Abstract
The Lyme disease spirochete Borrelia burgdorferi reduces the expression of outer surface protein C (OspC) in response to the development of an anti-OspC humoral response, leading to the hypothesis that the ability to repress OspC expression is critical for the pathogen to proceed to chronic infection. B. burgdorferi was genetically modified to constitutively express OspC by introducing an extra ospC copy fused with the borrelial flagellar gene (flaB) promoter. Such a genetic modification did not reduce infectivity or pathogenicity in severe combined immunodeficiency mice but resulted in clearance of infection by passively transferred OspC antibody. Spirochetes with constitutive ospC expression were unable to establish chronic infections in immunocompetent mice unless they had undergone very destructive mutations in the introduced ospC copy. Two escape mutants were identified; one had all 7 bp deleted between the putative ribosome-binding site and the start codon, ATG, causing a failure in translational initiation, and the other mutant had an insertion of 2 bp between nucleotides 315 and 316, resulting in a nonsense mutation at codon 108. Thus, the ability of B. burgdorferi to repress ospC expression during mammalian infection allows the pathogen to avoid clearance and to preserve the integrity of the important gene for subsequent utilization during its enzootic life cycle.
The genome of the Lyme disease spirochete Borrelia burgdorferi encodes more than 100 lipoproteins (6, 15). Many lipoproteins have been identified as targets of protective antibodies, including outer surface protein A (OspA) and OspC (14, 16, 34). Lipoproteins stimulate innate responses via Toll-like receptors, enhancing both humoral and cellular immune responses (1, 48). However, B. burgdorferi is able to cause persistent infection despite the development of vigorous immune responses against the pathogen (40).
Deliberate regulation of antigen expression is essential for spirochete survival in various environments. B. burgdorferi is maintained within a complex enzootic life cycle involving the tick vector Ixodes scapularis and a mammal (5). The up-regulation of OspA is crucial for colonization of ticks by B. burgdorferi (10, 39, 50), while the down-regulation of this lipoprotein during mammalian infection is important for continuation of the enzootic cycle, as its expression triggers an anti-OspA immune response effectively blocking acquisition by the vector (9, 46, 47).
B. burgdorferi up-regulates OspC when induced by a fresh blood meal, a response that may be essential for the organism to migrate into tick salivary glands and initiate a mammalian infection (17, 33). However, its expression is also affected by the host immune status. The antigen is abundantly expressed only in the absence of the adaptive immune response (7, 8, 24, 26, 28). ospC expression is dramatically reduced either by passive OspC antibody transfer in SCID mice or by the specific immune response induced during infection of an immunocompetent host (7, 24, 26, 28). We hypothesized that the down-regulation of OspC in response to the development of specific immune pressure is an effective strategy for B. burgdorferi to proceed to chronic infection. To test this hypothesis, the ospC coding region was fused with the flagellin gene (flaB) promoter, and the consequence of constitutive ospC expression was explored in both immunodeficient and immunocompetent murine models.
MATERIALS AND METHODS
Construction of pBBE22-ospC′.
A 251-bp fragment of the flaB promoter region was amplified by PCR with the use of the primer pair 5′-AGAAGTACGAAGATAGAGAGAGAAA-3 (forward) and 5′-AACACATATGTCATTCCTCCATGATAAA-3 (reverse). An 895-bp fragment extending from the ATG translational start codon to the 262-bp sequence downstream of the stop codon of the ospC gene was amplified with the use of another primer pair (forward, 5′-CCACCATATGAAAAAGAATACATTAAGT-3′; reverse, 5′-CCGTTTAAGCCTACTTAAAGTCT-3′). (The underlined sequences are NdeI restriction sites.) The two PCR products were pooled, purified by using a QIAquick PCR purification kit (QIAGEN Inc., Valencia, CA), digested with NdeI, repurified, and ligated. The resultant product was used as a template and amplified by nested PCR with the use of a third primer pair (forward, 5′-ATAGGATCCAAGATAGAGAGAGAAAAGT-3′; reverse, 5′-TTCCTCTAGAGAAGAGCTTAAAGTTAA-3′) (the underlined sequences are BamHI [forward] and XbaI [reverse] sites). The PCR product was purified, digested with BamHI and XbaI, and cloned into the recombinant plasmid pBBE22 (a gift from S. Norris) (35). The insert and flanking regions within the recombinant plasmid were sequenced to ensure that the construct was as designed. The generated plasmid was designated pBBE22-ospC′.
Generation of transformants.
B. burgdorferi B31 5A13 spirochetes (a gift from S. Norris) were transformed with the recombinant plasmid pBBE22 or pBBE22-ospC′ as described previously (38, 43, 49). Transformants were identified by PCR using a primer pair specific for the kanamycin cassette (49). The plasmid contents of transformants were surveyed by PCR and confirmed by microarray hybridization using previously reported primers and protocols (49).
Replacement of pBBE22-ospC′ with pGE22.
After the recombinant plasmid pBBE22 was digested with Acc65I, the BBE22 insert was purified and cloned into the shuttle vector pBSV2G (a gift from P. Rosa and P. Stewart), which carries a gentamicin cassette (11), to construct the recombinant plasmid pGE22. A transformant that contained the recombinant plasmid pBBE22-ospC′ was transformed with pGE22. Resultant transformants were screened in gentamicin-supplemented Barbour-Stoenner-Kelly H (BSK-H) complete medium (Sigma Chemical Co., St. Louis, MO) as described previously (49). Gentamicin-resistant clones were analyzed for the absence of pBBE22-ospC′ as described above. Only transformants that contained pGE22 but had lost pBBE22-ospC′ were examined for plasmid content by PCR as previously described (49).
Preparation of recombinant OspC and generation of mouse anti-OspC sera.
The coding region, excluding the signal peptide coding sequence, of the ospC gene was PCR amplified, cloned into the expression vector pET16b, and transformed into Escherichia coli strain BL21(DE3) (Novagen, La Jolla, CA). The recombinant protein was purified using a Hi-Trap affinity column (Amersham Pharmacia Biotech, Piscataway, NJ). The protein purity and concentration were determined using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and a protein assay kit (Bio-Rad Laboratories, Richmond, CA), respectively. Approximately 70 μg recombinant protein was dissolved in 100 μl of phosphate-buffered saline (PBS; pH 7.3), emulsified with 30 μl of Freund's complete (first injection) or incomplete (remaining injections) adjuvant, and subcutaneously administered into each BALB/c mouse (age, 5 to 8 weeks) at 3-week intervals. Mice were euthanized 3 weeks after the last immunization for antiserum preparation.
In vitro characterization of transformants and isolates recovered from infected mice.
