Abstract
Purpose:
T cell-based immunotherapy via the in vitro or in vivo expansion of prostate tumor-associated antigen (TAA)-specific T lymphocytes is one of the most promising therapeutic approaches to treat prostate cancer. T-cell alternate reading frame protein (TARP) is a mitochondrial protein that is specifically expressed in prostate epithelial cells. We have done experiments aimed at identifying helper T lymphocyte (HTL) epitopes for TARP for the design of T cell-based immunotherapy for prostate cancer.
Experimental Design:
Dendritic cells from normal donors were pulsed with synthetic peptides derived from TARP, which were predicted to serve as HTL epitopes. These dendritic cells were used to stimulate CD4+ T cells in vitro to trigger HTL responses against TARP. T-cell responses to these peptides were also studied with lymphocytes from prostate cancer patients.
Results:
The two peptides, TARP1-14 and TARP14-27, were shown to elicit effective in vitro HTL responses using lymphocytes from both normal volunteers and prostate cancer patients. Peptide TARP1-14-reactive HTLs were found restricted by HLA-DR53 and could recognize naturally processed protein antigen derived from tumor cells, which was presented by autologous dendritic cells. Most significantly, stimulation with peptide TARP14-27 generated four HTL lines restricted by HLA-DR1, HLA-DR9, HLA-DR13, and HLA-DR15, some of which capable of recognizing naturally processed antigens presented by dendritic cell or directly by TARP-positive tumor cells.
Conclusions:
Our results show that TARP constitutes a TAA that can be recognized by tumorreactive HTL. The newly described TARP epitopes could be used to optimize and improveT-cell epitope -based immunotherapy against prostate and other tumors expressing TARP.
Prostate cancer is one of the most common types of malignancies affecting adult men in the United States (1). When prostate adenocarcinoma is diagnosed in early stage, it is usually controlled locally by surgery and/or local radiation therapy. However, patients with tumor recurrences and those with advanced prostate cancer, including metastasis to bone and lymph nodes, are limited to hormonal ablation therapy. Unfortunately, there are no effective therapeutic measures once the disease reaches a hormone-refractory state. Therefore, alternative approaches for the control of advanced prostate cancer and for preventing tumor recurrences need to be explored. T cell -based immunotherapy, which has recently emerged as a promising therapeutic approach to eliminate some types of cancer such as malignant melanoma, depends on the identification of corresponding tumor-associated antigens (TAA; refs. 2, 3). Such TAA contain specific peptide epitopes, some recognized by cytotoxic T lymphocytes (CTL) and others by helper T lymphocytes (HTL) in the context of MHC molecules (4). A number of potential prostate TAA for developing T-cell immunotherapy have been reported (5-10). However, many of these products are also expressed by other tissues, including liver and kidney, raising safety concerns on the possibility of generating severe autoimmune pathology to vital organs. The T-cell receptor γ alternate reading frame protein (TARP) is a 7-kDa mitochondrial product that is specifically expressed by normal and transformed prostatic epithelial cells, which has also been found present in some breast cancer cell lines (11, 12). Recently, two peptide epitopes from TARP were described (TARP4-13 and TARP27-35), which were shown to induce antitumor CTL responses in vitro (13, 14). Furthermore, circulating CD8+ T lymphocytes from peripheral blood of prostate cancer patients were able to recognize these CTL epitopes, suggesting that immune responses to TARP are present in vivo (13). Thus, TARP seems an ideal TAA for the development therapeutic vaccines for both prostate and breast cancers.
Because there is strong evidence that antigen-specific CD4+ HTL are capable of potentiating antitumor CTL responses (15 -17), our laboratory has focused on the identification of MHC class II (MHC II) -restricted HTL peptide epitopes from various TAA (18-24). In the present study, we report that two synthetic peptides from TARP (TARP1-14 and TARP14-27) were capable of eliciting in vitro antitumor HTL in the context of several MHC II alleles both in normal individuals and prostate cancer patients. These TARP HTL epitopes could be used in combination with the previously described CTL epitopes to enhance the potency of prostate and breast cancer vaccines.
Materials and Methods
Cell lines. EBV-transformed lymphoblastoid cells (EBV-LCL) were produced from peripheral blood mononuclear cells (PBMC) of HLA-typed volunteers using culture supernatant from the EBV-producing B95-8 cell line from the American Type Culture Collection (Manassas, VA). Mouse fibroblasts cell lines (L cells) transfected and expressing individual human MHC II molecules were kindly provided by Dr. Robert W. Karr (Pfizer Global R&D, New London, CT) and Dr. Takehiko Sasazuki (Tokyo, Japan). The prostate cancer cell lines LNCaP and PC3 and the breast cancer cell lines MCF7 and SKBr3 were obtained from the American Type Culture Collection and were maintained in tissue culture as recommended by the supplier.
Synthetic peptides. Potential HLA-DR -restricted CD4+ T-cell epitopes were selected from the amino acid sequence of the TARP using the algorithm tables for three HLA-DR alleles (DRB1*0101, DRB1*0401, and DRB1*0701) described by Southwood et al. (25). The predicted peptide epitopes were synthesized by solid-phase organic chemistry and purified by high-performance liquid chromatography. The purity (>80%) and identity of peptides were assessed by high-performance liquid chromatography and mass spectrometry, respectively.
