Abstract
We have developed experimental murine Campylobacter infection models which demonstrate efficient establishment and reproducible, high-level colonization. Following oral inoculation, wild-type C3H mice with normal enteric flora were colonized inconsistently and inefficiently by C. jejuni strain 81-176. However, C3H mice with a limited gut flora (LF) were efficiently colonized at high levels (108 CFU/g of stool or large intestine tissue) followed by clearance after several weeks. Large intestine tissue showed minimal to mild inflammation at days 7 and 28 postinoculation. In striking contrast, C3H SCID mice with the same limited flora remained persistently colonized at a consistently high level until they were euthanized 8 months postinoculation. Lower gastrointestinal tract tissue from LF-SCID mice showed marked to severe inflammation in the colon and cecum at days 7 and 28 and intense inflammation of the stomach at day 28. These findings indicate that although the innate response alone cannot block colonization persistence, it is sufficient to orchestrate marked gut inflammation. Moreover, the adaptive immune response is critical to mediate C. jejuni clearance from the colonized gut. To validate our LF murine model, we verified that motility and chemotaxis are critical for colonization. Insertion-deletion mutations were generated in motB and fliI, which encode products essential for motility and flagellar assembly, and in the presumptive chemotaxis gene cheA (histidine kinase). All mutants failed to establish colonization in LF mice. Our limited flora murine colonization models serve as tractable, reproducible tools to define host responses to C. jejuni infection and to identify and characterize virulence determinants required for colonization.
Campylobacter jejuni has become the most frequently identified cause of acute infectious diarrhea in the developed world, with nearly 2 to 3 million cases estimated each year in the United States alone (2, 21). The hallmarks of infection are abdominal pain, fever, myalgias, and bloody or watery diarrhea. Campylobacter colonizes the distal small intestine and colon and causes varying degrees of mucosal inflammation, including a neutrophilic infiltrate, crypt abscesses, and patchy ulceration (13). Although the vast majority of infections are self-limited, chronic sequelae include Reiter's syndrome, a reactive polyarthropathy (14), and Guillain-Barre syndrome, a demyelinating disorder that leads to acute neuromuscular paralysis (1, 47). Campylobacter species live as commensals in the intestines of a wide variety of birds and mammals. The primary mode of transmission to humans is via contaminated and undercooked poultry; less frequent sources include unpasteurized milk, contaminated drinking water, and contact with pets and other animals (21). In contrast to Campylobacter enteritis in the industrialized world, infections in the developing world usually lead to only mild watery diarrhea or asymptomatic colonization. This is likely due to the much higher incidence of infection and the subsequent development of protective immunity (35).
Despite its obvious global importance, relatively little is known regarding mechanisms of C. jejuni pathogenesis and host responses to infection. The lack of high-resolution, tractable, and reproducible models for studying infection in vivo has clearly hampered progress. Attempts to establish a suitable animal model using newborn pigs, weanling ferrets, gnotobiotic canine pups, nonhuman primates, and other hosts have suffered from high costs, difficulties in handling and availability, lack of reproducibility, or inadequate biological characterization (3-5, 40, 50, 52). While the chick model of commensal infection is both reproducible and tractable (23, 24), the chick immune system is poorly defined and not readily amenable to genetic manipulation. Murine models of C. jejuni infection overcome some of these limitations, but available models suffer from sporadic colonization or an absence of consistent intestinal pathology or clinical signs (8, 10, 27). The use of germfree/gnotobiotic or immunocompromised mice has alleviated these shortcomings with varying degrees of success (25, 51, 53, 54). However, to date no particular model has been broadly and consistently employed to study Campylobacter infection and immunity in mammals.
