Abstract
Eimeria spp. are intracellular protozoa that infect intestinal epithelia of most vertebrates, causing coccidiosis. Intestinal intraepithelial lymphocytes (IEL) that reside at the basolateral site of epithelial cells (EC) have immunoregulatory and immunoprotective roles against Eimeria spp. infection. However, it remains unknown how IEL are involved in the regulation of epithelial barrier during Eimeria sp. infection. Here, we demonstrated two distinct roles of IEL against infection with Eimeria vermiformis, a murine pathogen: production of cytokines to induce protective immunity and expression of junctional molecules to preserve epithelial barrier. The number of IEL markedly increased when oocyst production reached a peak. During infection, IEL increased production of gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) and decreased transforming growth factor β (TGF-β) production. Addition of IFN-γ and TNF-α or supernatants obtained from cultured IEL from E. vermiformis-infected mice reduced transepithelial electrical resistance (TER) in a confluent CMT93 cell monolayer, a murine intestine-derived epithelial line, but antibodies against these cytokines suppressed the decline of TER. Moreover, TGF-β attenuated the damage of epithelial monolayer and changes in TER caused by IFN-γ and TNF-α. The expression of junctional molecules by EC was decreased when IEL produced a high level of IFN-γ and TNF-α and a low level of TGF-β in E. vermiformis-infected mice. Interestingly, IEL constantly expressed junctional molecules and a coculture of EC with IEL increased TER. These results suggest that IEL play important multifunctional roles not only in protection of the epithelium against E. vermiformis-induced change by cytokine production but also in direct interaction with the epithelial barrier when intra-EC junctions are down-regulated.
Eimeria spp., are intracellular protozoan parasites (phylum: Apicomplexa) closely related to infectious pathogens of humans such as Cryptosporidium spp. (38). Eimeria spp. infect the intestinal epithelial cells (EC) of numerous animals including rodents and livestock, and Eimeria sp.-infected animals often show growth retardation and morbidity and mortality (4, 16). Currently, an enormous economic problem in the livestock business is caused by infection with Eimeria spp., leading to intestinal coccidiosis involving a bloody diarrhea, sloughing of the epithelium, and death of the host (39). Although inflammatory tissue injury has been reported to closely correlate with the oocyst output from the host (3), the involvement of immunological events with disruption of barrier function in intestinal epithelium during Eimeria sp. infection has remained unclear.
Eimeria vermiformis, a murine pathogen, has been widely used as a coccidial model in the laboratory to elucidate the mechanism of host protection against primary and secondary infections. T cells bearing T-cell receptor αβ (TCRαβ) (αβ T cells) are necessary for protecting the host against infection, whereas γδ T cells are important for repairing lesions as regulatory cells (31). It is assumed that the effects of T cells in protection against E. vermiformis are partially mediated by cytokines, such as gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α), because these cytokines have been detected in E. vermiformis-infected hosts (27). IFN-γ-deficient or -depleted mice are highly susceptible to infection (40, 41). IFN-γ is produced in E. vermiformis-infected hosts, especially at the site of infection. Although intestinal intraepithelial lymphocytes (IEL) reside at a basolateral site of the intestinal epithelium, no studies have examined the role of cytokine production by IEL during E. vermiformis infection.
The epithelial lining of the gastrointestinal tract forms a regulated, selectively permeable barrier between the luminal contents and the underlying tissue compartments by junctional molecules. The junction complex constitutes the primary barrier against the paracellular penetration of intestinal microorganisms. In particular, tight junction complexes, which are formed by occludin, a plasma membrane protein, and zonula occludens (ZO), its cytoplasmic partner protein, are linked to the actin cytoskeleton (23). Selective disturbance of tight junction complexes by microorganisms results in the rapid decrease of transepithelial electrical resistance (TER) of the EC layer, resulting in increasing paracellular permeability (20). Recently, we demonstrated that IEL express a range of junctional molecules associated with EC (15). Both IFN-γ and TNF-α are elevated in the inflammatory mucosa, showing a contribution to the proinflammatory cascade, which may be involved in barrier disruption (24, 26). Hence, we examined cytokine production by IEL and their involvement in epithelial function to understand the contribution of IEL in maintaining an epithelial barrier during enteric infection.
MATERIALS AND METHODS
Mice.
Male C57BL/6 mice were purchased at the age of 8 weeks from the Japan SLC (Hamamatsu, Japan). All mice were used between 8 and 12 weeks of age, and protocols were approved by the institutional review board for animal experiments of the University of Miyazaki.
Infection by E. vermiformis.
E. vermiformis was passaged in mice, and oocysts were purified and sporulated (33). After microscopical scoring of stocks for sporulation, mice were given 100 or 500 sporulated oocysts in 100 μl of water by oral gavage. During infection, feces were collected every 3 days. Oocysts were counted on McMaster chambers after salt flotation.
Histological analysis.
Intestines were fixed with 4% paraformaldehyde in phosphate-buffered saline and embedded in paraffin. The paraffin sections were stained with hematoxylin-eosin.
Cell preparation.
Following E. vermiformis infection, mice were euthanized and both IEL and EC in the small intestine were isolated and prepared every 3 days according to a modification of previously published methods (12). In brief, after the small intestine was divided into the upper one-third (including the duodenum) and the lower two-thirds (from the jejunum to the ileum), dissected small segments of each part were incubated at 37°C for 30 min in RPMI 1640 medium (Sigma Chemical Co., Missouri) containing 10% fetal calf serum and 1 mM dithiothreitol with vigorous shaking. The tissue suspension was passaged through a nylon mesh to remove debris and centrifuged through a 25%-40%-75% discontinuous Percoll (Sigma) gradient at 600 × g at 20°C for 20 min. The cells collected from the interface of 40%-75% and 25%-40% were IEL and EC, respectively.
Cell culture and cytokine analysis.
Whole and sorted IEL (1 × 106/ml) were added to a 96-well plate precoated with 2.5 μg anti-CD3ɛ monoclonal antibody (MAb) (145-2C11; BD Pharmingen) and were cultured for 48 h in RPMI 1640 supplemented with 10% fetal calf serum, 100 U/ml penicillin, and 100 U/ml streptomycin. The supernatants were collected to estimate the cytokine contents. The contents of cytokines in the culture supernatant were assayed by an enzyme-linked immunosorbent assay (ELISA) system using mouse IFN-γ, interleukin-4 (IL-4), and TNF-α (e-bioscience, San Diego, Calif.) and transforming growth factor β (TGF-β) (Biosource International Inc., Camarillo, Calif.). ELISA systems were used according to the manufacturer's instructions.
Analysis of mRNA expression of junctional molecules.
