Abstract
Chlamydiae, which are obligate intracellular bacteria, replicate in a nonlysosomal vacuole, termed an inclusion. Although neither the host nor the chlamydial proteins that mediate the intracellular trafficking of the inclusion have been clearly identified, several enhanced green fluorescent protein (GFP)-tagged Rab GTPases, including Rab4A, are recruited to chlamydial inclusions. GFP-Rab4A associates with inclusions in a species-independent fashion by 2 h postinfection by mechanisms that have not yet been elucidated. To test whether chlamydial inclusion membrane proteins (Incs) recruit Rab4 to the inclusion, we screened a collection of chlamydial Incs for their ability to interact with Rab4A by using a yeast two-hybrid assay. From our analysis, we identified a specific interaction between Rab4A and Chlamydia trachomatis Inc CT229, which is expressed during the initial stages of infection. CT229 interacts with only wild-type Rab4A and the constitutively active GTPase-deficient Rab4AQ67L but not with the dominant-negative GDP-restricted Rab4AS22N mutant. To confirm the interaction between CT229 and Rab4A, we demonstrated that DsRed-CT229 colocalized with GFP-Rab4A in HeLa cells and more importantly wild-type and constitutively active GFP-Rab4A colocalized with CT229 at the inclusion membrane in C. trachomatis serovar L2-infected HeLa cells. Taken together, these data suggest that CT229 interacts with and recruits Rab4A to the inclusion membrane and therefore may play a role in regulating the intracellular trafficking or fusogenicity of the chlamydial inclusion.
Chlamydiae are obligate intracellular bacteria that are the leading cause of bacterially acquired sexually transmitted disease (6) as well as preventable blindness worldwide (43). Chlamydiae replicate within a nonlysosomal vacuole that they actively modify through the expression of chlamydial early genes (38). As a result, the cellular interactions of the inclusion are selectively altered such that the inclusion avoids fusion with endosomes and lysosomes, is trafficked to the peri-Golgi region of the cell, and acquires the ability to intercept and fuse with a subset of Golgi derived vesicles (38). Collectively, these events enable chlamydiae to establish a protective replication-competent niche from which chlamydiae can avoid destruction by host lysosomal hydrolases, access nutrients and metabolic precursors, and evade the host immune system. However, the molecular mechanisms that chlamydiae employ to regulate the intracellular trafficking and the fusogenic properties of the inclusion have only now begun to be elucidated.
The chlamydia-containing vacuoles of several Chlamydia species are actively trafficked from the periphery of the cell to the peri-Golgi region or microtubule organizing center (MTOC) in a microtubule- and dynein-dependent manner (8, 15, 33, 38). Unlike most host dynein-dependent trafficking pathways, which require the assistance of dynactin, a large multisubunit complex that facilitates cargo binding to dynein (34), Chlamydia trachomatis serovar L2 elementary body (EB) trafficking is dynactin independent (15). Failure to modify the inclusion during the first 2 h postinfection results in eventual death of chlamydiae as a result of lysosomal fusion (38). During the early stages of infection, up to 60 chlamydial genes, termed “immediate-early” or “early” genes, are transcribed (2, 40). Although many of these early genes function in basic metabolic processes, several including incD to incG and the open reading frames ct222 to ct229 encode inclusion membrane proteins (Incs) (1, 39). The temporal expression pattern of this subset of Incs suggests that they may play critical roles during this crucial remodeling process.
Incs comprise a large family of highly diverse chlamydia-specific proteins that are expressed only during infection and are exclusively localized to the inclusion membrane (30). Importantly, several Incs, including IncA and IncG, express domains that are exposed to the host cytosol (17, 29, 37), suggesting that they may function to exploit host trafficking and signaling pathways by specific recruitment of host factors to the inclusion membrane. This model is supported by the specific interaction of IncG with 14-3-3β at the inclusion membrane (37).
In addition, chlamydiae may also exploit host membrane trafficking pathways to regulate the intracellular trafficking and/or fusogenicity of the inclusion (reviewed in reference 13). Intracellular membrane and organelle biogenesis is tightly controlled by a variety of conserved membrane and soluble factors including N-ethylmaleimide-sensitive factor (NSF), NSF attachment receptor proteins (v-SNARE and t-SNARE), soluble NSF attachment proteins, and the small GTP binding proteins, such as ADP-ribosylating factors (ARFs) and Rab GTPases (reviewed in reference 31). Rab GTPases are the largest family of small GTP binding proteins that cycle between a cytosolic GDP-bound inactive state and a membrane-bound GTP-bound active state (26). They regulate many different aspects of vesicular trafficking including formation of transport vesicles at the donor membrane, trafficking and docking of transport vesicles, and fusion of transport vesicles at target membranes. Each of over 60 mammalian Rab GTPases localizes to a distinct organelle or organellar domain and functions in both constitutive and regulated secretory pathways (reviewed in references 9, 27, and 44). We recently demonstrated that a subset of green fluorescent protein (GFP)-tagged Rabs including both the early endosome (EE) Rabs, Rab4 and Rab11, and the Golgi-localized Rabs, Rab1, Rab6, and Rab10, specifically localized to chlamydial inclusions (32), demonstrating that chlamydiae can recruit key components of the host vesicular membrane trafficking pathways to the inclusion. However, the specific mechanism of Rab GTPase recruitment to the inclusion has not yet been investigated.
To begin to understand how Rab GTPases are recruited to chlamydial inclusions, we undertook experiments to further characterize the interaction of Rab4A with the inclusion and to specifically test whether Rab4A interacts with any known chlamydial Inc. In this paper, we demonstrate that Rab4A interacts with C. trachomatis CT229 in a guanine nucleotide-dependent manner, suggesting that CT229 mimics a Rab4A effector and functions to recruit Rab4A to the C. trachomatis inclusion membrane.
MATERIALS AND METHODS
Cell culture and organisms.
Monolayer cultures of HeLa 229 epithelial cells (CCL 1.2; American Type Culture Collection) were grown in RPMI 1640 (Mediatech, Inc., Herndon, Va.) supplemented with 10% fetal bovine serum (Atlas Biologicals, Fort Collins, Colo.) and 10 μg gentamicin (Invitrogen, Carlsbad, Calif.) per ml at 37°C in an atmosphere of 5% CO2 and 95% humidified air. C. trachomatis LGV 443 cells (serotype L2) were propagated in HeLa cells and purified by Renografin density gradient centrifugation (5). Stock cultures of C. trachomatis were provided by Harlan Caldwell (Rocky Mountain Laboratories [RML], National Institute of Allergy and Infectious Diseases [NIAID], National Institutes of Health [NIH], Hamilton, Mont.). Infections were carried out as previously described (5).
Reagents and antibodies.
Mouse antichlamydial lipopolysaccharide (LPS) was generously provided by Harlan Caldwell (RML, NIAID, NIH), and rabbit anti-CT229 was prepared commercially by Quality Controlled Biochemicals (Hopkinton, Mass.) using a peptide containing the amino acids TLSERLQVQASRRKK (corresponding to amino acids 201 to 215 of CT229) (Ted Hackstadt, RML, NIAID, NIH). Additional antibodies used were as follows: goat anti-rabbit GFP (Living Color) (Clontech, Palo Alto, Calif.), goat anti-mouse immunoglobulin G conjugated to Texas red (Jackson Immunochemicals, West Grove, Pa.) and goat anti-mouse immunoglobulin G conjugated to Cy5 (Zymed, South San Francisco, Calif.). Transferrin (Tfn) conjugated to Alexa 568 was purchased from Molecular Probes (Eugene, Oreg.), and brefeldin A (BFA) and nocodazole were purchased from CalBiochem (San Diego, Calif.).
Plasmid constructions.