Transformants or isolates were grown in BSK-H complete medium to late exponential phase at 23°C, 33°C, or 37°C and harvested by centrifugation. RNA samples were quantified for cDNA copy numbers of flaB and ospC by reverse transcription-quantitative PCR (RT-qPCR) as previously described (28). Spirochetes were dissolved in sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer, separated by electrophoresis, and electrotransferred onto a nitrocellulose membrane (23). Blots were probed with a mixture of FlaB and OspC monoclonal antibodies (MAbs) or a mixture of FlaB MAb and mouse antisera raised against a recombinant OspC antigen. The FlaB and OspC MAbs were developed by Barbour et al. (3) and Mbow et al. (30), respectively.
Infectivity and pathogenicity study.
Groups of 10 SCID mice in the BALB/c background (Harlan, Indianapolis, IN) were given single intradermal/subcutaneous injections of 104 transformed spirochetes. Animals were examined for the development of arthritis at 2-day intervals, starting at 2 weeks, and sacrificed at 1 month postinoculation. Joint, heart, and skin specimens (not from the inoculation site) were used for DNA and RNA preparation. DNAs were quantified for copy numbers of the flaB and murine actin genes by qPCR as previously described (49). The tissue spirochete burden was expressed as flaB DNA copies per 106 host cells (2 × 106 actin DNA copies). RNAs were converted to cDNAs and quantified for mRNA copy numbers of flaB and ospC by qPCR as described above.
Passive immunization of infected SCID mice with OspC MAb.
Groups of 10 SCID mice were inoculated with transformants as described above and received six subcutaneous injections of 100 μg OspC MAb (30) or mouse immunoglobulin G2a (IgG2a; Sigma) as a control on days 12, 14, 16, 18, 20, and 22. Mice were sacrificed 3 days after the last passive immunization. Heart, tibiotarsal joint, and skin specimens were collected aseptically for spirochete culture as previously described (49).
Acute infectivity study.
Groups of five BALB/c mice (provided by the Division of Laboratory Animal Medicine, Louisiana State University) were inoculated with transformants as described above. Retro-orbital blood was drawn to monitor the anti-OspC response on day 10 by an enzyme-linked immunosorbent assay (ELISA), as described below; mice were sacrificed at 19 days postinoculation. Joint, heart, and skin specimens (not from the inoculation site) were used for DNA and RNA preparation; sera were collected for ELISA. The tissue bacterial burden and ospC expression level were analyzed by qPCR and RT-qPCR as described above.
Chronic infectivity study.
Groups of three to eight BALB/c mice were inoculated with transformants as described above. In some experiments, ear biopsies were taken for spirochete culture by using a 2.0-mm punch, and retro-orbital blood was drawn to monitor the anti-OspC response at intervals of 2 to 4 weeks, starting on day 14 postinoculation. Mice were sacrificed 5 months later; heart, tibiotarsal joint, and skin specimens were collected aseptically for spirochete culture as previously described (49).
Measurement of anti-OspC humoral immune response.
Specific OspC antibody end-point titers were determined by ELISA. Ninety-six-well plates (Corning Inc., Corning, NY) were coated with 100 μl of 2.0-μg/ml recombinant OspC per well. Sera were serially diluted twofold, starting at 1/200. Five samples drawn from naive BALB/c mice were used as a control. ELISA was performed as previously described (23).
Identification of escape mutations.
Spirochetes were recovered from mice that had been inoculated with a transformant with constitutive ospC expression, grown to late exponential phase in BSK-H complete medium at either 23°C or 37°C, and harvested by centrifugation. Lysates were subjected to an immunoblot analysis of OspC expression as described above. Selected isolates were grown to stationary phase at 33°C, and total DNA was extracted by using a DNeasy tissue kit (QIAGEN). E. coli DH5α competent cells (Invitrogen Life Technologies, Carlsbad, CA) were transformed with spirochetal DNA by heat shock. Three to five kanamycin-resistant colonies were randomly selected from each transformation experiment and sequenced for the introduced ospC copy and the fused flaB promoter.
Statistical analysis.
Data were analyzed using Microsoft Excel (Redmond, WA) software. A two-tailed Student t test was used to analyze RT-qPCR and qPCR data. P values of ≤0.05 were considered significant.
RESULTS
Generation and in vitro characterization of transformants with constitutive ospC expression.
B. burgdorferi B31 5A13 spirochetes were transformed with the recombinant plasmid pBBE22 or pBBE22-ospC′; 12 and 9 transformants, respectively, were obtained from transformation experiments with the plasmids. Plasmid content analyses by PCR and microarray hybridization identified transformants 5A13/pBBE22, from the 12 clones that received pBBE22, and 5A13/pBBE22-ospC′, from the 9 clones that were transformed with pBBE22-ospC′, for further analysis because these two clones harbored all of the plasmids important for infection and also shared similar plasmid profiles. Both had lost cp9, lp25, lp56, and lp21; 5A13/pBBE22-ospC′ was also lacking lp5 (Table 1).
TABLE 1.
Constitutive ospC expression results in spirochetal clearance by passively transferred OspC MAb in SCID micea
| Transformant | Missing plasmids | Antibody received | No. of positive cultures/total no. of specimens examined
|
|||
|---|---|---|---|---|---|---|
| Heart | Joint | Skin | All sites | |||
| 5A13/pBBE22 | cp9, lp25, lp56, lp21 | IgG2a | 5/5 | 5/5 | 5/5 | 15/15 |
| OspC MAb | 5/5 | 5/5 | 5/5 | 15/15 | ||
| 5A13/pBBE22-ospC′ | cp9, lp25, lp56, lp21, lp5 | IgG2a | 5/5 | 5/5 | 5/5 | 15/15 |
| OspC MAb | 0/5 | 0/5 | 0/5 | 0/15 | ||
Groups of 10 BALB/c SCID mice were inoculated with transformant 5A13/pBBE22 or 5A13/pBBE22-ospC′, passively immunized with either six doses of OspC MAb or mouse IgG2a as a control, and sacrificed 3 days after the last passive transfer. Heart, tibiotarsal joint, and skin specimens were harvested and cultured for spirochetes in BSK-H complete medium.
