Abstract
One approach to microbial genotyping is to make use of sets of single-nucleotide polymorphisms (SNPs) in combination with binary markers. Here we report the modification and automation of a SNP-plus-binary-marker-based approach to the genotyping of Staphylococcus aureus and its application to 391 S. aureus isolates from southeast Queensland, Australia. The SNPs used were arcC210, tpi243, arcC162, gmk318, pta294, tpi36, tpi241, and pta383. These provide a Simpson's index of diversity (D) of 0.95 with respect to the S. aureus multilocus sequence typing database and define 61 genotypes and the major clonal complexes. The binary markers used were pvl, cna, sdrE, pT181, and pUB110. Two novel real-time PCR formats for interrogating these markers were compared. One of these makes use of “light upon extension” (LUX) primers and biplexed reactions, while the other is a streamlined modification of kinetic PCR using SYBR green. The latter format proved to be more robust. In addition, automated methods for DNA template preparation, reaction setup, and data analysis were developed. A single SNP-based method for ST-93 (Queensland clone) identification was also devised. The genotyping revealed the numerical importance of the “South West Pacific” and “Queensland” community-acquired methicillin-resistant S. aureus (MRSA) clones and the clonal complex 239 “Aus-1/Aus-2” hospital-associated MRSA. There was a strong association between the community-acquired clones and pvl.
Bacterial genotyping is a field in which there is continuous technological innovation. Many methods have been described, with pulsed-field gel electrophoresis, amplified fragment length polymorphism, multilocus sequence typing (MLST), and variable number tandem repeat analysis currently being used widely. In general, the whole-genome/electrophoresis-based methods such as pulsed-field gel electrophoresis and amplified fragment length polymorphism give very high resolution (13, 29) and are used for establishing epidemiological linkages in short time scales, MLST is used for research into large scale population structures (3, 4), while variable number tandem repeat analysis is showing some promise at being amenable to both classes of application (7, 14).
We have devised a systematic approach to the development of new bacterial genotyping methods. The central hypothesis is that, given a defined data set of genetic diversity within a bacterial species, an appropriate means of analyzing those data, and a numerical description of the required resolving power of a genotyping method, it is in principal always possible to identify a set of polymorphic sites that, if interrogated, will provide the required resolving power in an efficient manner. Robertson et al. (25) described the “Minimum SNPs” software that can derive highly informative sets of single-nucleotide polymorphisms (SNPs) from DNA sequence alignments and the application of this to the development of SNP-based genotyping methods for Neisseria meningitidis and Staphylococcus aureus. The SNPs used were derived from MLST databases. The most generally applicable SNP sets were optimized through maximization of Simpson's index of diversity (D). This work was extended by Stephens et al. (28), who showed that, in the case of S. aureus, the genotypes defined by a seven-member high-D SNP set were concordant with the population structure of the species, and that it is possible to identify a small set of binary markers (genes present in some isolates but not others) that efficiently add genotyping resolution.
Real-time PCR is a single-step closed tube method that is amenable to automated setup and data analysis. Real-time PCR devices are increasingly cost-effective to purchase and run, standardized real-time PCR-based procedures can incorporate the interrogation of different classes of polymorphisms (e.g., SNPs plus genes that exhibit binary variability), and real-time PCR-based procedures have the potential to incorporate primary diagnosis and target quantification. An inherent disadvantage of real-time PCR is that it is not very amenable to multiplexing, so interrogating multiple genetic targets normally requires multiple reactions. In our previous reports of the application of real-time PCR to SNP-based bacterial genotyping, the technique used was allele-specific PCR in the real-time format (kinetic PCR) (10, 25, 28). The advantage of kinetic PCR is that it is simple and robust and requires only generic mastermix and unlabeled primers. The disadvantage is that it requires at least two reactions per SNP, making the interrogation of multiple SNPs somewhat unwieldy and expensive. Therefore, an objective of this study was to develop variants of kinetic PCR that preserve the advantages but reduce the cost and the number of reactions required.
Here we report the development of two novel formats for allele-specific real-time PCR, the application of these methods to the genotyping of a large number of S. aureus isolates, and automated methods for DNA preparation, reaction setup, and data analysis. The utilities of these methods have been compared using S. aureus as a model system. This study has yielded a robust, cost-effective, and automated procedure for S. aureus genotyping. The small number of genetic targets interrogated means that this approach is especially amenable to adaptation to emerging technologies such as “lab-on-a-chip” devices and dedicated, fully automated real-time PCR machines.
MATERIALS AND METHODS
MLST clonal group analysis of S. aureus using SNP-based genotyping.
An eBURST analysis was done on 403 S. aureus sequence types (STs) (5). These STs were included when the eBURST facility at http://eburst.mlst.net/ was asked to analyze the entire S. aureus MLST database. Clonal complexes were defined using the program's default settings.
Bacterial isolates.
All S. aureus isolates used for the SNP genotyping were collected between June 2004 and November 2004 in southeast Queensland, Australia. They were obtained from 391 cases of inpatient and outpatient infections at the Princess Alexandra, Ipswich, Queen Elizabeth II, Logan, Redland, Boonah, and Beaudesert Hospitals. The identities of isolates were confirmed by nuc PCR, and methicillin resistance was confirmed by mecA PCR. A list of all isolates, together with genotyping results, is available upon request.
DNA extractions.
Cultivation of all S. aureus isolates and DNA extractions from a small subset of the isolates were as described by Stephens et al. (28). As part of the current study, an automated method for S. aureus DNA extraction based on the Corbett X-tractor Gene automated DNA extraction system (Corbett Robotics, Brisbane, Australia) was developed. The optimal protocol arrived at was as follows. Twelve colonies from overnight cultures on blood agar plates were suspended in 100 μl lysostaphin buffer (40 μg/ml) and incubated at room temperature for 30 min. Lysates were transferred to the X-tractor Gene (Corbett Robotics, Brisbane, Australia) and 200 μl liquid sample digest buffer (catalog no. B5810) using X-tractor Gene reagent pack (catalog no. XTR-1; Corbett Life Science, Brisbane, Australia) was added, and incubation at room temperature continued for a further 30 min. The program from this point on was identical to the whole-blood DNA extraction protocol in the X-tractor Gene product literature (http://www.corbettlifescience.com/control.cfm?page=X-tractor_Gene).
SNPs and binary markers.