In vitro induction of antigen-specific helper T lymphocyte lines with synthetic peptides. The procedure selected for the generation of TARP-reactive HTL lines using peptide-stimulated PBMC has been described in detail (22, 23). Briefly, dendritic cells were produced in tissue culture from purified CD14+ monocytes (using antibody-coated magnetic microbeads from Miltenyi Biotech, Auburn, CA) that were cultured for 7 days at 37jC in a humidified CO2 (5%) incubator in the presence of 50 ng/mL granulocyte macrophage colony-stimulating factor (GM-CSF) and 1,000 IU/mL interleukin 4. Peptide-pulsed dendritic cells (3 μg/mL for 2 hours at room temperature) were irradiated (4,200 rad) and cocultured with autologous purified CD4+ T cells (using antibody-coated magnetic microbeads from Miltenyi Biotech) in 96-well round-bottomed culture plates. One week later, the CD4+ T cells were restimulated with peptide-pulsed irradiated autologous PBMC and 2 days later, human rIL-2 was added at a final concentration of 10 IU/mL. One week later, the T cells were tested for antigen reactivity using a cytokine release assay as described below. Those cultures exhibiting a significant response of cytokine release to peptide (at least 2.5-fold over background) were expanded in 24- or 48-well plates by weekly restimulation with peptides and irradiated autologous PBMC. Complete culture medium for all procedures consisted of AIM-V medium (Invitrogen Life Technologies, Carlsbad, CA) supplemented with 3% human male AB serum. All blood samples were obtained after the appropriate informed consent.
Measurement of antigen-specific responses with helper T lymphocyte lines. CD4+ T cells (3 × 104 per well) were mixed with irradiated antigen-presenting cells (APC) in the presence of various concentrations of antigen (peptides and tumor lysates), in 96-well culture plates. APC consisted of either autologous PBMC (1 105 per well), HLA-DR -expressing L cells (3 × 104 per well), MHC-type × EBV-LCL (3 × 104 per well), autologous dendritic cells (5 103 per well), or prostate and breast tumor cell lines (3×104 per well) that were treated with IFN-γ (500 units/mL for 48 hours) to enhance MHC antigen expression. The expression of HLA-DR molecules on tumor cells was evaluated by flow cytometry using anti -HLA -DR (L243) monoclonal antibody (mAb) conjugated with fluorescein isothiocynate. Tumor cell lysates were prepared by three freeze-thaw cycles of 1 × 108 tumor cells, resuspended in 1 mL of serum-free RPMI 1640. Lysates were used as a source of antigen at 5×105 cell equivalents per mL. Culture supernatants were collected after 48 hours for measuring antigen-induced lymphokine (GM-CSF or IFN-γ) production by the HTL using commercially available ELISA kits (BD PharMingen, San Diego, CA). To show antigen specificity and MHC restriction, blocking of the antigen-induced response was assessed by adding anti – HLA-DR mAb L243 (IgG2a, prepared from supernatants of the hybridoma HB-55 obtained from the American Type Culture Collection), or anti -HLA-A, HLA-B, HLA-C mAb W6/32(IgG2a, American Type Culture Collection) at 10 μg/mL throughout the 48-hour incubation period. All assessments of ELISA were carried out at least in triplicate and results correspond to the mean values with the SD of the mean.
Measurement of peptide-specific responses in prostate cancer patients. PBMC were isolated from fresh heparinized blood by Ficoll-Hypaque centrifugation and washed twice with RPMI 1640. PBMC were resuspended in complete medium and plated at 1.5 105 cells per well and cultured in triplicate in 96-well round-bottomed × plates in the presence of 10 μg/mL peptide. Negative controls, in the absence of peptide were done in six replicate samples. One week later, the cultures were restimulated with peptide-pulsed (10 μg/mL) irradiated autologous PBMC (5 × 104 cells per well). After 7 days of restimulation, supernatant were harvested for examining the ability of peptide-induced lymphokine production by the patient’s PBMC. The appropriate informed consent for blood donation was obtained from all patients.
Results
Identification of potential helper T lymphocyte epitopes from T-cell alternate reading frame protein. We first selected and synthesized three peptides from the TARP sequence, TARP1-14 (MQMFPPSPLFFFLQ), TARP14-27 (QLLKQSSRRLEHTF), and TARP27-41 (FVFLRNFSLMLLRYI), based on results (data not shown) derived a prediction algorithm that identifies potential promiscuous MHC II binding peptides for three common human allele products, HLA-DR1, HLA-DR4, and HLA-DR7 (25). In the past, this approach has allowed us to identify several HTL epitopes for renowned TAA, including HER-2/neu, gp100, MAGE-A3, PSA, EBNA2, CEA, and the HTLV-1 envelope protein (18-24). It is noteworthy that some of the HTL epitopes derived from the use of this algorithm were found to be restricted by MHC II molecules other than HLA-DR1, HLA-DR4, and HLA-DR7, such as HLA-DR9, HLA-DR15, HLA-DR52, HLA-DR53, HLA-DQ2, and HLA-DQ6, indicating that this prediction approach extends beyond the original three MHC alleles.