Enhanced GFP (EGFP)-Rab4A was constructed as previously described (32). Site-directed mutants were constructed using QuikChange (Stratagene, San Diego, Calif.). EGFP-Rab4AQ67L and EGFP-Rab4AS22N were constructed using EGFP-Rab4A as template with the mutagenic 5′ oligonucleotide TGGGATACAGCAGGACTAGAACGATTCAGGTCC and the mutagenic 3′ oligonucleotide GGACCTGAATCGTTCTAGTCCTGCTGTATCCCA, and the mutagenic 5′ oligonucleotide GCAGGAACTGGCAAAAATTGCTTACTTCATCAG and the mutagenic 3′ oligonucleotide CTGATGAAGTAAGCAATTTTTGCCAGTTCCTGC, respectively. EGFP-CT229 was constructed by PCR amplification using a 5′ gene-specific primer designed with an EcoRI site (GAATTCATGAGCTGTTCTAATATTAATTCAGG) and a 3′ gene-specific primer designed with a BamHI site (GGATCCTTTTTTACGACGGGATGCCTG) using C. trachomatis serovar L2 genomic DNA as template. The PCR product was digested with EcoRI and BamHI and cloned into the EcoRI and BamHI sites of EGFP-C2 (Clontech). EGFP fusion proteins are referred to as GFP fusion proteins. pGAD-ct229 (101-215) was constructed by PCR amplification using a 5′ gene-specific primer with a NcoI site (CCATGGAGCGTCACTTAGAAGTTGTCTCTATGC) and the same 3′ gene-specific primer containing a BamHI site described above using C. trachomatis serovar L2 genomic DNA as template. The PCR product was digested with NcoI and BamHI and cloned into the NcoI and BamHI sites of pGadT7 (Clontech). pGAD-ct229 (101-197) was constructed by digesting pGAD-ct229 (101-215) with BamHI and BglII and religating the plasmid. GAL4 binding domain fusions containing the indicated constitutively active Rab GTPases deleted for the carboxy-terminal lipid modification site were constructed by site-directed mutagenesis (QuikChange; Stratagene) using EGFP-tagged plasmids described in the work of Rzomp et al. (32) as templates. Each gene was subsequently PCR amplified with 5′ gene-specific primers and 3′ gene-specific primers, digested with the appropriate restriction enzymes whose specific DNA restriction endonuclease sites had been incorporated into the 5′ ends of each primer, and cloned into the corresponding sites of pGBKT7 (Clontech).
Eukaryotic transfections.
HeLa 229 cells grown on 12-mm-diameter coverslips (no. 1 thickness) in 24-well plates were washed once in serum-free RPMI 1640 (Mediatech) and transfected with Lipofectamine using a total of 0.4 μg of DNA per well according to the manufacturer's protocol (Invitrogen, Carlsbad, Calif.). Twenty-four hours posttransfection, cells were infected with C. trachomatis serovar L2 at the indicated multiplicity of infection (MOI) and incubated for the indicated times at 37°C.
LSCM.
Cells were fixed in 4% formaldehyde in phosphate-buffered saline (PBS) for 60 min at room temperature (RT). For antibody labeling, fixed cells were permeabilized in PBS containing 0.05% saponin and 0.2% bovine serum albumin for 10 min at RT, and primary and secondary antibodies were incubated in permeabilization buffer sequentially for 60 min each at RT. Coverslips were mounted onto glass slides using Prolong Antifade (Molecular Probes) and viewed by laser scanning confocal microscopy (LSCM). An Olympus Fluoview 500 confocal laser-scanning imaging system equipped with krypton, argon, and He-Ne lasers on an Olympus IX70 inverted microscope with a PLAPO 60× objective was used (Olympus America, Inc., Melville, NY). Confocal images were processed using Adobe Photoshop 8.0 (Adobe Systems, Inc., Mountain View, Calif.).
Yeast two-hybrid screening.
Yeast two-hybrid assays were performed with the Matchmaker Two-Hybrid System 3 (Clontech) as previously described (37). DNA-containing pGBK fusion plasmids were transformed into Saccharomyces cerevisiae strain AH109 (Clontech), while DNA-containing pGAD fusions were transformed into S. cerevisiae strain Y187 (Clontech) using the S.c. Easycomp Transformation kit (Invitrogen), and transformants were selected on synthetic complete (SC) plates lacking appropriate nutrients. Yeast matings between Y187 and AH109 strains were performed as described by Clontech. To identify possible interacting clones, diploids were diluted into sterile water and transferred to synthetic complete plates lacking leucine (Leu), tryptophan (Trp), histidine (His), and adenine (Ade) using a multipronged liquid transfer tool (V & P Scientific, Inc., San Diego, Calif.).
GST pull-down.
Escherichia coli strain BL21 expressing glutathione S-transferase (GST)-Rab4A was grown to an optical density at 600 nm of 0.4 prior to induction of the culture with 100 μM isopropyl-β-d-thiogalactopyranoside. Induced cultures were grown at 37°C for 4 h and then harvested by centrifugation at 500 × g. The pelleted bacteria were lysed in B-PER (Pierce, Rockford, Ill.) for 20 min at RT, and soluble protein was collected by centrifugation at 27,000 × g for 15 min at 4°C. GST-Rab4A was purified by affinity chromatography using glutathione agarose beads (BD Biosciences, San Diego, Calif.). Soluble protein extracts were incubated with glutathione agarose beads overnight at 4°C with rocking. Unbound proteins were removed by washing GST-Rab4A-bound glutathione agarose beads five times with buffer containing 20 mM Tris-HCl, pH 7.4, and 150 mM NaCl. GST pull-down experiments were done essentially as described in reference 4. To activate the GST-Rab4A fusion protein, glutathione beads bound with GST-Rab4A were incubated in small GTPase loading buffer (20 mM Tris-HCl, pH 7.4, 2 mM EDTA, and 25 mM NaCl) in a microspin column (Pierce) containing freshly prepared GTPγS or GDP at a final concentration of 2 mM for 20 min at 37°C. Tubes were chilled on ice, prior to addition of 1 M MgCl2 to a final concentration of 10 mM. HeLa cells were lysed in PBS containing 0.5% NP-40 and protease inhibitors. MgCl2 (1 M) was added to the soluble protein extracts at a final concentration of 0.1 mM, and the lysates were precleared with glutathione agarose beads for 60 min at 4°C. Guanine-nucleotide-loaded GST-Rab4A bound to glutathione agarose beads was incubated with precleared HeLa cell lysates overnight at 4°C. Bound beads were washed six times in wash buffer containing 20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.2% Triton X-100, and 2.5 mM MgCl2 and three times in buffer containing 20 mM Tris-HCl, pH 7.4, and 2.5 mM MgCl2 at 4°C. Bound proteins were eluted off the glutathione beads by incubation at 85°C for 5 min. Eluted proteins were identified by immunoblot analysis using ECL plus reagent (Amersham Biosciences, Piscataway, NJ) as the detection system.
RESULTS
GFP-Rab4A associates with C. trachomatis inclusions early during the chlamydial developmental cycle.