The SNPs used in this study were derived from the S. aureus MLST database available at http://www.mlst.net. The SNPs were identified using the software package Minimum SNPs, v.2.0415. This program is as described by Robertson et al. (25) but with the added feature that the user can specify that certain SNPs are excluded from or included in the SNP set (24). This greatly facilitates the assembly of SNP sets with reference to primer design constraints. The eight-member SNP set ultimately used in this study provides a Simpson's index of diversity (D) of 0.95 with respect to the entire S. aureus MLST database. The SNPs are acrC210, tpi241, tpi243, arcC162, gmk318, pta294, tpi36, and pta383. The binary markers interrogated in this study were pvl, cna, sdrE, pUB110, and pT181.
Primer sequences.
The sequences of all primers used in this study are shown in Table 1. Light upon extension (LUX) primers were obtained from Invitrogen Life Technologies, while the unlabeled primers were obtained from Proligo (Lismore, NSW, Australia). Primer design was carried out using Primer Express 2.0.0 (Applied Biosystems) and the LUX primer design software (Invitrogen).
TABLE 1.
Primer sequences for fluorescent (LUX) and unlabeled primers
| Primer name | Sequencea |
|---|---|
| L1 | FAM-caaccaCAGGGTATGATAGGCTATTGGtTG |
| L2 | JOE-gacattgTTGGATACTTGTGGTGCAATGtC |
| L4 | JOE-cacaaaGCCCCATCAATATCAGTTTGtG |
| L5 | FAM-cacaaaGCCCCATCAATATCAGTTTGtG |
| L1A | FAM-gtatccCGGCAATGCCATTGGAtAC |
| L2B | JOE-gacattgTTGGATACTTGTGGTGCAATGtC |
| L6 | FAM-caccttACGTCAAATGCGTGAAGGtG |
| L7 | JOE-catcaaTGCGTGAAGGTGAAGTTGAtG |
| L8 | FAM-caatccCCTTGTGAATCAAGTTCTGGAtTG |
| L9 | JOE-caatccCCTTGTGAATCAAGTTCTGGAtTG |
| L10 | JOE-caatcgTTGATGATTTACCAGTTCCGAtTG |
| L11 | FAM-caatcgTTGATGATTTACCAGTTCCGAtTG |
| L12 | JOE-cagcttCTGCGACAAGTGAATTGAAAGCtG |
| L13 | FAM-cagcttCTGCGACAAGTGAATTGAAAGCtG |
| L20 | JOE-gatctgTGAAGGGAATGCAGCAGAtC |
| L14 | FAM-gtacttcaCGGGATTTGCAGAATTTGAAGtAC |
| L18A | JOE-cacgcTAGGTGTTGTGCCTATGCGtG |
| L16 | FAM-cacctgTATCTCTAACGGCTTGTCAGGtG |
| L22 | JOE-caccgAACTTCTAATGACACTGGCGGtG |
| U1 | CGTATAAAAAGGACCAATTGGTCTG* |
| U2 | CGTATAAAAAGGACCAATTGGTCTA* |
| U3 | CGTATAAAAAGGACCAATTGGTCTT* |
| U4 | GTAAATCATCAACATCTGAAGATATG |
| U5 | GTAAATCATCAACATCTGAAGATGTG |
| U6 | GTAAATCATCAACATCTGAAGATGTA |
| U7 | GATCATCTTTATCTACTTCCACACGTGCA* |
| U8 | GATCATCTTTATCTACTTCCACACGTGCT* |
| U9 | ACCTACTAATCGCTCTCTCAAGTAA* |
| U10 | ACCTACTAATCGCTCTCTCAAGTAT* |
| U11 | TGCAGCACATTCAACAGAA |
| U12 | TGCAGCACATTCAACAGAC |
| U13 | TGCAGCACATTCAACAGAT |
| U14 | GATGAAGAAATTAACAAAAAAGCGCCT |
| U15 | GATGAAGAAATTAACAAAAAAGCGCCC |
| U16 | TTGCACAATCACCAAAGATGTATTATA* |
| U17 | TTGCACAATCACCAAAGATGTATTATT* |
| U18 | TTGAGCTGTCTTGGTTCATTGATT |
| U19 | AAACACTACTTGTTCCCGCTTCA |
| U20 | GGTCAGAGCCACGTTCACCA |
| U21 | TGCTTCAACATCCCAACCAA |
| U22 | TGGACACCGTCTTTATCAACGTCT |
| S1 | CAGGGTATGATAGGCTATTGGTTG |
| S2 | GCCCCATCAATATCAGTTTGTG |
| S3 | ACGTCAAATGCGTGAAGGTG |
| S4 | CCTTGTGAATCAAGTTCTGGATTG |
| S5 | TTGATGATTTACCAGTTCCGATTG |
| S6 | CTGCGACAAGTGAATTGAAAGCTG |
| aroE252GF | GGTATAATACAGATGGTATCGGTTATGTG |
| aroE252GR | ACCTGCGCCCAAAATTAAAA |
| spaF | AGCACCAAAAGAGGAAGACAA |
| spaR | GTTTAACGACATGTACTCCGT |
| mecAF | GATCGCAACGTTCAATTTAATTTTG |
| mecAR | GCTTTGGTCTTTCTGCATTCCT |
Boldface type indicates a polymorph at the SNP; lowercase type indicates hairpin loop structures of LUX primers. *, SNP is in reverse primer.
Real-time PCRs.
For reactions using LUX primers, each reaction tube contained 2× platinum quantitative PCR SuperMix-UDG (Invitrogen Life Technologies), LUX labeled primers, and unlabeled primers, all at final concentrations of 0.5 μM, 2 μl of DNA template, and distilled water to give a final volume of 20 μl. The Rotor-Gene 3000 multiplexing system (Corbett Life Science) was used to amplify all samples. Cycling conditions were as follows: 1 cycle at 50°C for 1 min, 1 cycle at 95°C for 2 min, 30 cycles of 95°C for 5 s, 55°C for 20 s, 72°C for 20 s, and 60°C for 10 s, with fluorescent acquisition after the 55°C and 60°C steps.