Total RNA was extracted from isolated cells using the RNeasy Mini kit (QIAGEN, Valencia, Calif.) and primed with 20 pmol of a random primer in mixtures for reverse transcription. The synthesized cDNA was amplified by PCR using primers specific for the murine junctional molecules and β-actin cDNA sequence. The primer sets were described as previously reported for reverse transcription-PCR (RT-PCR) (15): ZO-1, sense, 5′-CAA AGC CCA CCA AGG TCA C-3′, and antisense, 5′-TCT CTT TCC GAG GCA TTA GCA-3′; occludin, sense, 5′-CAG GGC TCT TTG GAG GAA-3′, and antisense, 5′-TAC ACG ATC GTG GCA ATA AAC-3′; junctional adhesion molecule (JAM), sense, 5′-GAC CCG GAA GGA CAA TGG AGA-3′, and antisense, 5′-AGG ACA GCT GCC ACG ATG C-3′; β-catenin, sense, 5′-ACT GTT CTA CGC CAT CAC GAC-3′, and antisense, 5′-CCT CTA TGC CAC CCA CTT G-3′; E-cadherin, sense, 5′-GCA CAT ATG TAG CTC TCA TC-3′, and antisense, 5′-CCT TCA CAG TCA CAC ACA TG-3′; desmoglein 2, sense, 5′-GGA CTT TGG AAA CGG ACT TC-3′, and antisense, 5′-TCT GTA ATT CCC TTC CCA GTG-3′; connexin 26, sense, 5′-GCT CAC GGT CCT CTT CAT CT-3′, and antisense, 5′-ACC CTT CGA TAC GGA CCT TCT-3′; β-actin, sense, 5′-TGG AAT CCT GTG GCA TCC ATG AAA C-3′, and antisense, 5′-TAA AAC GCA GCT CAG TAA CAG TCC G-3′. For quantitative analysis of mRNA, the synthesized cDNA was amplified by using primers and probes specific for ZO-1 and occludin and β-actin cDNA sequence (TaqMan gene expression assays; Applied Biosystems, Foster City, Calif.) according to the manufacturer's instructions. Quantitative real-time PCR was performed by using an ABI Prism 7000 sequence detector system (Applied Biosystems, Foster City, Calif.). Results are expressed as difference (n-fold) from the expression of β-actin.
Western blotting analysis.
Western blotting analysis was performed as previously reported (15). In brief, isolated EC and IEL were washed with cold phosphate-buffered saline and lysed in sample buffer (125 mM Tris, pH 6.8, 2% sodium dodecyl sulfate, 10% glycerol, and 2% 2-mercaptoethanol) at 95°C for 7 min, followed by sonication for 1 min. Proteins were electrophoresed and transferred to a polyvinylidene difluoride membrane. The membrane was blocked with 10% skimmed milk in Tris-buffered saline-Tween for 1 h, incubated with each Ab against junctional proteins overnight at 4°C, and then incubated with appropriate horseradish peroxidase-labeled secondary Abs (affinity-purified goat anti-rabbit, goat anti-mouse, or goat anti-rat immunoglobulin G) that were obtained from Jackson ImmunoResearch Labs Inc. (West Grove, Pa.). Bound antibodies were visualized by enhancement with chemiluminescence substrate (Pierce, Rockford, Ill.). The primary Abs against junctional molecules used in Western blotting were as follows: rabbit antioccludin (Zymed; cytoplasmic domain specific), rabbit antioccludin (Santa Cruz; extracellular/cytoplasmic domain), mouse anti-β-catenin (BD Transduction Laboratories), goat anti-JAM1 (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.), goat anti-E-cadherin (extracellular domain specific; R&D Systems Inc., Minneapolis, MN), and mouse anti-desmoglein (BD Transduction Laboratories). Mouse anti-β-actin Ab was purchased from Sigma (St. Louis, Mo.).
Fluorescence-activated cell sorting analysis.
IEL were stained with fluorescein isothiocyanate-conjugated TCRγδ (GL3), phycoerythrin-conjugated TCRβ (H57-597), and allophycocyanin-conjugated CD3ɛ (145-2C11) MAbs as described previously (13). Flow cytometry analysis was performed on a FACScalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ). All MAbs were purchased from BD Pharmingen (San Jose, Calif.).
Measurement of TER.
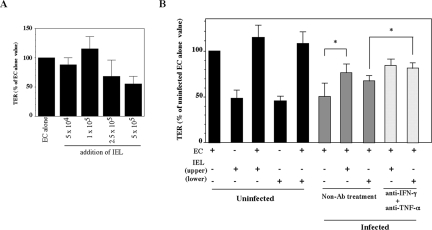
Functional integrity of tight junctions in cell layers established on filter inserts was assessed by measuring TER using a Millicel ERS volt-ohmmeter (Millipore Corp., Bedford, Mass.). The CMT93 cell line, a mouse intestine-derived epithelial line, was obtained from the American Type Culture Collection (CCL223). CMT93 cells were seeded on the apical chamber of a transwell by using the BD BioCoat Intestinal Epithelium Differentiation Environment (BD Biosciences, Bedford, Mass.) for 72 h in a 5% CO2 incubator according to the manufacturer's instructions and developed a TER around 450 Ω/cm2. After that, culture medium was removed from the apical and basolateral chamber and replaced with either fresh medium or medium containing IFN-γ (1, 10, or 100 ng/ml) (Chemicon, Temecula, Calif.), TNF-α (1, 10, or 100 ng/ml) (Peprotech EC, London, United Kingdom), or TGF-β (2.5 ng/ml) (Roche Diagnostics, Mannheim, Germany) and cultured for 48 h. To examine the effect of cytokines produced by IEL in E. vermiformis-infected mice on the barrier of CMT93 cells, mice were euthanized at each day after E. vermiformis infection and IEL were prepared. IEL were stimulated with plate-bound anti-CD3 MAb for 48 h, and the supernatant was recovered. The culture medium from the apical and basolateral chamber was removed and replaced with the supernatant. For neutralization of IFN-γ and TNF-α, anti-IFN-γ and anti-TNF-α MAbs (BioLegend, San Diego, Calif.) were added to culture wells at 10 μg/ml. The TER without a cellular monolayer was consistently less than 70 Ω/cm2. For primary EC culture, EC were isolated as described previously (12) and 5 × 105 cells were seeded on the apical chamber of a transwell by using the BD BioCoat Intestinal Epithelium Differentiation Environment for 6 days to develop a TER around 200 Ω/cm2. A varying number of IEL were added onto cultured EC on day 4 after the start of culture, and 2 days later, TER was measured. Values of TER are expressed as percentages of the initial resistance as follows: % of the initial resistance = [(resistance from each point) − (resistance from a blank)/(resistance from nontreated cells) − (resistance from a blank)] × 100.
Depletion of IFN-γ or TNF-α in vivo.
Five hundred micrograms of anti-IFN-γ MAb (R4-6A2) (21) or 50 μg of anti-TNF-α MAb (1F3F3; Bender MedSystems Products, Vienna, Austria) or rat immunoglobulin G (Cappel, North Carolina) was administered intraperitoneally into the mice on days −1, 3, and 7 after infection.
Statistical analysis.
Student's t test was used to determine the significant differences. A P value of less than 0.05 was taken as significant.
RESULTS
Preference of E. vermiformis for infecting the small intestine.
