Abstract
A vaccine that effectively protects immunocompromised patients against invasive aspergillosis is a novel approach to a universally fatal disease. Here we present a rationale for selection and in vivo testing of potential protein vaccine candidates, based on the modification of an immunodominant fungal allergen for which we demonstrate immunoprotective properties. Pulmonary exposure to viable Aspergillus fumigatus conidia as well as vaccination with crude hyphal extracts protects corticosteroid-immunosuppressed mice against invasive aspergillosis (J. I. Ito and J. M. Lyons, J. Infect. Dis. 186:869-871, 2002). Sera from the latter animals contain antibodies with numerous and diverse antigen specificities, whereas sera from conidium-exposed mice contain antibodies predominantly against allergen Asp f 3 (and some against Asp f 1), as identified by mass spectrometry. Subcutaneous immunization with recombinant Asp f 3 (rAsp f 3) but not with Asp f 1 was protective. The lungs of Asp f 3-vaccinated survivors were free of hyphae and showed only a patchy low-density infiltrate of mononuclear cells. In contrast, the nonimmunized animals died with invasive hyphal elements and a compact peribronchial infiltrate of predominately polymorphonuclear leukocytes. Three truncated versions of rAsp f 3, spanning amino acid residues 15 to 168 [rAsp f 3(15-168)], 1 to 142, and 15 to 142 and lacking the known bipartite sequence required for IgE binding, were also shown to be protective. Remarkably, vaccination with either rAsp f 3(1-142) or rAsp f 3(15-168) drastically diminished the production of antigen-specific antibodies compared to vaccination with the full-length rAsp f 3(1-168) or the double-truncated rAsp f 3(15-142) version. Our findings point to a possible mechanism in which Asp f 3 vaccination induces a cellular immune response that upon infection results in the activation of lymphocytes that in turn enhances and/or restores the function of corticosteroid-suppressed macrophages to clear fungal elements in the lungs.
Invasive pulmonary aspergillosis (IPA) is a rapidly progressive and most often fatal disease that is common among severely immunocompromised individuals, including patients with hematologic malignancies, neutropenia, chronic granulomatous disease, solid-organ transplants (SOT), and allogeneic hematopoietic cell transplants (HCT). IPA is frequently observed in HCT and SOT recipients following the prolonged corticosteroid immunosuppression used to treat graft-versus-host disease in HCT and required to avoid graft rejection in SOT (10, 11, 19, 23, 32, 44, 46). The ubiquitous mold Aspergillus fumigatus is the most frequently isolated causative agent of invasive aspergillosis (28). It is also involved in allergic bronchopulmonary aspergillosis (ABPA) and other fungal diseases. Healthy individuals rarely contract respiratory fungal infections, being protected against inhaled spores (conidia) through innate immunity provided by alveolar macrophages and neutrophils (41). Opsonizing antibodies were previously suggested to play a role in enhancing phagocytosis of conidia and in B-cell-mediated memory immunity (33).
Currently available antifungal agents have had only limited success in treating IPA (18) and are also associated with serious toxicities, for example, nephrotoxicity and hepatotoxicity (15, 16, 18). It is therefore attractive to propose and test methods to induce, maintain, and/or rapidly restore specific antifungal immunity in immunocompromised patients. The restoration of the immune system is the key challenge for hematopoietic cell transplant recipients in which immunopathological effects, namely, graft-versus-host disease, need to be suppressed. Several authors have encouraged the development of antifungal vaccines or immunotherapies that would enhance or restore protective antifungal immunity (1, 8, 12, 42, 43), and in vitro cell-based and animal studies have been undertaken to support the feasibility of such an approach. Experiments include specific T-cell enhancing (2, 9) and dendritic cell-pulsing vaccination methods (3, 4), both in mice with IPA. Previously, we have shown that mice vaccinated subcutaneously with crude fungal protein extracts or by intranasal inoculation of viable conidia (VC) survive an otherwise lethal pulmonary challenge under corticosteroid immunosuppression (21). Although crude protein mixtures or deliberate exposure to Aspergillus would not be suitable for use in humans due to safety concerns related to toxicity and allergenicity, the use of a recombinant protein vaccine is both attractive and feasible. Such a vaccine could be produced in large amounts at low costs and with straightforward quality and safety controls designed to avoid allergenicity.
Several strategies to identify potential vaccine candidates exist. The recent availability of the complete genomes of the A. fumigatus strain Af293 (34), Aspergillus nidulans (13), and Aspergillus oryzae (31) can enable “reverse vaccinology” projects in which putative immunogenic antigens are first computationally predicted (36, 38). Such endeavors will likely lead to hundreds of vaccine candidates being tested, presumably in animal experiments. Recombinant antigens, including allergens, can be selected from expression libraries and screened for their ability to induce a protective immune response. Here, we have undertaken an immunochemical and mass spectrometric approach to identify the dominant antigen to which antibodies are produced in naïve immunocompetent mice following nasopulmonary exposure to viable A. fumigatus conidia, as previously described (21). We demonstrate that mice protectively immunized in this way elicit a specific immunoglobulin G2a (IgG2a) response against allergen Asp f 3. Subcutaneous injection of various versions of recombinant Asp f 3 (rAsp f 3), with or without deletion of the “allergenic” IgE-binding epitope, provides a significant degree of protection in corticosteroid-immunosuppressed mice.
MATERIALS AND METHODS
Chemicals and solvents were of the highest grade available and purchased from Sigma-Aldrich unless noted otherwise. Oligonucleotides were from Integrated DNA Technologies, Coralville, IA. Purchased antibodies used in this study were mouse monoclonal anti-Asp f 1, type IgG1 (Indoor Biotechnologies catalog no. 4A6), horseradish peroxidase (HRP)-conjugated rat anti-mouse IgG2a heavy chain (Serotec catalog no. MCA1588P), and HRP-conjugated goat anti-mouse IgG heavy plus light chains (Bio-Rad catalog no. 170-6516).
Aspergillus fumigatus.
A strain of A. fumigatus, AFCOH1, isolated from an IPA patient at the City of Hope National Medical Center (Duarte, CA) was used for vaccine preparations and infection as described previously (21). Conidium stock suspensions were prepared by collecting spores from day 5 to day 7 cultures on potato dextrose agar (BD/Difco) grown at 37°C into sterile 0.9% saline containing 0.1% Tween 80. Clumps of conidia were dispersed with 3-mm glass beads, and the suspension was washed twice and suspended to the desired concentration with 0.9% saline containing 0.01% Tween 80 (or alternatively 1% n-octyl-β-d-glucopyranoside) and 30% glycerol. Aliquots were frozen at −80°C and quick thawed to 37°C prior to use. This procedure gave mycelium-free suspensions of conidia, with >95% single conidia. Conidia were enumerated with a hemacytometer, and viability was assessed by agar plating.
Crude antigen and vaccine preparations.
Crude hyphal extract was prepared by sonication of hyphal mass from 72-h cultures grown in Czapek Dox medium supplemented with 1% Tryptone (BD/Difco). A 50-ml conical centrifuge tube containing 25 ml of washed hyphal mass was sonicated for a total of 4 min (four 1-min cycles) on ice by use of a Misonix Sonicator 3000 fitted with a 0.5-inch horn at an intensity setting of 10. The hyphal sonicate (HS) is not sterile and contains some viable hyphal fragments in a complex mixture of released hyphal proteins and other cellular components.
Fractionation.