Kinetic PCR for SNP genotyping of S. aureus using SYBR green was conducted for the most part as per Stephens et al. (28), with format modifications as described in Results and Discussion. The reactions were performed on a Corbett Rotor-Gene 3000 or an Applied Biosystems ABI7000 sequence detection system. One microliter of extracted DNA (1 to 3 ng) was added to 19 μl of reaction mastermix containing 10 μl of the 2× SYBR green PCR Mastermix (Applied Biosystems) and 8 pmol of each primer. Temperature cycling for these reactions was as follows: 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, 56°C for 20 s, and 72°C for 35 s. The SNPs interrogated and primer sequences for these reactions are shown in Tables 2 and 3.
TABLE 2.
Recipe for DMKP embodiment for S. aureus genotyping using SNPs plus binary genes: distribution of fluorescent (LUX) and unlabeled primers into 11 tubes
| Tube no. | LUX primer no. | Fluorescent label | Unlabeled primer name | Gene/SNP + polymorph |
|---|---|---|---|---|
| 1 | L1 | FAM | U1 | arcC210 C |
| 1 | L4 | JOE | U4 | tpi241 + 243 A+G |
| 2 | L2 | JOE | U2 | arcC210 T |
| 2 | L5 | FAM | U5 | tpi241 + 243 G+G |
| 3 | L1 | FAM | U3 | arcC210 A |
| 3 | L4 | JOE | U6 | tpi241 + 243 G+A |
| 4 | L1A | FAM | U7 | arcC162 T |
| 4 | L7 | JOE | U10 | gmk318 A |
| 5 | L2B | JOE | U8 | arcC162 A |
| 5 | L6 | FAM | U9 | gmk318 T |
| 6 | L8 | FAM | U11 | pta294 A |
| 6 | L10 | JOE | U14 | tpi36 T |
| 7 | L9 | JOE | U12 | pta294 C |
| 7 | L11 | FAM | U15 | tpi36 C |
| 8 | L8 | FAM | U13 | pta294 T |
| 8 | L12 | JOE | U16 | pta383 T |
| 9 | L13 | FAM | U17 | pta383 A |
| 9 | L18A | JOE | U20 | pUB110 |
| 10 | L14 | FAM | U19 | cna |
| 10 | L22 | JOE | U22 | sdrE |
| 11 | L16 | FAM | U21 | pvl |
| 11 | L20 | JOE | U18 | pT181 |
TABLE 3.
STKP embodiment for S. aureus genotyping using SNPs: distribution of primers into eight tubesa
| Tube no. | Locus | Possible polymorphs | Polymorph for STKP | Primer combination |
|---|---|---|---|---|
| 1 | arcC210 | C,T | C | S1, U1 |
| 2 | tpi241 + 243 | GA,GG,AG | GA | S2, U6 |
| 3 | arcC162 | A,T | A | S1, U8 |
| 4 | gmk318 | A,T | T | S3, U9 |
| 5 | pta294 | A,C | A | S4, U11 |
| 6 | pta383 | A,T | T | S6, U16 |
| 7 | tpi36* | C,T | C | S5, U15 |
| 8 | tpi36* | C,T | T | S5, U14 |
Conventional kinetic PCR requires the same number of reactions as there are polymorphs at a particular locus. In the full S. aureus kinetic PCR genotyping method, 17 reactions are required—two for each of 7 loci, except for arcC210, tpi241 + 243, and pta294, where three polymorphs are possible and consequently three reactions are required (see third column). Single-tube kinetic PCR uses a universal control reaction—in this case tpi36 in tubes 7 and 8—to normalize cycle times obtained so that polymorphs can be deduced from a single reaction per locus. *, “universal” control reaction.
MLST.
MLST was performed on selected isolates as specified by Enright et al. (4).
RESULTS AND DISCUSSION
Informative power of a set of eight SNPs derived from the S. aureus MLST database.
The overarching purpose of this study was to develop and compare novel automated methods for interrogating eight highly discriminatory SNPs defined by the S. aureus MLST database. This set of SNPs is similar to the seven-member set described by Stephens et al. (28), with minor modifications introduced to facilitate implementation of the novel SNP interrogation formats. The SNPs were identified using the computer program “Minimum SNPs” which can derive, from DNA sequence alignments, sets of SNPs that have a high Simpson's index of diversity (D) with respect to the alignment being analyzed. It was previously determined by Stephens et al. (28) that a set of seven high-D SNPs defines genotypes that are concordant with the major S. aureus clonal complexes. This finding was made with a relatively small subset of STs that were all derived from methicillin-resistant S. aureus (MRSA) isolates. It was therefore of interest to determine if this is also the case with the modified SNP set and a much larger set of STs that included both MRSA and methicillin-susceptible S. aureus (MSSA).
The relationship between SNP profiles and the S. aureus population structure as defined by eBURST analysis (5) is available at http://www.ihbi.qut.edu.au/research/cells_tissue/phil_giffard/. These data depict the relationship between SNP profiles and eBURST clonal complexes for the 403 STs included in the eBURST analysis and the relationship between SNP profiles and STs for all STs in the database. Consistent with the findings of Stephens et al. (28), the major clonal complexes and a subset of singletons are discriminated, and there is little or no discrimination within clonal complexes. It was concluded that these SNPs are suitable for assigning S. aureus isolates to major clonal complexes. Interrogation of these SNPs can also be combined with methods that interrogate rapidly evolving markers in “phylogenetic hierarchical resolving assays using nucleic acids”-like genotyping procedures, as described by Keim et al. (12).
The eight SNPs define 61 extant S. aureus genotypes. This is a consequence of the selection of the SNPs on the basis of their states being as uncorrelated as possible and therefore having maximal informative power in combination with each other. We have previously speculated that these SNPs are very ancient in origin and so have had time to be reassorted by horizontal gene transfer and recombination (28). This is consistent with other studies that have shown that, in clonal organisms, a SNP set defines about as many genotypes as there are SNPs; conversely, in nonclonal organisms, the number of genotypes can be much more than the number of SNPs (6, 9, 11, 12, 15). With S. aureus, the SNPs that discriminate clonal complex founders from single-locus variants are likely of recent origin, have not been reassorted by recombination, and in consequence, are polymorphic in a tiny fraction of the species' diversity and have little informative power when interrogated.
Automation of genome DNA extraction from S. aureus isolates.