Generation, specificity, and MHC II restriction of T-cell alternate reading frame protein -reactive helper T lymphocytes. Because one of the three peptides predicted to function as HTL epitopes that were prepared for these studies (TARP27-41) could not be solubilized (even in 100% DMSO), we were only able to assess the in vitro immunogenicity of the remaining two peptides (TARP1-14 and TARP14-27). Purified CD4+ T cells derived from five healthy male donors were stimulated in vitro with each of the other two TARP peptides, which were pulsed onto autologous dendritic cells in 96 replicate microcultures using 96-well plates. The microcultures were individually restimulated for three weekly cycles with peptide and freshly prepared g-irradiated autologous PBMCs. Seven days after the last restimulation, the T-cell cultures were tested for their ability to produce cytokine (GM-CSF or IFN-γ) upon stimulation with peptide and autologous PBMCs. Those cultures that exhibited at least a 2-fold increase lymphokine production to antigen compared with in the absence of peptide (data not presented) were expanded for further study. We obtained a total of four different HTL lines (some which were subsequently cloned) from these donors summarized in Table 1. For peptide TARP1-14, we obtained HTL line 7D from an HLA-DR4/DR9, HLA-DR53 normal male individual, and for peptide TARP14-27, we obtained two HTL lines: K18 and TNG4, from two normal male individuals expressing HLA-DR1/DR15 and HLA-DR9/DR14, respectively. Lastly, two HTL clones (9H12 and 6E5) reactive with peptide TARP14-27 were obtained from two normal male individuals (HLA-DR9/DR13 and HLA-DR4/DR15). These HTL lines and clones were analyzed in detail for their antigen specificity and MHC II restriction patterns.
Table 1.
MHCclass II-restricted helper T-cell responses to TARP peptides
| TARP-specific HTL | MHC class II | TARP epitope | Restriction molecule | Recognition of naturally processed antigen |
|---|---|---|---|---|
| 7D | DR4/9 DQ7/8 | TARP1-14 | DR53 | Tumor lysate |
| K18 | DR1/15 DQ5/6 | TARP14-27 | DR1 | Tumor lysate |
| TNG4 | DR9/14 DQ5/9 | TARP14-27 | DR9 | Tumor lysate |
| 9H12 | DR9/13 DQ6/9 | TARP14-27 | DR13 | Tumor lysate, tumor cell line |
| 6E5 | DR4/15 DQ1/4 | TARP14-27 | DR15 | Tumor lysate, tumor cell line |
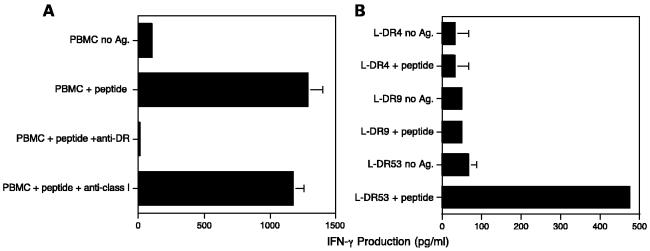
As shown in Fig. 1, peptide TARP1-14 was able to induce an antigen specific. HTL line (7D) from an HLA-DR4/DR9, HLA-DR53 normal male individual. Moreover, the peptide-induced response of this HTL line was inhibited by anti-HLA-DR mAb (L243) but not by anti -HLA class I mAb (W6/32), indicating that this HTL line recognized the peptide TARP1-14 presented in the context of MHC II (Fig. 1A). Because the L243 mAb does not react with HLA-DQ or HLA-DP, we assumed that HLA-DR molecules were the MHC II-restricting elements. To confirm this assumption and to more specifically determine which of the DR allele products was responsible for peptide presentation, several mouse fibroblasts cell lines (L cells) expressing well-characterized HLA-DR molecules were used as APC. As shown in Fig. 1B, only the L cells expressing DR53 were capable of presenting peptide TARP1-14 to the HTL line 7D. Thus, in this case, the response to peptide TARP1-14 was clearly restricted by the HLA-DR53 allelic product.
Fig. 1.
HLA-DR53-restricted HTL responses to peptide TARP1-14. An HTL line was selected from an HLA-DR4/DR9, HLA-DR53 normal male individual by weekly stimulation of CD4+ Tcells with peptide and APCas described in Materials and Methods. A, using autologous PBMCas APC, the T-cell response to peptide TARP1-14 was inhibited by anti – HLA-DR mAb L243 but not by anti -HLA class I mAb W6/32 (both used at 10 μg/mL). B, when mouse fibroblast (L cells) transfected with HLA-DR genes were used as APC, it became evident that the HTL line recognized peptide TARP1-14 (used at 3 μg/mL) in the context of HLA-DR53. Columns, triplicate determinations; bars, SD. Columns without bars had SD<10% the value of the mean. Representative of at least two to three experiments that were done with the same samples.
Four distinct HTL responses derived from four separate donors were obtained towards peptide TARP14-27. The results presented in Fig. 2 show that the recognition of peptide TARP14-27 by the corresponding HTL lines (or clones) was restricted by four different HLA-DR molecules (HLA-DR1 for HTL K18, HLA-DR9 for HTL TNG4, HLA-DR13 for HTL 9H12, and HLA-DR15 for HTL 6E5). These results indicate that the HTL epitope represented by peptide TARP14-27 is highly promiscuous with respect to its MHC II restriction pattern.
Fig. 2.
