Yoda et al. (45). After evaluating a number of methods for the removal of GroEL, we identified the B-PER wash of the affinity column-bound fusion proteins to be the most effective. We believe the removal of GroEL is necessary for the development of a highly specific antibody detection EIA because of the relatively high prevalence of anti-GroEL antibodies in human sera (2, 11, 24, 25).
A further challenge was the sensitivity of the SV capsid proteins to proteases factor Xa and thrombin. For this reason, full-length fusion proteins were used for hyperimmune antiserum production and as antigen for detecting anti-SV antibodies in human serum samples.
Hyperimmune sera generated against the SV capsid fusion proteins reacted strongly with the homologous antigens expressed in both baculovirus and E. coli but only moderately (8 times weaker) with a heterologous strain within the genogroup and weakly (64 to 128 times) with strains in heterologous genogroups (Fig. 3), indicating that the antigenic relationships among SVs correlate well with genetic relationships, similar to that recently reported by Hansman et al. (12). In contrast to the highly specific reactivity of antibodies generated against baculovirus-expressed recombinant NV VLPs (18), Yoda et al. reported broader intergenogroup reactivities of hyperimmune serum generated against E. coli-expressed NV capsid fusion proteins (45). These results postulate the role of epitopes on the more conserved N-terminal region of the capsid protein, which was supported by the epitope mapping of two broadly reactive monoclonal antibodies (44, 46). In our study, the two hyperimmune sera generated against E. coli-expressed SV capsid fusion proteins showed little cross-reactivity with strains representing heterologous genogroups; however, a direct comparison of antibodies generated against a panel of SV VLPs and E. coli fusion proteins remains to be elucidated.
The most interesting finding of this study is the significantly lower seroprevalence of SVs (23%) than that of NVs (93%) in children between 0 and 3 months of age. Since 97% of the samples examined from this age group were collected within the first week of life, this likely represents the prevalence of maternal antibody against these viruses. Similarly, a low prevalence of antibodies against SVs in children of <5 months of age was also reported in Japan and Kenya (29, 36), although another study reported a 100% seroprevalence to the Sapporo virus among children 0 to 3 months of age in Houston, TX, with a sharp drop to 25% between 4 and 11 months of age (28). When serum samples collected from U.S. military personnel were studied, we found a 63% prevalence of SV antibodies and 63 to 100% prevalence in adults was reported in Asian countries and the United States by others (26, 28). This discrepancy between the high prevalence of antibody to SVs in adults and the low prevalence of maternal antibody in infants indicates some unique feature of SV infection and immunity which needs to be addressed in future studies.
The high prevalence of SV antibodies by 2 years of age in Mexican children indicates a high frequency of SV infections in early childhood in this community. The outcome of these infections (clinical or subclinical) and the role of antibodies acquired by the first infection in protection against subsequent infections or clinical disease by the same or different antigenic types are unknown and need to be evaluated. One early study indicates that the presence of SV-specific serum antibodies correlates with resistance to SV gastroenteritis (27). In one of our previous studies, 5.2% of the diarrhea and 3% of the nondiarrhea stool samples collected from Mexican children contained SV-specific sequences (8), indicating that SVs may cause a significant number of subclinical infections. Thus, future studies to better understand SV infection and immunity are warranted.
Acknowledgments
We thank Irene Hofmann for helping with the EM and Weiming Zhong for laboratory assistance.
This study was supported by a Trusty grant from the Cincinnati Children's Hospital Research Foundation and by the NIH (R01 AI37093 and PO1 HD 13021).