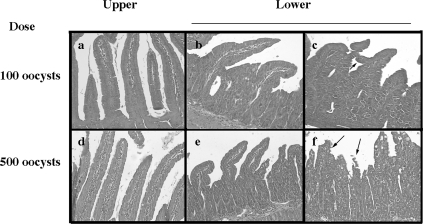
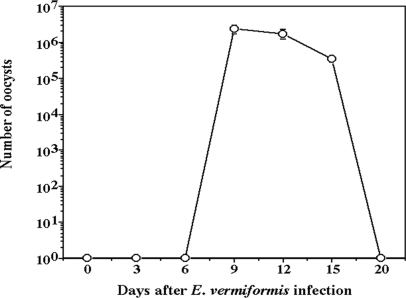
E. vermiformis infects the lower part (jejunum to ileum) of the murine small intestine (46), confirmed in the present study in C57BL/6 mice with 100 oocysts (Fig. 1). Alteration of the structure, such as enlarged crypt EC, breakage at the villus tip (arrows in Fig. 1f), and mound-like villi (arrowhead in Fig. 1c), was observed in the lower segment of the small intestine in an E. vermiformis dose-dependent manner. We noted that when mice were infected with a higher dose (>1,000 oocysts) of E. vermiformis, some succumbed before the infection had spread throughout the jejunum (data not shown). In addition, the body weight of the mice infected with 100 oocysts was not altered. Based on these observations, we used 100 oocysts of E. vermiformis to infect mice in subsequent experiments. Oocyst production increased from days 6 to 9 postinfection (p.i.) with E. vermiformis and then gradually decreased (Fig. 2).
FIG. 1.
Hematoxylin-and-eosin-stained transverse sections of the small intestine on day 9 p.i. Mice were given 100 or 500 oocysts of E. vermiformis. On day 9 p.i., the small intestine was removed and divided into parts, the upper (including duodenum [a and d]) and the lower (jejunum [b and e] and ileum [c and f]) (magnification, ×200).
FIG. 2.
Oocyst output of E. vermiformis in C57BL/6 mice. Mice were orally infected with 100 oocysts of E. vermiformis, and the oocyst output was determined every 3 days. Three mice were used in each point. Values represent the mean ± standard deviation of three individual experiments.
Changes in the kinetics of IEL subsets following E. vermiformis infection.
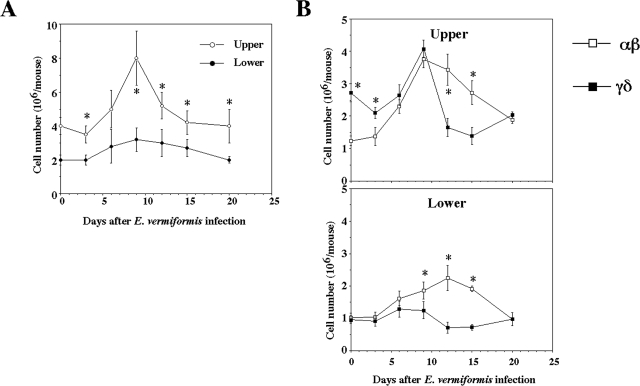
While staining the tissue sections with hematoxylin and eosin, we noticed that the number of IEL in the upper one-third was much higher than that in the lower two-thirds of the small intestine in healthy mice (26/100 EC in the upper segment versus 11/100 EC in the lower segment). In addition to the regulatory function of IEL for EC (12, 18, 49), IEL have been shown to change in response to E. vermiformis infection (8). We confirmed these findings. The difference in the infection pattern with E. vermiformis between the upper and lower segments of the small intestine (46) (Fig. 1) allowed assessment of compartmentalization in the response induced by infection of the lower small intestine. Although E. vermiformis infection was not observed in the upper segment, the total number of IEL seen in infected mice increased temporally on day 9 p.i. and then decreased to the preinfection levels, while the number in the lower segment did not change significantly (Fig. 3A). Murine IEL comprise an approximately equal frequency of T cells bearing TCRαβ (αβ IEL) and TCRγδ (γδ IEL) (10). We also examined the changes in the population of αβ IEL and γδ IEL during E. vermiformis infection. As shown in Fig. 3B, a larger number of γδ IEL were present in the upper segment before infection and then decreased until day 15 p.i., whereas αβ IEL increased following infection. Both αβ and γδ IEL in the upper segment almost recovered to the preinfection level on day 20 p.i. In the lower segment, αβ IEL and γδ IEL were nearly equal in number. αβ IEL increased until day 15 p.i., whereas γδ IEL decreased. The number of γδ IEL was lower than that of αβ IEL during infection except for the early stage of infection in the upper segment (days 0 to 3 p.i.). Both αβ and γδ IEL in the lower segment of the small intestine recovered to the initial level on day 20 p.i. These results indicate that the total number of IEL increased at around a peak of oocyst production, which was due to an increase in αβ IEL rather than γδ IEL.
FIG. 3.
Cell number of IEL following infection with E. vermiformis. (A) Total IEL number was counted by staining with trypan blue. (B) Population of IEL bearing TCRαβ and TCRγδ of the upper and lower parts following infection. IEL were stained with fluorescein isothiocyanate-conjugated TCRγδ MAb, PE-conjugated TCRβ MAb, and allophycocyanin-conjugated CD3ɛ MAb. The TCRαβ and TCRγδ expression was gated on CD3+ cells. Three mice were used in each point. Values represent the mean ± standard deviation of three individual experiments in a triplicate assay. Asterisks represent statistically significant differences (P < 0.05).
Production of preventive cytokines by IEL in E. vermiformis-infected mice.
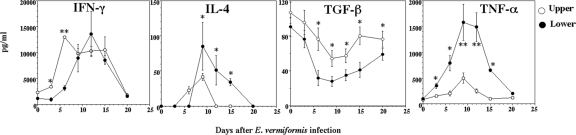
Cytokine balance is important for protection against or susceptibility to infection. IFN-γ, a typical Th1-type cytokine, has been shown to be important against primary infection with E. vermiformis (35, 41). E. vermiformis development occurs within EC; hence, IEL, which reside between EC, are well placed to influence parasite development and to maintain the integrity of the EC barrier. IEL are reported to produce both Th1 and Th2 cytokines (48); thus, it is important to determine which cytokines are produced by IEL during E. vermiformis infection. We examined the production of cytokines IFN-γ, IL-4 (a typical Th2-type cytokine), and TNF-α (an inflammatory cytokine) by IEL in the upper and lower segments of the gut during infection. As shown in Fig. 4, IFN-γ, IL-4, and TNF-α were increased in IEL after infection, reached a peak on days 6 to 12 p.i., and then recovered to the initial level on day 20 p.i. In contrast, TGF-β (a Th3-type and immunoregulatory cytokine) decreased and reached a minimum on days 12 to 15 p.i and recovered to the initial level on day 20 p.i. These changes in the cytokines occurred after oocyst shedding (Fig. 2). TGF-β was produced in greater quantities in the upper segment than in the lower segment of the small intestine, and large quantities of TNF-α were produced in the lower segment during infection.
FIG. 4.
Changes in cytokine production of IEL following infection with E. vermiformis. Freshly isolated IEL at each time point were cultured with plate-bound anti-CD3 MAb for 48 h, and the supernatants were collected to determine the concentration of each cytokine by ELISA for IFN-γ, IL-4, TNF-α, and TGF-β. Three mice were used in each point. Values represent the mean ± standard deviation of three individual experiments in a triplicate assay. Asterisks represent statistically significant differences (*, P < 0.05; **, P < 0.01).
Effects of cytokines produced by IEL on the epithelial barrier.