HS was prefractionated by subsequent ultrafiltration through Centricon devices (Millipore) with a 30-kDa cutoff membrane and then with a 10-kDa cutoff membrane. Crude culture filtrate (CF) as well as prefractionated HS retentate and filtrate of the 10-kDa cutoff membrane fraction was analyzed by Western blot analysis and further fractionated by reversed-phase high-pressure liquid chromatography (HPLC) (Jupiter column, 5-μm, C18 paricles with 300-Å pores, 250 by 4.6 mm; Phenomenex, Torrance, California) with a gradient of acetonitrile-water in 0.1% trifluoroacetic acid (ÄKTA purifier; GE Healthcare). Fractions were spotted on nitrocellulose membrane (Bio-Rad), and dot blots were developed with sera from VC- and HS-immunized mice or monoclonal anti-Asp f 1 antibodies and HRP-conjugated anti-mouse IgG, diluted 1:3,000, for chemiluminescent detection. Positive fractions were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and stained with GelCode blue (Pierce).
Mass spectrometric protein identification.
Gel bands were excised, placed on needle-punctured V-shaped microtiter plates (Greiner), and robotically processed using a Genesis Proteam 100 liquid handling system (Tecan) with a customized procedure encompassing gel destaining in 50% acetonitrile and 100 mM ammonium bicarbonate, protein reduction in 10 mM tris(carboxyethyl phosphine) (Pierce) and 50 mM ammonium bicarbonate, and alkylation with iodoacetamide followed by 8 h of digestion with trypsin at 37°C. Digest peptides were captured on reversed-phase Poros 20 R 2 beads (Applied Biosystems, Inc.), collected on ZipTips (Millipore), eluted onto stainless steel sample plates, and cocrystallized with α-cyano-4-hydroxy cinnamic acid as the matrix-assisted laser desorption ionization (MALDI) mass spectrometry (MS) matrix. Single-stage mass spectrometric analyses were performed with a Protof2000 MALDI-quadrupole (MALDI-Q)-time-of-flight instrument (PerkinElmer/Sciex), and multistage mass spectrometric fragmentation spectra were obtained with a self-built MALDI-Q-ion trap essentially as previously described (22, 25). Spectra were analyzed by database searching using Profound, Xproteo, and the GPM X! Tandem.
Recombinant proteins.
Asp f 1 (GenBank accession no. P67875 and AAB07779) was expressed from the pQEMW1 plasmid, kindly provided by Frank Ebel and Jürgen Heesemann at the LMU Munich, Germany, by use of an M15 Escherichia coli host strain containing the repressor plasmid pREP4 (QIAGEN) as described previously (45). Recombinant, His-tagged Ubc9 was a gift from Jing Song and Yuan Chen and has been described earlier (30). Asp f 3 (GenBank accession no. O43099) and its truncated forms were cloned and expressed using a pQE30Xa vector (QIAGEN). In brief, total mRNA was obtained from ground hyphae by use of an RNeasy mini kit (QIAGEN), reverse transcribed with a Superscript II kit (Invitrogen), and PCR amplified with the specific primers 1 (GAGCTCATGTCTGGACTCAAGGCCGGTGACA) and 2 (GGTACCTTACAGGTGCTTGAGGACGGTCTCG), containing a SacI and a KpnI site, respectively (underlined). pQE30Xa and the primers were restricted with SacI/KpnI and ligated using T4 ligase (all from New England Biolabs) (40). The resulting plasmid was named pMK2Aspf3, transformed into E. coli M15(pREP4), and selected on Luria-Bertani agar plates with 100 μg/ml ampicillin and 25 μg/ml kanamycin. The N-terminal deletion of Asp f 3, containing residues 15 to 168 [Asp f 3(15-168)], was produced by partial amplification of the insert sequence from pMK2Aspf3 with primers 2 and 3 (AGGCCTGTCTTCTCTTACATCCCC), the latter beginning with a StuI site (underlined). pQE30Xa and the PCR product were digested with StuI/KpnI, ligated, and selected as described above, yielding pMK2Aspf3(15-168). C-terminally truncated rAsp f 3(1-142) and the bipartite N- and C-terminal truncation rAsp f 3(15-142) were obtained by introduction of a stop codon at K143 into the sequences of pMK2Aspf3 and pMK2Aspf3(15-168), respectively, using a QuikChange kit (Stratagene) with PCR primers ATTGACCACGGCTAGATTACCTACG and CGTAGGTAATCTAGCCGTGGTCAAT, which mismatch the Asp f 3 sequence in the underlined base pair. DNA sequencing, performed at the DNA Sequencing Core Lab of the City of Hope, verified the construct sequences. Proteins were expressed at 37°C in 1-liter E. coli cultures with LB medium after IPTG (isopropyl-β-d-thiogalactopyranoside) induction (40) and purified from lysed cells by use of self-packed Ni-nitrilotriacetic acid agarose columns and urea-containing lysis, wash, and elution buffers (QIAGEN). Identities of the purified recombinant proteins were confirmed by peptide mass fingerprinting of gel-separated products (see above). Protein concentrations were determined by use of a Bradford (5) protein assay (Bio-Rad).
Fungal protein extracts and Western blots.
HS was subjected to electrophoresis on reducing Bis-Tris SDS Nu-PAGE gels (4 to 10%; Invitrogen). rAsp f 3, its sequence variants, rAsp f 1, and rUbc9 were electrophoretically separated in the same way after approximately 0.4 or 0.25 μg protein was loaded per lane. Proteins were transferred to polyvinylidene difluoride membrane (0.22 μm; Bio-Rad) by use of an Xcell II blot module (Invitrogen). Membranes were blocked at 4°C overnight in 5% milk, 0.24% Tris base, 0.8% NaCl, and 0.01% Tween 20, adjusted to pH 7.6 with ∼1.2 mM HCl (final concentration). To analyze sera from multiple individuals, membranes blotted with HS from a single-slot SDS-polyacrylamide gel were cut into strips of 5 mm in width (cut alongside the direction of separation) and probed in 1.2-ml volumes of serum in milk, 1:2,500, in Accutran disposable incubation trays with multiple channels (Schleicher & Schuell, Inc., Keene, NH). HRP-conjugated secondary antibodies (see above) were used in dilutions of 1:3,000 to 1:20,000 in accordance with the manufacturer's instructions for chemiluminescent detection on X-ray films.
Mice.
CF-1 female mice (H2-k major histocompatibility complex class I haplotype; Charles River Labs) were purchased at 7 weeks of age and were allowed to acclimate for at least 1 week prior to use. All experiments were conducted in a biosafety level 2 containment facility in compliance with animal care regulations and under care and use protocols approved by the institutional research animal care committee.
Vaccinations.
Mice were vaccinated twice, 2 weeks apart (see Fig. 3A), subcutaneously at the base of the tail with 40 μl of the following vaccine preparations: HS, rAsp f 1, and rAsp f 3. The hyphal sonicate was administered neat and rAsp f 1 was administered neat as a 1:1 (vol/vol) emulsion in TiterMax (TM) (TiterMax, Inc., Norcross, Georgia) prepared according to the manufacturer's instructions or in a particulate form. The particulate, adjuvant-free Asp f 3 vaccine was prepared by precipitation with trichloroacetic acid and resuspension of the protein pellet in the original volume of phosphate-buffered saline (PBS) with 0.5% methylcellulose. Vortexing in the presence of glass beads produced sufficiently small protein particles that passed through a 25-gauge injection needle.