REFERENCES
- 1.Atmar, R. L., and M. K. Estes. 2001. Diagnosis of noncultivatable gastroenteritis viruses, the human caliciviruses. Clin. Microbiol. Rev. 14:15-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bencina, D., B. Slavec, and M. Narat. 2005. Antibody response to GroEL varies in patients with acute Mycoplasma pneumoniae infection. FEMS Immunol. Med. Microbiol. 43:399-406. [DOI] [PubMed] [Google Scholar]
- 3.Chiba, S., Y. Sakuma, R. Kogasaka, M. Akihara, K. Horino, T. Nakao, and S. Fukui. 1979. An outbreak of gastroenteritis associated with calicivirus in an infant home. J. Med. Virol. 4:249-254. [DOI] [PubMed] [Google Scholar]
- 4.Deng, Y., C. A. Batten, B. L. Liu, P. R. Lambden, M. Elschner, H. Gunther, P. Otto, P. Schnurch, W. Eichhorn, W. Herbst, and I. N. Clarke. 2003. Studies of epidemiology and seroprevalence of bovine noroviruses in Germany. J. Clin. Microbiol. 41:2300-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duizer, E., K. J. Schwab, F. H. Neill, R. L. Atmar, M. P. Koopmans, and M. K. Estes. 2004. Laboratory efforts to cultivate noroviruses. J. Gen. Virol. 85:79-87. [DOI] [PubMed] [Google Scholar]
- 6.Farkas, T., S. Nakajima, M. Sugieda, X. Deng, W. Zhong, and X. Jiang. 2005. Seroprevalence of noroviruses in swine. J. Clin. Microbiol. 43:657-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farkas, T., W. M. Zhong, Y. Jing, P. W. Huang, S. M. Espinosa, N. Martinez, A. L. Morrow, G. M. Ruiz-Palacios, L. K. Pickering, and X. Jiang. 2004. Genetic diversity among sapoviruses. Arch. Virol. 149:1309-1323. [DOI] [PubMed] [Google Scholar]
- 8.Farkas, T., X. Jiang, M. L. Guerrero, W. Zhong, N. Wilton, T. Berke, D. O. Matson, L. K. Pickering, and G. Ruiz-Palacios. 2000. Prevalence and genetic diversity of human caliciviruses (HuCVs) in Mexican children. J. Med. Virol. 62:217-223. [PubMed] [Google Scholar]
- 9.Green, K. Y., T. Ando, M. S. Balayan, T. Berke, I. N. Clarke, M. K. Estes, D. O. Matson, S. Nakata, J. D. Neill, M. J. Studdert, and H. J. Thiel. 2000. Taxonomy of the caliciviruses. J. Infect. Dis. 181(Suppl. 2):S322-S330. [DOI] [PubMed] [Google Scholar]
- 10.Guo, M., Y. Qian, K. O. Chang, and L. J. Saif. 2001. Expression and self-assembly in baculovirus of porcine enteric calicivirus capsids into virus-like particles and their use in an enzyme-linked immunosorbent assay for antibody detection in swine. J. Clin. Microbiol. 39:1487-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Handley, H. H., J. Yu, D. T. Yu, B. Singh, R. S. Gupta, and J. H. Vaughan. 1996. Autoantibodies to human heat shock protein (hsp)60 may be induced by Escherichia coli groEL. Clin. Exp. Immunol. 103:429-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansman, G. S., K. Natori, T. Oka, S. Ogawa, K. Tanaka, N. Nagata, H. Ushijima, N. Takeda, and K. Katayama. 2005. Cross-reactivity among sapovirus recombinant capsid proteins. Arch. Virol. 150:21-36. [DOI] [PubMed] [Google Scholar]
- 13.Huang, P., T. Farkas, S. Marionneau, W. Zhong, N. Ruvoen-Clouet, A. L. Morrow, M. Altaye, L. K. Pickering, D. S. Newburg, J. LePendu, and X. Jiang. 2003. Noroviruses bind to human ABO, Lewis, and secretor histo-blood group antigens: identification of 4 distinct strain-specific patterns. J. Infect. Dis. 188:19-31. [DOI] [PubMed] [Google Scholar]
- 14.Jiang, X., D. Y. Graham, K. N. Wang, and M. K. Estes. 1990. Norwalk virus genome cloning and characterization. Science 250:1580-1583. [DOI] [PubMed] [Google Scholar]
- 15.Jiang, X., J. Wang, and M. K. Estes. 1995. Characterization of SRSVs using RT-PCR and a new antigen ELISA. Arch. Virol. 140:363-374. [DOI] [PubMed] [Google Scholar]