An automated procedure for extracting and purifying S. aureus DNA using the Corbett Robotics X-tractor Gene was developed, optimized, and ultimately used to extract DNA from all isolates used in this study. The procedure was based around the method for DNA extraction from whole blood (Buffy Coat DNA extraction protocol, Corbett Life Sciences, Australia), so the optimization focused on cell lysis. Lysostaphin is the most costly reagent in the extraction procedure, so the amount of lysostaphin required has been minimized. Additionally, it was shown that the incubations can all be conducted at room temperature, which means that all manipulations subsequent to suspension of the colonies in the lysis buffer can be carried out by the X-tractor Gene. The combinations of lysostaphin, proteinase K, and incubation temperatures that were tested, together with the yields and quality of nucleic acid are available (see Materials and Methods for optimal protocol). Agarose gel electrophoresis revealed that the purified DNA is intact and free of RNA (data not shown). The X-tractor Gene program is available from us. The cost and time of the extraction procedure were calculated as $1.60 and 3.5 min per isolate, including all reagent costs.
Novel real-time PCR-based approach to SNP interrogation based upon fluorescent primers.
A central aim of this study was to develop new kinetic PCR formats that will allow the streamlining of SNP-based bacterial genotyping.
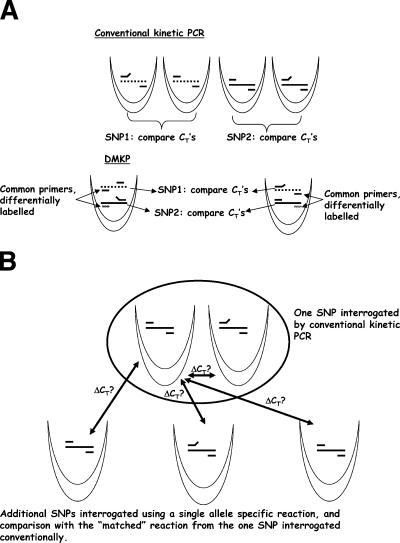
LUX real-time PCR primers increase in fluorescence when incorporated into PCR products (Invitrogen product literature) and so allow amplimer detection and quantification. They are available labeled with 6-carboxyfluorescein (FAM) and 6-carboxy-4′,5′-dichloro-2′,7′-dimethoxyfluorescein (JOE) and so can be used in biplexed real-time PCR procedures. The most straightforward way of using these primers for kinetic PCR is to make allele-specific LUX primers and carry out both allele-specific reactions in the same tube. However, this strategy is flawed for two reasons. First, carrying out both the allele-specific reactions for a particular SNP in a single tube is competitive priming rather than standard allele-specific PCR. In competitive priming, the increase in amplimer as the reaction proceeds provides more opportunities for mispriming by the mismatched primer and so reduces the reaction specificity (8). Second, we have observed on many occasions that adding labels to allele-specific primers changes their priming efficiencies unpredictably, making complete reoptimization of the assay necessary and often difficult or impossible (data not shown). Accordingly, we devised a biplexing approach in which the allele-specific reactions for a particular SNP are carried out in separate tubes and the allele-specific primers are unlabeled. In this method, a given reaction tube contains single allele-specific reactions for two different SNPs, the LUX primers are the non-allele-specific “common” primers that are in effect used to identify the target, and the allele-specific primers are unlabeled and essentially identical to the primers used in our prototype SYBR green-based procedures. We have termed this format “distributed multiplexed kinetic PCR” (DMKP), and it is depicted in Fig. 1A.
FIG. 1.
Novel real-time PCR formats. (A) DMKP. The different SNPs or templates are shown as solid and dashed lines. Mismatched primers are depicted with detached 5′ ends. (B) Single-tube kinetic PCR. A single allele-specific reaction for each SNP is compared with a universal control reaction—the “perfectly matched” reaction from a conventional kinetic PCR SNP interrogation.
An 11-tube DMKP method for interrogating the 8 high-D SNPs and the binary markers pvl, cna, sdrE, pUB110, and pT181 was devised. The primer sequences are shown in Table 1, and the primer combinations are shown in Tables 2 and 3. During optimization, it was found to be necessary to include subterminal mismatches in the allele-specific primers to obtain reasonable allele specificity (data not shown). A typical set of cycle threshold (CT) values from the prototype, manually setup assay can be seen in Table 4. The CT values are in the correct orientation. It was concluded that this method showed sufficient promise for it to be automated.
TABLE 4.
Allele-specific real-time PCR cycle times for known STsa
| Gene + polymorphb | CT for ST:
|
||
|---|---|---|---|
| 1 | 30 | 93 | |
| arcC210GCF | 19.30 | 28.26 | 31.62 |
| arcC210GTJ | 31.53 | 18.99 | 23.29 |
| arcC210GAF | Undet | 32.42 | 36.44 |
| tpi241 + 243ATGJ | Undet | 21.93 | 25.35 |
| tpi241 + 243GTGF | 26.84 | 16.97 | 20.72 |
| tpi241 + 243GTAJ | 19.11 | 26.60 | 30.13 |
| arcC162CTF | 18.44 | 28.28 | 19.07 |
| arcC162CAJ | 28.35 | 17.73 | 31.46 |
| gmk318AAJ | 23.78 | 21.7 | 24.51 |
| gmk318ATF | Undet | 20.96 | 20.37 |
| tpi36TJ | 30.45 | 21.92 | 20.93 |
| tpi36CF | 19.65 | 18.3 | 24.95 |
| pta294AAF | 20.75 | Undet | Undet |
| pta294ACJ | 24.38 | 20.07 | 18.88 |
| pta294ATF | 36.27 | 19.65 | 21.41 |
| pta383CTJ | 23.04 | 21.92 | 21.98 |
| pta383CAF | 32.47 | 19.82 | 21.38 |
Boldface type indicate reactions with perfectly matched allele-specific primers. Undet, undetermined.
5′ bases and subterminal mismatches are indicated. F, FAM labeling; J, JOE labeling.
The reaction setup was automated using the CAS-1200 precision liquid handling system (Corbett Robotics), and the data were analyzed using spreadsheet software. The CAS-1200 was programmed to first make the primer mixes and then assemble the reaction mixtures with the samples being added last. For details of the reactant quantities, see Materials and Methods. The total time for setting up 6 complete genotyping assays was 27 min, with the incidence of machine malfunction essentially zero. The CAS-1200 program is available from us.