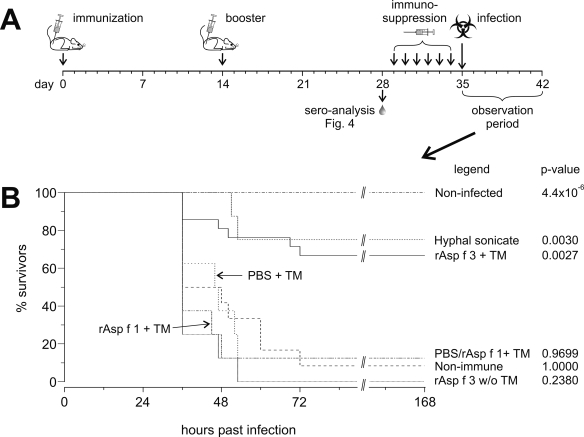
FIG. 3.
(A) Experimental scheme of immunization, immunosuppression, and challenge. (B) Survival curves recorded during the observation period. The numbers of animals per group were 16 noninfected controls, 8 mice given HS, 21 mice given rAsp f 3 plus TM adjuvant (rAsp f 3 + TM), 8 mice given PBS plus TM, 8 mice given rAsp f 1 plus TM, and 12 nonimmune controls that received PBS injections instead of antigens or adjuvant. w/o, without.
Serological analysis.
Plasma was obtained 2 weeks after the second immunization and prior to the initiation of immunosuppression (see Fig. 3A). Blood was taken from a small tail vein incision and diluted 1:20 in PBS, and the diluted plasma was separated by centrifugation, pooled, and frozen at −80°C until tested by Western or dot blotting.
Immunosuppression, antibiotic treatments, and infection.
Cortisone acetate was administered subcutaneously in 2.5-mg doses for six consecutive days prior to challenge, commencing 2 weeks after the second immunization (see Fig. 3A). To reduce the risk of bacterial infection associated with immunosuppression, mice were prophylactically provided acidified water containing sulfamethoxazole-trimethoprim (Sulfatrim; Alpharma) and were administered 200 μg of levofloxacin (Levaquin; Ortho-McNeil) subcutaneously 1 h prior to infection. Under light ketamine-xylazine anesthesia, mice were intranasally inoculated with 30 μl of conidial suspension containing 3 × 106 viable conidia while being held in the vertical position and were placed on their backs during recovery from anesthesia.
Assessment of infection.
After inoculation, all animals fully recovered within 1 to 2 h and were normal in appearance until signs of disease became apparent 24 to 30 h after infection. Mice were observed on a regular basis during the day and were weighed each morning, and body temperature was taken in the morning and evening with a digital thermometer inserted into the vagina. Time of death or premature euthanasia was recorded, and deaths that occurred at night were assigned a time of death midway between the last evening observation and the first morning observation. Criteria for premature euthanasia were labored breathing, a 20% weight loss, and severe hypothermia (<32°C). Time-to-death data were analyzed by the Mann-Whitney U test (equivalent to the Wilcoxon rank sum test) using Leon Avery's web-based U test. Disease pathology and assessment of the fungal distribution within the lung parenchyma were performed with formalin-fixed, paraffin-embedded sections of lung tissue using standard hematoxylin and eosin and Gomori methenamine silver staining. Microscopy was performed with an Olympus AX70 model U-MPH microscope (Tokyo, Japan) with a QImaging RETIGA EXi camera and ImageProPlus 5.1 software.
RESULTS
Potential vaccine candidates.
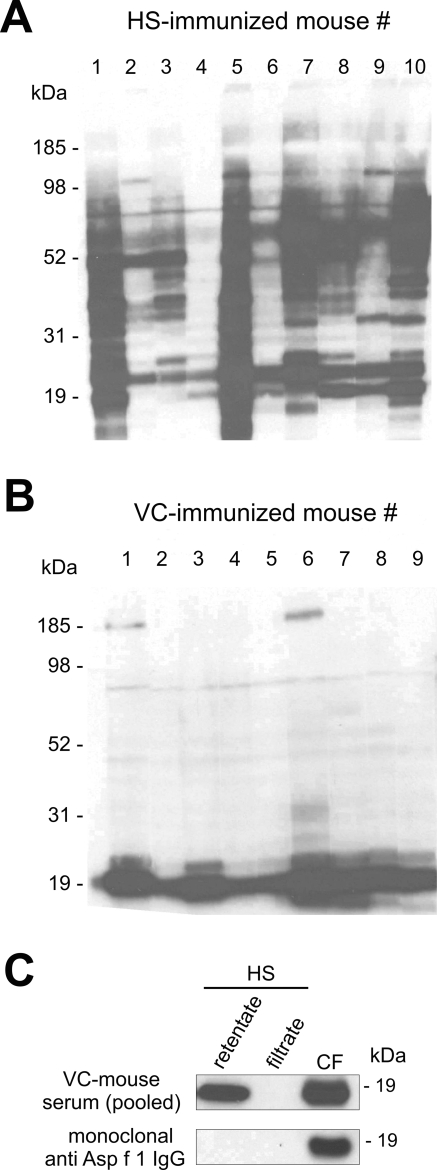
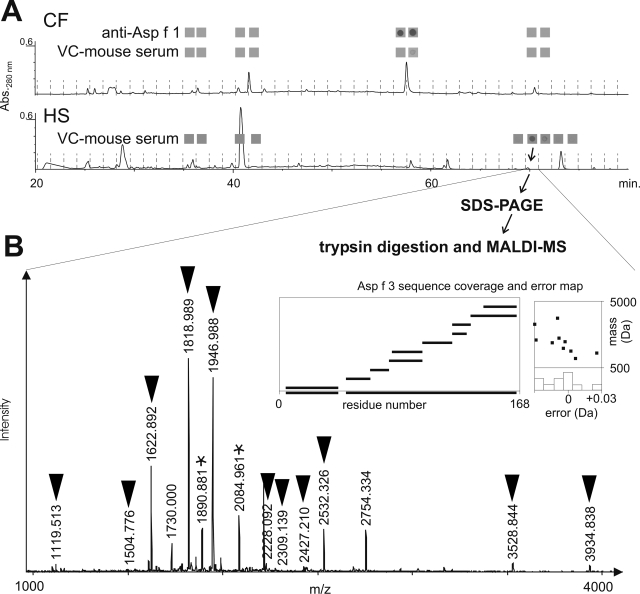
To identify promising protein vaccine candidates, we analyzed individual sera from immunized mice that were notably protected against IPA for content of antigen-specific immunoglobulin. As previously shown, subcutaneous injections of HS as well as nasopulmonary exposure to VC in CF-1 mice are protective under cortisone acetate-induced immunosuppressive conditions (21). Sera from HS-vaccinated mice contain IgG antibodies with various specificities to A. fumigatus antigens (demonstrated by Western blot in Fig. 1A). In contrast, IgG from the VC-vaccinated animals reacted predominantly with antigen molecules at approximately 19 kDa (Fig. 1B). The presence of antibodies against Asp f 3, dipeptidyl peptidase, and catalase in sera pooled from mice surviving A. fumigatus infections (after nasopulmonary exposure to viable conidia) was observed earlier in immunoprecipitation experiments that utilized immobilized protein A as the antibody-capturing matrix (data not shown). Attempts to immunoprecipitate fungal protein with much smaller antibody amounts from single mice produced inconsistent results, but such antibodies proved useful for the tracing of antigens during fractionation and chromatography. CF and HS were prefractionated by dual-stage ultrafiltration through membranes with nominal cutoffs of 30 and 10 kDa. The sera from VC-exposed mice clearly react with a 19-kDa antigen found in the 10- to 30-kDa fraction of CF and HS (Fig. 1C). The antigen from HS is not identical with Asp f 1, another known 19-kDa antigen and major allergen detected only extracellularly in CF by use of a monoclonal anti-Asp f 1 antibody (Fig. 1C). The HS retentate of the 10-kDa membrane was further separated by HPLC, and individual fractions were subjected to dot blot analysis (Fig. 2A). Asp f 1 was detected by dot blotting with 10-kDa retentate fractions of CF eluting at ∼57 min by use of the monoclonal anti-Asp f 1 antibody. The same fraction also showed a weak reaction with sera from VC-exposed mice. A 70-min fraction of the HS retentate reacted strongly with IgG from VC-immune mice. This fraction was further separated by SDS-PAGE (not shown), and the protein content of an 18- to 20-kDa band was reduced, alkylated, trypsin digested, and analyzed by MALDI-Q-time-of-flight and MALDI-Q-ion trap mass spectrometry (Fig. 2B). Peptide mass fingerprinting as well as tandem MS data (see the supplemental material) identified the known allergen Asp f 3 (GenBank accession no. XP_747849) as the major component of the IgG-binding HPLC fraction, with an unusually high sequence coverage of about 93%. A few digest peptides of peptidylprolyl cis-trans isomerase (cyclophilin, PPIase, Asp f 11 [GenBank accession no. XP_749504]) and cofilin (GenBank accession no. XP_753587) were also detected in the same band (see the supplemental material), indicating the presence of these proteins as minor impurities. Taken together, our data indicate that mice infected with viable conidia produce specific antibodies predominantly against Asp f 3 and at lower levels against Asp f 1.