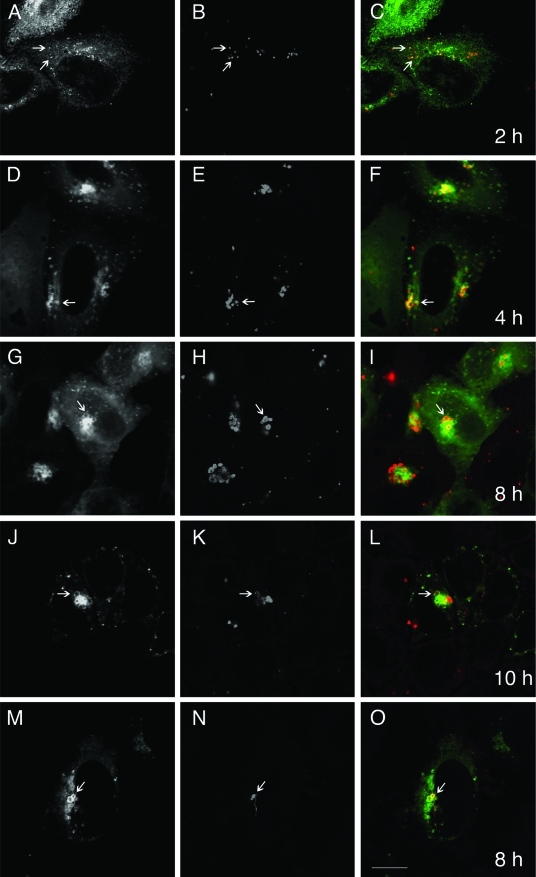
Although we have recently demonstrated that several GFP-Rab GTPases are specifically recruited to chlamydial inclusions (32), the biological significance of Rab GTPase recruitment to the inclusion has not yet been elucidated. The role of each Rab GTPase may differ depending on its specific intracellular localization as well as the timing of recruitment to the inclusion. To begin to understand the role of Rab4A recruitment to the chlamydial inclusion, we analyzed the intracellular distribution of GFP-Rab4A at different times postinfection. HeLa cells transiently expressing GFP-Rab4A were infected with C. trachomatis serovar L2 at an MOI of approximately 20. At different times postinfection, the intracellular localization of GFP-Rab4A was then determined by LSCM. By 2 h postinfection, GFP-Rab4A begins to colocalize with individual EB-containing vacuoles and remains associated with the chlamydial inclusion during the remainder of the chlamydial developmental cycle (Fig. 1 and data not shown). GFP-Rab4A localizes to EB-containing vacuoles prior to arrival at the peri-Golgi region, suggesting that recruitment of GFP-Rab4A may play a role in intracellular trafficking. Finally, recruitment of GFP-Rab4A to early EB-containing vacuoles is not due to the high MOIs used in these experiments since GFP-Rab4A is also recruited to EB-containing vacuoles in cells infected at an MOI of less than 1 (Fig. 1M to O).
FIG. 1.
GFP-Rab4A colocalizes with EB-containing vacuoles by 2 h postinfection. HeLa cells transiently expressing GFP-Rab4A (A, D, G, J, and M) were infected with C. trachomatis serovar L2 at an MOI of 20 (A to L) or less than 1 (M to O). At the indicated times postinfection, the cells were fixed and stained with antichlamydial LPS (B, E, H, K, and N) and viewed by LSCM. Panels C, F, I, L, and O show GFP-Rab4A (green) and antichlamydial LPS (red) merged. Arrows indicate selected chlamydia-containing vacuoles that colocalize with GFP-Rab4A. Bar, 5 μm.
GFP-Rab4A association with C. trachomatis inclusions is independent of microtubules and an intact Golgi apparatus but dependent on early chlamydial gene expression.
As shown previously, GFP-Rab4A remains associated with mature chlamydial inclusions even when Rab4A-containing pericentriolar recycling endodomes (REs) are redistributed to the cellular periphery due to disassembly of the microtubule network (32). These data demonstrated that the ability of GFP-Rab4A to remain localized to the inclusion does not require an intact microtubule network but did not address whether an intact microtubule network is required for the initial recruitment of GFP-Rab4A to the inclusion. To examine this question, microtubules were depolymerized prior to chlamydial infection by pretreating HeLa cells transiently expressing GFP-Rab4A with nocodazole for 3 h prior to chlamydial infection and also during the subsequent 8-h infection period. In order to visualize early events during the chlamydial developmental cycle, cells were infected at an MOI of approximately 20. Infected cells were fixed and labeled with antichlamydial LPS and visualized by LSCM. As previously reported, in the presence of nocodazole, chlamydial EBs remain dispersed throughout the host cytosol in nonfused vacuoles (33). In cells treated with nocodazole, although the vacuoles are no longer localized to the MTOC and are instead localized to the periphery of the cell, GFP-Rab4A still colocalized with EB-containing vacuoles (Fig. 2A to C). In addition, GFP-Rab4A also localizes to EB-containing vacuoles in cells treated with BFA (Fig. 2D to F). Collectively these data demonstrate that neither an intact microtubule network nor an intact Golgi apparatus is required for the recruitment of GFP-Rab4A to chlamydial inclusions.
FIG. 2.
GFP-Rab4A recruitment to the chlamydial inclusion. (A to F) Three hours prior to infection with C. trachomatis serovar L2 at an MOI of approximately 20, HeLa cells transiently expressing GFP-Rab4A (A and D) were pretreated with nocodazole (NOC) (A to C) or BFA (D to F). Eight hours postinfection, cells were fixed and stained with antichlamydial LPS (B and E) and then viewed by LSCM. (C and F) GFP-Rab4A (green) and antichlamydial LPS (red) merged. (G to I) HeLa cells transiently expressing GFP-Rab4A (G) were infected with C. trachomatis serovar L2 at an MOI of approximately 20 and then incubated at 37°C for 24 h in the presence of chloramphenicol (CAM). Cells were then fixed and stained with antichlamydial LPS (H) and viewed by LSCM. (I) GFP-Rab4A (green) and antichlamydial LPS (red) merged. Small arrows indicate selected chlamydia-containing vacuoles that colocalize with GFP-Rab4A. Large arrows indicate chlamydia-containing vacuoles that do not colocalize with GFP-Rab4A. Bar, 10 μm.
Chlamydial inclusions do not fuse with host endosomes or lysosomes (18, 35, 36, 42, 47). Instead, the inclusion is trafficked to the peri-Golgi region of the cell by a mechanism that requires early chlamydial protein synthesis (38). To determine whether the recruitment of GFP-Rab4A also requires early chlamydial protein synthesis, we examined the intracellular localization of GFP-Rab4A in infected HeLa cells grown in the presence of chloramphenicol. Transiently transfected cells expressing GFP-Rab4A were infected with C. trachomatis serovar L2 at an MOI of 20 in the presence of chloramphenicol. Twenty-four hours postinfection cells were fixed and stained with antichlamydial LPS and viewed by LSCM. As shown, in Fig. 2G to I, in the presence of chloramphenicol, EBs remain dispersed throughout the host cytosol and do not colocalize with GFP-Rab4A, demonstrating that early chlamydial protein synthesis is required for recruitment of GFP-Rab4A to chlamydial inclusions. These data suggest that active remodeling of the inclusion through expression and localization of inclusion membrane proteins is required for the recruitment of GFP-Rab4A to the inclusion.
GFP-Rab4A is recruited to chlamydial inclusions in a guanine nucleotide-dependent manner.
Since Rab GTPases interact with their respective effector proteins in a guanine nucleotide-dependent manner, we wanted to determine whether GFP-Rab4A interacted with the inclusion in a similar manner (7). To test this, we constructed GTPase-deficient (GFP-Rab4AQ67L) and GDP-restricted (GFP-Rab4AS22N) GFP-Rab4A fusion proteins and analyzed their intracellular localization in infected cells (22). HeLa cells were transiently transfected with DNA containing each GFP-Rab4A fusion as well as with GFP alone (data not shown). Twenty-four hours posttransfection, cells were infected with C. trachomatis serovar L2 at an MOI of approximately 1. Eighteen hours postinfection cells were fixed and examined by LSCM. As shown in Fig. 3, both GFP-Rab4A (Fig. 3A) and GFP-Rab4AQ67L (Fig. 3B) localized to chlamydial inclusions as demonstrated by the distinct rim-like staining pattern of each fusion protein surrounding the inclusion. In contrast, GDP-restricted GFP-Rab4AS22N (Fig. 3C) did not localize to inclusions, demonstrating that only active GTP-bound Rab4A but not inactive GDP-bound Rab4A is recruited to chlamydial inclusions. Similar results were observed with Rab4B fusion proteins (data not shown).
FIG. 3.
>GFP-Rab4A is recruited to the chlamydial inclusion in a guanine nucleotide-dependent manner. HeLa cells transiently expressing GFP-Rab4A (A), GFP-Rab4AQ67L (B), and GFP-Rab4AS22N (C) were infected with C. trachomatis serovar L2 at an MOI of 1. At 18 h postinfection, cells were fixed and stained with antichlamydial LPS (data not shown) and viewed by LSCM. Only wild-type (A) and constitutively active (B) but not dominant-negative (C) Rab4A fusion proteins localize to the inclusion. Similar results were obtained with Rab4B fusion proteins (data not shown). Arrows indicate inclusions. Bar, 10 μm.