The LUX primer-based genotyping with automated setup was tested extensively for reproducibility using isolates of known genotype as determined by full sequence typing and/or by an adaptation of the SYBR green-based SNP plus binary gene method described by Stephens et al. (28) and also by carrying out duplicate assays on unknown isolates. In total, 391 isolates were genotyped, of which 44 were initially of known genotype, and 260 were done in duplicate. Significant difficulties were experienced in achieving high levels of reproducibility. It was necessary to empirically develop quite complex criteria for calling SNPs and the presence or absence of binary genes. The criteria were different for each SNP, reflecting differences in the relative priming efficiencies of the allele-specific primers. In general, criteria were developed to determine cycle time cutoff and ΔCT values. Extensive examination of the data has led us to conclude that the very small fluorescence increases that take place when LUX primers are incorporated into PCR products (<0.2-fold) result in unreliability in the signal normalization. It is also possible that the multiplexing and/or variation in automated reagent quantities were impacting the results, but it was not possible to unambiguously discriminate these effects from signal normalization issues. Ultimately, it was found to be necessary to analyze the data with the “slope correct” and “ignore first 10 cycles” functions inactivated to avoid large artifacts in the normalization of the SNP interrogation reactions, while the binary gene interrogation was most reliable with these functions switched on. The criteria for SNP calling are available at http://www.ihbi.qut.edu.au/research/cells_tissue/phil_giffard/. When these were used, the accuracy of SNP calling was 98% when full MLST determination or SYBR green-based SNP interrogation were used as the gold standard. This figure of 98% is for single SNPs, so the incidence of correct genotypes was less than that.
These criteria for SNP calling were incorporated into a Microsoft Excel macro. Raw CT values may be pasted into the macro, which outputs the SNP profile and also the clonal complex if the SNP profile matches one of the major clonal complexes. This macro is available from us. A similar degree of accuracy was found with the binary markers, with 98% of the LUX data points in accordance with the SYBR green method. A cycle time value of <27 was used as the criterion to call the presence or absence of binary markers using LUX.
It was concluded that the DMKP using LUX primers is a method that shows considerable promise. However, it would be significantly more attractive if the LUX primers displayed a greater fluorescence increase when incorporated into PCR products. As a consequence, we set out to develop an alternative strategy to streamlining SNP-based genotyping.
A novel approach to streamlining SYBR green-based kinetic PCR.
Despite intense efforts, we were unable to achieve the accuracy of DMKP using LUX primers higher than 98%. Therefore, an alternative strategy was devised. We have previously observed that kinetic PCR using SYBR green is very robust (25, 28). Also, we have observed that the relationships between the kinetics of reactions interrogating different SNPs are stable from experiment to experiment (data not shown). Therefore, it was reasoned that it should be possible to call the SNPs using just one reaction per SNP, provided that each reaction was compared with a single “universal” control reaction (Fig. 1B.) This was initially tested by reanalyzing our collection of SYBR green kinetic PCR data to determine if the SNPs could be called using just one of the allele-specific reactions and comparing the CTs to a single arbitrarily chosen reaction from within the assay. It was found that, in essentially all cases, the SNP could indeed be called accurately (data not shown). Accordingly, a standardized format and data analysis protocol were developed. In this format, one of the eight SNPs (tpi36) is interrogated using the conventional “two-reaction” method. The reaction that gives the lowest CT (and so has the perfectly matched allele-specific primer) is used as the control. This avoided the necessity of finding a new invariant target to use as the control, and it also allowed one of the eight SNPs to be interrogated using the exceptionally robust “two-tube” method. The conventional “two-tube” interrogation of tpi36 gives the smallest ΔCT (i.e., weakest SNP signal) of any of the “two-tube” reactions (data not shown), so this was used as the control. We have named this approach “single-tube kinetic PCR” (STKP). The primers used in the current STKP method for interrogating the eight S. aureus high-D SNPs are shown in Table 3.
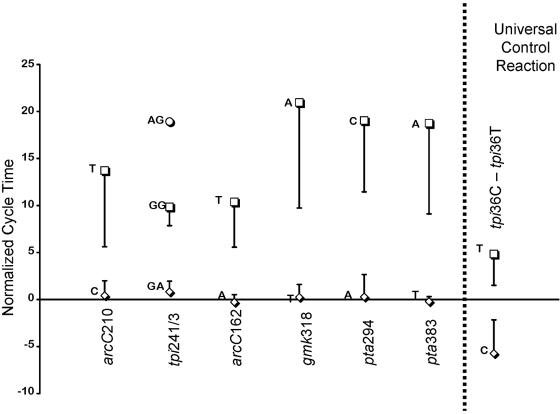
The robustness of the STKP reaction was determined by genotyping in duplicate 44 S. aureus isolates. All of these isolates had been previously genotyped using the conventional “two-tube” kinetic PCR and/or complete ST determination. This constituted 704 SNP interrogations. Just one of these 704 reactions gave a result that was inconsistent with the other genotyping methods. Therefore, this procedure is robust to a degree that makes duplicates virtually unnecessary. The reproducibility of the CT values is shown in Fig. 2. It can be seen that the different alleles of a given SNP give rise to signals that are separated by >3 standard deviations.
FIG. 2.
Reproducibility of STKP interrogation of the 8 high-D S. aureus SNPs. The bars represent 2.58 standard deviations of the ΔCT for each polymorph, calculated against the tpi36 universal control reactions. This was derived from 80 STKP assays.
It was concluded that this method for assigning S. aureus isolates to major clonal complexes is suitable for high-throughput routine applications. It is straightforward, as it requires only generic SYBR green mastermix and unlabeled primers. It is also very simple to set up and interpret because there is just one primer set per reaction tube. The automated PCR setup program and Microsoft Excel data analysis macro were adapted to the STKP procedure, and these are both available from us.
As previously stated (28), our view is that the SNP-based genotyping is of greatest utility when combined with interrogation of rapidly evolving markers. This STKP reaction could easily be combined with reactions designed to interrogate the presence or absence of binary markers. This could be done either by SYBR green (28), DMKP using LUX primers (this article), or indeed, other chemistries, such as TaqMan. Given our current experience, the best compromise between robustness and economy of time and reagents would be obtained by using STKP to interrogate the SNPs and a multiplexed fluorescent primer- or probe-based method to interrogate the binary markers. This is because our data do not currently indicate that a combined DMKP/STKP method in which there are two SNPs interrogated per reaction tube would be sufficiently robust. Therefore, the SYBR green STKP remains the best choice, pending improvements in chemistry or data analysis software. In the case of the binary markers, the adoption of an STKP strategy is not a meaningful concept because there is only one reaction per polymorphic site. Therefore, multiplexing remains the only available strategy for reducing the number of “binary marker” reaction tubes. Determining the presence or absence of binary markers is an inherently simple task. Although we only achieved 98% accuracy using the LUX primers, the great bulk of our optimization efforts were focused on the SNPs, so that figure could probably be improved, particularly if the primers fluoresced more intensely. In addition, there is a wide choice of highly effective real-time PCR chemistries for readily determining the presence or absence of genes in multiplexed reactions, and any of these could be used (16, 19, 23).