FIG. 1.
Nineteen Western blots of hyphal protein extracts probed with sera from individual mice after immunization either (A) with HS or (B) through nasopulmonary exposure to VC. HRP-conjugated goat anti-mouse IgG was used for chemiluminescent detection. (C) Western blots of prefractionated HS and CF. HS was passed through a 30-kDa cutoff membrane and was then separated into filtrate and retentate by use of a 10-kDa cutoff membrane.
FIG. 2.
(A) Reversed-phase HPLC separation and dot blot analysis of CF and prefractionated HS. The input was the 10- to 30-kDa HS fraction from ultrafiltration. The 70-min HPLC faction of HS reacts positively with sera from VC-immunized mice. Abs.280 nm, absorbance at 280 nm. (B) MALDI MS analysis of a 19-kDa band of this fraction identifies A. fumigatus Asp f 3 as the major protein component. Black inverted triangles mark assigned peptide ions that match the Asp f 3 sequence. Stars mark two peptide ions from cofilin that were assigned based on tandem MS data of these ions (see the supplemental material).
Recombinant Asp f 3 as a vaccine.
Purified, His-tagged rAsp f 3 and rAsp f 1 were produced and tested as vaccines in a murine model of IPA. The vaccination scheme is depicted in Fig. 3A. Vaccinations with HS served as a positive control (protection expected), whereas mock immunizations with either PBS or the TM adjuvant alone were used as negative controls (no protection expected). Approximately 65% of the rAsp f 3-vaccinated mice survived (P < 0.003) (Fig. 3B), a degree of protection comparable to that achieved with crude HS vaccinations (P = 0.003). None of the immunizations was fatal (see noninfected controls, P = 4.4 × 10−6 [Fig. 3B]). The rAsp f 3 vaccine induced a protective immune response only in the presence of the TM adjuvant. Subcutaneous injections of Asp f 3 without TM as well as Asp f 1 with TM and the mock immunizations with either PBS or TM alone were not protective. Differences in survival times between the latter four groups were not statistically relevant.
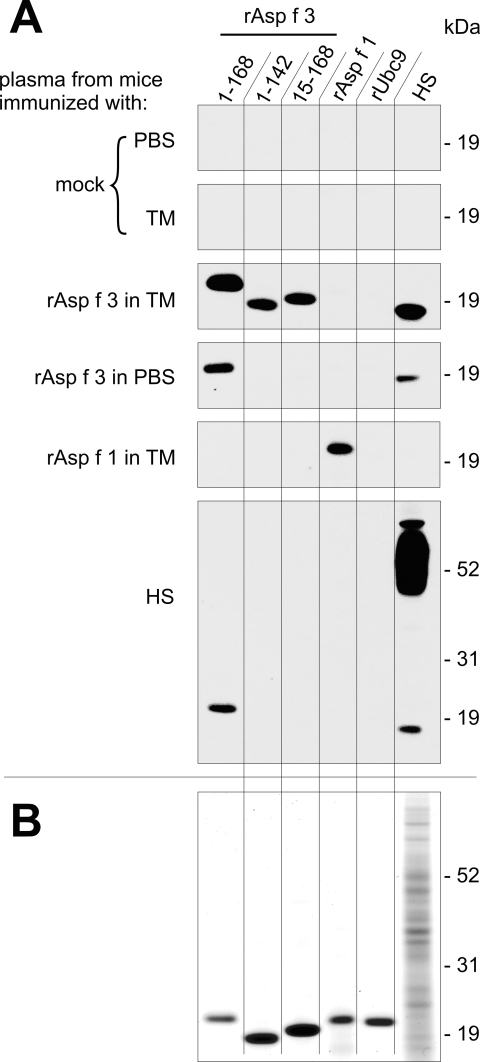
Plasma obtained from all animals on the day before immunosuppression (Fig. 3A) was used to probe Western blots with different versions of rAsp f 3, rAsp f 1, rUbc9, and HS (Fig. 4A). The recombinant proteins were loaded at comparable levels (0.4 μg/lane) as determined by Bradford (5) and as indicated by the GelCode blue-stained gel shown in Fig. 4B. Antigen-specific IgG2a was detected in initial experiments (not reported here) to be the dominant immunoglobulin subclass in the sera of immunized animals. IgG2a induction is the immunoglobulin marker of a TH1-type immune response that is expected when using the TM adjuvant. TM is a proprietary synthetic formulation of TiterMax, Inc., that contains the block copolymer CRL89-41, squalene, and a microparticulate stabilizer and that, in contrast to Freund's adjuvant, is free of mycobacterium extracts. We therefore developed the Western blots with a monoclonal HRP-conjugated anti-mouse IgG2a antibody (Fig. 4A). IgG2a against full-length rAsp f 3 (168 amino acids plus His tag) was detected in the sera of animals vaccinated with rAsp f 3 or HS. Natural non-His-tagged Asp f 3 is responsible for the signal below 19 kDa on the lanes of blotted hematoxylin and eosin. The C- and N-terminally truncated versions of rAsp f 3, spanning residues 1 to 142 and 15 to 168, respectively, reacted only with sera from mice vaccinated with rAsp f 3 plus adjuvant. Similar truncated versions were reported to lack the ability to bind human IgE from ABPA patients (37). Accordingly, such engineered proteins no longer possess the IgE-binding property by which most A. fumigatus allergens have been defined (24). IgG2a from sera of HS-immunized animals reacts with full-length rAsp f 3 but not with the truncated versions, suggesting that the IgG2a epitope responded to in these mice might be similar (if not identical) to the IgE-binding conformational epitope in sera from ABPA patients (37). A His-tagged recombinant mouse protein, Ubc9, was included in the blots to test if any of the recombinant His-tagged vaccines would induce anti-His-tag antibody production. No such antibodies were observed. Mice immunized with the major allergen rAsp f 1 produce antibodies only against rAsp f 1, and the associated immune response was not protective.