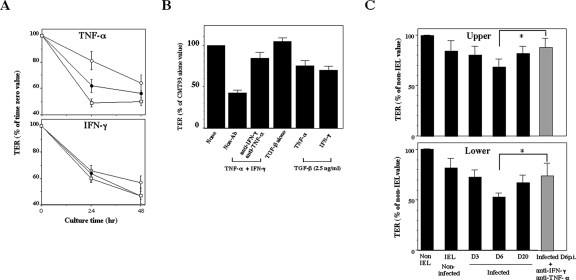
We next examined whether cytokines produced by IEL directly affect the epithelial barrier function. In order to examine the functional integrity of the epithelial barrier, we measured TER in CMT93 cells, which were cultured in a transwell. The CMT93 cell layer progressively lost TER in a dose-dependent manner when the cells were cultured with IFN-γ or TNF-α (Fig. 5A). TER was further decreased when CMT93 cells were cultured in combination with IFN-γ and TNF-α (Fig. 5B). As TGF-β has been known not only to repair mucosal injury but also to preserve the integrity of the intestinal mucosa (6, 28), we examined the effect of TGF-β on TER in CMT93 cells. Although TER in CMT93 cells was decreased by the addition of TNF-α or IFN-γ alone (Fig. 5A), it was increased by the addition of TNF-α plus TGF-β or IFN-γ plus TGF-β (Fig. 5B). TER was not affected by addition of TGF-β alone. Antibodies against TNF-α or IFN-γ suppressed the decline of TER in CMT93 cells. Furthermore, when CMT93 cells were cultured with the supernatant of IEL derived from the upper or lower segment of the small intestine from noninfected mice, TER decreased to 84% or 82%, respectively (Fig. 5C). TER in CMT93 cells decreased to 68% when CMT93 cells were cultured with the supernatant obtained from IEL from the upper segment of the infected mice on day 6 p.i. In contrast, TER was more intensively reduced (54%) by exposure to the supernatant of IEL from the lower segment of the infected mice. The decreased TER in CMT93 cells was significantly reversed by the addition of anti-IFN-γ and anti-TNF-α Abs, confirming that these cytokines directly affect TER. These results indicate that the epithelial barrier could be impaired through inflammatory cytokines produced by IEL in E. vermiformis-infected mice.
FIG. 5.
Abolishment of TER in an epithelial cell line by inflammatory cytokines produced by IEL. (A) CMT93 cells were exposed to 1 (open circles), 10 (closed circles), and 100 (open squares) ng/ml of recombinant IFN-γ or TNF-α for 24 and 48 h. (B) CMT93 cells were cultured in combination with 10 ng/ml of IFN-γ and TNF-α. for 48 h. CMT93 cells were also cultured with 2.5 ng/ml of TGF-β1 and IFN-γ or TNF-α (10 ng/ml) for 48 h. (C) CMT93 cells were cultured with supernatants of IEL prepared from mice infected with E. vermiformis on days 3, 6, and 20 p.i. in the presence of immobilized anti-CD3 MAb. Supernatant prepared from mice infected with E. vermiformis on day 6 p.i. was added with 10 μg/ml of anti-IFN-γ and anti-TNF-α Abs to CMT93 cells. These cells were then incubated for 48 h at 37°C for the determination of TER. Values represent the mean ± standard deviation of three individual experiments in a triplicate assay. Asterisks represent statistically significant differences (*, P < 0.05).
Decreased expression of junctional molecules in EC during E. vermiformis infection.
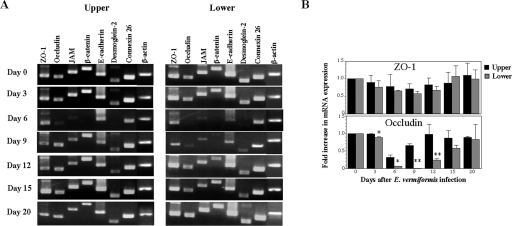
An established clinical manifestation of coccidiosis is a reproducible fluid loss at the peak of infection (30), suggesting involvement of the physical damage and disruption of the epithelial barrier as shown in Fig. 1. To maintain the integrity of the intestinal barrier, EC express junctional molecules such as tight junction (ZO-1, occludin, and JAM), adherens junction (β-catenin and E-cadherin), desmosome (desmoglein 2), and gap junction (connexin 26) at the apical-to-basolateral sites to prevent paracellular mutual crossing of a body fluid and of a variety of luminal contents (2, 36). In order to determine how the epithelial barrier is impaired in the E. vermiformis-infected mice, we examined the expression level of specific mRNA for a variety of junctional molecules in small intestinal EC. In the upper segment of the small intestine, the mRNA expression of occludin, JAM, β-catenin, and desmoglein 2 remarkably decreased on day 6 p.i. (Fig. 6A). In the lower segment, in addition to these junctional molecules, the expression of E-cadherin was also decreased. The expression of these molecules was partially recovered on day 9 p.i. and completely on day 12 p.i. in the upper segment. In contrast, the recovery of these molecules in the lower segment was delayed in comparison with that in the upper part. Since ZO-1 and occludin are crucially important for formation of TER, we further examined these proteins' expression by real-time RT-PCR. The expression of occludin mRNA in the lower segment is less than that in the upper segment, whereas expression of ZO-1 was not altered, consistent with RT-PCR data in Fig. 6A.
FIG. 6.
Decreased mRNA expression of junctional molecules in small intestinal EC following infection with E. vermiformis. (A) Total RNA was prepared from EC of small intestine and amplified by RT-PCR. (B) mRNA expression of ZO-1 and occludin was analyzed quantitatively by real-time PCR. Data are presented as the ratio of each time point to day 0 in the upper and lower segment. Asterisks represent statistically significant differences between the upper and lower segments (*, P < 0.05; **, P < 0.01).
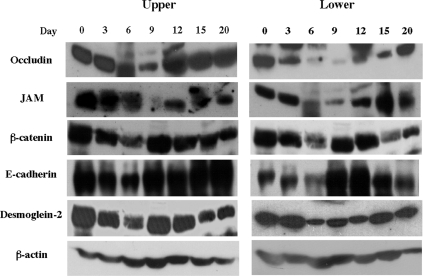
We further confirmed the expression of occludin, JAM, β-catenin, E-cadherin, and desmoglein 2 in EC at the protein level (Fig. 7). In the lower segment, the expression of occludin, one of the tight junction molecules, continued to decrease from day 3 p.i. to day 9 p.i. and then recovered. The expression of JAM also decreased on days 6 and 9 p.i. The expression of β-catenin, E-cadherin, and desmoglein, which are located basolaterally in the enterocytes, was shown to decrease on day 6 p.i. and then increased until day 15 p.i. In the upper segment, although JAM and β-catenin transiently decreased, expression was rapidly recovered. These findings suggest a strong relationship between the impairment of expression of junctional molecules by EC and the severity of the infection-induced lesions.
FIG. 7.
Western blotting analysis for the expression of junctional molecules at the protein level in EC of small intestine. Cell lysate was prepared from small intestinal EC in mice and analyzed by Western blotting as described in Materials and Methods.
Expression of junctional molecules in IEL during E. vermiformis infection and maintenance of the epithelial barrier by IEL.