Rab4A interacts with C. trachomatis Inc CT229 in a guanine nucleotide-dependent manner.
Based upon the observation that active GTP-bound Rab4A (GFP-Rab4AQ67L) but not inactive GDP-bound Rab4A (GFP-Rab4AS22N) is recruited to the inclusion, Rab4A is likely to be recruited to the inclusion via a direct interaction with a chlamydial protein that mimics a Rab effector or via an indirect interaction mediated by a Rab4A effector protein. If Rab4A directly interacts with the inclusion membrane, the Incs are logical targets to mediate this interaction. To determine whether any known inclusion membrane protein directly interacts with Rab4A, we screened a collection of Incs for their ability to interact with Rab4A using the yeast two-hybrid assay. Portions of 27 different inc genes were cloned individually into the GAL4 DNA activation domain plasmid pGADT7 and expressed in a MATα yeast strain, while constitutively active Rab4AQ67L was cloned into the GAL4 DNA binding domain plasmid pGBKT7 and expressed in a yeast strain of the opposite mating type. To prevent membrane localization of the GAL4BD-Rab4A fusion protein, amino acids mediating the membrane localization of Rab4A were deleted from the carboxy terminus of the fusion protein (12). Pairwise crosses were performed between each of the pGAD-inc-expressing MATα yeast strains and the pGBK-Rab4AQ67L-expressing MATa yeast strain. As negative controls, pGAD-inc strains were also crossed with yeast strains containing pGBKT7 alone or pGBK-p53. Diploids containing both plasmids were selected on the appropriate selective media and screened for the induction of the GAL4-dependent activation of the HIS3 and ADE2 reporter fusions.
Activation of the HIS3 and ADE2 reporter gene fusions, which permits growth on medium lacking His and Ade, indicates a positive interaction between the GAL4AD and GAL4BD fusion proteins. Failure to grow under these selective conditions indicates no interaction. None of the fusion proteins individually activated transcription of the reporter genes (Fig. 4A). Of the Incs tested, only one, CT229, interacted with Rab4A (Fig. 4A) and Rab4B (data not shown) in this system. In this assay, we used a GAL4BD fusion expressing the hydrophilic carboxy-terminal domain of CT229 containing amino acids 101 to 215 (1) since expression of full-length CT229 was toxic to yeast (data not shown). As shown in Fig. 4A, strains expressing pGAD-ct229 (101-215) grew on media lacking His and Ade only in the presence of pGBK-Rab4A and not in the presence of vector alone or with pGBK-p53. These data demonstrate the specificity of the interaction between GAL4AD-CT229 (101-215) and GAL4BD-Rab4A. GAL4AD-CT229 (101-215) also interacted with constitutively active GAL4BD-Rab4B (data not shown).
FIG. 4.
Rab4 interacts with C. trachomatis CT229 in a guanine nucleotide-dependent manner. (A) S. cerevisiae strain AH109 containing pGBKT7, pGBKBD-p53, or the indicated constitutively active pGBK-Rab constructs was mated with S. cerevisiae strain Y187 expressing pGADT7, pGAD-ct229 (101-215), or pGAD-ct229 (101-197). To identify possible interacting clones, diploids, which were selected on plates lacking Leu and Trp, were diluted into sterile water and transferred to plates lacking Leu, Trp, His, and Ade using a multipronged liquid transfer tool. GAL4AD-CT229 (101-215), but not GAL4AD-CT229 (101-197), interacted with only GAL4BD-Rab4AQ67L and not any of the other Rab GTPases tested. (B) S. cerevisiae strain Y187 expressing either pGADT7, pGAD-ct229 (101-215), or pGAD-ct229 (101-197) was mated with S. cerevisiae strain AH109 expressing pGBK-Rab4A, pGBK-Rab4AQ67L, and pGBK-Rab4AS22N. Diploids were selected and screened as described for panel A. GAL4AD-CT229 (101-215) interacted with constitutively active GAL4BD-Rab4AQ67L, and to a lesser extent with wild-type GAL4BD-Rab4A (data not shown), but not with inactive GDP-restricted GAL4BD-Rab4AS22N.
To further demonstrate the specificity of the interaction between CT229 and Rab4A, we tested whether GAL4AD-CT229 (101-215) interacted with any additional Rab GTPases including Rab1, Rab3, Rab6, Rab8, Rab10, and Rab11 (Fig. 4A and data not shown). As shown in Fig. 4A, CT229 interacted only with Rab4 isoforms. If CT229 is mimicking a Rab4 effector, then it should interact only with Rab4 in its activated GTP-bound state. To confirm this, we constructed both wild-type and dominant-negative GDP-restricted GAL4BD-Rab4A fusion proteins and tested whether GAL4AD-CT229 (101-215) interacted with GAL4BD-Rab4A in a guanine nucleotide-dependent fashion. Similar to other well-characterized Rab effector proteins, GAL4AD-CT229 (101-215) interacted with only GAL4BD-Rab4AQ67L and not GAL4BD-Rab4AS22N (Fig. 4B). Prolonged incubation demonstrated that GAL4AD-CT229 (101-215) also interacted with GAL4BD-Rab4A but with a much lower affinity (data not shown). These data demonstrate that amino acids 101 to 215 of CT229 are required for the interaction of CT229 and Rab4. Interestingly, deletion of the carboxy-terminal 18 amino acids (GAL4AD-CT229 [101-197]) prevented interaction with GAL4BD-Rab4AQ67L. However, a GAL4AD fusion containing amino acids 198 to 215 did not interact with GAL4BD-Rab4AQ67L (data not shown). Therefore, these data demonstrate that amino acids 198 to 215 are necessary but not sufficient for interaction with Rab4A in the yeast two-hybrid system.
Since another Inc, IncA, was shown to interact with itself, we also examined whether CT229 interacted with itself. As shown in Fig. 4A, CT229 interacts with itself and this interaction is mediated by amino acids 101 to 197. Therefore, although amino acids 198 to 215 are required to mediate interaction with Rab4, these amino acids are not required to mediate CT229-CT229 interactions in this system.
To confirm our yeast two-hybrid results, we characterized the interaction of CT229 and Rab4A using several additional assays. First, we examined the interaction of CT229 and Rab4A using in vitro GST pull-down experiments. Detergent extracts derived from HeLa cells transiently expressing GFP-CT229 or GFP were isolated and incubated with GST-Rab4AGTPγS or GST overnight at 4°C (Materials and Methods). As shown in Fig. 5A, only GST-Rab4AGTPγS (lane 6) but not GST (lane 5) was able to interact with GFP-CT229. As expected, neither fusion protein interacted with GFP (lanes 3 and 4). To confirm that only activated GST-Rab4A interacted with CT229, we repeated the experiment with inactive GDP-bound GST-Rab4A. For this experiment, we probed the immunoblot with anti-CT229 (Fig. 5B). In contrast to what we observed for GST-Rab4AGTPγS (lane 3), GST-Rab4A-GDP did not interact with CT229 (lane 4), thus confirming that interaction between GST-Rab4A and GFP-CT229 was dependent on active GTP-loaded GST-Rab4A. Unfortunately, we were unable to perform GST pull-down experiments with GFP-CT229 (101-215) due to the aggregation of this fusion protein in HeLa cells (data not shown) and therefore could not confirm that the carboxy-terminal domain of CT229 was sufficient for the interaction between the two proteins in this system.
FIG. 5.