HTL responses to peptideTARP14-27. HTL lines were selected from (A) an HLA-DR1/DR15 normal male individual (K18) and from (B)anHLA-DR9/DR14 normal male individual (TNG4) and HTL clones were selected from (C) an HLA-DR9/DR13 normal male individual (9H12) and from (D) an HLA-DR4/DR15 normal male individual (6E15) by weekly stimulation of CD4+ Tcells with peptide and APCas described in Materials and Methods.When autologous PBMC were used as APC, all HTL responses to peptide TARP14-27 were inhibited by anti – HLA-DR mAb L243 but not by anti -HLA class I mAbW6/32 (both at used10 μg/mL).When mouse L-cell fibroblasts transfected with HLA-DR genes were used as APC, HTL line K18 recognized the peptide in the context of HLA-DR1 allele (A), HTL line TNG4 recognized the peptide in the context of HLA-DR9 allele (B), HTL clone 9H12 recognized the peptide in the context of HLA-DR13 allele (C), and HTL clone 6E5 recognized the peptide in the context of HLA-DR15 allele (D). Columns, triplicate determinations; bars, SD. Columns without bars had SD < 10% the value of the mean. Representative of at least two to three experiments that were done with the same samples.
Recognition of naturally processed tumor antigen by autologous antigen-presenting cell. We believe that to use a synthetic peptide to generate antitumor T-cell responses in the clinical setting, it is necessary to show that the corresponding T-cell epitope is naturally produced by the APC’s antigen processing machinery. Thus, peptide-reactive T cells must be able to recognize naturally processed tumor cell -derived antigen. The capacity of a T cell to become stimulated by APCs that have naturally processed antigen will depend on two major factors: first, whether the peptide epitope is processed as such by the APC’s proteolytic machinery and second, whether there is sufficient affinity of the T-cell receptor for the peptide/MHC complex. Thus, we first evaluated the overall affinity (i.e., avidity) of the HTL for their corresponding peptide epitopes. Peptide titration curves using autologous PBMCs or dendritic cells as APCs revealed that antigen-mediated HTL responses occurred in a dose-dependent manner (Fig. 3). Furthermore, the peptide concentrations required to obtain half of the maximal response were in most cases below 1μg/mL, suggesting that the HTL have a relatively high avidity for their corresponding epitopes TARP1-14 and TARP14-27.
Fig. 3.
Peptide dose-response curves were done to estimate the avidity of these HTL for peptide TARP1-14 (A)and TARP14-27 (B-E) using autologous PBMCs and dendritic cells (DC) as APC. Points, triplicate determinations; bars, SD. Points without bars had SD < 10% the value of the mean. Representative of at least two to three experiments that were done with the same samples.
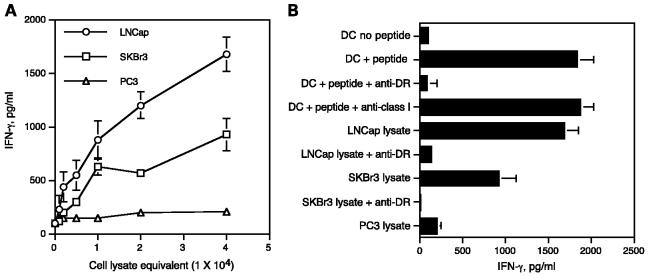
Next, to investigate whether the two TARP peptides described above truly represent naturally processed T-cell epitopes, we assessed the capacity of the TARP peptide-reactive HTLs to respond autologous dendritic cells pulsed with lysates from TARP-expressing tumor cells. Under these circumstances the dendritic cells would have to generate the T-cell epitopes via the conventional MHC II antigen-processing pathway. When the DR53-restricted HTL line specific for peptide TARP1-14 was cultured with autologous dendritic cells that were previously pulsed with cell lysates from TARP-expressing tumors (LNCaP and SKBr3), it became evident that the HTLs responded to the tumor cell lysates in a dose-dependent manner (Fig. 4A). On the other hand, no response was observed towards dendritic cells presenting lysate from the TARP-negative tumor line (PC3) indicating that the response to tumor lysate was antigen specific. Antibody blocking experiments were done to show that the recognition of the tumor lysate -derived antigen occurred via the presentation of naturally processed peptide in the context of MHC II. As shown in Fig. 4B, the addition of anti – HLA-DR mAb abolished the HTL response to synthetic peptide as well as to tumor lysates. These results validate that the HTL epitope represented by peptide TARP1-14 is processed by professional APCs via the exogenous MHC II pathway and that it is efficiently presented to antigen-specific HTLs.
Fig. 4.
Recognition of naturally processed antigen by the HLA-DR53 -restricted,TARP1-14-specific HTL line. A, when autologous DC were used as APC, the peptide-reactive HTL were able to respond to various amounts of cell lysates derived from LNCaP and SKBr3 tumor cells expressing TARP but not to TARP-negative PC3 tumor cells. B, recognition of tumor lysates via autologous dendritic cell (DC) was inhibited by anti – HLA-DR mAb L243 at 10 μg/mL. Triplicate determinations; bars, SD. Columns and symbols without bars had SD < 10% the value of the mean. Representative of at least two to three experiments that were done with the same samples.
Similarly, we next assessed whether the HTL epitope recognized by peptide TARP14-27-specific HTL was also naturally processed by APCs and presented by the MHC II molecules of lysate-pulsed autologous dendritic cells. As shown in Fig. 5, all the peptide-induced HTL lines were able to respond to antigen in a dose-dependent fashion when autologous dendritic cells that pulsed with TARP-positive tumor lysates (LNCaP, SKBr3 and MCF7) were used as APCs. Again, these responses were blocked by anti – HLA-DR mAb (Fig. 6), indicating that the HTL epitope TARP14-27 can also be generated through the exogenous antigen-processing MHC II pathway.
Fig. 5.