FIG. 4.
Serological analysis of immunized mice. (A) Western blots with recombinant proteins, full-length and truncated rAsp f 3, rAsp f 1, rUbc9, and HS were probed with pooled plasma from mice obtained on the day before initiating immunosuppression. HRP-conjugated rat anti-mouse IgG2a served as the secondary antibody. (B) Coomassie-stained SDS-polyacrylamide gel loaded with comparable amounts of recombinant proteins that served as the input for the Western blots shown in panel A.
Truncated nonallergen versions of rAsp f 3 as vaccines.
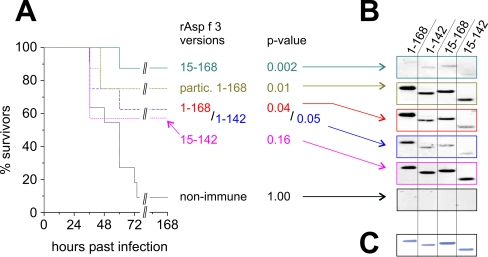
We tested whether truncated versions of rAsp f 3, namely, rAsp f 3(15-168), rAsp f 3(1-142), and rAsp f 3(15-142), still function as vaccines. Using the same murine model for IPA and TM as the adjuvant, we found that the N-terminal (residues 15 to 168) and C-terminal (residues 1 to 142) truncations were similarly protective, with 87% (P < 0.002) and 62% (P < 0.05) survivors, respectively (Fig. 5A). Double-truncated rAsp f 3(15-142) was somewhat protective (57% survivors, P < 0.16), suggesting a trend. It can be stated that the truncated versions of rAsp f 3 elicit protection that is comparable to and in some cases even better than that elicited by full-length rAsp f 3(1-168), as was demonstrated for the N-terminal truncation. Western blotting with plasma samples from the immunized animals indicated that vaccinations with full-length rAsp f 3(1-168) and double-truncated rAsp f 3(15-142) induce strong specific IgG responses against all four tested versions of rAsp f 3 (Fig. 5B). Remarkably, these antibody responses are significantly diminished in animals vaccinated with either the N-terminal (residues 15 to 168) or the C-terminal (residues 1 to 142) deletion. IgG from animals vaccinated with the C-terminal truncation reacts more strongly with full-length Asp f 3 than with the truncated versions, but IgG from those vaccinated with the N-terminal truncation yields only weak signals on Western blots with rAsp f 3(1-142) and rAsp f 3(15-168) (Fig. 5B).
FIG. 5.
(A) Survival curves for mice vaccinated with truncated versions of Asp f 3 with TM adjuvant (as indicated by each first and last amino acid residue number) and particulate, adjuvant-free full-length rAsp f 3 (partic. 1-168). Eight animals were used in each group, except for Asp f 3(1-142) with 7 mice and the control group with 11 mice. (B) Serological analysis of pooled plasma from these mice, obtained by Western blotting (around 19 kDa). (C) The Coomassie-stained gel indicates comparable loading amounts of the rAsp f 3 versions (250 ng/band).
Particulate rAsp f 3 as an adjuvant-free vaccine.
Since TM is not suitable for human use and the soluble form of rAsp f 3 was not protective, we tested an adjuvant-free, particulate form of full-length rAsp f 3 that was prepared by trichloroacetic acid precipitation and resuspension in PBS with methylcellulose. The particulate rAsp f 3 was found to be as immunoprotective as the rAsp f 3/TM preparation (Fig. 5A), and the induction of specific anti-Asp f 3 antibodies was comparable (Fig. 5B).
Histopathology.
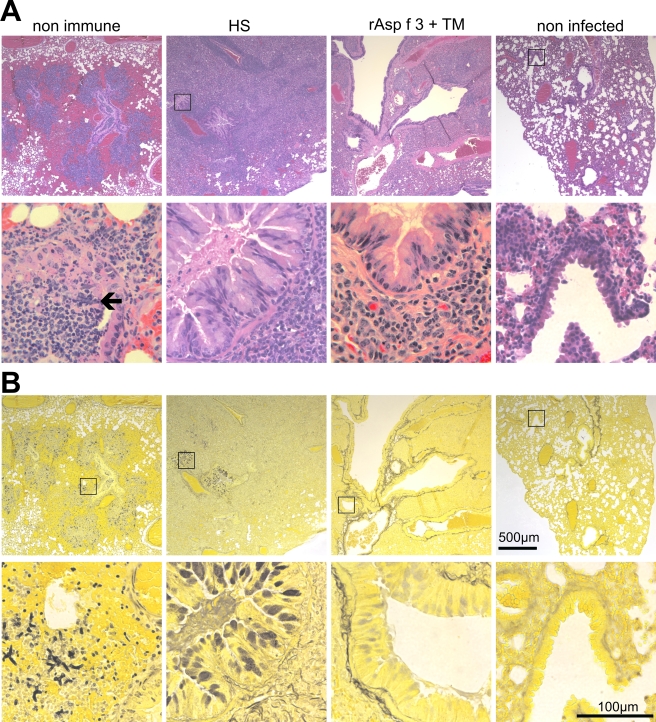
We examined the lungs of the following groups of mice: succumbed nonimmune animals, HS-immunized survivors, rAsp f 3/TM-vaccinated survivors, and normal noninfected animals (Fig. 6). Terminally infected nonimmune individuals had numerous hyphal elements within the bronchi and invading the peribronchial tissues. This was accompanied by a compact peribronchial infiltrate consisting predominately of polymorphonuclear leukocytes, a few histiocytes, and lymphoid cells. The adjacent pulmonary parenchyma showed edema and focal hemorrhage (Fig. 6, first column). The lungs from HS-immunized survivors have a very dense peribronchial mononuclear cell infiltrate composed predominately of large reactive lymphoid cells, small lymphocytes, plasma cells, and histiocytes. The bronchial mucosal epithelium was hypersecretory, with mucin filling many of the bronchial lumens. No hyphal element could be identified by Gomori silver staining (Fig. 6, second column). Mice immunized with Asp f 3/TM were also free of hyphal elements but had fewer intrabronchial secretions than the HS-vaccinated animals. The peribronchial infiltrate was also less dense, and the cellular composition showed fewer of the large lymphoid cells and was composed predominately of small lymphocytes, plasma cells, and histiocytes (Fig. 6, third column).
FIG. 6.
Histopathology. Hematoxylin and eosin (A) and Gomori silver (B) staining of consecutive slices of formalin-fixed lungs of a fatally infected nonimmune animal (first column), an HS-vaccinated survivor (second column), an rAsp f 3- plus TM-vaccinated survivor (third column), and a noninfected mouse (far right column). Magnifications, ×20 for the top row and ×200 for the bottom row in each panel. Square insets, when displayed in the top rows, mark the regions chosen for the higher-magnification image shown in the bottom row. The black arrow points to a hyphal structure that is readily visible in the hematoxylin and eosin-stained tissue.