GST-Rab4A interacts with GFP-CT229. GST-Rab4A and GST were expressed in E. coli and purified by affinity chromatography using glutathione agarose beads (data not shown). (A) Glutathione-bound GST-Rab4A was loaded with GTPγS and incubated overnight with protein extracts from HeLa cells expressing GFP or GFP-CT229. Beads were washed, and bound protein was eluted and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Immunoblot analysis was performed using anti-GFP. Lane 1, total cellular lysate from GFP-expressing HeLa cells. Lane 2, total cellular lysate from GFP-CT229-expressing HeLa cells. GST-Rab4-GTPγS (lane 6) but not GST (lane 5) interacted with GFP-CT229. Neither GST-Rab4-GTPγS (lane 4) nor GST (lane 3) pulled down GFP. The thin arrow indicates GFP-CT229. The thick arrow indicates GFP. (B) Glutathione-bound GST-Rab4A was loaded with GTPγS or GDP and incubated overnight with protein extracts from HeLa cells expressing GFP-CT229. Beads were washed, and bound proteins were eluted and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Immunoblot analysis was performed using anti-CT229. Lane 1, total cellular extract of GFP-CT229-expressing HeLa cells; lane 2, total cellular extract of GFP-expressing HeLa cells. GST-Rab4-GTPγS (lane 3) but not GST-Rab4-GDP (lane 4) interacted specifically with GFP-CT229. The arrow indicates GFP-CT229. Lane 5, total cellular extract of GST-expressing HeLa cells. The asterisk indicates cross-reacting protein present in HeLa cells.
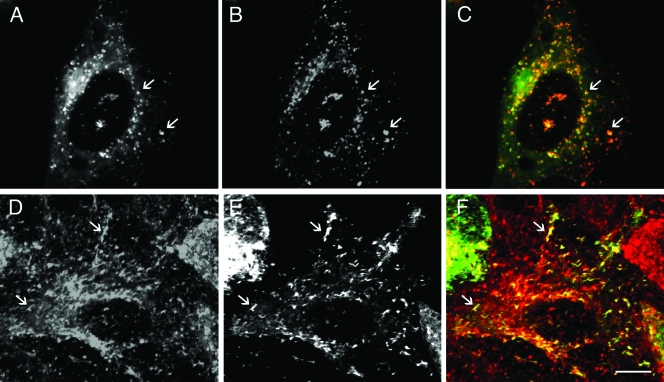
As a second approach, we examined whether ectopically expressed CT229 colocalized with GFP-Rab4A in HeLa cells. For these studies, we constructed and coexpressed DsRed-CT229 and GFP-Rab4A and examined the intracellular localization of the two fusion proteins in uninfected HeLa cells by LSCM. GFP-CT229 and DsRed-CT229 colocalize in HeLa cells, demonstrating that these separate CT229 fusion proteins localize in a similar manner and can be used interchangeably in our experiments (data not shown). As shown in Fig. 6A to C, extensive colocalization of the two fusion proteins was observed. In addition, we also examined whether ectopically expressed GFP-CT229 colocalized with Tfn, a marker for REs, by incubating cells with Tfn-Alexa 568. As shown in Fig. 6D to F, GFP-CT229 also colocalized with Tfn-Alexa 568, demonstrating that fluorescent fusion proteins containing CT229 colocalized with two separate markers of REs, Rab4A and Tfn.
FIG. 6.
CT229-tagged fluorescent fusion proteins colocalize with GPF-Rab4A and Tfn. (A and B) HeLa cells were cotransfected with GFP-Rab4A (A) and DsRed-CT229 (B). Twenty-four hours posttransfection, cells were fixed and viewed by LSCM. (C) GFP-Rab4A (green) and DsRed-CT229 (red) merged. Arrows indicate selected vesicles that colocalize DsRed-CT229 and GFP-Rab4A. (D and E) HeLa cells transiently expressing GFP-CT229 were serum starved for 2 h (D) and then incubated with Tfn conjugated to Alexa 568 for 60 min at 37°C (E). (F) GFP-CT229 (green) and Tfn-Alexa 568 (red) merged. Cells were viewed by LSCM. Arrows indicate selected regions where GFP-CT229 colocalizes with Tfn-Alexa 568. Bar, 10 μm.
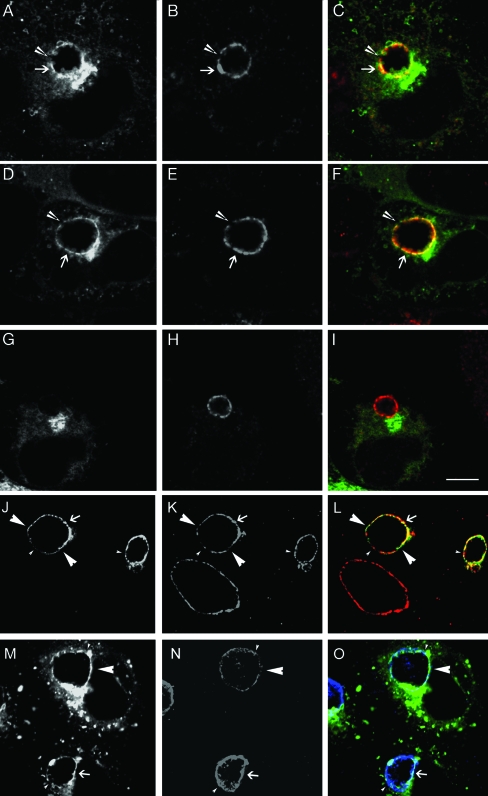
As a final approach, we examined whether GFP-Rab4A colocalized with endogenous CT229 in infected HeLa cells. HeLa cells transiently expressing GFP-Rab4A, GFP-Rab4AQ67L, or GFP-Rab4AS25N were infected with C. trachomatis serovar L2 for 18 h. Cells were fixed and stained with anti-CT229. Both GFP-Rab4A and GFP-Rab4AQ67L colocalized exclusively with CT229 at the inclusion membrane (Fig. 7A to F), while the inactive GFP-Rab4AS22N fusion protein did not (Fig. 7G to I). Similar to what has been shown for other Incs (1, 39), CT229 is not localized uniformly within the inclusion membrane but is instead localized in distinct patches within the membrane. Consistent with the two proteins interacting, GFP-Rab4A localized only to the inclusion membrane at regions where CT229 was present and not to regions where CT229 was absent. However, because of the limited resolution of fluorescence microscopy and the fact that Rab4 and CT229 may colocalize at the inclusion membrane simply because they are both localized to the inclusion membrane, we performed two additional control assays. First, we examined whether GFP-Rab4A colocalized with IncG, which does not interact with Rab4A (data not shown), by staining GFP-Rab4A-expressing HeLa cells with anti-IncG. As expected, since both proteins localize to the inclusion membrane, we did observe colocalization between GFP-Rab4A and IncG at the inclusion membrane. However, in contrast to what was observed between GFP-Rab4A and CT229, there were several regions where both proteins localized to the inclusion membrane in the absence of the other (Fig. 7M to O), demonstrating that just because two proteins localize to the inclusion membrane, they do not always colocalize with each other when examined by LSCM. Similar results were obtained when we stained GFP-Rab11A-expressing cells with anti-CT229 (Fig. 7J to L), demonstrating that much less colocalization is observed between Rab GTPases and Incs that do not interact with each other than between Rab GTPases and Incs that do interact. These in vivo data support the in vitro data that demonstrate that Rab4 and CT229 interact with each other. Collectively, these data provide evidence that Rab4A and CT229 specifically interact and that CT229 most likely functions to recruit Rab4A to the chlamydial inclusion.
FIG. 7.
GFP-Rab4A colocalizes with endogenous CT229 at the inclusion membrane. HeLa 229 cells transiently expressing GFP-Rab4A (A and M), GFP-Rab4AQ67L (D), GFP-Rab4AS22N (G), and GFP-Rab11A (J) were infected with C. trachomatis serovar L2 at an MOI of approximately 1. Eighteen hours postinfection cells were fixed and stained with anti-CT229 (B, E, H, and K) or anti-IncG (N) and viewed by LSCM. (C, F, and I) GFP-Rab4A (green) and anti-CT229 (red) merged; (L) GFP-Rab11A (green) and anti-CT229 (red) merged; (O) GFP-Rab4A (green) and anti-IncG (blue) merged. (A to I) Arrows indicate selected areas of colocalization between GFP-Rab4A and CT229. Arrowheads indicate selected regions of the inclusion devoid of both GFP-Rab4A and CT229. (J to O) Arrows indicate selected areas of colocalization between the indicated proteins; large arrowheads indicate selected areas where the indicated GFP fusion localizes to the inclusion in the absence of the indicated Inc protein; small arrowheads indicate selected areas where the indicated Inc protein localizes to the inclusion in the absence of the indicated GFP fusion. Bar, 10 μm.