Recognition of naturally processed antigen by TARP14-27-reactive HTL. The HTL derived from different four donors were able to recognize various amounts of TARP-positive tumor cell lysates (LNCaP, SKBr3, and MCF7) but not TARP-negative PC3 tumor cells by autologous dendritic cells (A-D). Points, triplicate determinations; bars, SD. Points without bars had SD < 10% the value of the mean. Representative of at least two to three experiments that were done with the same samples.
Fig. 6.
Indirect recognition of peptide TARP14-27 via exogenous antigen presentation by autologous dendritic cells (DC). The HTLs derived from different four donors were able to recognize the TARP-positive tumor cell lysates (LNCaP, SKBr3, and MCF7) but not TARP-negative PC3 tumor cell lysate by autologous dendritic cells and antigen recognition in all cases was inhibited by anti – HLA-DR mAb L243 at 10 μg/mL (A-D). Columns, triplicate determinations; bars, SD. Columns without bars had SD < 10% the value of the mean. Representative of at least two to three experiments that were done with the same samples.
Direct recognition of T-cell alternate reading frame protein expressing tumor cells by the T-cell alternate reading frame protein -specific helper T lymphocytes. Having shown that the TARP peptide -reactive HTLs were effective in recognizing naturally processed antigen by dendritic cells through the exogenous MHC II presentation pathway, we explored whether TARP-positive tumor cells that express MHC II molecules could themselves generate the antigenic peptide/MHC II complex on their cell surface and directly stimulate the TARP-reactive HTL. To carry out these experiments, tumor cells were cultured for 48 hours before the assay with IFN-γ to increase MHC II surface expression (Fig. 7A). The data presented in Fig. 7B indicates that the HLA-DR13 -restricted, TARP14-27-specific HTLs were capable of recognizing antigen directly on SKBr3 tumor cells (DR13+, TARP+) but not on PC3 tumor cells or autologous EBV-LCL (DR13+, TARP-negative). Most importantly, the reactivity against the SKBr3 tumor cells by the HTL was inhibited by anti – HLA-DR mAb, indicating that the interaction of the HTL and the tumor cells is via the T-cell receptor and the tumor’s MHC II molecules. Similarly, as shown in Fig. 7C the HLA-DR15 -restricted, TARP14-27 reactive HTL produced IFN-γ when directly stimulated with MCF7 tumor cells (DR15+, TARP+) but not with SKBr3 (DR15, TARP+), which express an unrelated HLA-DR allele or with × autologous EBV-LCL (DR15+, TARP×). The recognition of MCF7 cells by the HTL was also inhibited by anti – HLA-DR mAb (L243). However, the HLA-DR53 -restricted, TARP1-14-specific HTLs were not capable of directly recognizing the SKBr3 tumor cells (TARP+, DR53+; data not shown). These results indicate that under some circumstances the T-cell epitopes derived from the TARP protein can be processed endogenously by the tumor cells to be expressed on their own MHC II molecules for direct presentation to HTLs.
Fig. 7.
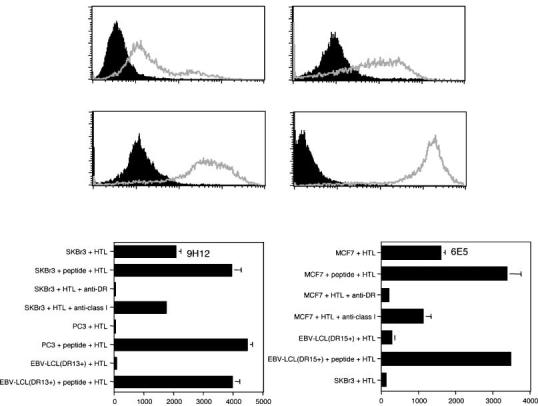
Direct recognition of MHCII/TARP14-27 on tumor cells by HTL. A, cell surface expression of HLA-DR molecules on tumor cells.Tumor cell lines (SKBr3, PC3, and MCF7) were cultured with IFN-γ (500 units/mL) for 48 hours to enhance MHCantigen expression. The expression of HLA-DR molecules on tumor cells was evaluated by staining with anti – HLA-DR (L243) mAb conjugated with fluorescein isothiocyanate (grey line, open histograms). Staining with the isotype negative control (black-filled histograms). B, HLA-DR13 -restricted HTL clone produced IFN-γ as the result of recognizing antigen on HLA-DR13+ TARP+ SKBr3 tumor cells presenting naturally processed epitope. C, similarly, the HLA-DR15 -restricted HTL clone also produced IFN, as the result of recognizing antigen on HLA-DR15+ TARP+ MCF7 tumor cells. In both cases, production of IFN-γ by the HTL clones was inhibited by anti – HLA-DR mAb L243 at10 μg/mL. Columns, triplicate determinations; bars, SD. Columns without bars had SD < 10% the value of the mean. Representative of at least two to three experiments that were done with the same samples.