DISCUSSION
Defined by IgE binding, about 58 allergens from A. fumigatus have been characterized thus far and at least nine additional allergens have been predicted based on similarity with other fungal allergens (27, 28, 34, 37, 39). Although most allergens react with IgE antibodies from ABPA patients, it is not clear whether subcutaneous injections with purified forms of such allergens would actually hypersensitize nonallergic individuals. In fact, it has been shown that total murine IgE production induced through repeated pulmonary exposure to recombinant allergens, including Asp f 3, reached levels that were only 20% or less than those obtained through exposure to crude A. fumigatus extracts (26). Exploration of potential protective properties of so-called “allergens” may therefore be worthwhile. Bozza et al. demonstrated a protective effect for the recombinant A. fumigatus allergen Asp f 16 but did not find Asp f 3 to be protective following intranasal vaccination in the presence of CpG oligodeoxynucleotides as adjuvants (2). However, Bozza's approach and ours are not comparable. The routes of vaccination—subcutaneous versus intranasal and TM adjuvant or particulate forms versus oligodeoxynucleotides—differ significantly, as do the animal models used. The subcutaneous route of immunization used in this study was chosen because earlier experiments with fungal extracts demonstrated that the subcutaneous route was more effective at inducing protection than nasopulmonary exposure (21). We chose a corticosteroid immunosuppression model, whereas Bozza et al. used a cyclophosphamide-induced neutropenic model. Our rationale for the use of the corticosteroid-induced immunosuppression mouse model for vaccine testing was to simulate the effects of the prolonged immunosuppression that recipients of hematopoietic cell transplants experience to control graft-versus-host disease. Such prolonged corticosteroid immunosuppression is the number one risk factor for invasive fungal infections. The degree of protection observed in our study, although below 100%, is comparable to that obtained with subcutaneous injections of hyphal sonicate or exposure to viable conidia. In addition, the CF-1 mice used in our study are considered outbred, and it is possible that genetic variation is responsible for individual differences in the levels of protection induced by an rAsp f 3-based vaccine.
Independently of us, and while this paper was being written, Orsborn et al. recently showed that vaccinations with recombinant Pmp1 can protect mice against Coccidioides posadasii infections (35). Asp f 3 is a homologue to Pmp1 from C. posadasii, with which it shares ∼68% sequence identity. Both proteins have some sequence homology with two presumed peroxisomal matrix proteins, PMPA and PMPB (PMP20), from Candida boidinii (14), with which Asp f 3 was reported to share a common IgE-binding epitope (17). Several genes of other fungi encode sequences that are nearly identical to A. fumigatus Asp f 3. These include PMP20 (Asp f 3) from Aspergillus nidulans FGSC A4 (GenBank accession no. AN8692.2) with 90% identity, a cDNA from Aspergillus oryzae RIB40 (GenBank accession no. AN8692.2) with 86% identity, Pen c 3 from Penicillium citrinum (GenBank accession no. AF144753) with 81% identity, and others with significant similarity, such as a peroxisomal-like protein mRNA sequence from Paracoccidioides brasiliensis (GenBank accession no. AY376436) with 67% identity and a putative alkyl hydroperoxide reductase from Candida albicans SC5314 (GenBank accession no. XM_715419) with 38% identity. It is therefore possible that an Asp f 3-based vaccine could provide cross-protection against various fungal pathogens.
The four recombinant versions of rAsp f 3 tested here appear to be processed differently during immunization. It should also be noted that both the full-length Asp f 3(1-168) and the double-truncated version rAsp f 3(15-142) could be purified from E. coli lysates in the absence of urea. In contrast, the N- and C-terminal truncations, comprising residues 15 to 168 and 1 to 142, respectively, are not very soluble and needed to be purified from the lysates with urea. These findings suggest distinct structural properties for the various versions of rAsp f 3. Such conformational differences could influence phagocytosis, proteasomal processing, and major histocompatibility complex display and, as a consequence, might result in different immunogenic properties. This speculation is supported by the protective effect of the adjuvant-free particulate rAsp f 3 compared to the observed lack of protection when soluble adjuvant-free rAsp f 3 was tested. The use of particulate vaccines is the basis for various immunization strategies, including the use of alum and emulsions as the particle-forming matrix for soluble vaccine candidates. In this context, particulate recombinant vaccines against hepatitis viruses have been produced and immunogenic differences between particulate and nonparticulate antigens have been found (20, 29). Although we originally exploited a specific antibody response to identify the immunodominant Asp f 3 antigen, it turned out that protective N- or C-terminally truncated versions of the same protein induce only weak antibody responses. A potential protective effect of anti-Asp f 3 antibodies alone seems therefore unlikely. Considering the patchy infiltrate of mononuclear cells found in the hypha-free lungs of Asp f 3-vaccinated mice, we hypothesize that such vaccinations may induce adaptive cell-based immunity. In a likely but yet to be tested scenario, “vaccine-trained” lymphocytes (presumably T cells) could be activated upon infection and in turn perhaps enhance and/or restore the function of corticosteroid-suppressed macrophages (6, 7), allowing them to efficiently clear fungal elements from the lungs. Currently there are insufficient data to test such a hypothesis, and further experiments will be required to analyze the mechanisms by which an Asp f 3-based vaccine functions.
Conclusion.
We have demonstrated that a combined immunochemical and mass spectrometric approach was useful in identifying Asp f 3 as a potential vaccine candidate against IPA. Full-length recombinant Asp f 3 and some truncated versions that lack the human “allergenic” IgE-binding epitope are immunoprotective. We propose that such modified, recombinant Asp f 3-based vaccines are potentially suitable for human use.
Supplementary Material
Acknowledgments
Financial support for this work was provided in part by the Hermann Foundation.
We thank Frank Ebel and Jürgen Heesemann from the Max von Pettenkofer-Institut/Bakteriologie, LMU Munich, Germany, for the pQEMW1 plasmid and Jing Song and Yuan Chen from the City of Hope, Duarte, CA, for rUbc9.