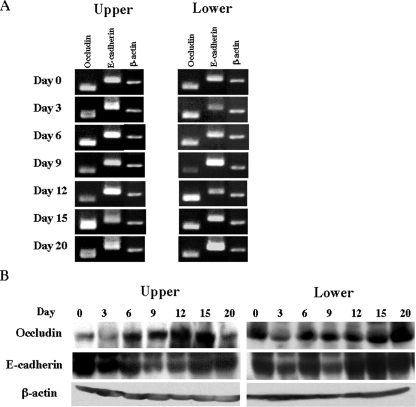
We recently reported that IEL constitutively express junctional molecules, similar to EC, especially occludin and E-cadherin, strongly suggesting that IEL bind to and interact with EC (15). We examined the expression of occludin and E-cadherin in IEL during infection with E. vermiformis. Unlike EC, occludin and E-cadherin expressed by IEL did not decrease following infection at either the mRNA or the protein level (Fig. 8A and B). These results indicate that the junctional molecules in IEL, but not in EC, are maintained during infection.
FIG. 8.
Sustained mRNA expression of junctional molecules in small intestinal IEL following infection with E. vermiformis. After small intestine was separated into upper and lower segments, fresh IEL were obtained. (A) Total RNA was prepared from IEL in the upper and lower parts and amplified by RT-PCR. (B) Western blotting analysis for the expression of junctional molecules at protein level in IEL of small intestine. Cell lysate was prepared from small intestinal IEL in the upper and lower segments and analyzed by Western blotting.
We further examined whether IEL can maintain an epithelial barrier function of EC by measuring TER of freshly isolated EC. Freshly isolated EC of the small intestine reached a maximal TER on day 6 after the start of the culture (data not shown). Titration of the number of IEL applied to EC on day 4 of culture revealed that maximum TER on day 6 was achieved with 1 × 105 IEL (Fig. 9A). As shown in Fig. 9B, TER did not change significantly when EC alone or EC plus IEL from noninfected mice were cultured. In contrast, TER markedly decreased when EC prepared from infected mice were used. Interestingly, TER of EC recovered when cocultured with IEL in the upper segment of the small intestine of infected mice but not with IEL in the lower segment. The decreased TER in EC recovered after the treatment of mice with anti-IFN-γ and anti-TNF-α Abs confirmed that these cytokines decrease TER in EC in vivo. IEL die easily when separated and cultured in isolation (13). However, we observed that approximately 60% of IEL remained alive at 48 h in the transwell under the present experimental conditions (data not shown). Collectively, these results suggest that IEL contribute to the maintenance of an epithelial barrier in E. vermiformis-infected mice.
FIG. 9.
Increased TER in freshly isolated EC cocultured with IEL from E. vermiformis-infected mice on day 6 p.i. (A) Effect of a varying number of IEL on TER of EC. On day 4 after EC were cultured, IEL were added to EC and TER was measured on day 6. Values represent the mean ± standard deviation of three individual experiments in a triplicate assay. (B) On day 4 after EC were cultured, 1 × 105 IEL were added to EC, and TER was measured with or without 10 μg/ml of anti-IFN-γ and anti-TNF-α Abs on day 6. Values represent the mean ± standard deviation of three individual experiments in a triplicate assay. Asterisks represent statistically significant differences (P < 0.05).
Effects of IFN-γ and TNF-α on inhibition of growth of E. vermiformis and in exaggeration of epithelial damage.
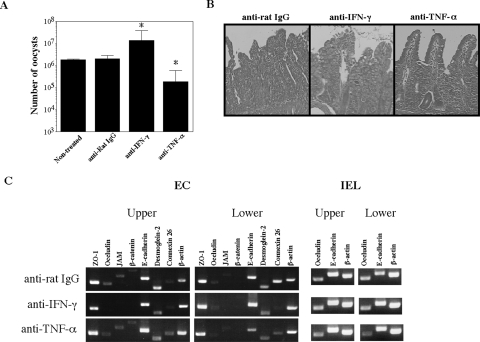
IFN-γ and TNF-α were increased in E. vermiformis-infected mice (Fig. 4), and these cytokines diminished epithelial barrier function (Fig. 5). We examined the role of IFN-γ and TNF-α in modulation of intestinal epithelial barrier against E. vermiformis infection by using IFN-γ- or TNF-α-depleted mice. As previously reported (41), anti-IFN-γ Ab-treated mice produced approximately 10-fold-more oocysts than control Ab-treated mice on day 9 p.i. (Fig. 10A). By contrast, anti-TNF-α Ab-treated mice showed markedly lower levels of oocyst output. Histological analysis revealed that anti-IFN-γ Ab-treated mice exhibited more severe intestinal damage than control immunoglobulin G or anti-TNF-α Ab-treated mice did in the lower segment (Fig. 10B). We further examined expression of junctional molecules by EC in anti-IFN-γ or -TNF-α Ab-treated mice. Treatment with anti-IFN-γ Ab or anti-TNF-α Ab alone failed to recover expression of junctional molecules, which are suppressed by Eimeria infection (Fig. 10C), compared with the level of the expression in noninfected mice (Fig. 6A, day 0 panel). These results suggest that IFN-γ is an important cytokine in protective immunity against Eimeria infection and that TNF-α may exacerbate infection. Epithelial damage was affected by the level of infection and also influenced by the activity of cytokines in vivo.
FIG. 10.
Modulation of junctional molecules due to depletion of IFN-γ or TNF-α in vivo. (A) Oocyst output of E. vermiformis in mice treated with anti-IFN-γ Ab or anti-TNF-α Ab in vivo. Mice were orally infected with 100 oocysts of E. vermiformis, and the oocyst output was determined on day 9 p.i. Values represent the mean ± standard deviation of three mice in each group. Asterisks represent statistically significant differences (*, P < 0.05). (B) Hematoxylin-and-eosin-stained transverse sections of the small intestine on day 9 p.i. (magnification, ×200). (C) mRNA expression of junctional molecules in small intestinal EC following infection with E. vermiformis. Total RNA was prepared from EC of small intestine and amplified by RT-PCR. IgG, immunoglobulin G.
DISCUSSION
The disruption of the epithelial barrier has been reported in intestinal diseases including those induced by infection. IEL are located to play protective roles against infection, repair of a damaged mucosal barrier, and maintenance of the barrier function in the intestine. The present study focused upon IEL as a defender of the host against the consequences of E. vermiformis infection, and we found that IEL play an important role in sustaining the barrier function via maintaining the expression of junctional molecules as well as by producing cytokines against E. vermiformis infection. These data strongly suggest that IEL function both as constituent cells of the physical epithelial barrier during infection and in killing pathogens in local intracellular infection.