DISCUSSION
As obligate intracellular bacteria, chlamydiae exploit host cellular processes most likely through specific interactions between chlamydial proteins localized to the cytosolic face of the inclusion and host cytosolic or membrane-associated proteins. In this paper, using the yeast two-hybrid system, we identified a novel interaction between a chlamydial Inc, C. trachomatis CT229, and the mammalian GTPase Rab4A. The interaction between these two proteins was confirmed by both GST pull-down experiments and colocalization studies in C. trachomatis-infected HeLa cells. These data are consistent with the idea that chlamydial Incs function to recruit specific host proteins to the chlamydial inclusion.
The chlamydia-directed inclusion remodeling that occurs during the first 2 h postinfection is essential for the intracellular survival of chlamydiae. The identities of the specific chlamydial proteins that are required for the inclusion remodeling have not been determined. ct229 is one of six C. trachomatis inc genes expressed during the first hour postinfection (2, 40). The early expression of ct229 suggests that CT229 plays an important early role in the biogenesis and remodeling of the inclusion. Unfortunately, the function of CT229 has remained elusive due to both the lack of homology of CT229 with other known proteins (1) and the inability to genetically manipulate the chlamydial genome. However, an essential, but as yet unidentified, role for CT229 is supported by the ability of microinjected antibodies generated against the carboxy-terminal 15 amino acids of CT229 (TLSERLQVQASRRKK) to inhibit the development of C. trachomatis serovar L2 in HeLa cells (16). The data presented in this paper suggest that at least one function of CT229 is to recruit Rab4 to the C. trachomatis inclusion.
Rab4, which is localized to EEs, controls receptor recycling from the EE to the RE or from the EE to the plasma membrane (25, 28, 45, 46) as well as apical membrane transport in polarized epithelial cells (23). Active GTP-bound Rab4 functions by interacting with numerous downstream effector molecules, such as Rab coupling factor (21), syntaxin 4 (20), rabaptin 4 (24), rabaptin 5 (48), rabip4 (10), rabip4′ (14), rabensosyn 5 and Rabex 5 (11), and cytoplasmic dynein light intermediate chain 1 (3). Similar to the interaction between Rab4 and its cellular downstream effectors, interaction between CT229 and Rab4A requires that Rab4A be in its activated GTP-bound state. Therefore, these data confirm the specificity of interaction between CT229 and Rab4A and suggest that CT229 functions by mimicking a Rab4A effector molecule, resulting in recruitment of Rab4A to the C. trachomatis inclusion. Since dynein, a component of which is a Rab4 effector molecule, has been shown previously to be both recruited to the inclusion and required for the intracellular trafficking of EBs (8, 15), it is possible that recruitment of Rab4A to the inclusion facilitates the recruitment of dynein to the inclusion. Other Rab4 effectors may also be recruited to the inclusion and play a role in chlamydial pathogenesis, but the localization of additional Rab4 effectors to the inclusion has not yet been addressed.
We have previously demonstrated that Rab4 localizes to C. trachomatis, Chlamydia muridarum, and Chlamydia pneumoniae inclusions (32). However, except for a homologue present in C. muridarum, no homologues of CT229 have been detected in the C. pneumoniae genome (19). This confronts us with an apparent contradiction. If CT229 recruits Rab4 to C. trachomatis and C. muridarum, how is Rab4 recruited to the C. pneumoniae inclusion in the absence of a CT229 homologue? We have not yet addressed this issue experimentally, but we can envision several possible models for how Rab4 is recruited to the C. pneumoniae inclusion in the absence of a CT229 homologue. First, C. pneumoniae may encode a functional homologue of CT229 that shares no primary amino acid sequence identity or only limited sequence homology with CT229, thus eluding identification in homology searches. Second, as mentioned above, Rab4 interacts with at least eight different downstream effectors, and since the predicted profile of inclusion membrane proteins differs dramatically between C. trachomatis and C. pneumoniae (1), Rab4 may be recruited to the C. trachomatis and C. pneumoniae inclusions via interaction with a functionally distinct chlamydial Inc protein.
Time course analysis revealed that GFP-Rab4A colocalizes with EBs by 2 h postinfection. At this early time point, EBs are still situated at the periphery of the cell and have not yet been trafficked to the peri-Golgi region of the cell. Recruitment of GFP-Rab4A to the inclusion was not observed at earlier times (data not shown), suggesting that Rab4A does not associate with the nascent inclusion during the entry process and is not recruited as a result of uptake into a classical EE. Previous data are consistent with this hypothesis, as no EE markers have been shown to be incorporated into the early chlamydial vacuole (35). Therefore, Rab4A is recruited to the chlamydial vacuole by a microtubule-independent mechanism after the nascent vacuole has formed. In contrast to the early recruitment of both GFP-Rab4A and GFP-Rab11A (32), other GFP-Rabs, such as the Golgi-localized Rab6, do not associate with inclusions until at least 8 h postinfection (A. R. Moorhead and M. A. Scidmore, unpublished data). These data suggest that Rab GTPases most likely function at several points during the chlamydial developmental cycle. The recruitment of Rab4 to vacuoles prior to their redistribution to the MTOC suggests that Rab4 may play a role in the intracellular redistribution of EBs. Considering that both Tfn-containing REs associate with chlamydial inclusions (35, 36, 47) and that Rab4- and Rab11-positive REs are transported in a microtubule-dependent manner to the peri-Golgi region, Rab4 and/or Rab11 may function to recruit REs to the inclusion. As a result, EB-containing vacuoles may be trafficked to the MTOC by exploitation of recycling endosomal trafficking to the MTOC. Although endosomes containing Tfn are still recruited to the inclusion and EBs are still trafficked to the peri-Golgi region in the presence of a dominant-negative Rab4 (data not shown), Rab4 may still function in these processes as discussed below.
Based upon the yeast two-hybrid results, the carboxy-terminal domain of CT229 contained by amino acids 101 to 215 mediates the interaction between CT229 and Rab4. These results are consistent with previous microinjection data that demonstrated that the carboxy-terminal domain is exposed to the host cell cytosol (16). Thus, the Rab4-interacting domain that we identified in this study is present within the cytosolically exposed domain of CT229. Further analysis revealed that deletion of amino acids 198 to 215 of CT229 prevented interaction between CT229 and Rab4. However, fusion of amino acids 198 to 215 of CT229 to GAL4AD did not confer the ability of this fusion protein to bind to GAL4BD-Rab4 nor did fusions containing only these amino acids colocalize with full-length CT229 fusion proteins or Tfn in HeLa cells (data not shown). These data suggest that amino acids 198 to 215 of CT229 are necessary but not sufficient for interaction with Rab4. The failure of truncated GAL4AD-CT229 (101-197) to interact with Rab4 may be due to the instability of this fusion protein or possibly due to a deletion of a specific Rab4 binding domain. However, GAL4AD-CT229 (101-197) interacts with GAL4BD-CT229 (101-215) in the two-hybrid assay, demonstrating that GAL4AD-CT229 (101-197) is indeed stably expressed. Therefore, amino acids 198 to 215 may constitute part of a single Rab4 binding site or one of multiple Rab4 binding sites or may be needed to confer the proper tertiary structure that is required for exposure of a Rab4 binding site. As previously mentioned, antiserum raised against a peptide containing amino acids 201 to 215 inhibits chlamydial development when microinjected into infected cells during the first 2 h postinfection (16). Although untested, these data suggest that binding of anti-CT229 to CT229 may physically occlude a Rab4 binding site.