- 16.Jiang, X., J. Wang, D. Y. Graham, and M. K. Estes. 1992. Detection of Norwalk virus in stool by polymerase chain reaction. J. Clin. Microbiol. 30:2529-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang, X., M. Wang, D. Y. Graham, and M. K. Estes. 1992. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J. Virol. 66:6527-6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang, X., N. Wilton, W. M. Zhong, T. Farkas, P. W. Huang, E. Barrett, M. Guerrero, G. Ruiz-Palacios, K. Y. Green, J. Green, A. D. Hale, M. K. Estes, L. K. Pickering, and D. O. Matson. 2000. Diagnosis of human caliciviruses by use of enzyme immunoassays. J. Infect. Dis. 181(Suppl. 2):S349-S359. [DOI] [PubMed] [Google Scholar]
- 19.Jiang, X., W. Zhong, M. Kaplan, L. K. Pickering, and D. O. Matson. 1999. Expression and characterization of Sapporo-like human calicivirus capsid proteins in baculovirus. J. Virol. Methods 78:81-91. [DOI] [PubMed] [Google Scholar]
- 20.Jiang, X., W. M. Zhong, T. Farkas, P. W. Huang, N. Wilton, E. Barrett, D. Fulton, R. Morrow, and D. O. Matson. 2002. Baculovirus expression and antigenic characterization of the capsid proteins of three Norwalk-like viruses. Arch. Virol. 147:119-130. [DOI] [PubMed] [Google Scholar]
- 21.Jing, Y., Y. Qian, Y. Huo, L. P. Wang, and X. Jiang. 2000. Seroprevalence against Norwalk-like human caliciviruses in Beijing, China. J. Med. Virol. 60:97-101. [DOI] [PubMed] [Google Scholar]
- 22.Johansson, P. J., K. Bergentoft, P. A. Larsson, G. Magnusson, A. Widell, M. Thorhagen, and K. O. Hedlund. 2005. A nosocomial sapovirus-associated outbreak of gastroenteritis in adults. Scand. J. Infect. Dis. 37:200-204. [DOI] [PubMed] [Google Scholar]
- 23.Kapikian, A. Z., R. G. Wyatt, R. Dolin, T. S. Thornhill, A. R. Kalica, and R. M. Chanock. 1972. Visualization by immune electron microscopy of a 27-nm particle associated with acute infectious nonbacterial gastroenteritis. J. Virol. 10:1075-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lemos, J. A., M. Giambiagi-Demarval, and A. C. Castro. 1998. Expression of heat-shock proteins in Streptococcus pyogenes and their immunoreactivity with sera from patients with streptococcal diseases. J. Med. Microbiol. 47:711-715. [DOI] [PubMed] [Google Scholar]
- 25.Mayr, M., B. Metzler, S. Kiechl, J. Willeit, G. Schett, Q. Xu, and G. Wick. 1999. Endothelial cytotoxicity mediated by serum antibodies to heat shock proteins of Escherichia coli and Chlamydia pneumoniae: immune reactions to heat shock proteins as a possible link between infection and atherosclerosis. Circulation 99:1560-1566. [DOI] [PubMed] [Google Scholar]
- 26.Nakata, S., S. Chiba, H. Terashima, and T. Nakao. 1985. Prevalence of antibody to human calicivirus in Japan and Southeast Asia determined by radioimmunoassay. J. Clin. Microbiol. 22:519-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakata, S., S. Chiba, H. Terashima, T. Yokoyama, and T. Nakao. 1985. Humoral immunity in infants with gastroenteritis caused by human calicivirus. J. Infect. Dis. 152:274-279. [DOI] [PubMed] [Google Scholar]
- 28.Nakata, S., M. K. Estes, and S. Chiba. 1988. Detection of human calicivirus antigen and antibody by enzyme-linked immunosorbent assays. J. Clin. Microbiol. 26:2001-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakata, S., S. Honma, K. Numata, K. Kogawa, S. Ukae, N. Adachi, X. Jiang, M. K. Estes, Z. Gatheru, P. M. Tukei, and S. Chiba. 1998. Prevalence of human calicivirus infections in Kenya as determined by enzyme immunoassays for three genogroups of the virus. J. Clin. Microbiol. 36:3160-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Numata, K., M. E. Hardy, S. Nakata, S. Chiba, and M. K. Estes. 1997. Molecular characterization of morphologically typical human calicivirus Sapporo. Arch. Virol. 142:1537-1552. [DOI] [PubMed] [Google Scholar]
- 31.Pang, X. L., S. Honma, S. Nakata, and T. Vesikari. 2000. Human caliciviruses in acute gastroenteritis of young children in the community. J. Infect. Dis. 181(Suppl. 2):S288-S294. [DOI] [PubMed] [Google Scholar]
- 32.Pang, X. L., J. Joensuu, and T. Vesikari. 1999. Human calicivirus-associated sporadic gastroenteritis in Finnish children less than two years of age followed prospectively during a rotavirus vaccine trial. Pediatr. Infect. Dis. J. 18:420-426. [DOI] [PubMed] [Google Scholar]