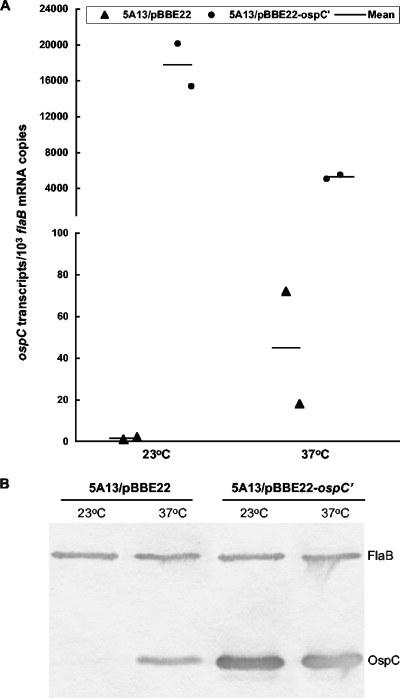
Next, we examined whether the fused flaB promoter functioned properly. It is well documented that the activity of the native ospC promoter is temperature dependent when spirochetes are grown in vitro (2, 37, 42), providing a scheme for assessing the contribution of the fused flaB promoter to lipoprotein expression. 5A13/pBBE22 and 5A13/pBBE22-ospC′ spirochetes were grown to late exponential phase at 23°C and 37°C; the accumulation of ospC and flaB transcripts was then analyzed by RT-qPCR. The 5A13/pBBE22 spirochetes matched every 1,000 flaB transcripts with only 1.5 ospC mRNAs on average, indicating that the native promoter is essentially silent, at 23°C, but the number increased to 45 ospC transcripts when spirochetes were grown at 37°C (Fig. 1A). In contrast, 5A13/pBBE22-ospC′ spirochetes matched every 1,000 flaB mRNAs with 17,800 and 5,300 ospC transcripts at 23°C and 37°C, respectively, indicating that the fused flaB promoter is very active at both temperatures (Fig. 1A). The addition of the flaB promoter increased ospC mRNA copy numbers 12,000- and 120-fold (P values were 0.01 and 0.02, respectively) when spirochetes were grown at 23°C and 37°C, respectively. OspC expression was also analyzed at the translational level by immunoblotting. When grown at 23°C, 5A13/pBBE22 produced too few OspC molecules for immunoblot detection; however, when the temperature was increased to 37°C, expression increased dramatically (Fig. 1B), consistent with the RT-qPCR data (Fig. 1A). 5A13/pBBE22-ospC′ accumulated a significant amount of the antigen at both temperatures, a result that not only is consistent with the RT-qPCR data but also indicates that mRNAs transcribed from the introduced ospC copy are appropriately translated into protein.
FIG. 1.
Fused flaB promoter leads to constitutive ospC expression in cultured B. burgdorferi. (A) Constitutive ospC expression at the transcriptional level. Transformants 5A13/pBBE22 and 5A13/pBBE22-ospC′ were grown to late exponential phase at 23°C and 37°C. RNA samples were analyzed by RT-qPCR for flaB and ospC mRNA copy numbers. Each datum point was calculated from a triplicate experiment and is presented as the mean ospC mRNA copy number per 1,000 flaB transcripts. (B) Constitutive ospC expression at the translational level. Spirochetes were prepared as described for panel A, and lysates were subjected to immunoblotting with a mixture of FlaB and OspC MAbs.
Constitutive ospC expression does not reduce infectivity or pathogenicity in SCID mice.
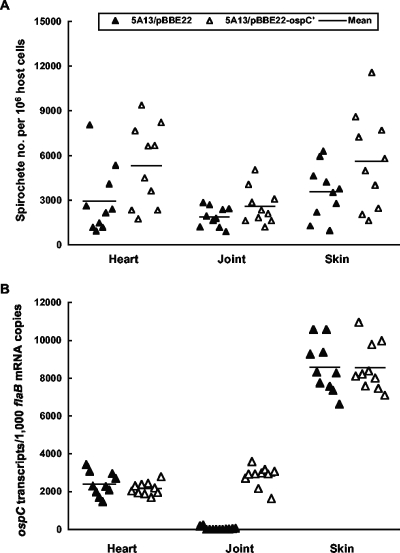
An immunodeficient host was used to address the specific issue of whether ospC expression reduces infectivity or pathogenicity because 5A13/pBBE22-ospC′ spirochetes might not be able to effectively evade adaptive immunity. Groups of 10 SCID mice were challenged with either 5A13/pBBE22 or 5A13/pBBE22-ospC′ spirochetes. Severe joint swelling evolved in each of the 20 mice between 2 and 3 weeks postinoculation, regardless of whether they received 5A13/pBBE22 or 5A13/pBBE22-ospC′ spirochetes (data not shown). The tissue spirochete burdens were determined as an indication of infectivity. DNAs were prepared from heart, joint, and skin specimens from the 20 mice and quantified by qPCR (Fig. 2A). The 5A13/pBBE22-ospC′ spirochete load was 40% higher in the heart tissue than the 5A13/pBBE22 load (P = 0.05), while there was no significant difference in the joint or skin tissues from the two groups (P values were 0.14 and 0.10, respectively). This study indicates that constitutive ospC expression does not reduce infectivity in either joint or skin tissues, but rather increases the spirochete load in the heart when the adaptive immune response is absent.
FIG. 2.
Infectivity and ospC expression of transformants in SCID mice. (A) Groups of 10 BALB/c SCID mice were infected with either transformant 5A13/pBBE22 or 5A13/pBBE22-ospC′ for 1 month. DNA samples were prepared from heart, joint, and skin specimens and analyzed for spirochete flaB and murine actin DNA copies by qPCR. The data are expressed as spirochete numbers per 106 host cells. (B) RNA samples were prepared from the heart, joint, and skin tissues and quantified for flaB and ospC expression by RT-qPCR. The data are presented as ospC mRNA copy numbers per 1,000 flaB transcripts.
The in vivo activity of the fused flaB promoter was also assessed by analyzing the relative copy numbers of ospC and flaB mRNAs by RT-qPCR. Transformant 5A13/pBBE22 exhibited differential tissue expression of the lipoprotein gene (Fig. 2B). The native ospC promoter was very active in both heart and skin tissues, resulting in ratios of ospC to flaB transcripts of approximately 2.4:1 to 8.5:1. The native ospC promoter's low activity in the joint tissue, where the ratio of ospC to flaB transcripts measured in 5A13/pBBE22 was only 0.05:1, showed a significant contribution of the fused flaB promoter to ospC expression. In the same type of tissue, the amount of ospC mRNA accumulated by 5A13/pBBE22-ospC′ spirochetes was 56-fold more than that accumulated by 5A13/pBBE22 spirochetes (P < 1.6 × 10−13), indicating that the fused promoter functions well. Due to the high activity of the native ospC promoter, however, no significant difference in the amounts of ospC mRNA produced in the two transformants could be detected for either the heart or skin tissue (P values were 0.30 and 0.99, respectively).
5A13/pBBE22-ospC′ spirochetes are unable to evade clearance by passively transferred OspC MAb in SCID mice.