The reagent cost of the real-time PCR procedures was calculated at A$19.53 per isolate for the LUX-based method (includes binary markers) and A$13.36 per isolate for the SYBR green-based method (SNPs only). The great majority of the cost is in the mastermixes, so there is scope for considerable cost reductions through volume reductions and identification of cheaper mastermix products.
Development of an assay for the identifications of S. aureus ST-93: the “Queensland clone.”
It is now clear that community acquired MRSA (CA-MRSA) is caused by strains belonging to a variety of S. aureus clonal complexes, with the phage-borne pvl gene being an important pathogenicity determinant (27). In Australia, the major CA-MRSA clones belong to ST-1, ST-30, and ST-93. The ST-1 and ST-30 clones are found over large parts of the world, but ST-93 is a singleton (not closely related to other STs) that, to date, has been found only in Australia (2, 20). This, in combination with the fact that it appears to be highly virulent and responsible for a large proportion of CA-MRSA infections in Australia, makes it of particular interest to clinical and public health microbiologists. Accordingly, we set out to develop an identification procedure for this clone.
It was found that ST-93 gives the same SNP genotype as the ST-59 and ST-121 clonal complexes and a number of other singletons. This is not optimal, as the unrelated CA-MRSA SF25:P clone that is mainly found on the U.S. West Coast is ST-59 (7). However, as has been reported by Stephens et al. (28), we were able to use Minimum SNPs to reveal that a G at position 252 of the MLST locus aroE discriminates ST-93 from all other known STs. A kinetic PCR method for interrogating the aroE252G SNP was developed and shown to be robust using isolates of known ST. This assay was used to screen 49 isolates from our collection of 391 that gave the SNP profile characteristic of ST-59 and ST-93. Forty-six of these gave a result characteristic of ST-93. Of these, 30 were methicillin resistant and pvl positive and thus consistent with the Queensland clone. The remaining 16 were methicillin susceptible, and 12 of these were also pvl positive. Only one of the isolates that were negative for the ST-93-specific SNP was pvl positive.
It was concluded that this procedure is useful for screening for the ST-93 “Queensland clone” either on its own or in combination with the high-D SNPs and/or the pvl gene.
Genotyping results.
The genotypes of all isolates were established on the basis of either completely consistent duplicate DMKP results or from STKP results. The complete data set is available at http://www.ihbi.qut.edu.au/research/cells_tissue/phil_giffard/, and a summary is shown in Table 5. The results are consistent with earlier reports. For example, clonal complex 239 (Aus-1/Aus-2 strain) is known to be the most abundant hospital-acquired MRSA strain in southeast Queensland. As expected, this was the most abundant SNP profile. It is of interest that the majority of the isolates are MRSA, which suggests that the clonal complex 239 isolates in this region are purely of epidemic origin. Consistent with this, clonal complex 239 MRSA are very common worldwide (1-3), and its evolutionary origin has been hypothesized to have resulted from recombination of ST-8 and ST-30 (26). The two major community acquired clones in southeast Queensland are the Southwest Pacific/Western Samoan phage pattern/Oceania strain (clonal complex 30) and the Queensland clone (ST-93) (2, 17, 20, 21). The SNP profile corresponding to these clones was abundant, and as expected, the pvl gene was present in a large proportion of these isolates. The somewhat unexpected abundance of MSSA isolates with the same SNP and pvl genotypes is significant because it suggests that the establishment of these lineages in this region predates their acquisition of SCC-mec. ST-93 is worthy of continued focus. It is a singleton according to eBURST analysis and, to date, appears to have been found only in Australia. It very commonly harbors the pvl gene and has been associated with fatal necrotizing pneumonia (22). The other abundant genotypes that include significant numbers of MRSA isolates correspond to clonal complexes 1, 5, and 8, and this is consistent with the previously reported abundance of these clonal complexes worldwide. Clonal complexes 5 and 8 are not generally associated with community-acquired infections, and as expected, the isolates with these SNP profiles are largely pvl negative. Clonal complex 1 is associated with community-acquired infections in Australia and the United States (18). The Australian representatives of this lineage are assigned to the Western Australia clone. It has previously been reported that the pvl gene is uncommon in the Western Australia clone (20), and our results are consistent with this. Finally, this study provided data that justifies ongoing surveillance of S. aureus. The presence of the pvl gene in representatives of clonal complex 5 and 239 is cause for concern.
TABLE 5.