- 33.Parker, S. P., W. D. Cubitt, X. J. Jiang, and M. K. Estes. 1994. Seroprevalence studies using a recombinant Norwalk virus protein enzyme immunoassay. J. Med. Virol. 42:146-150. [DOI] [PubMed] [Google Scholar]
- 34.Phan, T. G., M. Okame, T. A. Nguyen, N. Maneekarn, O. Nishio, S. Okitsu, and H. Ushijima. 2004. Human astrovirus, norovirus (GI, GII), and sapovirus infections in Pakistani children with diarrhea. J. Med. Virol. 73:256-261. [DOI] [PubMed] [Google Scholar]
- 35.Rohman, M., and K. J. Harrison-Lavoie. 2000. Separation of copurifying GroEL from glutathione-S-transferase fusion proteins. Protein Expr. Purif. 20:45-47. [DOI] [PubMed] [Google Scholar]
- 36.Sakuma, Y., S. Chiba, R. Kogasaka, H. Terashima, S. Nakamura, K. Horino, and T. Nakao. 1981. Prevalence of antibody to human calicivirus in general population of northern Japan. J. Med. Virol. 7:221-225. [DOI] [PubMed] [Google Scholar]
- 37.Sano, K., K. Maeda, M. Oki, and Y. Maeda. 2002. Enhancement of protein expression in insect cells by a lobster tropomyosin cDNA leader sequence. FEBS Lett. 532:143-146. [DOI] [PubMed] [Google Scholar]
- 38.Simpson, R., S. Aliyu, M. Iturriza-Gomara, U. Desselberger, and J. Gray. 2003. Infantile viral gastroenteritis: on the way to closing the diagnostic gap. J. Med. Virol. 70:258-262. [DOI] [PubMed] [Google Scholar]
- 39.Tan, M., and X. Jiang. 2005. The P domain of norovirus capsid protein forms a subviral particle that binds to histo-blood group antigen receptors. J. Virol. 79:14017-14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan, M., W. Zhong, D. Song, S. Thornton, and X. Jiang. 2004. E. coli-expressed recombinant norovirus capsid proteins maintain authentic antigenicity and receptor binding capability. J. Med. Virol. 74:641-649. [DOI] [PubMed] [Google Scholar]
- 41.Thain, A., K. Gaston, O. Jenkins, and A. R. Clarke. 1996. A method for the separation of GST fusion proteins from co-purifying GroEL. Trends Genet. 12:209-210. [DOI] [PubMed] [Google Scholar]
- 42.Vinje, J., R. A. Hamidjaja, and M. D. Sobsey. 2004. Development and application of a capsid VP1 (region D) based reverse transcription PCR assay for genotyping of genogroup I and II noroviruses. J. Virol. Methods 116:109-117. [DOI] [PubMed] [Google Scholar]
- 43.Yan, H., T. Abe, T. G. Phan, T. A. Nguyen, T. Iso, Y. Ikezawa, K. Ishii, S. Okitsu, and H. Ushijima. 2005. Outbreak of acute gastroenteritis associated with group A rotavirus and genogroup I sapovirus among adults in a mental health care facility in Japan. J. Med. Virol. 75:475-481. [DOI] [PubMed] [Google Scholar]
- 44.Yoda, T., Y. Suzuki, Y. Terano, K. Yamazaki, N. Sakon, T. Kuzuguchi, H. Oda, and T. Tsukamoto. 2003. Precise characterization of Norovirus (Norwalk-like virus)-specific monoclonal antibodies with broad reactivity. J. Clin. Microbiol. 41:2367-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoda, T., Y. Terano, A. Shimada, Y. Suzuki, K. Yamazaki, N. Sakon, I. Oishi, E. T. Utagawa, Y. Okuno, and T. Shibata. 2000. Expression of recombinant Norwalk-like virus capsid proteins using a bacterial system and the development of its immunologic detection. J. Med. Virol. 60:475-481. [DOI] [PubMed] [Google Scholar]
- 46.Yoda, T., Y. Terano, Y. Suzuki, K. Yamazaki, I. Oishi, T. Kuzuguchi, H. Kawamoto, E. Utagawa, K. Takino, H. Oda, and T. Shibata. 2001. Characterization of Norwalk virus GI specific monoclonal antibodies generated against Escherichia coli expressed capsid protein and the reactivity of two broadly reactive monoclonal antibodies generated against GII capsid towards GI recombinant fragments. BMC Microbiol. 1:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zintz, C., K. Bok, E. Parada, M. Barnes-Eley, T. Berke, M. A. Staat, P. Azimi, X. Jiang, and D. O. Matson. 2005. Prevalence and genetic characterization of caliciviruses among children hospitalized for acute gastroenteritis in the United States. Infect. Genet. Evol. 5:281-290. [DOI] [PubMed] [Google Scholar]