It has long been considered that IEL serve a critical role in the mucosal immune system by performing a variety of regulatory functions, including cytokine production, cytotoxic activity, and induction of apoptosis in EC (11, 14, 44, 48). E. vermiformis infects the lower segment of the small intestine in mice (46), and infection-induced inflammation and damage of tissue were restricted to this segment (Fig. 1). We found that the total number of IEL in the upper segment is relatively larger than that in the lower segment even before infection and increases further during E. vermiformis infection (Fig. 3A). Overall the IEL in the murine small intestine contain approximately equal populations bearing TCRαβ and TCRγδ (10); however, the results of these studies indicated rationalization of the IEL populations with greater numbers of γδ IEL in the upper part of the small intestine than in more distal regions (Fig. 3). We have reported that γδ T-cell-deficient (Cδ−/−) mice are susceptible to trinitrobenzene sulfonic acid-induced colitis and show a fragile small intestine (12), suggesting that the residence of fewer γδ IEL in the lower part of the small intestine might cause fragility of the intestinal epithelium. Interestingly, these data might support the possibility that IEL function to prevent the spread of infection or damage to the upper segment because the number of IEL, especially αβ IEL in the upper segment, is higher from day 6 to day 9 p.i. (Fig. 3), and this alteration occurs concurrently with oocyst production (Fig. 1). Our data support the previous report that in adult mice, CD4+ αβ T cells play the role of effector cells against primary infection of E. vermiformis, while γδ T cells play the role of regulatory cells (31). Moreover, the dynamics of IEL in noninfected regions of the small intestine reveal a level of coordination within the gut T-cell responses more extensive than that previously reported in any system. The effects of this coordination may be an important proactive response to protect uninfected regions of the gut from the potential of an infection spreading within the small intestine.
A transmission electrical microscopy study revealed that the sporozoites of Eimeria spp. actively invaded intestinal EC, which resulted in the disruption of internal cellular organization and extensive tissue damage, including disrupted microvilli (5). Interestingly, sporozoites likely moved from EC to EC at lateral sites because a junction structure, such as the tight junction, was not disturbed at the apical site, whereas that at the lateral or basolateral site was. In this study, decreased expression of occludin and JAM recovered from day 12 to day 15 p.i.; in contrast, E-cadherin and desmoglein 2 decreased from day 3 to day 6 p.i. (Fig. 6 and 7), suggesting that, during the schizogony stage, sporocysts and merozoites might move among adjacent cells, causing the destruction of the junction structure at the lateral site. Furthermore, it is possible that many EC are easily broken and damaged when oocysts break out of the host EC. Our study provides evidence that the expression of junctional proteins at the apical site, such as occludin and JAM, was reduced. We have recently demonstrated that IEL constitutively express junctional molecules, such as occludin and E-cadherin, and that the expression of these proteins is higher in γδ IEL than in αβ IEL (15). We showed in this study that IEL, unlike EC, constantly express E-cadherin and occludin during all periods of E. vermiformis infection (Fig. 8). It would be interesting to determine why and how the sporozoites of E. vermiformis prefer to move within restricted sites of the epithelium. For example, Clostridium perfringens enterotoxin binds to claudin 3 and 4, the molecules in the tight junction (9, 43). The surface of Listeria monocytogenes interacts with the extradomain of E-cadherin (19, 25, 37). These reports suggest that junctional molecules may be receptors for invading microorganisms. Therefore, it is likely that E. vermiformis could also bind to certain junctional molecules to move between EC. Further information is needed to prove these hypotheses.
Although eimerian infection is controlled by IFN-γ and CD4+ T cells restricted to major histocompatibility complex class II (41), it is noteworthy that IL-4 mRNA was up-regulated during infection. The production of antigen-specific IL-5 was also noted with ex vivo assays (32), and it is clear that a Th1/Th2 mixed cytokine profile is induced by infection. Nonetheless, unlike IFN-γ, IL-4 deficiency does not alter the level of oocyst output (41) and the role of Th2-type cytokines in the biology of eimerian infections is unknown, although it might be speculated that these could reduce the level of Th1-type cytokines in the gut, performing a regulatory role to reduce immune-mediated damage.
The disruption of the EC barrier might be also mediated through cytokines that are produced by IEL, a hypothesis supported by the findings of the present study. During the eimerian infection, IEL transiently produced high amounts of IFN-γ and TNF-α associated with a decrease in production of TGF-β, suggesting an interaction between production of these cytokines by IEL and that by EC. IFN-γ is well established as an important anti-Eimeria molecule in vitro (34) and in vivo (35, 41), as is the anti-inflammatory role for TGF-β (17, 29). Hence, our model is that the production of a high amount of IFN-γ is involved in parasite killing, whereas the infection is controlled and then resolution of the intestinal lesions is coordinated by IEL and production of TGF-β. These cytokines are known to be involved in the alteration of junctional molecules. The expression of junctional molecules on the IEL would also help to provide a structural framework for EC recovery and promote close juxtaposition between IEL and the damaged/recovering EC.
IFN-γ down-regulates the occludin promoter of the human intestinal cell line HT-29/B6 and modulates junction integrity (24, 26). On the other hand, it is well known that TGF-β regulates multiple physiological functions in various tissues, including intestinal EC, and that γδ IEL can produce TGF-β (7). Recently, we reported that the transfer of γδ IEL to Cδ−/− mice ameliorated trinitrobenzene sulfonic acid-induced colitis mediated by TGF-β (12). TGF-β was shown to enhance the barrier function of T84 intestinal epithelial monolayers and to promote intestinal epithelial restitution (6, 28). In our data, the IFN-γ-mediated loss of TER in CMT93 cells was normalized by addition of TGF-β (Fig. 4B). These results strongly support the hypothesis that TGF-β is necessary for the maintenance of intestinal epithelial integrity. Indeed, TNF-α induces damage in the lower segment of the small intestine after infection and was produced in the greatest amounts from IEL in the infected segment of the small intestine (Fig. 4). Taken together, our results suggest that IEL positively produce Th1-type cytokines, such as IFN-γ, to protect the host against E. vermiformis. Moreover, IEL might function to prevent the excessive disruption of the epithelial layer through IEL-derived cytokines by maintaining the expression of junctional molecules and production of TGF-β. It would also be interesting to determine why junctional molecules expressed by EC are remarkably down-regulated, while those in IEL are maintained during E. vermiformis infection (Fig. 7 and 8). This might be explained by the fact that intestinal EC possess a TNF receptor (TNFR) as well as an IFN-γ receptor (1). TNF-α has been shown to down-regulate occludin but not ZO-1 in astrocytes (47). This down-regulation of occludin was entirely mediated by TNFR1. TNFR consists of TNFR1 and TNFR2 (22, 42). TNF-α binds to both receptors; however, TNFR1 is mainly expressed on nonlymphocytes, such as EC and fibroblasts (45). Further studies will be needed to elucidate what kinds of signals from the cytokine receptor are linked to the gene expression of junctional molecules in EC and IEL and how this occurs.
Acknowledgments
This work was supported by grants-in-aid for scientific research (C) from the Japan Society for the Promotion of Science (17590373), the Takeda Science Foundation (K.I.-O.), and the 21st Century COE program (University of Miyazaki) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. A.L.S. received support from BBSRC and DEFRA of the United Kingdom.
We thank Goro Matsuzaki (University of the Ryukyus, Japan) for carefully reading and providing critical comments. We also thank Noriko Kanemaru for technical assistance and Joko Shigenobu and Ayumi Tanaka for their steadfast support.