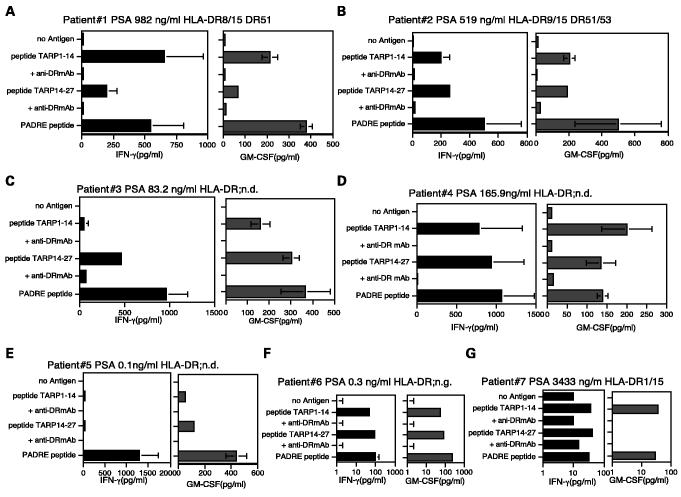
Recognition of peptide TARP1-14 and TARP14-27 by peripheral blood mononuclear cells from prostate cancer patients. Lastly, we wished to determine if T-cell responses to the TARP peptide epitopes TARP1-14 and TARP14-27 could be detected in prostate cancer patients. Because only small blood samples from these patients were available, we were unable to establish long-term T cell lines and to obtain HLA typing for some of the patients. Thus, direct T-cell responses were studied by culturing PBMCs with peptide in 96-well round-bottomed plate, and 1 week later, they were restimulated once using the left over irradiated autologous PBMCs and peptides. Seven days after the second peptide restimulation, culture supernatants were collected for measuring lymphokines secreted (IFN-γ and GM-CSF) as a response to antigen. The results show that the majority of the patients (6 of 7; Fig. 8) had a measurable T-cell response to peptides TARP1-14 and/or TARP14-27. The ability of anti – HLA-DR antibodies to inhibit the response is an indication that the T cells recognize these peptides in the context of MHC class II. However, in one case (patient 7, Fig. 8G), the specificity of the response was only observed in the GM-CSF release assay, as the background response with the IFN-γ assay was too high. As a positive control, we included in these experiments the peptide “PADRE” (Pan DR epitope), which is well known for its ability to eliciting HTL responses to the majority of MHC class II alleles (26). It should be noted that all of these responding patients had advanced prostate cancer (bone metastasis) and the level of serum PSA was relatively high. These results suggest that T-cell responses to peptides TARP1-14 and TARP14-27 may be present in some prostate cancer patients during the course of their disease.
Fig. 8.
T-cell responses to peptideTARP1-14 and TARP14-27 in patients with prostate cancer. PBMCs were isolated from seven prostate cancer patients and were stimulated with peptides as described in Materials and Methods. The peptide-stimulated PBMCs derived from patients 1-7 (A-G) produced lymphokines (IFN-γ and/or GM-CSF), and production of lymphokines by PBMCs was inhibited by adding anti – HLA-DR mAb L243 at 10 μg/mL during culture. Column, triplicate determinations; bars, SD. Columns without bars had SD < 10% the value of the mean. ND, not determined.
Discussion
The identification of antigenic peptides that are naturally processed and presented by APCs and by tumor cells themselves, which can elicit antitumor T-cell responses is important for the development of cancer immunotherapy. Because CTLs are thought to be the main effector elements of the immune system capable of destroying tumors, most emphasis to date has been devoted to the identification of CTL epitopes. Consequently, a large number of MHC class I (MHC I) -restricted peptide epitopes from TAA expressed by melanoma, breast, and colon cancers have been identified using predictive, or reverse immunology approaches. Being aware that MHC II-restricted HTLs play an important role in antitumor responses such as enhancing CTL activity or by displaying direct effector function against tumors (15-17, 19, 27), various groups have used reverse immunology approaches to identify MHC II peptide epitopes from TAA. Although prostate cancer is a common type of malignancy, very few CTL and HTL epitopes have been thus far identified (5-10, 23, 28, 29). TARP seems an ideal TAA for developing immunotherapy for prostate cancer because of its preferential expression on prostatic epithelial cells (11, 12). In fact, recent work has resulted in the identification of MHC I-restricted CTL epitopes from TARP (13, 14).
The results presented herein establish that HTLs from normal individuals and prostate cancer patients are also capable of recognizing peptide epitopes derived from TARP. Most importantly is the demonstration that TARP peptide -reactive HTL could effectively recognize naturally processed antigen either in the form of tumor lysates processed by APCs, or in some cases directly on tumor cells expressing TARP and MHC II molecules. The naturally processed epitope-recognized TAA-specific HTL may be derived from different sources, such as dead/apoptotic tumor cells and tumor lysates, and may be presented by different APC types, including monocytes, professional APCs (dendritic cells), and MHC II+ tumor cells that express the TAA. In present study, TARP14-27-specific HTLs were able to recognize both TARP-expressing tumor cells directly and antigen captured and processed by autologous dendritic cells. In contrast, the TARP1-14-specific HTLs failed to directly recognize TARP+ tumor cells expressing the appropriate restriction MHC allele but were able to recognize autologous dendritic cells that had taken up the tumor lysates. These results suggest that the epitope TARP1-14 may not be endogenously processed as such by the tumor cells, or may not be expressed at sufficient levels to allow direct recognition by the HTLs. However, this may be a premature conclusion because this observation is based on a single HTL isolate. Notwithstanding, both TARP1-14 and TARP14-27 epitopes could be effectively presented to the HTLs by professional APCs that exogenously captured the TAA, suggesting that in in vivo circumstances at the tumor site, TAA-specific HTLs would be able to recognize APCs that have taken up dead tumor cells or debris. Consequently, antigen-activated HTLs at the tumor site would likely enhance CTL function or exhibit direct effector activity towards the tumor cells (17, 27, 30). Although the proteasomal and endosomal processing of TARP, which is mitochondrial protein, has not yet been examined in detail, the present study indicates that the TARP1-14 epitope is exogenously processed and the TARP14-27 epitope is both endogenously and exogenously processed and presented by the restricted MHC II molecules. A possible explanation for different recognition pattern in endogenously and exogenously processing pathways would be that peptides bind to MHC molecules in a different conformation when they are loaded exogenously or when they are generated endogenously, as shown by others (31, 32).