Clonal complexes identified from genotyping of all S. aureus isolatesa
| Clonal complex | SNP profile (allele) | No. of isolates
|
|
|---|---|---|---|
| MSSA (pvl+) | MRSA (pvl+) | ||
| 1 | CGATAACT | 11 (2) | 19 (1) |
| 5 | CGATTACA | 36 (3) | 9 (0) |
| 8 | TGATACCA | 10 (0) | 20 (0) |
| 9 | TGATAACT | 6 (0) | 0 (0) |
| 509 (or 12) | CGGTTCCA | 10 (0) | 2 (1) |
| 15 | CGATAACA | 17 (0) | 0 (0) |
| 20 | CGATTACT | 13 (1) | 1 (0) |
| 22 | CGGTTACA | 3 (1) | 4 (0) |
| 25 | CGGTAACA | 4 (0) | 1 (0) |
| 30 | TGGATCCA | 17 (6) | 17 (14) |
| 45 | CGGATCCA | 10 (0) | 1 (1) |
| 72 | CGATTCCA | 1 (0) | 0 (0) |
| 78 | TGATTACA | 21 (1) | 4 (0) |
| 93 | TGGTTCTA (aroE252G) | 16 (12) | 30 (30) |
| Non-93/None | TGGTTCTA (aroE252A/T) | 1 (1) | 2 (0) |
| 97 | TGGTAACT | 2 (1) | 0 (0) |
| 239 | TGAAACCA | 1 (0) | 72 (1) |
| CC1 outliers | TGATTACT | 5 (0) | 0 (0) |
| None | TGATTCCA | 5 (0) | 2 (1) |
| None | CGGATCTA | 1 (1) | 0 (0) |
| None | TGGTACCA | 0 (0) | 1 (0) |
| None | TGGTTCCA | 4 (1) | 7 (6) |
| None | TGAATCCA | 0 (0) | 3 (0) |
| Not analyzed | TGAATACA | 1 (0) | 0 (0) |
| Not analyzed | TGGAACCA | 1 (0) | 0 (0) |
Clonal complexes are named in accordance with the clonal complex founder that corresponds to the SNP profile. STs are allocated to clonal complexes on the basis of eBURST default settings (5). The SNP profiles are indicated in the order arcC210, tpi241, tpi243, arcC162, gmk318, pta294, tpi36, pta383. Clonal complex 509 is better known as clonal complex 12, but eBURST analysis indicates that CC509 is the founder. “93” indicates that the isolates have been confirmed as ST-93 by interrogation of aroE252. “CC1 outliers” indicates that the SNP profile corresponds to a small number of outliers in clonal complex 1. “None” indicates that the profile does not correspond to a dominant clonal complex. “Not analyzed” means that the profiles do not match any ST included in the eBURST analysis (but they do match STs in the database). Complete information regarding the relationship between SNP profiles and STs is available at http://www.ihbi.qut.edu.au/research/cells_tissue/phil_giffard/.
The resolving power of the genotyping was determined by calculating D with respect to the collection of isolates. SNP analysis alone provided a D of 0.91, and this was increased to 0.95 by interrogation of the binary markers. The genotypes obtained are similar to those reported by Stephens et al. (28).
In conclusion, we have developed an efficient means of genotyping S. aureus. The SNP profiles were able to discriminate the major clones extant in southeast Queensland. Additional resolution may be obtained by interrogating binary markers. Even more resolution could potentially be obtained by measuring the melting temperatures of tandem repeat loci in conjunction with SNP and/or binary marker interrogation. We are currently determining the informative power of this approach, which is, in effect, a three-stage “phylogenetic hierarchical resolving assays using nucleic acids”-like procedure.
Acknowledgments
This work was supported by the Cooperative Research Centres Program of the Australian Federal Government, the Scientific, Educational, and Research Trust Fund of Queensland Health Pathology Service, the Ipswich Hospital Foundation, and Corbett Life Science.
REFERENCES
- 1.Chongtrakool, P., T. Ito, X. X. Ma, Y. Kondo, S. Trakulsomboon, C. Tiensasitorn, M. Jamklang, T. Chavalit, J.-H. Song, and K. Hiramatsu. 2006. Staphylococcal cassette chromosome mec (SCCmec) typing of methicillin-resistant Staphylococcus aureus strains isolated in 11 Asian countries: a proposal for a new nomenclature for SCCmec elements. Antimicrob. Agents Chemother. 50:1001-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coombs, G. W., G. R. Nimmo, J. M. Bell, F. Huygens, P. M. Giffard, F. O'Brien, and the Australian Group for Antimicrobial Resistance. 2004. Community methicillin-resistant Staphylococcus aureus in Australia: genetic diversity in strains causing outpatient infections. J. Clin. Microbiol. 42:4735-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Enright, M. C., D. A. Robinson, G. Randle, E. J. Feil, H. Grundmann, and B. G. Spratt. 2002. The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA). Proc. Natl. Acad. Sci. USA 99:7687-7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Enright, M. C., N. P. Day, C. E. Davies, S. J. Peacock, and B. G. Spratt. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 38:1008-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feil, E. J., B. C. Li, D. M. Aanensen, W. P. Hanage, and B. G. Spratt. 2004. eBURST: Inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J. Bacteriol. 186:1518-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filliol, I., A. S. Motiwala, M. Cavatore, W. Qi, M. H. Hazbon, M. Bobadilla del Valle, J. Fyfe, L. Garcia-Garcia, N. Rastogi, C. Sola, T. Zozio, M. I. Guerrero, C. I. Leon, J. Crabtree, S. Angiuoli, K. D. Eisenach, R. Durmaz, M. L. Joloba, A. Rendon, J. Sifuentes-Osornio, A. Ponce de Leon, M. D. Cave, R. Fleischmann, T. S. Whittam, and D. Alland. 2006. Global phylogeny of Mycobacterium tuberculosis based on single nucleotide polymorphism (SNP) analysis: insights into tuberculosis evolution, phylogenetic accuracy of other DNA fingerprinting systems, and recommendations for a minimal standard SNP set. J. Bacteriol. 188:759-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Francois, P., A. Huyghe, Y. Charbonnier, M. Bento, S. Herzig, I. Topolski, B. Fleury, D. Lew, P. Vaudaux, S. Harbarth, W. van Leeuwen, A. van Belkum, D. S. Blanc, D. Pittet, and J. Schrenzel. 2005. Use of an automated multiple-locus, variable-number tandem repeat-based method for rapid and high-throughput genotyping of Staphylococcus aureus isolates. J. Clin. Microbiol. 43:3346-3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giffard, P. M., J. A. McMahon, H. M. Gustafson, R. T. Barnard, and J. Voisey. 2001. Comparison of competitively primed and conventional allele-specific nucleic acid amplification. Anal. Biochem. 292:207-215. [DOI] [PubMed] [Google Scholar]