We attempted to examine the function of Rab4 in infected cells by examining infected cells transiently expressing dominant-negative or constitutively activated mutants of Rab4. However, no defect was observed in the overall ability of chlamydiae to grow in nonpolarized epithelial cells as evidenced by the growth of inclusions in cells expressing either the constitutively active or dominant-negative Rab4 mutant. Because Rab4 is localized to Tfn-containing endosomes and plays a major role in Tfn receptor recycling, we also examined the trafficking and the recruitment of Tfn-containing REs to the inclusion in infected cells expressing each Rab4 mutant. However, Tfn was still trafficked to either the 6-h or the mature 18-h inclusion in infected cells expressing Rab4A or Rab4B mutants (data not shown). Taken together, these data suggest that neither Rab4 isoform alone is necessary for the intracellular development of chlamydiae or the recruitment of Tfn-containing endosomes to the inclusion. In addition, EBs were still redistributed to the peri-Golgi region at 6 h postinfection in cells expressing dominant-negative Rab4, suggesting that neither Rab4 isoform alone plays a role in the intracellular redistribution of EBs. However, Rab4A and Rab4B may be functionally redundant, and since inhibition of both isoforms simultaneously has not been examined, the possibility remains that Rab4 functions in one of these cellular processes. Finally, dominant-negative Rab mutants prevent activation of wild-type Rab GTPases by stably binding guanine exchange factors (GEFs), thereby titrating out the GEFs and thus preventing wild-type Rab GTPases from being loaded with GTP. Therefore, if chlamydiae express a protein that mimics a GEF, wild-type Rab4 may still be activated at the inclusion membrane even in the presence of a dominant-negative Rab mutant. Although no chlamydial proteins with homologies to known GEFs have been identified in the completed chlamydial genomes, we cannot yet rule out this possibility since Salmonella spp. have genes encoding two proteins, SopE and SopE2, which lack any detectable sequence homology with known Rho GEFs and yet have been shown to function as Rho GEFs (41). Therefore, in the future, the potential functions of Rab4 will be examined in infected cells by depleting the cell of Rab4 isoforms individually and in combination using small interfering RNA.
The interaction between CT229 and Rab4 at the inclusion membrane provides a second example of how Incs function by recruiting host proteins to the chlamydial inclusion. Importantly, this newly identified interaction between a chlamydial Inc and a host protein suggests that identifying additional interacting host ligands of other Incs may help to elucidate the function of this unique class of chlamydial proteins. Further work is needed in order to elucidate the exact role of CT229-Rab4 interactions in C. trachomatis-infected cells as well as to address how Rab4 is recruited to C. pneumoniae inclusions.
Acknowledgments
We thank Harlan D. Caldwell and Ted Hackstadt for providing chlamydial organisms and antisera. We also thank Helene Marquis and members of the Scidmore lab for critical reading of the manuscript.
A.R.M. was supported by NIHT32RR07059.
Editor: R. P. Morrison
REFERENCES
- 1.Bannantine, J. P., R. S. Griffiths, V. Viratyosin, W. J. Brown, and D. D. Rockey. 2000. A secondary structural motif predictor of protein localization to the chlamydial inclusion membrane. Cell. Microbiol. 2:35-47. [DOI] [PubMed] [Google Scholar]
- 2.Belland, R. J., G. Zhong, D. D. Crane, D. Hogan, D. Sturdevant, J. Sharma, W. L. Beatty, and H. D. Caldwell. 2003. Genomic transcriptional profiling of the developmental cycle of Chlamydia trachomatis. Proc. Natl. Acad. Sci. USA 100:8478-8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bielli, A., P. O. Thornquist, A. G. Hendrick, R. Finn, K. Fitzgerald, and M. W. McCaffrey. 2001. The small GTPase Rab4A interacts with the central region of cytoplasmic dynein light intermediate chain-1. Biochem. Biophys. Res. Commun. 281:1141-1153. [DOI] [PubMed] [Google Scholar]
- 4.Brymora, A., V. A. Valova, and P. J. Robinson. 2004. Protein-protein interactions identified by pull-down experiments and mass spectrometry, p. 17.5.1-17.5.24. In M. D. J. S. Bonifacino, J. B. Harford, J. Lippincott-Schwartz, and K. M. Yamada (ed.), Current protocols in cell biology, vol. 2. John Wiley and Sons, Inc., New York, N.Y. [DOI] [PubMed] [Google Scholar]
- 5.Caldwell, H. D., J. Kromhout, and J. Schachter. 1981. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect. Immun. 31:1161-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 1997. Chlamydia trachomatis genital tract infections—United States, 1995. Morb. Mortal. Wkly. Rep. 46:193-198. [PubMed] [Google Scholar]
- 7.Chavrier, P., and B. Goud. 1999. The role of ARF and Rab GTPases in membrane transport. Curr. Opin. Cell Biol. 11:466-475. [DOI] [PubMed] [Google Scholar]
- 8.Clausen, J. D., G. Christiansen, H. U. Holst, and S. Birklund. 1997. Chlamydia trachomatis utilizes the host cell microtubule network during early events of infection. Mol. Microbiol. 25:441-449. [DOI] [PubMed] [Google Scholar]
- 9.Collins, R. N., and P. Brennwald. 2000. Rab proteins. Front. Mol. Biol. 24:137-175. [Google Scholar]
- 10.Cormont, M., M. Mari, A. Galmiche, P. Hofman, and Y. Le Marchand-Brustel. 2001. A FYVE-finger-containing protein, Rabip4, is a Rab4 effector involved in early endosomal trafficking. Proc. Natl. Acad. Sci. USA 98:1637-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Renzis, S., B. Sonnichsen, and M. Zerial. 2002. Divalent Rab effectors regulate the sub-compartmental organization and sorting of early endosomes. Nat. Cell Biol. 4:124-133. [DOI] [PubMed] [Google Scholar]
- 12.Farnsworth, C. C., M. C. Seabra, L. H. Ericsson, M. H. Gelb, and J. A. Glomset. 1994. Rab geranylgeranyl transferase catalyzes the geranylgeranylation of adjacent cysteines in the small GTPases Rab1A, Rab3A and Rab5A. Proc. Natl. Acad. Sci. USA 91:11963-11967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fields, K. A., and T. Hackstadt. 2002. The chlamydial inclusion: escape from the endocytic pathway. Annu. Rev. Cell Dev. Biol. 18:221-245. [DOI] [PubMed] [Google Scholar]
- 14.Fouraux, M. A., M. Deneka, V. Ivan, A. van der Heijden, J. Raymackers, D. van Suylekom, W. J. van Venrooij, P. van der Sluijs, and G. J. M. Pruijn. 2004. Rabip4′ is an effector of rab5 and rab4 and regulates transport through early endosomes. Mol. Biol. Cell 15:611-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grieshaber, S. S., N. A. Grieshaber, and T. Hackstadt. 2003. Chlamydia trachomatis utilizes host cell dynein to traffic to the microtubule-organizing center in a p50 dynamitin-independent process. J. Cell Sci. 116:3793-3802. [DOI] [PubMed] [Google Scholar]
- 16.Hackstadt, T., M. A. Scidmore-Carlson, and C. A. Dooley. 1999. Chlamydia trachomatis inclusion membrane protein required for intracellular development. Mol. Biol. Cell 10(Suppl. S):182A. [Google Scholar]
- 17.Hackstadt, T., M. A. Scidmore-Carlson, E. I. Shaw, and E. R. Fischer. 1999. The Chlamydia trachomatis IncA protein is required for homotypic fusion. Cell. Microbiol. 1:119-130. [DOI] [PubMed] [Google Scholar]