The consequence of constitutive ospC expression was first investigated in a passive transfer study. Two groups of 10 SCID mice each were inoculated with either transformant 5A13/pBBE22 or 5A13/pBBE22-ospC′. Two weeks later, after spirochetes disseminated and infection was established, five mice from each group were administered either OspC MAb or mouse IgG2a as a control. Spirochetes were recovered from each specimen from all 10 mice that had been inoculated with 5A13/pBBE22, regardless of whether they received the OspC MAb or IgG2a, but were only recovered from the specimens of the five 5A13/pBBE22-ospC′-infected mice that were given the isotype control (Table 1). Transferred OspC antibody cleared the 5A13/pBBE22-ospC′ infection in each tissue of all five mice, indicating that constitutive ospC expression abrogates the ability of spirochetes to evade clearance by specific antibody.
5A13/pBBE22-ospC′ spirochetes evade the immune system less effectively than 5A13/pBBE22 spirochetes during early infection of immunocompetent mice.
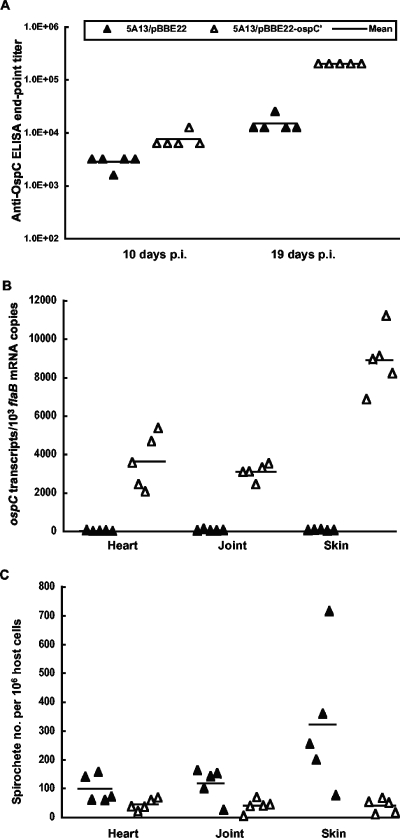
Ten BALB/c mice were inoculated with either 5A13/pBBE22 or 5A13/pBBE22-ospC′ to examine the influence of constitutive ospC expression on early infection in immunocompetent mice. Retro-orbital blood was drawn for assessment of the immune response on day 10; mice were sacrificed at 19 days postinoculation. 5A13/pBBE22-ospC′ elicited a 2- to 4-fold stronger anti-OspC humoral response than did 5A13/pBBE22 within 10 days postinoculation, which increased to a 16-fold difference at the end of the experiment (Fig. 3A), suggesting that increased OspC expression resulted from the introduction of the extra ospC copy. Consistent with this explanation, RT-qPCR showed that 5A13/pBBE22-ospC′ spirochetes maintained constitutive ospC expression (Fig. 3B). In contrast, ospC transcription in 5A13/pBBE22 was dramatically reduced by the immune response elicited during infection. 5A13/pBBE22-ospC′ spirochetes accumulated ospC transcripts 140, 48, and 119 times more than did 5A13/pBBE22 spirochetes in the heart (P = 4.4 × 10−5), joint (P = 1.7 × 10−7), and skin (P = 1.6 × 10−6) tissues, respectively (Fig. 3B). As a result of constitutive ospC expression, the 5A13/pBBE22-ospC′ spirochete burden was 54%, 66%, and 87% lower than the 5A13/pBBE22 burden in the heart (P = 0.047), joint (P = 0.021), and skin (P = 0.033) tissues, respectively (Fig. 3C).
FIG. 3.
5A13/pBBE22-ospC′ spirochetes evade the immune system less effectively than 5A13/pBBE22 spirochetes during early infection. (A) Groups of 10 BALB/c mice were inoculated with either transformant 5A13/pBBE22 or 5A13/pBBE22-ospC′ and sacrificed on day 19 postinoculation. The anti-OspC humoral response was titrated by an end-point ELISA, using sera collected on days 10 and 19 postinoculation (p.i.). (B) RNA samples were prepared from heart, joint, and skin specimens from mice sacrificed on day 19 postinoculation and quantified for flaB and ospC expression by RT-qPCR. The data are presented as ospC mRNA copy numbers per 1,000 flaB transcripts. (C) DNA samples were prepared from heart, joint, and skin specimens and analyzed for spirochete flaB and actin DNA copies by qPCR. The data are expressed as spirochete numbers per 106 host cells.
Spirochetes with constitutive ospC expression either are cleared or have undergone escape mutations.
Two groups of eight BALB/c mice each were inoculated with either 5A13/pBBE22 or 5A13/pBBE22-ospC′ to further examine the consequence of constitutive ospC expression in immunocompetent mice. Ear biopsies were taken for bacterial culture, and retro-orbital blood was drawn for assessment of the anti-OspC humoral response every 2 to 4 weeks postinoculation. 5A13/pBBE22-ospC′ elicited an 8- to 16-fold stronger anti-OspC humoral response than did 5A13/pBBE22 at each time point (data not shown). Spirochetes were successfully isolated from all of the mice that received 5A13/pBBE22 at each of the time points; however, from the mice that were inoculated with 5A13/pBBE22-ospC′, spirochetes were recovered consistently only during the first 8 weeks (data not shown). Six of the eight mice that had been inoculated with the transformant became negative by culture after 11 weeks of infection. However, B. burgdorferi was consistently recovered from the remaining two mice, designated BC13 and BC18, for the remaining time points, indicating that chronic infection had been established.
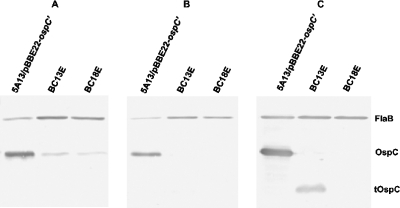
To determine whether escape mutations might have occurred in the introduced gene copy, the OspC expression of spirochetes isolated from the ear biopsies of mice BC13 and BC18 was analyzed by immunoblotting. When grown at 37°C, both isolates BC13E and BC18E, recovered from mice BC13 and BC18, respectively, produced much less antigen reactive with OspC MAb than the original inoculum (Fig. 4A), suggesting that mutations may have occurred in the extra ospC copy. To eliminate the contribution to OspC expression by the native gene copy, cells were grown to late exponential phase at 23°C. As presented in Fig. 4B, in contrast to the initial inoculum, both isolates lacked an OspC antigen reactive with the MAb, indicating that mutations had occurred. Since a MAb recognizes a single epitope, a mild mutation, such as an amino acid substitution, insertion, or deletion, may completely abolish antigenic reactivity. To assess the extent of mutation, lysates prepared from the isolates grown at 23°C were probed with antisera raised against a recombinant full-length OspC protein. Isolate BC13E, but not BC18E, did indeed abundantly produce a short version of the OspC antigen (Fig. 4C).