Lastly, the present results have practical implications for designing peptide-based vaccines for prostate cancer. Peptide TARP1-14 was found restricted by HLA-DR53, coexpressed by individuals bearing the HLA-DR4, HLA-DR7, and HLA-DR9 alleles, which would cover a large proportion (f50%) of individuals. On the other hand, peptide TARP14-27 was presented in a promiscuous fashion by several MHC II alleles (DR1, DR9, DR13, and DR15), which comprise near 64% and 81% of the alleles expressed in Caucasians and Japanese, respectively (25). Together, both peptides would offer coverage to the majority of the population. It is noteworthy that the two HTL epitopes, TARP1-14 and TARP14-27, are located in close proximity to two recently described HLA-A2 restricted CTL epitopes, TARP4-13 and TARP27-35 (13, 14). Thus, it becomes feasible to produce vaccines with combined HTL and CTL epitopes, such as a synthetic peptide of TARP14-35 or TARP1-35, which could result in inducing potent and long-term antitumor responses in patients with TARP-positive cancers.
Footnotes
Grant support: NIH grants R01CA82677, P50CA91956, and RR00585 (E. Celis) and Ministry of Education, Science, Sports, and Culture of Japan grant-in-aid 16790220 (H. Kobayashi).
The costs of publication of this article were defrayed in part by the payment of page charges.This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Jermal A, Tiwari RC, Murray T, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.van der Bruggen P, Zhang Y, Chaux P, et al. Tumorspecific shared antigenic peptides recognized by humanTcells. Immunol Rev. 2002;188:51–64. doi: 10.1034/j.1600-065x.2002.18806.x. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg SA. Progress inhuman tumorimmunology andimmunotherapy. Nature (Lond) 2001;411:380–4. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 4.Renkvist N, Castelli C, Robbins PF, Parmiani G. A listing of human tumor antigens recognized by T cells. Cancer Immunol Immunother. 2001;50:3–15. doi: 10.1007/s002620000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Correale P, Walmsley K, Nieroda C, et al. In vitro generation of human cytotoxicT lymphocytes specific for peptides derived from prostate-specific antigen. J Natl Cancer Inst. 1997;89:293–300. doi: 10.1093/jnci/89.4.293. [DOI] [PubMed] [Google Scholar]
- 6.Harada M, Kobayashi K, Matsueda S, Nakagawa M, Noguchi M, Ito K. Prostate-specific antigen-derived epitopes capable of inducing cellular and humoral responses in HLA-A24+ prostate cancer patients. Prostate. 2003;57:152–9. doi: 10.1002/pros.10280. [DOI] [PubMed] [Google Scholar]
- 7.Lu J, Celis E. Recognition of prostate tumor cells by cytotoxicT lymphocytes specific for prostate-specific membrane antigen. Cancer Res. 2002;62:5807–12. [PubMed] [Google Scholar]
- 8.Peahwa MV, Shi JD, Ruegg C, Laus R, vanSchooten WC. Induction of prostate tumor-specific CD8+ cytotoxic T lymphocytes in vitro using antigen-presenting cells pulsed with prostatic acid phosphatase peptides. Prostate. 1998;36:129–38. doi: 10.1002/(sici)1097-0045(19980701)36:2<129::aid-pros8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 9.Dannull J, Diener PA, Prikler L, et al. Prostate stem cell antigen is a promising candidate for immunotherapy of advanced prostate cancer. Cancer Res. 2000;60:5522–8. [PubMed] [Google Scholar]
- 10.Francini G, Scardino A, Kosmatopoulos K, et al. High-affinity HLA-A*0201 peptides from parathyroid hormone-related protein generated in vitro and in vivo antitumor CTL response without autoimmune side effects. J Immunol. 2002;169:4840–9. doi: 10.4049/jimmunol.169.9.4840. [DOI] [PubMed] [Google Scholar]
- 11.Wolfgang CD, Essand M, Vincent JJ, Lee B, Pastan I. TARP: a nuclear protein expressed in prostate and breast cancer cells derived from an alternate reading frame of the T cell receptor g chain locus. Proc Natl Acad Sci U S A. 2000;97:9437–42. doi: 10.1073/pnas.160270597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maeda H, Nagata S, Wolfgang CD, et al. The T cell receptor chain alternate reading frame protein (TARP), a prostate-specific protein localized in mitochondria. J Biol Chem. 2004;279:24561–8. doi: 10.1074/jbc.M402492200. [DOI] [PubMed] [Google Scholar]
- 13.Oh S, Terabe M, Pendleton CV, et al. Human CTLs to wild-type and enhanced epitopes of a novel prostate and breast tumor-associated protein,TARP, lysehuman breast cancer cells. Cancer Res. 2004;64:2610–8. doi: 10.1158/0008-5472.can-03-2183. [DOI] [PubMed] [Google Scholar]