Editor: A. Casadevall
Footnotes
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Bellocchio, S., S. Bozza, C. Montagnoli, K. Perruccio, R. Gaziano, L. Pitzurra, and L. Romani. 2005. Immunity to Aspergillus fumigatus: the basis for immunotherapy and vaccination. Med. Mycol. 43(Suppl. 1):S181-S188. [DOI] [PubMed] [Google Scholar]
- 2.Bozza, S., R. Gaziano, G. B. Lipford, C. Montagnoli, A. Bacci, P. Di Francesco, V. P. Kurup, H. Wagner, and L. Romani. 2002. Vaccination of mice against invasive aspergillosis with recombinant Aspergillus proteins and CpG oligodeoxynucleotides as adjuvants. Microbes Infect. 4:1281-1290. [DOI] [PubMed] [Google Scholar]
- 3.Bozza, S., C. Montagnoli, R. Gaziano, G. Rossi, G. Nkwanyuo, S. Bellocchio, and L. Romani. 2004. Dendritic cell-based vaccination against opportunistic fungi. Vaccine 22:857-864. [DOI] [PubMed] [Google Scholar]
- 4.Bozza, S., K. Perruccio, C. Montagnoli, R. Gaziano, S. Bellocchio, E. Burchielli, G. Nkwanyuo, L. Pitzurra, A. Velardi, and L. Romani. 2003. A dendritic cell vaccine against invasive aspergillosis in allogeneic hematopoietic transplantation. Blood 102:3807-3814. [DOI] [PubMed] [Google Scholar]
- 5.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 6.Brummer, E., J. H. Choi, and D. A. Stevens. 2005. Interaction between conidia, lung macrophages, immunosuppressants, proinflammatory cytokines and transcriptional regulation. Med. Mycol. 43(Suppl. 1):S177-S179. [DOI] [PubMed] [Google Scholar]
- 7.Brummer, E., M. Kamberi, and D. A. Stevens. 2003. Regulation by granulocyte-macrophage colony-stimulating factor and/or steroids given in vivo of proinflammatory cytokine and chemokine production by bronchoalveolar macrophages in response to Aspergillus conidia. J. Infect. Dis. 187:705-709. [DOI] [PubMed] [Google Scholar]
- 8.Casadevall, A., and L. A. Pirofski. 2005. Feasibility and prospects for a vaccine to prevent cryptococcosis. Med. Mycol. 43:667-680. [DOI] [PubMed] [Google Scholar]
- 9.Cenci, E., A. Mencacci, A. Bacci, F. Bistoni, V. P. Kurup, and L. Romani. 2000. T cell vaccination in mice with invasive pulmonary aspergillosis. J. Immunol. 165:381-388. [DOI] [PubMed] [Google Scholar]
- 10.Denning, D. W. 1998. Invasive aspergillosis. Clin. Infect. Dis. 26:781-803, 804-805. [DOI] [PubMed] [Google Scholar]
- 11.Duthie, R., and D. W. Denning. 1995. Aspergillus fungemia: report of two cases and review. Clin. Infect. Dis. 20:598-605. [DOI] [PubMed] [Google Scholar]
- 12.Feldmesser, M. 2005. Prospects of vaccines for invasive aspergillosis. Med. Mycol. 43:571-587. [DOI] [PubMed] [Google Scholar]
- 13.Galagan, J. E., S. E. Calvo, C. Cuomo, L. J. Ma, J. R. Wortman, S. Batzoglou, S. I. Lee, M. Basturkmen, C. C. Spevak, J. Clutterbuck, V. Kapitonov, J. Jurka, C. Scazzocchio, M. Farman, J. Butler, S. Purcell, S. Harris, G. H. Braus, O. Draht, S. Busch, C. D'Enfert, C. Bouchier, G. H. Goldman, D. Bell-Pedersen, S. Griffiths-Jones, J. H. Doonan, J. Yu, K. Vienken, A. Pain, M. Freitag, E. U. Selker, D. B. Archer, M. A. Penalva, B. R. Oakley, M. Momany, T. Tanaka, T. Kumagai, K. Asai, M. Machida, W. C. Nierman, D. W. Denning, M. Caddick, M. Hynes, M. Paoletti, R. Fischer, B. Miller, P. Dyer, M. S. Sachs, S. A. Osmani, and B. W. Birren. 2005. Sequencing of Aspergillus nidulans and comparative analysis with A. fumigatus and A. oryzae. Nature 438:1105-1115. [DOI] [PubMed] [Google Scholar]
- 14.Garrard, L. J., and J. M. Goodman. 1989. Two genes encode the major membrane-associated protein of methanol-induced peroxisomes from Candida boidinii. J. Biol. Chem. 264:13929-13937. [PubMed] [Google Scholar]
- 15.Gupta, A. K., and E. Tomas. 2003. New antifungal agents. Dermatol. Clin. 21:565-576. [DOI] [PubMed] [Google Scholar]
- 16.Hamza, N. S., M. A. Ghannoum, and H. M. Lazarus. 2004. Choices aplenty: antifungal prophylaxis in hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 34:377-389. [DOI] [PubMed] [Google Scholar]
- 17.Hemmann, S., K. Blaser, and R. Crameri. 1997. Allergens of Aspergillus fumigatus and Candida boidinii share IgE-binding epitopes. Am. J. Respir. Crit. Care Med. 156:1956-1962. [DOI] [PubMed] [Google Scholar]
- 18.Herbrecht, R., D. W. Denning, T. F. Patterson, J. E. Bennett, R. E. Greene, J. W. Oestmann, W. V. Kern, K. A. Marr, P. Ribaud, O. Lortholary, R. Sylvester, R. H. Rubin, J. R. Wingard, P. Stark, C. Durand, D. Caillot, E. Thiel, P. H. Chandrasekar, M. R. Hodges, H. T. Schlamm, P. F. Troke, B. de Pauw, et al. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 347:408-415. [DOI] [PubMed] [Google Scholar]
- 19.Ho, P. L., and K. Y. Yuen. 2000. Aspergillosis in bone marrow transplant recipients. Crit. Rev. Oncol. Hematol. 34:55-69. [DOI] [PubMed] [Google Scholar]
- 20.Huang, C. F., S. S. Lin, Y. C. Ho, F. L. Chen, and C. C. Yang. 2006. The immune response induced by hepatitis B virus principal antigens. Cell. Mol. Immunol. 3:97-106. [PubMed] [Google Scholar]
- 21.Ito, J. I., and J. M. Lyons. 2002. Vaccination of corticosteroid immunosuppressed mice against invasive pulmonary aspergillosis. J. Infect. Dis. 186:869-871. [DOI] [PubMed] [Google Scholar]
- 22.Kalkum, M., G. J. Lyon, and B. T. Chait. 2003. Detection of secreted peptides by using hypothesis-driven multistage mass spectrometry. Proc. Natl. Acad. Sci. USA 100:2795-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kibbler, C. 2003. Aspergillus: the invisible threat. Nurs. Times 99:48-50. [PubMed] [Google Scholar]
- 24.Kodzius, R., C. Rhyner, Z. Konthur, D. Buczek, H. Lehrach, G. Walter, and R. Crameri. 2003. Rapid identification of allergen-encoding cDNA clones by phage display and high-density arrays. Comb. Chem. High Throughput Screen. 6:147-154. [DOI] [PubMed] [Google Scholar]
- 25.Krutchinsky, A. N., M. Kalkum, and B. T. Chait. 2001. Automatic identification of proteins with a MALDI-quadrupole ion trap mass spectrometer. Anal. Chem. 73:5066-5077. [DOI] [PubMed] [Google Scholar]
- 26.Kurup, V. P., J. Q. Xia, R. Crameri, D. A. Rickaby, H. Y. Choi, S. Fluckiger, K. Blaser, C. A. Dawson, and K. J. Kelly. 2001. Purified recombinant A. fumigatus allergens induce different responses in mice. Clin. Immunol. 98:327-336. [DOI] [PubMed] [Google Scholar]
- 27.Latge, J. P. 1999. Antigen and DNA patterns characteristic of Aspergillus fumigatus. Contrib. Microbiol. 2:69-87. [DOI] [PubMed] [Google Scholar]