FIG. 4.
Recovered spirochetes no longer produce an antigen reactive with OspC MAb from the introduced ospC copy. Spirochetes were isolated from ear biopsies of mice BC13 and BC18, which had been infected with transformant 5A13/pBBE22-ospC′ for 17 weeks, designated BC13E and BC18E, respectively, and grown to late exponential phase at either 37°C (A) or 23°C (B and C). The initial inoculum, 5A13/pBBE22-ospC′, was used as a control. Lysates were subjected to immunoblot analysis with either a mixture of FlaB and OspC MAbs (A and B) or a mixture of FlaB MAb and mouse antisera raised against recombinant OspC (C). tOspC, truncated OspC antigen.
To determine whether the mutant gene copies were actively transcribed, total RNA was extracted from spirochetes grown at 23°C, and ospC mRNA transcripts were quantified by RT-qPCR. Both isolates and the original inoculum accumulated the transcripts at comparable levels (data not shown), indicating that the mutations did not affect the transcriptional process. The failure of the mutant ospC mRNA to be translated into an antigen reactive with OspC antibodies could be due to a lack of the ribosome-binding site and/or initial start codon, or the mutant mRNA might be translated into an unrelated polypeptide that does not share any antigenicity with OspC.
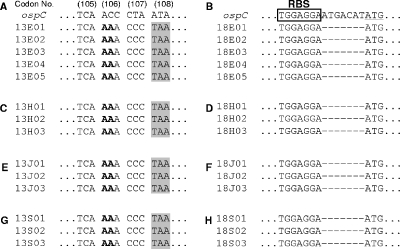
To identify mutations present in the pBBE22-ospC′ plasmids of BC13E and BC18E, total DNA was prepared and used to transform E. coli. Five clones transformed with each of the DNA preparations were randomly selected, and DNA sequencing showed that both BC13E and BC18E had undergone destructive yet different mutations (Fig. 5A and B), consistent with the interpretation of the immunoblotting results. The BC13E mutant contained a 2-bp insertion between nucleotides 315 and 316, resulting in a nonsense mutation at codon 108, and it encoded a polypeptide of only 107 amino acids, including the 19-amino-acid signal peptide. After the leader sequence was cleaved, the mutated OspC protein was less than one-half the normal length of 191 amino acids. All 7 bp between the putative ribosome-binding site and the start codon were deleted in BC18E, resulting in a mutation sufficient to abrogate translational initiation, although mutated ospC mRNA was abundantly accumulated. These changes apparently allowed the spirochetes to effectively avoid immune clearance and to establish chronic infection. All 16 mice were sacrificed at 5 months postinoculation. B. burgdorferi was recovered from each of the heart, joint, and skin specimens from all eight 5A13/pBBE22-inoculated mice but was recovered from the tissues of only mice BC13 and BC18 of the 5A13/pBBE22-ospC′ group (Table 2), consistent with the ear biopsy results. DNA sequencing indicated that all of the isolates from either BC13 or BC18 might be derived from an individual mutant (Fig. 5C to H).
FIG. 5.
Escape mutants have destructive mutations in the introduced ospC copy. Spirochetes were isolated from ear biopsies of mice BC13 and BC18 at 17 weeks postinoculation. The two mice were sacrificed after 5 months of infection; spirochetes were recovered from the heart, joint, and skin specimens. DNAs were extracted from these eight isolates and used to transform E. coli. Three to five kanamycin-resistant clones transformed with each DNA preparation were randomly selected and sequenced. In the four panels of the left column, the sequences represent isolates recovered from the ear biopsy (A) and the heart (C), joint (E), and skin (G) specimens of mouse BC13. All isolates obtained from this mouse had a 2-bp insertion (in bold) between nucleotides 315 and 316, producing a nonsense mutation, TAA (shaded), at codon 108. In the four panels of the right column, the sequences represent isolates from the ear biopsy (B) and the heart (D), joint (F), and skin (H) specimens of mouse BC18. All isolates recovered from this animal had 7 bp deleted between the putative ribosome-binding site (RBS) and the initial start codon, ATG (underlined).
TABLE 2.
Constitutive ospC expression diminishes the ability of B. burgdorferi to establish chronic infections in immunocompetent micea
| Transformant | No. of positive cultures/total no. of specimens examined
|
|||
|---|---|---|---|---|
| Heart | Joint | Skin | All sites | |
| 5A13/pBBE22 | 8/8 | 8/8 | 8/8 | 24/24 |
| 5A13/pBBE22-ospC′ | 2/8 | 2/8 | 2/8 | 6/24 |
Groups of eight BALB/c mice were inoculated with transformant 5A13/pBBE22 or 5A13/pBBE22-ospC′ and sacrificed at 5 months postinoculation. Heart, tibiotarsal joint, and skin specimens were harvested and cultured for spirochetes in BSK-H complete medium.
Restoration of chronic infectivity by abolishing constitutive ospC expression.
5A13/pBBE22-ospC′ spirochetes were transformed with the recombinant plasmid pGE22 to replace pBBE22-ospC′. pGE22 was constructed from the shuttle vector pBSV2G by inserting a copy of the BBE22 gene. Eighteen transformants were obtained from a single experiment; eight of them contained both recombinant plasmids and were discarded. A plasmid content survey of the remaining 10 transformants that had lost pBBE22-ospC′ revealed the following results: 5 maintained the same plasmid profile as the parent, 5A13/pBBE22-ospC′; 3 had lost lp28-1 and were discarded because they were not expected to establish infection in immunocompetent mice (21, 36); and the remaining 2 lacked either lp28-4 or cp32-3 and were designated 5A13/pGE22/07 and 5A13/pGE22/16, respectively. Three transformants, including 5A13/pGE22/07, 5A13/pGE22/16, and one clone that was randomly selected from the five clones that shared the same plasmid profile as the parental clone and designated 5A13/pGE22/03, were each inoculated into five BALB/c mice. Spirochetes were recovered from all 45 heart, joint, and skin specimens from the 15 mice after 5 months of infection (Table 3), indicating a full restoration of chronic infectivity once constitutive ospC expression had been abolished.
TABLE 3.