- 18.Heinzen, R. A., M. A. Scidmore, D. D. Rockey, and T. Hackstadt. 1996. Differential interaction with endocytic and exocytic pathways distinguish parasitophorous vacuoles of Coxiella burnetii and Chlamydia trachomatis. Infect. Immun. 64:796-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalman, S., W. Mitchell, R. Marathe, C. Lammel, J. Fan, R. W. Hyman, L. Olinger, J. Grimwood, R. W. Davis, and R. S. Stephens. 1999. Comparative genomes of Chlamydia pneumoniae and C. trachomatis. Nat. Genet. 21:385-389. [DOI] [PubMed] [Google Scholar]
- 20.Li, L., W. Omata, I. Kojima, and H. Shibata. 2001. Direct interaction of Rab4 with syntaxin 4. J. Biol. Chem. 276:5265-5273. [DOI] [PubMed] [Google Scholar]
- 21.Lindsay, A. J., A. G. Hendrick, G. Cantalupo, F. Senic-Matuglia, B. Goud, C. Bucci, and M. W. McCaffrey. 2002. Rab coupling protein (RCP), a novel Rab4 and Rab11 effector protein. J. Biol. Chem. 277:12190-12199. [DOI] [PubMed] [Google Scholar]
- 22.McCaffrey, M. W., A. Bielli, G. Cantalupo, S. Mora, V. Roberti, M. Santillo, F. Drummond, and C. Bucci. 2001. Rab4 affects both recycling and degradative endosomal trafficking. FEBS Lett. 495:21-30. [DOI] [PubMed] [Google Scholar]
- 23.Mohrmann, K., R. Leijendekker, L. Gerez, and P. van der Sluijs. 2002. Rab4 regulates transport to the apical plasma membrane in Madin-Darby canine kidney cells. J. Biol. Chem. 277:10474-10481. [DOI] [PubMed] [Google Scholar]
- 24.Nagelkerken, B., E. Van Anken, M. Van Raak, L. Gerez, K. Mohrmann, N. Van Uden, J. Holthuizen, L. Pelkmans, and P. Van Der Sluijs. 2000. Rabaptin4, a novel effector of the small GTPase rab4a, is recruited to perinuclear recycling vesicles. Biochem. J. 346:593-601. [PMC free article] [PubMed] [Google Scholar]
- 25.Pagano, R. E., P. Crottet, C. Prescianotto-Baschong, and M. Spiess. 2004. In vitro formation of recycling vesicles from endosomes requires adaptor protein-1/clathrin and is regulated by Rab4 and the connector Rabaptin-5. Mol. Biol. Cell 15:4990-5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pereira-Leal, J. B., and M. C. Seabra. 2000. The mammalian Rab family of small GTPases: definition of family and subfamily sequence motifs suggests a mechanism for functional specificity in the Ras superfamily. J. Mol. Biol. 301:1077-1087. [DOI] [PubMed] [Google Scholar]
- 27.Pfeffer, S. R. 2001. Rab GTPases: specifying and deciphering organelle identity and function. Trends Cell Biol. 11:487-491. [DOI] [PubMed] [Google Scholar]
- 28.Roberts, M., S. Barry, A. Woods, P. van der Sluijs, and L. Norman. 2001. PDGF-regulated rab4-dependent recycling of alphavbeta3 integrin from early endosomes is necessary for cell adhesion and spreading. Curr. Biol. 11:1392-1402. [DOI] [PubMed] [Google Scholar]
- 29.Rockey, D. D., D. Grosenbach, D. E. Hruby, M. G. Peacock, R. A. Heinzen, and T. Hackstadt. 1997. Chlamydia psittaci IncA is phosphorylated by the host cell and is exposed on the cytoplasmic face of the developing inclusion. Mol. Microbiol. 24:217-228. [DOI] [PubMed] [Google Scholar]
- 30.Rockey, D. D., M. A. Scidmore, J. P. Bannantine, and W. J. Brown. 2002. Proteins in the chlamydial inclusion membrane. Microbes Infect. 4:333-340. [DOI] [PubMed] [Google Scholar]
- 31.Rothman, J. E. 1994. Mechanisms of intracellular protein transport. Nature 372:55-63. [DOI] [PubMed] [Google Scholar]
- 32.Rzomp, K. A., L. D. Scholtes, B. J. Briggs, G. R. Whittaker, and M. A. Scidmore. 2003. Rab GTPases are recruited to chlamydial inclusions in both a species-dependent and species-independent manner. Infect. Immun. 71:5855-5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schramm, N., and P. B. Wyrick. 1995. Cytoskeletal requirements in Chlamydia trachomatis infection in host cells. Infect. Immun. 63:324-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schroer, T. A. 2004. Dynactin. Annu. Rev. Cell Dev. Biol. 20:759-799. [DOI] [PubMed] [Google Scholar]
- 35.Scidmore, M. A., E. R. Fischer, and T. Hackstadt. 2003. Restricted fusion of Chlamydia trachomatis vesicles with endocytic compartments during the initial stages of infection. Infect. Immun. 71:973-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scidmore, M. A., E. R. Fischer, and T. Hackstadt. 1996. Sphingolipids and glycoproteins are differentially trafficked to the Chlamydia trachomatis inclusion. J. Cell Biol. 134:363-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scidmore, M. A., and T. Hackstadt. 2001. Mammalian 14-3-3beta associates with the Chlamydia trachomatis inclusion membrane via its interaction with IncG. Mol. Microbiol. 39:1638-1650. [DOI] [PubMed] [Google Scholar]
- 38.Scidmore, M. A., D. D. Rockey, E. R. Fischer, R. A. Heinzen, and T. Hackstadt. 1996. Vesicular interactions of the Chlamydia trachomatis inclusion are determined by chlamydial early protein synthesis rather than route of entry. Infect. Immun. 64:5366-5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scidmore-Carlson, M. A., E. I. Shaw, C. A. Dooley, E. R. Fischer, and T. Hackstadt. 1999. Identification and characterization of a Chlamydia trachomatis early operon encoding four novel inclusion membrane proteins. Mol. Microbiol. 33:753-765. [DOI] [PubMed] [Google Scholar]
- 40.Shaw, E. I., C. A. Dooley, E. R. Fischer, M. A. Scidmore, K. A. Fields, and T. Hackstadt. 2000. Three temporal classes of gene expression during the Chlamydia trachomatis developmental cycle. Mol. Microbiol. 37:913-925. [DOI] [PubMed] [Google Scholar]
- 41.Stender, S., A. Friebel, S. Linder, M. Rhode, S. Mirold, and W. D. Hardt. 2000. Identification of SopE2 from Salmonella typhimurium, a conserved guanine nucleotide exchange factor for CDC42 of the host cell. Mol. Microbiol. 36:1206-1221. [DOI] [PubMed] [Google Scholar]
- 42.Taraska, T., D. M. Ward, R. S. Ajioka, P. B. Wyrick, S. R. Davis-Kaplan, C. H. Davis, and J. Kaplan. 1996. The late chlamydial inclusion membrane is not derived from the endocytic pathway and is relatively deficient in host proteins. Infect. Immun. 64:3713-3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thylefors, B., A. D. Negrel, R. Pararajasegaram, and K. Y. Dadzie. 1995. Global data on blindness. Bull. W. H. O. 73:115-121. [PMC free article] [PubMed] [Google Scholar]
- 44.Tuvim, M. J., R. Adachi, S. Hoffenberg, and B. F. Dickey. 2001. Traffic control: Rab GTPases and the regulation of interorganellar transport. News Physiol. Sci. 16:56-61. [DOI] [PubMed] [Google Scholar]
- 45.van der Sluijs, P., M. Hull, P. Webster, P. Male, B. Goud, and I. Mellman. 1992. The small GTP-binding protein rab4 controls an early sorting event on the endocytic pathway. Cell 70:729-740. [DOI] [PubMed] [Google Scholar]
- 46.van der Sluijs, P., M. Hull, A. Zahraoui, A. Tavitian, B. Goud, and I. Mellman. 1991. The small GTP-binding protein rab4 is associated with early endosomes. Proc. Natl. Acad. Sci. USA 88:6313-6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Ooij, C., G. Apodaca, and J. Engel. 1997. Characterization of the Chlamydia trachomatis vacuole and its interaction with the host endocytic pathway in HeLa cells. Infect. Immun. 65:758-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vitale, G., V. Rybin, S. Christoforidis, P. O. Thornquist, J. M. McCaffery, H. Stenmark, and M. Zerial. 1998. Distinct Rab-binding domains mediate interaction of Rabaptin-5 with GTP-bound rab4 and rab5. EMBO J. 17:1941-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]