- 28.Latge, J. P. 1999. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12:310-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, S. W., J. Zhang, Y. M. Li, S. H. Ou, G. Y. Huang, Z. Q. He, S. X. Ge, Y. L. Xian, S. Q. Pang, M. H. Ng, and N. S. Xia. 2005. A bacterially expressed particulate hepatitis E vaccine: antigenicity, immunogenicity and protectivity on primates. Vaccine 23:2893-2901. [DOI] [PubMed] [Google Scholar]
- 30.Liu, Q., C. Jin, X. Liao, Z. Shen, D. J. Chen, and Y. Chen. 1999. The binding interface between an E2 (UBC9) and a ubiquitin homologue (UBL1). J. Biol. Chem. 274:16979-16987. [DOI] [PubMed] [Google Scholar]
- 31.Machida, M., K. Asai, M. Sano, T. Tanaka, T. Kumagai, G. Terai, K. Kusumoto, T. Arima, O. Akita, Y. Kashiwagi, K. Abe, K. Gomi, H. Horiuchi, K. Kitamoto, T. Kobayashi, M. Takeuchi, D. W. Denning, J. E. Galagan, W. C. Nierman, J. Yu, D. B. Archer, J. W. Bennett, D. Bhatnagar, T. E. Cleveland, N. D. Fedorova, O. Gotoh, H. Horikawa, A. Hosoyama, M. Ichinomiya, R. Igarashi, K. Iwashita, P. R. Juvvadi, M. Kato, Y. Kato, T. Kin, A. Kokubun, H. Maeda, N. Maeyama, J. Maruyama, H. Nagasaki, T. Nakajima, K. Oda, K. Okada, I. Paulsen, K. Sakamoto, T. Sawano, M. Takahashi, K. Takase, Y. Terabayashi, J. R. Wortman, O. Yamada, Y. Yamagata, H. Anazawa, Y. Hata, Y. Koide, T. Komori, Y. Koyama, T. Minetoki, S. Suharnan, A. Tanaka, K. Isono, S. Kuhara, N. Ogasawara, and H. Kikuchi. 2005. Genome sequencing and analysis of Aspergillus oryzae. Nature 438:1157-1161. [DOI] [PubMed] [Google Scholar]
- 32.Maschke, M., U. Dietrich, M. Prumbaum, O. Kastrup, B. Turowski, U. W. Schaefer, and H. C. Diener. 1999. Opportunistic CNS infection after bone marrow transplantation. Bone Marrow Transplant. 23:1167-1176. [DOI] [PubMed] [Google Scholar]
- 33.Montagnoli, C., S. Bozza, A. Bacci, R. Gaziano, P. Mosci, J. Morschhauser, L. Pitzurra, M. Kopf, J. Cutler, and L. Romani. 2003. A role for antibodies in the generation of memory antifungal immunity. Eur. J. Immunol. 33:1193-1204. [DOI] [PubMed] [Google Scholar]
- 34.Nierman, W. C., A. Pain, M. J. Anderson, J. R. Wortman, H. S. Kim, J. Arroyo, M. Berriman, K. Abe, D. B. Archer, C. Bermejo, J. Bennett, P. Bowyer, D. Chen, M. Collins, R. Coulsen, R. Davies, P. S. Dyer, M. Farman, N. Fedorova, T. V. Feldblyum, R. Fischer, N. Fosker, A. Fraser, J. L. Garcia, M. J. Garcia, A. Goble, G. H. Goldman, K. Gomi, S. Griffith-Jones, R. Gwilliam, B. Haas, H. Haas, D. Harris, H. Horiuchi, J. Huang, S. Humphray, J. Jimenez, N. Keller, H. Khouri, K. Kitamoto, T. Kobayashi, S. Konzack, R. Kulkarni, T. Kumagai, A. Lafton, J. P. Latge, W. Li, A. Lord, C. Lu, W. H. Majoros, G. S. May, B. L. Miller, Y. Mohamoud, M. Molina, M. Monod, I. Mouyna, S. Mulligan, L. Murphy, S. O'Neil, I. Paulsen, M. A. Penalva, M. Pertea, C. Price, B. L. Pritchard, M. A. Quail, E. Rabbinowitsch, N. Rawlins, M. A. Rajandream, U. Reichard, H. Renauld, G. D. Robson, S. Rodriguez de Cordoba, J. M. Rodriguez-Pena, C. M. Ronning, S. Rutter, S. L. Salzberg, M. Sanchez, J. C. Sanchez-Ferrero, D. Saunders, K. Seeger, R. Squares, S. Squares, M. Takeuchi, F. Tekaia, G. Turner, C. R. Vazquez de Aldana, J. Weidman, O. White, J. Woodward, J. H. Yu, C. Fraser, J. E. Galagan, K. Asai, M. Machida, N. Hall, B. Barrell, and D. W. Denning. 2005. Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature 438:1151-1156. [DOI] [PubMed] [Google Scholar]
- 35.Orsborn, K. I., L. F. Shubitz, T. Peng, E. M. Kellner, M. J. Orbach, P. A. Haynes, and J. N. Galgiani. 2006. Protein expression profiling of Coccidioides posadasii by two-dimensional differential in-gel electrophoresis and evaluation of a newly recognized peroxisomal matrix protein as a recombinant vaccine candidate. Infect. Immun. 74:1865-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pizza, M., V. Scarlato, V. Masignani, M. M. Giuliani, B. Arico, M. Comanducci, G. T. Jennings, L. Baldi, E. Bartolini, B. Capecchi, C. L. Galeotti, E. Luzzi, R. Manetti, E. Marchetti, M. Mora, S. Nuti, G. Ratti, L. Santini, S. Savino, M. Scarselli, E. Storni, P. Zuo, M. Broeker, E. Hundt, B. Knapp, E. Blair, T. Mason, H. Tettelin, D. W. Hood, A. C. Jeffries, N. J. Saunders, D. M. Granoff, J. C. Venter, E. R. Moxon, G. Grandi, and R. Rappuoli. 2000. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science 287:1816-1820. [DOI] [PubMed] [Google Scholar]
- 37.Ramachandran, H., V. Jayaraman, B. Banerjee, P. A. Greenberger, K. J. Kelly, J. N. Fink, and V. P. Kurup. 2002. IgE binding conformational epitopes of Asp f 3, a major allergen of Aspergillus fumigatus. Clin. Immunol. 103:324-333. [DOI] [PubMed] [Google Scholar]
- 38.Rappuoli, R., and A. Covacci. 2003. Reverse vaccinology and genomics. Science 302:602. [DOI] [PubMed] [Google Scholar]
- 39.Rementeria, A., N. Lopez-Molina, A. Ludwig, A. B. Vivanco, J. Bikandi, J. Ponton, and J. Garaizar. 2005. Genes and molecules involved in Aspergillus fumigatus virulence. Rev. Iberoam. Micol. 22:1-23. [DOI] [PubMed] [Google Scholar]
- 40.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 41.Schaffner, A., H. Douglas, and A. Braude. 1982. Selective protection against conidia by mononuclear and against mycelia by polymorphonuclear phagocytes in resistance to Aspergillus. Observations on these two lines of defense in vivo and in vitro with human and mouse phagocytes. J. Clin. Investig. 69:617-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Segal, B. H., J. Kwon-Chung, T. J. Walsh, B. S. Klein, M. Battiwalla, N. G. Almyroudis, S. M. Holland, and L. Romani. 2006. Immunotherapy for fungal infections. Clin. Infect. Dis. 42:507-515. [DOI] [PubMed] [Google Scholar]
- 43.Stevens, D. A. 2004. Vaccinate against aspergillosis! A call to arms of the immune system. Clin. Infect. Dis. 38:1131-1136. [DOI] [PubMed] [Google Scholar]
- 44.Subira, M., R. Martino, T. Franquet, C. Puzo, A. Altes, A. Sureda, S. Brunet, and J. Sierra. 2002. Invasive pulmonary aspergillosis in patients with hematologic malignancies: survival and prognostic factors. Haematologica 87:528-534. [PubMed] [Google Scholar]
- 45.Weig, M., M. Frosch, K. Tintelnot, A. Haas, U. Gross, B. Linsmeier, and J. Heesemann. 2001. Use of recombinant mitogillin for improved serodiagnosis of Aspergillus fumigatus-associated diseases. J. Clin. Microbiol. 39:1721-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wiederhold, N. P., R. E. Lewis, and D. P. Kontoyiannis. 2003. Invasive aspergillosis in patients with hematologic malignancies. Pharmacotherapy 23:1592-1610. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.