Full restoration of chronic infectivity once constitutive ospC expression is abolisheda
| Transformant | Missing plasmids | No. of positive cultures/total no. of specimens examined
|
|||
|---|---|---|---|---|---|
| Heart | Joint | Skin | All sites | ||
| 5A13/pGE22/03 | cp9, lp25, lp56, lp21, lp5 | 5/5 | 5/5 | 5/5 | 15/15 |
| 5A13/pGE22/07 | cp9, lp25, lp28-4, lp56, lp21, lp5 | 5/5 | 5/5 | 5/5 | 15/15 |
| 5A13/pGE22/16 | cp9, lp25, lp56, lp21, lp5, cp32-3 | 5/5 | 5/5 | 5/5 | 15/15 |
Groups of five BALB/c mice were inoculated with transformant 5A13/pGE22/03, 5A13/pGE22/07, or 5A13/pGE22/16 and sacrificed at 5 months postinoculation. Heart, tibiotarsal joint, and skin specimens were harvested and cultured for spirochetes in BSK-H complete medium.
DISCUSSION
B. burgdorferi causes persistent infection despite the development of vigorous immune responses against the pathogen. One potential strategy B. burgdorferi uses to effectively evade the immune system is to selectively reduce the expression of antibody-targeted antigens, exemplified by OspC, in response to immune pressure (7, 24, 26, 28). To explore this hypothesis, B. burgdorferi was genetically modified to constitutively express OspC by introducing an extra ospC copy fused with a flaB promoter. Such a modification did not reduce infectivity or pathogenicity but completely abrogated the ability of B. burgdorferi to avoid clearance by passively transferred OspC antibody in SCID mice. Spirochetes with constitutive ospC expression were unable to establish chronic infection unless they had undergone escape mutation during the infection of immunocompetent mice.
B. burgdorferi 5A13 harbors 20 of the 21 borrelial plasmids (36). The lack of lp25 makes spirochetes highly transformable because the plasmid carries BBE02, a putative restriction-modification gene (20, 22). The plasmid also harbors BBE22, which encodes a nicotinamidase essential for mammalian infection (21, 35). Transformation of 5A13 spirochetes with a shuttle vector containing a copy of BBE22 restores infectivity and pathogenicity (35). Because of the essential role of BBE22, any gene associated with the shuttle vector would be maintained in the mammalian environment. By using this system, we generated a constitutively ospC-expressing variant with full infectivity and pathogenicity in the absence of adaptive immune responses. The activity of the fused flaB promoter was first shown in vitro. The introduction of the extra copy increased the ospC mRNA copy number 120- and 12,000-fold at 37°C and 23°C, respectively, consistent with the results of immunoblot analysis, which showed a dramatic increase in the OspC antigen's accumulation.
The introduction of an extra gene copy significantly increased ospC mRNA accumulation in the joint tissues only but elevated the tissue spirochete burden in the hearts of infected SCID mice. Our previous study showed that OspC antibody represses ospC expression in all tissues but significantly reduces the bacterial load only in the heart, suggesting that the antigen may play an important role in spirochetal colonization in this specific tissue (28). We have not been able to assess in vivo gene expression at the translational level; it remains to be determined whether the presence of the fused flaB promoter increases OspC antigen synthesis in the heart. The addition of an extra ospC copy may increase the lipoprotein's expression, even though RT-qPCR did not show a substantial increase in ospC mRNA accumulation, enhancing spirochetal colonization in the heart.
OspC antibody is able to effectively prevent initial infection (16, 30) but is unable to clear an established infection (4) because B. burgdorferi has the ability to repress the antigen's expression (7, 24, 26, 28). With the introduction of an extra ospC copy fused with the flaB promoter, B. burgdorferi was forced to constitutively express the antigen regardless of the immune status of the host. This modification resulted in clearance of infection when the passive transfer of OspC antibodies occurred in SCID mice. The immune response induced during infection of immunocompetent mice cleared most of the infections. Although spirochetes with constitutive ospC expression triggered a strong humoral response to the lipoprotein, this response did not appear to be the reason why chronic infection did not develop in the majority of infected mice. Once spirochetes had undergone escape mutation in the introduced ospC copy, persistent infection was readily established in the same strong immune environment. It is unknown whether recombination occurred between the introduced and native ospC copies.
The 5A13/pBBE22-ospC′ clone was selected from a total of nine transformants that received the recombinant plasmid pBBE22-ospC′. It was first shown to be fully infectious and pathogenic in the absence of adaptive immune responses. The fact that all infections of SCID mice initiated with this clone were cleared by passively transferred OspC MAb could potentially be attributed to unnoted genetic defects that had been introduced specifically into this clone and that abolished its ability to avoid immune clearance. However, only two escape mutants were selected during infections of immunocompetent mice; both had a very destructive mutation in the constitutively expressed copy, which clearly associated the failure to evade specific humoral immunity with the presence of the introduced ospC copy. Moreover, once pBBE22-ospC′ was replaced with the recombinant plasmid pGE22, all three transformants derived from clone 5A13/pBBE22-ospC′ established chronic infections in immunocompetent mice, confirming the consequence of constitutive ospC expression.
B. burgdorferi dynamically modifies its surface antigenic architecture in response to environmental changes, including the development of specific immune responses. It actively expresses OspA in unfed ticks (10, 31, 39), consistent with an essential role of the lipoprotein in colonization of the vector by B. burgdorferi (32, 50). A fresh blood meal induces the down-regulation of OspA and the up-regulation of OspC (31, 39). It is crucial for maintaining the enzootic cycle to repress OspA expression during mammalian infection because its strong immunogenicity would elicit an immune response, effectively blocking acquisition of the spirochetes by the tick (9, 46, 47). In contrast, active OspC expression is required only for initial mammalian infection (17, 45). To avoid clearance by OspC-specific humoral immunity induced during infection, B. burgdorferi represses ospC expression once an infection has been established (7, 24, 28). In the immune environment, after ospC expression is repressed, the pathogen abundantly expresses a group of surface-exposed lipoprotein antigens (28), including VlsE, which undergoes antigenic variation (51), DbpA, which potentially interacts with host decorin (18), and BBF01 (12), whose function remains to be identified. Unlike OspC, these antigens are allowed to be persistently expressed or even dramatically up-regulated during chronic infection, probably because specific immune responses to any of them are not able to affect spirochete infection in the mammalian host (13, 19, 23, 25, 27).