Editor: J. L. Flynn
REFERENCES
- 1.Baumgart, D. C., W. A. Olivier, T. Reya, D. Peritt, J. L. Rombeau, and S. R. Carding. 1998. Mechanisms of intestinal epithelial cell injury and colitis in interleukin 2 (IL2)-deficient mice. Cell. Immunol. 187:52-66. [DOI] [PubMed] [Google Scholar]
- 2.Berkes, J., V. K. Viswanathan, S. D. Savkovic, and G. Hecht. 2003. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut 52:439-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blagburn, B. L., and K. S. Todd, Jr. 1984. Pathological changes and immunity associated with experimental Eimeria vermiformis infections in Mus musculus. J. Protozool. 31:556-561. [DOI] [PubMed] [Google Scholar]
- 4.Chapman, H. D. 2001. Use of anticoccidial drugs in broiler chickens in the USA: analysis for the years 1995 to 1999. Poult. Sci. 80:572-580. [DOI] [PubMed] [Google Scholar]
- 5.Chobotar, B., H. D. Danforth, and R. Entzeroth. 1993. Ultrastructural observations of host-cell invasion by sporozoites of Eimeria papillata in vivo. Parasitol. Res. 79:15-23. [DOI] [PubMed] [Google Scholar]
- 6.Dignass, A. U., and D. K. Podolsky. 1993. Cytokine modulation of intestinal epithelial cell restitution: central role of transforming growth factor beta. Gastroenterology 105:1323-1332. [DOI] [PubMed] [Google Scholar]
- 7.Ehrhardt, R. O., W. Strober, and G. R. Harriman. 1992. Effect of transforming growth factor (TGF)-beta 1 on IgA isotype expression. TGF-beta 1 induces a small increase in sIgA+ B cells regardless of the method of B cell activation. J. Immunol. 148:3830-3836. [PubMed] [Google Scholar]
- 8.Findly, R. C., S. J. Roberts, and A. C. Hayday. 1993. Dynamic response of murine gut intraepithelial T cells after infection by the coccidian parasite Eimeria. Eur. J. Immunol. 23:2557-2564. [DOI] [PubMed] [Google Scholar]
- 9.Fujita, K., J. Katahira, Y. Horiguchi, N. Sonoda, M. Furuse, and S. Tsukita. 2000. Clostridium perfringens enterotoxin binds to the second extracellular loop of claudin-3, a tight junction integral membrane protein. FEBS Lett. 476:258-261. [DOI] [PubMed] [Google Scholar]
- 10.Guy-Grand, D., N. Cerf-Bensussan, B. Malissen, M. Malassis-Seris, C. Briottet, and P. Vassalli. 1991. Two gut intraepithelial CD8+ lymphocyte populations with different T cell receptors: a role for the gut epithelium in T cell differentiation. J. Exp. Med. 173:471-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guy-Grand, D., M. Malassis-Seris, C. Briottet, and P. Vassalli. 1991. Cytotoxic differentiation of mouse gut thymodependent and independent intraepithelial T lymphocytes is induced locally. Correlation between functional assays, presence of perforin and granzyme transcripts, and cytoplasmic granules. J. Exp. Med. 173:1549-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inagaki-Ohara, K., T. Chinen, G. Matsuzaki, A. Sasaki, Y. Sakamoto, K. Hiromatsu, F. Nakamura-Uchiyama, Y. Nawa, and A. Yoshimura. 2004. Mucosal T cells bearing TCRγδ play a protective role in intestinal inflammation. J. Immunol. 173:1390-1398. [DOI] [PubMed] [Google Scholar]
- 13.Inagaki-Ohara, K., H. Nishimura, A. Mitani, and Y. Yoshikai. 1997. Interleukin-15 preferentially promotes the growth of intestinal intraepithelial lymphocytes bearing gamma delta T cell receptor in mice. Eur. J. Immunol. 27:2885-2891. [DOI] [PubMed] [Google Scholar]
- 14.Inagaki-Ohara, K., H. Nishimura, T. Sakai, D. H. Lynch, and Y. Yoshikai. 1997. Potential for involvement of Fas antigen/Fas ligand interaction in apoptosis of epithelial cells by intraepithelial lymphocytes in murine small intestine. Lab. Investig. 77:421-429. [PubMed] [Google Scholar]
- 15.Inagaki-Ohara, K., A. Sawaguchi, T. Suganuma, G. Matsuzaki, and Y. Nawa. 2005. Intraepithelial lymphocytes express junctional molecules in murine small intestine. Biochem. Biophys. Res. Commun. 331:977-983. [DOI] [PubMed] [Google Scholar]
- 16.Jenkins, M. C. 2001. Advances and prospects for subunit vaccines against protozoa of veterinary importance. Vet. Parasitol. 101:291-310. [DOI] [PubMed] [Google Scholar]
- 17.Kitani, A., I. J. Fuss, K. Nakamura, O. M. Schwartz, T. Usui, and W. Strober. 2000. Treatment of experimental (trinitrobenzene sulfonic acid) colitis by intranasal administration of transforming growth factor (TGF)-beta1 plasmid: TGF-beta1-mediated suppression of T helper cell type 1 response occurs by interleukin (IL)-10 induction and IL-12 receptor beta2 chain downregulation. J. Exp. Med. 192:41-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Komano, H., Y. Fujiura, M. Kawaguchi, S. Matsumoto, Y. Hashimoto, S. Obana, P. Mombaerts, S. Tonegawa, H. Yamamoto, S. Itohara, et al. 1995. Homeostatic regulation of intestinal epithelia by intraepithelial gamma delta T cells. Proc. Natl. Acad. Sci. USA 92:6147-6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lecuit, M., S. Dramsi, C. Gottardi, M. Fedor-Chaiken, B. Gumbiner, and P. Cossart. 1999. A single amino acid in E-cadherin responsible for host specificity towards the human pathogen Listeria monocytogenes. EMBO J. 18:3956-3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li, E., W. F. Stenson, C. Kunz-Jenkins, P. E. Swanson, R. Duncan, and S. L. Stanley, Jr. 1994. Entamoeba histolytica interactions with polarized human intestinal Caco-2 epithelial cells. Infect. Immun. 62:5112-5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li, Y., K. Ishii, H. Hisaeda, S. Hamano, M. Zhang, K. Nakanishi, T. Yoshimoto, H. Hemmi, K. Takeda, S. Akira, Y. Iwakura, and K. Himeno. 2004. IL-18 gene therapy develops Th1-type immune responses in Leishmania major-infected BALB/c mice: is the effect mediated by the CpG signaling TLR9? Gene Ther. 11:941-948. [DOI] [PubMed] [Google Scholar]
- 22.Loetscher, H., Y. C. Pan, H. W. Lahm, R. Gentz, M. Brockhaus, H. Tabuchi, and W. Lesslauer. 1990. Molecular cloning and expression of the human 55 kd tumor necrosis factor receptor. Cell 61:351-359. [DOI] [PubMed] [Google Scholar]
- 23.Madara, J. L. 1998. Regulation of the movement of solutes across tight junctions. Annu. Rev. Physiol. 60:143-159. [DOI] [PubMed] [Google Scholar]
- 24.Mankertz, J., S. Tavalali, H. Schmitz, A. Mankertz, E. O. Riecken, M. Fromm, and J. D. Schulzke. 2000. Expression from the human occludin promoter is affected by tumor necrosis factor alpha and interferon gamma. J. Cell Sci. 113:2085-2090. [DOI] [PubMed] [Google Scholar]