- 14.Carlsson B, Totterman TT, Essand M. Generation of cytotoxicT lymphocytes specific for the prostate and breast tissue antigenTARP. Prostate. 2004;61:161–70. doi: 10.1002/pros.20091. [DOI] [PubMed] [Google Scholar]
- 15.Pardoll DM, Topalian SL. The role of CD4+ Tcell responses in antitumor immunity. Curr Opin Immunol. 1998;10:588–94. doi: 10.1016/s0952-7915(98)80228-8. [DOI] [PubMed] [Google Scholar]
- 16.Hung K, Hayashi R, Lafond-Walker A, et al. The central role of CD4 (+) T cells in the antitumor immune response. J Exp Med. 1998;188:2357–68. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giuntoli RL, II, Lu J, Kobayashi H, Kennedy R, Celis E. Direct costimulation of tumor-reactive CTL by helper Tcells potentiate their proliferation, survival, and effector function. Clin Cancer Res. 2002;8:922–31. [PubMed] [Google Scholar]
- 18.Kobayashi H, Lu J, Celis E. Identification of helper T-cell epitopes that encompass or lie proximal to cytotoxic T-cell epitopes in the gp100 melanoma tumor antigen. Cancer Res. 2001;61:7577–84. [PubMed] [Google Scholar]
- 19.Omiya R, Buteau S, Kobayashi H, Paya CV, Celis E. Inhibition of EBV-induced lymphoproliferation by CD4+ Tcells specific for an MHCclass II promiscuous epitope. J Immunol. 2002;169:2172–9. doi: 10.4049/jimmunol.169.4.2172. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi H, Omiya R, Ruiz M, et al. Identification of an antigenic epitope for helper T lymphocytes from carcinoembryonic antigen. Clin Cancer Res. 2002;8:3219–25. [PubMed] [Google Scholar]
- 21.Kobayashi H, Song Y, Hoon DS, Appella E, Celis E. Tumor-reactiveT helper lymphocytes recognize a promiscuous MAGE-A3 epitope presented by various major histocompatibility complex class II alleles. Cancer Res. 2001;61:4773–8. [PubMed] [Google Scholar]
- 22.Kobayashi H, Wood M, Song Y, Appella E, Celis E. Defining promiscuous MHCclass II helperT-cell epitopes for the HER2/neu tumor antigen. Cancer Res. 2000;60:5228–36. [PubMed] [Google Scholar]
- 23.Kobayashi H, Omiya R, Sodey B, et al. Identification of naturally processed helper T-cell epitopes from prostate-specific membrane antigen using peptidebased in vitro stimulation. Clin Cancer Res. 2003;9:5386–93. [PubMed] [Google Scholar]
- 24.Kobayashi H, Nagato T, Yanai M, et al. Recognition of adult T-cell leukemia/lymphoma cells by CD4+ helper T lymphocytes specific for humanT-cell leukemia virus type I envelope protein. Clin Cancer Res. 2004;10:7053–62. doi: 10.1158/1078-0432.CCR-04-0897. [DOI] [PubMed] [Google Scholar]
- 25.Southwood S, Sidney J, Kondo A, et al. Several common HLA-DR types share largely overlapping peptide binding repertoires. J Immunol. 1998;160:3363–73. [PubMed] [Google Scholar]
- 26.Alexander J, Sidney J, Southwood S, et al. Development of high potency universal DR-restricted helper epitopes by modification of high affinity DR-blocking peptides. Immunity. 1994;1:751–61. doi: 10.1016/s1074-7613(94)80017-0. [DOI] [PubMed] [Google Scholar]
- 27.Zang Y, Chaux P, Stroobant V, et al. A MAGE-3 peptide presented by HLA-DR1 to CD4+ T cells that were isolated from a melanoma patient vaccinated with a MAGE-3 protein. JImmunol. 2003;171:219–25. doi: 10.4049/jimmunol.171.1.219. [DOI] [PubMed] [Google Scholar]
- 28.Hural JA, Friedman RS, McNabb A, et al. Identification of naturally processed CD4 T cell epitopes from the prostate-specific antigen kallikrein 4 using peptide-based in vitro stimulation. J Immunol. 2002;169:557–65. doi: 10.4049/jimmunol.169.1.557. [DOI] [PubMed] [Google Scholar]
- 29.Schroers R, Shen L, Rollins L, et al. Identification of MHCclass II-restricted T-cell epitopes in prostate-specific membrane antigen. Clin Cancer Res. 2003;9:3260–71. [PubMed] [Google Scholar]
- 30.Atanackovic D, Altorki NK, Stockert E, et al. Vaccine-induced CD4+ T cell responses to MAGE-3 protein in lung cancer patients. J Immunol. 2004;172:3289–96. doi: 10.4049/jimmunol.172.5.3289. [DOI] [PubMed] [Google Scholar]
- 31.Viner NJ, Nelson CA, Deck B, Unanue E. Complexes generated by the binding of free peptides to class II MHCmolecules are antigenically diverse compared with those generated by intracellular processing. JImmunol. 1996;156:2365–8. [PubMed] [Google Scholar]
- 32.Peterson DA, DiPaolo RJ, Kanagawa O, Unanue E. A single MHCanchor residue alters. the conformation of a peptide-MHCcomplex inducingTcells that survive negative selection. J Immunol. 2001;166:5874–7. doi: 10.4049/jimmunol.166.10.5874. [DOI] [PubMed] [Google Scholar]