Nothing is known about the molecular mechanisms governing antibody-induced antigen down-regulation. B. burgdorferi may sense a specific antibody and selectively down-regulate an antigen via an as yet unidentified signaling pathway(s) or generate multiple phenotypes, such as cells that abundantly express OspC and others that do not. OspC antibodies may eliminate phenotypes actively expressing the antigen but allow others to persist. Although the ospC gene is polymorphic among B. burgdorferi isolates (29, 44), its sequence is well maintained during chronic infections of immunocompetent mice (41). While mutation is an effective way to evade the immune system, it may cause the functional loss of affected genes. Regardless of how the specific immune response represses ospC expression, our current study clearly shows that B. burgdorferi must undergo escape mutation in order to establish chronic infection if it is unable to repress ospC expression in the event of the immune response being mounted. By down-regulating ospC rather than mutating, B. burgdorferi is able to avoid clearance and to preserve the gene's integrity for subsequent utilization during its enzootic life cycle.
Acknowledgments
We thank S. Norris for providing clonal isolate 5A13 and the recombinant plasmid pBBE22, P. Rosa and P. Stewart for providing the shuttle vector pBSV2G, K. DePonte and N. Marcantonio for providing the FlaB hybridoma cell line, and M. Mbow and R. Gilmore for providing the OspC hybridoma cell line.
This work was supported in part by an NIH/NIAMS career development award and an Arthritis Foundation Investigators award.
Editor: D. L. Burns
REFERENCES
- 1.Alexopoulou, L., V. Thomas, M. Schnare, Y. Lobet, J. Anguita, R. T. Schoen, R. Medzhitov, E. Fikrig, and R. A. Flavell. 2002. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat. Med. 8:878-884. [DOI] [PubMed] [Google Scholar]
- 2.Alverson, J., S. F. Bundle, C. D. Sohaskey, M. C. Lybecker, and D. S. Samuels. 2003. Transcriptional regulation of the ospAB and ospC promoters from Borrelia burgdorferi. Mol. Microbiol. 48:1665-1677. [DOI] [PubMed] [Google Scholar]
- 3.Barbour, A. G., S. F. Hayes, R. A. Heiland, M. E. Schrumpf, and S. L. Tessier. 1986. A Borrelia-specific monoclonal antibody binds to a flagellar epitope. Infect. Immun. 52:549-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bockenstedt, L. K., E. Hodzic, S. Feng, K. W. Bourrel, A. de Silva, R. R. Montgomery, E. Fikrig, J. D. Radolf, and S. W. Barthold. 1997. Borrelia burgdorferi strain-specific OspC-mediated immunity in mice. Infect. Immun. 65:4661-4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgdorfer, W., and J. E. Keirans. 1983. Ticks and Lyme disease in the United States. Ann. Intern. Med. 99:121. [DOI] [PubMed] [Google Scholar]
- 6.Casjens, S., N. Palmer, R. van Vugt, W. M. Huang, B. Stevenson, P. Rosa, R. Lathigra, G. Sutton, J. Peterson, R. J. Dodson, D. Haft, E. Hickey, M. Gwinn, O. White, and C. M. Fraser. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:490-516. [DOI] [PubMed] [Google Scholar]
- 7.Crother, T. R., C. I. Champion, J. P. Whitelegge, R. Aguilera, X. Y. Wu, D. R. Blanco, J. N. Miller, and M. A. Lovett. 2004. Temporal analysis of the antigenic composition of Borrelia burgdorferi during infection in rabbit skin. Infect. Immun. 72:5063-5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crother, T. R., C. I. Champion, X. Y. Wu, D. R. Blanco, J. N. Miller, and M. A. Lovett. 2003. Antigenic composition of Borrelia burgdorferi during infection of SCID mice. Infect. Immun. 71:3419-3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Silva, A. M., D. Fish, T. R. Burkot, Y. Zhang, and E. Fikrig. 1997. OspA antibodies inhibit the acquisition of Borrelia burgdorferi by Ixodes ticks. Infect. Immun. 65:3146-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Silva, A. M., S. R. Telford III, L. R. Brunet, S. W. Barthold, and E. Fikrig. 1996. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J. Exp. Med. 183:271-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elias, A. F., J. L. Bono, J. J. Kupko III, P. E. Stewart, J. G. Krum, and P. A. Rosa. 2003. New antibiotic resistance cassettes suitable for genetic studies in Borrelia burgdorferi. J. Mol. Microbiol. Biotechnol. 6:29-40. [DOI] [PubMed] [Google Scholar]
- 12.Feng, S., E. Hodzic, and S. W. Barthold. 2000. Lyme arthritis resolution with antiserum to a 37-kilodalton Borrelia burgdorferi protein. Infect. Immun. 68:4169-4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng, S., E. Hodzic, K. Freet, and S. W. Barthold. 2003. Immunogenicity of Borrelia burgdorferi arthritis-related protein. Infect. Immun. 71:7211-7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fikrig, E., S. W. Barthold, F. S. Kantor, and R. A. Flavell. 1990. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science 250:553-556. [DOI] [PubMed] [Google Scholar]
- 15.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J. F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. C. Venter, et al. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 16.Gilmore, R. D., Jr., K. J. Kappel, M. C. Dolan, T. R. Burkot, and B. J. Johnson. 1996. Outer surface protein C (OspC), but not P39, is a protective immunogen against a tick-transmitted Borrelia burgdorferi challenge: evidence for a conformational protective epitope in OspC. Infect. Immun. 64:2234-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grimm, D., K. Tilly, R. Byram, P. E. Stewart, J. G. Krum, D. M. Bueschel, T. G. Schwan, P. F. Policastro, A. F. Elias, and P. A. Rosa. 2004. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc. Natl. Acad. Sci. USA 101:3142-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo, B. P., E. L. Brown, D. W. Dorward, L. C. Rosenberg, and M. Höök. 1998. Decorin-binding adhesins from Borrelia burgdorferi. Mol. Microbiol. 30:711-723. [DOI] [PubMed] [Google Scholar]
- 19.Hagman, K. E., X. Yang, S. K. Wikel, G. B. Schoeler, M. J. Caimano, J. D. Radolf, and M. V. Norgard. 2000. Decorin-binding protein A (DbpA) of Borrelia burgdorferi is not protective when immunized mice are challenged via tick infestation and correlates with the lack of DbpA expression by B. burgdorferi in ticks. Infect. Immun. 68:4759-4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawabata, H., S. J. Norris, and H. Watanabe. 2004. BBE02 disruption mutants of Borrelia burgdorferi B31 have a highly transformable, infectious phenotype. Infect. Immun. 72:7147-7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Labandeira-Rey, M., J. Seshu, and J. T. Skare. 2003. The absence of linear plasmid 25 or 28-1 of Borrelia burgdorferi dramatically alters the kinetics of experimental infection via distinct mechanisms. Infect. Immun. 71:4608-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawrenz, M. B., H. Kawabata, J. E. Purser, and S. J. Norris. 2002. Decreased electroporation efficiency in Borrelia burgdorferi containing linear plasmids lp25 and lp56: impact on transformation of infectious B. burgdorferi. Infect. Immun. 70:4798-4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang, F. T., A. L. Alvarez, Y. Gu, J. M. Nowling, R. Ramamoorthy, and M. T. Philipp. 1999. An immunodominant conserved region within the variable domain of VlsE, the variable surface antigen of Borrelia burgdorferi. J. Immunol. 163:5566-5573. [PubMed] [Google Scholar]