- 25.Mengaud, J., H. Ohayon, P. Gounon, R. M. Mege, and P. Cossart. 1996. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell 84:923-932. [DOI] [PubMed] [Google Scholar]
- 26.Oshima, T., F. S. Laroux, L. L. Coe, Z. Morise, S. Kawachi, P. Bauer, M. B. Grisham, R. D. Specian, P. Carter, S. Jennings, D. N. Granger, T. Joh, and J. S. Alexander. 2001. Interferon-gamma and interleukin-10 reciprocally regulate endothelial junction integrity and barrier function. Microvasc. Res. 61:130-143. [DOI] [PubMed] [Google Scholar]
- 27.Ovington, K. S., L. M. Alleva, and E. A. Kerr. 1995. Cytokines and immunological control of Eimeria spp. Int. J. Parasitol. 25:1331-1351. [DOI] [PubMed] [Google Scholar]
- 28.Planchon, S. M., C. A. Martins, R. L. Guerrant, and J. K. Roche. 1994. Regulation of intestinal epithelial barrier function by TGF-beta 1. Evidence for its role in abrogating the effect of a T cell cytokine. J. Immunol. 153:5730-5739. [PubMed] [Google Scholar]
- 29.Powrie, F., J. Carlino, M. W. Leach, S. Mauze, and R. L. Coffman. 1996. A critical role for transforming growth factor-beta but not interleukin 4 in the suppression of T helper type 1-mediated colitis by CD45RB(low) CD4+ T cells. J. Exp. Med. 183:2669-2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramsburg, E., R. Tigelaar, J. Craft, and A. Hayday. 2003. Age-dependent requirement for γδ T cells in the primary but not secondary protective immune response against an intestinal parasite. J. Exp. Med. 198:1403-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts, S. J., A. L. Smith, A. B. West, L. Wen, R. C. Findly, M. J. Owen, and A. C. Hayday. 1996. T-cell alpha beta + and gamma delta + deficient mice display abnormal but distinct phenotypes toward a natural, widespread infection of the intestinal epithelium. Proc. Natl. Acad. Sci. USA 93:11774-11779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rose, M. E., P. Hesketh, R. K. Grencis, and A. J. Bancroft. 2000. Vaccination against coccidiosis: host strain-dependent evocation of protective and suppressive subsets of murine lymphocytes. Parasite Immunol. 22:161-172. [DOI] [PubMed] [Google Scholar]
- 33.Rose, M. E., D. G. Owen, and P. Hesketh. 1984. Susceptibility to coccidiosis: effect of strain of mouse on reproduction of Eimeria vermiformis. Parasitology 88:45-54. [DOI] [PubMed] [Google Scholar]
- 34.Rose, M. E., A. L. Smith, and D. Wakelin. 1991. Gamma interferon-mediated inhibition of Eimeria vermiformis growth in cultured fibroblasts and epithelial cells. Infect. Immun. 59:580-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rose, M. E., D. Wakelin, and P. Hesketh. 1989. Gamma interferon controls Eimeria vermiformis primary infection in BALB/c mice. Infect. Immun. 57:1599-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneeberger, E. E., and R. D. Lynch. 2004. The tight junction: a multifunctional complex. Am. J. Physiol. Cell Physiol. 286:C1213-C1228. [DOI] [PubMed] [Google Scholar]
- 37.Schubert, W. D., C. Urbanke, T. Ziehm, V. Beier, M. P. Machner, E. Domann, J. Wehland, T. Chakraborty, and D. W. Heinz. 2002. Structure of internalin, a major invasion protein of Listeria monocytogenes, in complex with its human receptor E-cadherin. Cell 111:825-836. [DOI] [PubMed] [Google Scholar]
- 38.Shields, J. M., and B. H. Olson. 2003. Cyclospora cayetanensis: a review of an emerging parasitic coccidian. Int. J. Parasitol. 33:371-391. [DOI] [PubMed] [Google Scholar]
- 39.Shirley, M. W. 1992. Research on avian coccidia: an update. Br. Vet. J. 148:479-499. [DOI] [PubMed] [Google Scholar]
- 40.Smith, A. L., and A. C. Hayday. 2000. An alphabeta T-cell-independent immunoprotective response towards gut coccidia is supported by gammadelta cells. Immunology 101:325-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith, A. L., and A. C. Hayday. 2000. Genetic dissection of primary and secondary responses to a widespread natural pathogen of the gut, Eimeria vermiformis. Infect. Immun. 68:6273-6280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith, C. A., T. Davis, D. Anderson, L. Solam, M. P. Beckmann, R. Jerzy, S. K. Dower, D. Cosman, and R. G. Goodwin. 1990. A receptor for tumor necrosis factor defines an unusual family of cellular and viral proteins. Science 248:1019-1023. [DOI] [PubMed] [Google Scholar]
- 43.Sonoda, N., M. Furuse, H. Sasaki, S. Yonemura, J. Katahira, Y. Horiguchi, and S. Tsukita. 1999. Clostridium perfringens enterotoxin fragment removes specific claudins from tight junction strands: evidence for direct involvement of claudins in tight junction barrier. J. Cell Biol. 147:195-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taguchi, T., W. K. Aicher, K. Fujihashi, M. Yamamoto, J. R. McGhee, J. A. Bluestone, and H. Kiyono. 1991. Novel function for intestinal intraepithelial lymphocytes. Murine CD3+, gamma/delta TCR+ T cells produce IFN-gamma and IL-5. J. Immunol. 147:3736-3744. [PubMed] [Google Scholar]
- 45.Tartaglia, L. A., and D. V. Goeddel. 1992. Two TNF receptors. Immunol. Today 13:151-153. [DOI] [PubMed] [Google Scholar]
- 46.Todd, K. S., Jr., and D. L. Lepp. 1971. The life cycle of Eimeria vermiformis Ernst, Chobotar and Hammond, 1971 in the mouse Mus musculus. J. Protozool. 18:332-337. [DOI] [PubMed] [Google Scholar]
- 47.Wachtel, M., M. F. Bolliger, H. Ishihara, K. Frei, H. Bluethmann, and S. M. Gloor. 2001. Down-regulation of occludin expression in astrocytes by tumour necrosis factor (TNF) is mediated via TNF type-1 receptor and nuclear factor-κB activation. J. Neurochem. 78:155-162. [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto, M., K. Fujihashi, M. Amano, J. R. McGhee, K. W. Beagley, and H. Kiyono. 1994. Cytokine synthesis and apoptosis by intestinal intraepithelial lymphocytes: signaling of high density alpha beta T cell receptor+ and gamma delta T cell receptor+ T cells via T cell receptor-CD3 complex results in interferon-gamma and interleukin-5 production, while low density T cells undergo DNA fragmentation. Eur. J. Immunol. 24:1301-1306. [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto, M., K. Fujihashi, K. Kawabata, J. R. McGhee, and H. Kiyono. 1998. A mucosal intranet: intestinal epithelial cells down-regulate intraepithelial, but not peripheral, T lymphocytes. J. Immunol. 160:2188-2196. [PubMed] [Google Scholar]