- 9.Gutacker, M. M., M. Barun, H. Soini, E. Shashkina, B. N. Kreiswirth, E. Graviss, and J. M. Musser. 2006. Single-nucleotide polymorphism based population genetic analysis of Mycobacterium tuberculosis strains from 4 geographic sites. J. Infect. Dis. 193:121-128. [DOI] [PubMed] [Google Scholar]
- 10.Hammond, D. S., J. M. Schooneveldt, G. R. Nimmo, F. Huygens, and P. M. Giffard. 2005. blaSHV genes in Klebsiella pneumoniae: different allele distributions are associated with different promoters within individual isolates. Antimicrob. Agents Chemother. 49:256-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hommais, F., S. Pereira, C. Acquaviva, P. Escobar-Paramo, and E. Denamur. 2005. Single-nucleotide polymorphism phylotyping of Escherichia coli. Appl. Environ. Microbiol. 71:4784-4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keim, P., M. N. van Ert, T. Pearson, A. J. Vogler, L. Y. Huynh, and D. M. Wagner. 2004. Anthrax molecular epidemiology and forensics: using the appropriate marker for different evolutionary scales. Infect. Gen. Evol. 4:205-213. [DOI] [PubMed] [Google Scholar]
- 13.Keys, C., S. Kemper, and P. Keim. 2005. Highly diverse variable number tandem repeat loci in the E. coli O157:H7 genomes for high-resolution molecular typing. J. Appl. Microbiol. 98:928-940. [DOI] [PubMed] [Google Scholar]
- 14.Malachowa, N., A. Sabat, M. Gniadkowski, J. Krzyszton-Russjan, J. Empel, J. Miedzobrodzki, K. Kosowska-Schick, P. C. Appelbaum, and W. Hryniewicz. 2005. Comparison of multiple-locus variable tandem-repeat analysis with pulsed-field gel electrophoresis, spa typing, and multilocus sequence typing for clonal characterization of Staphylococcus aureus isolates. J. Clin. Microbiol. 43:3095-3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morales, G., L. Wiehlmann, P. Gudowius, C. van Delden, B. Tummler, J. L. Martinez, and F. Rojo. 2004. Structure of Pseudomonas aeruginosa populations analyzed by single nucleotide polymorphism and pulsed-field gel electrophoresis genotyping. J. Bacteriol. 186:4228-4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris, R., B. Robertson, and M. Gallagher. 1996. Rapid reverse transcription-PCR detection of hepatitis C virus RNA in serum by using TaqMan fluorogenic detection system. J. Clin. Microbiol. 34:2923-2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munckhof, W. J., J. Schooneveldt, G. W. Coombs, J. Hoare, and G. R. Nimmo. 2003. Emergence of community-acquired methicillin-resistant Staphylococcus aureus (MRSA) infection in Queensland, Australia. Int. J. Infect. Dis. 7:259-264. [DOI] [PubMed] [Google Scholar]
- 18.Naimi, T. S., K. H. Le Dell, D. J. Boxrud, A. V. Groom, C. D. Steward, S. K. Johnson, J. M. Besser, C. O'Boyle, R. N. Danila, J. E. Cheek, M. T. Osterholm, K. A. Moore, and K. E. Smith. 2001. Epidemiology and clonality of community-acquired methicillin-resistant Staphylococcus aureus in Minnesota, 1996-1998. Clin. Infect. Dis. 33:990-996. [DOI] [PubMed] [Google Scholar]
- 19.Neoh, S. H., M. J. Brisco, F. A. Firgaira, K. J. Trainor, D. R. Turner, and A. A. Morley. 1999. Rapid detection of the factor V Leiden (1691 G>A) haemochromatosis (845 G>A) mutation of fluorescence resonance energy transfer (FRET) and real-time PCR. J. Clin. Pathol. 52:766-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nimmo, G. R., G. W. Coombs, P. C. Pearson, F. G. O'Brien, K. J. Christiansen, J. D. Turnidge, I. B. Gosbell, P. Collignon, and M.-L. McLaws. 2006. MRSA in the Australian community: an evolving epidemic. Med. J. Aust. 184:384-388. [DOI] [PubMed] [Google Scholar]
- 21.Nimmo, G. R., J. Schooneveldt, G. O'Kane, B. McCall, and A. Vickery. 2000. Community acquisition of gentamicin-sensitive methicillin-resistant Staphylococcus aureus in southeast Queensland, Australia. J. Clin. Microbiol. 38:3926-3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peleg, A. Y., W. Munckhof, S. L. Kleinschmidt, A. J. Stephens, and F. Huygens. 2005. Life-threatening community-acquired methicillin-resistant Staphylococcus aureus infection in Australia. Eur. J. Clin. Microbiol. Infect. Dis. 24:384-387. [DOI] [PubMed] [Google Scholar]
- 23.Piatek, A. S., S. Tyagi, A. D. Pol, A. Telenti, L. P. Miller, F. R. Kramer, and D. Alland. 1998. Molecular beacon sequence analysis for detecting drug resistance in Mycobacterium tuberculosis. Nat. Biotechnol. 16:359-363. [DOI] [PubMed] [Google Scholar]
- 24.Price, E. P., V. Thiruvenkataswamy, L. Mickan, L. Unicomb, R. E. Rios, F. Huygens, and P. M. Giffard. 2006. Genotyping of Campylobacter jejuni using seven single nucleotide polymorphisms in combination with flaA short variable region sequencing. J. Med. Microbiol. 55:1061-1070. [DOI] [PubMed] [Google Scholar]
- 25.Robertson, G. A., V. Thiruvenkataswamy, H. Shilling, E. P. Price, F. Huygens, F. A. Henskens, and P. M. Giffard. 2004. Identification and interrogation of highly informative single nucleotide polymorphism sets defined by bacterial multilocus sequence typing databases. J. Med. Microbiol. 53: 35-45. [DOI] [PubMed] [Google Scholar]
- 26.Robinson, D. A., and M. C. Enright. 2004. Evolution of Staphylococcus aureus by large chromosomal replacements. J. Bacteriol. 186:1060-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Said-Salim, B., B. Mathema, K. Braughton, S. Davis, D. Sinsimer, W. Eisner, Y. Likhoshvay, F. R. DeLeo, and B. N. Kreiswirth. 2005. Differential distribution and expression of Panton-Valentine Leucocidin among community-acquired methicillin-resistant Staphylococcus aureus strains. J. Clin. Microbiol. 43:3373-3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stephens, A. J., F. Huygens, J. Inman-Bamber, E. P. Price, G. R. Nimmo, J. Schooneveldt, W. Munckhof, and P. M. Giffard. 2006. Methicillin-resistant Staphylococcus aureus genotyping using a small set of polymorphisms. J. Med. Microbiol. 55:43-51. [DOI] [PubMed] [Google Scholar]
- 29.van Belkum, A., A. M. Struelens, A. de Visser, H. Verburgh, and M. Tibayrenc. 2001. Role of genomic typing in taxonomy, evolutionary genetics and microbial epidemiology. Clin. Microbiol. Rev. 14:547-560. [DOI] [PMC free article] [PubMed] [Google Scholar]