- 24.Liang, F. T., M. B. Jacobs, L. C. Bowers, and M. T. Philipp. 2002. An immune evasion mechanism for spirochetal persistence in Lyme borreliosis. J. Exp. Med. 195:415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang, F. T., M. B. Jacobs, and M. T. Philipp. 2001. C-terminal invariable domain of VlsE may not serve as target for protective immune response against Borrelia burgdorferi. Infect. Immun. 69:1337-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang, F. T., F. K. Nelson, and E. Fikrig. 2002. Molecular adaptation of Borrelia burgdorferi in the murine host. J. Exp. Med. 196:275-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang, F. T., J. M. Nowling, and M. T. Philipp. 2000. Cryptic and exposed invariable regions of VlsE, the variable surface antigen of Borrelia burgdorferi sl. J. Bacteriol. 182:3597-3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang, F. T., J. Yan, M. L. Mbow, S. L. Sviat, R. D. Gilmore, M. Mamula, and E. Fikrig. 2004. Borrelia burgdorferi changes its surface antigenic expression in response to host immune responses. Infect. Immun. 72:5759-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marconi, R. T., D. S. Samuels, and C. F. Garon. 1993. Transcriptional analyses and mapping of the ospC gene in Lyme disease spirochetes. J. Bacteriol. 175:926-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mbow, M. L., R. D. Gilmore, Jr., and R. G. Titus. 1999. An OspC-specific monoclonal antibody passively protects mice from tick-transmitted infection by Borrelia burgdorferi B31. Infect. Immun. 67:5470-5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohnishi, J., J. Piesman, and A. M. de Silva. 2001. Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks. Proc. Natl. Acad. Sci. USA 98:670-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pal, U., A. M. de Silva, R. R. Montgomery, D. Fish, J. Anguita, J. F. Anderson, Y. Lobet, and E. Fikrig. 2000. Attachment of Borrelia burgdorferi within Ixodes scapularis mediated by outer surface protein A. J. Clin. Investig. 106:561-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pal, U., X. Yang, M. Chen, L. K. Bockenstedt, J. F. Anderson, R. A. Flavell, M. V. Norgard, and E. Fikrig. 2004. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J. Clin. Investig. 113:220-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Probert, W. S., and R. B. LeFebvre. 1994. Protection of C3H/HeN mice from challenge with Borrelia burgdorferi through active immunization with OspA, OspB, or OspC, but not with OspD or the 83-kilodalton antigen. Infect. Immun. 62:1920-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Purser, J. E., M. B. Lawrenz, M. J. Caimano, J. K. Howell, J. D. Radolf, and S. J. Norris. 2003. A plasmid-encoded nicotinamidase (PncA) is essential for infectivity of Borrelia burgdorferi in a mammalian host. Mol. Microbiol. 48:753-764. [DOI] [PubMed] [Google Scholar]
- 36.Purser, J. E., and S. J. Norris. 2000. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 97:13865-13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramamoorthy, R., and D. Scholl-Meeker. 2001. Borrelia burgdorferi proteins whose expression is similarly affected by culture temperature and pH. Infect. Immun. 69:2739-2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samuels, D. S. 1995. Electrotransformation of the spirochete Borrelia burgdorferi. Methods Mol. Biol. 47:253-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 92:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steere, A. C. 2001. Lyme disease. N. Engl. J. Med. 345:115-125. [DOI] [PubMed] [Google Scholar]
- 41.Stevenson, B., L. K. Bockenstedt, and S. W. Barthold. 1994. Expression and gene sequence of outer surface protein C of Borrelia burgdorferi reisolated from chronically infected mice. Infect. Immun. 62:3568-3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stevenson, B., T. G. Schwan, and P. A. Rosa. 1995. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect. Immun. 63:4535-4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stewart, P. E., R. Thalken, J. L. Bono, and P. Rosa. 2001. Isolation of a circular plasmid region sufficient for autonomous replication and transformation of infectious Borrelia burgdorferi. Mol. Microbiol. 39:714-721. [DOI] [PubMed] [Google Scholar]
- 44.Theisen, M., B. Frederiksen, A. M. Lebech, J. Vuust, and K. Hansen. 1993. Polymorphism in ospC gene of Borrelia burgdorferi and immunoreactivity of OspC protein: implications for taxonomy and for use of OspC protein as a diagnostic antigen. J. Clin. Microbiol. 31:2570-2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tilly, K., J. G. Krum, A. Bestor, M. W. Jewett, D. Grimm, D. Bueschel, R. Byram, D. Dorward, M. J. Vanraden, P. Stewart, and P. Rosa. 2006. Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect. Immun. 74:3554-3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsao, J., A. G. Barbour, C. J. Luke, E. Fikrig, and D. Fish. 2001. OspA immunization decreases transmission of Borrelia burgdorferi spirochetes from infected Peromyscus leucopus mice to larval Ixodes scapularis ticks. Vector Borne Zoonotic Dis. 1:65-74. [DOI] [PubMed] [Google Scholar]
- 47.Tsao, J. I., J. T. Wootton, J. Bunikis, M. G. Luna, D. Fish, and A. G. Barbour. 2004. An ecological approach to preventing human infection: vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. Proc. Natl. Acad. Sci. USA 101:18159-18164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wooten, R. M., Y. Ma, R. A. Yoder, J. P. Brown, J. H. Weis, J. F. Zachary, C. J. Kirschning, and J. J. Weis. 2002. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J. Immunol. 168:348-355. [DOI] [PubMed] [Google Scholar]
- 49.Xu, Q., S. V. Seemanapalli, L. Lomax, K. McShan, X. Li, E. Fikrig, and F. T. Liang. 2005. Association of linear plasmid 28-1 with an arthritic phenotype of Borrelia burgdorferi. Infect. Immun. 73:7208-7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang, X. F., U. Pal, S. M. Alani, E. Fikrig, and M. V. Norgard. 2004. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J. Exp. Med. 199:641-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang, J. R., J. M. Hardham, A. G. Barbour, and S. J. Norris. 1997. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell 89:275-285. [DOI] [PubMed] [Google Scholar]