In this study, we demonstrate that oral dosing of C. jejuni into C3H mice with a defined, limited gut flora (LF) leads to efficient establishment and reproducible colonization, with the highest levels localized to the large intestine. LF-SCID mice remain persistently colonized at this high level, while immunocompetent LF mice gradually resolve the infection. Moreover, LF-SCID mice suffer from intense inflammation of the cecum, colon, and stomach; in contrast, immunocompetent LF mice show little or no inflammation at corresponding time points. Since <103 CFU of wild-type C. jejuni are sufficient to colonize the gut, our murine models should prove sensitive enough to identify mutants attenuated or defective for establishing colonization. Indeed, we demonstrate that C. jejuni mutants deficient in motility or chemotaxis fail to colonize LF mice. These models should find broad applicability in characterizing colonization determinants and defining elements of the host response to infection.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Campylobacter jejuni strain 81-176 is a clinical isolate shown to cause diarrheal disease in human volunteer studies (9, 29) and was kindly provided by Patricia Guerry. NCTC 11168 has been sequenced (37) and was obtained from the Central Public Health Laboratory (London, United Kingdom). C. jejuni was routinely cultured at 42°C in a microaerophilic environment (BBL CampyPak and Oxoid CampyGen) for 36 to 48 h on Campylobacter agar base (Difco) supplemented with amphotericin B (2 μg/ml), cephalothin (15 μg/ml), trimethoprim (5 μg/ml), vancomycin (10 μg/ml), polymyxin B (2.5 units/ml), and 10% (vol/vol) defibrinated sheep blood (Mission Lab, Rosemead, CA). C. jejuni deletion-insertion mutants were grown under the above conditions on plates additionally supplemented with kanamycin (30 μg/ml).
Animals and animal care.
Five- to six-week-old BALB/c and C3H mice with normal gut flora were purchased from Charles River Laboratories. LF and LF-SCID C3H mice of the same age were purchased from an animal facility maintained by the UCLA Department of Radiation Oncology. This colony of mice was founded at UCLA 13 years ago by conventionalization of germfree founders with several nonpathogenic species of Clostridium. The composition of the gut flora has grown more complex over the years; while Firmicutes species predominate, members of Lactobacillus and Acinetobacter taxa have been identified in the large intestines by using oligonucleotide fingerprinting of rRNA genes (41). These mice are bred and maintained under strict, barrier-contained specific-pathogen-free conditions. Sentinel animals are tested regularly for colonization by other microbes in this facility. Following inoculation, mice were housed in groups of four in sterile polycarbonate microisolator cages with autoclaved bedding and provided with sterilized water and food ad lib. All experiments were approved by the UCLA Animal Research Committee.
Experimental infection.
C. jejuni used for inoculations was recovered from freezer stocks, plated on selective medium, and incubated for 36 to 48 h, as described above. A bacterial lawn was then prepared from these freshly grown plates and incubated overnight. C. jejuni was harvested from these plates with a sterile Dacron swab and suspended in brucella broth (Difco) The concentration of bacteria was estimated spectrophotometrically and subsequently confirmed by serial dilutions and plating on selective medium. Mice were given 100 μl of 5% sodium bicarbonate (wt/vol) by direct oral inoculation with a ball-tipped inoculating needle (Fisher), followed 15 min later by the C. jejuni inoculum in 200 μl of brucella broth.
Quantitation of colonization.
At postinoculation (p.i.) days 3 or 4, 7, and 14 and weekly thereafter, a fresh fecal pellet was collected from each mouse, weighed, and homogenized in 500 μl of sterile phosphate-buffered saline. Appropriate serial dilutions were plated on selective medium and incubated in a microaerophilic environment for 36 to 48 h. At select time points postinoculation, a subset of mice were euthanized and their gastrointestinal (GI) tracts aseptically removed. Sections from the stomach (both glandular and aglandular), duodenum, small intestine, large intestine, and cecum were harvested following gentle extrusion of their lumenal contents, weighed, homogenized in sterile phosphate-buffered saline, serially diluted, plated on selective medium, and incubated for 36 to 48 h. C. jejuni recovery is expressed as CFU per gram of stool or tissue.
Plasmids and construction of insertion-deletion mutants.
The pRY plasmid series was kindly provided by Patricia Guerry (49). The Campylobacter aphA-3 cassette (30), which confers kanamycin resistance, was amplified by PCR from plasmid pRY107. The 5′ and 3′ portions of cheAWY, motB, and fliI genes were amplified from C. jejuni 81-176 genomic DNA using PCR; primers were derived from the published genome sequence (37). Insertion-deletion mutation plasmids were constructed into pRY112 with aphA-3 flanked by the 5′ and 3′ fragments; the structures of these plasmids were verified by restriction digest mapping and PCR and electroporated into strain 81-176. Double-crossover homologous recombinants, which were Kmr Cms, were identified and underwent further PCR analysis to verify disruption of the appropriate chromosomal gene.
Motility assay.
Wild-type and mutant C. jejuni 81-176 colonies were picked from a freshly grown plate with a needle and stabbed into brucella broth medium containing 0.4% agar. Plates were incubated at 42°C overnight under microaerophilic conditions and then assessed for their ability to form a swarm colony versus a compact colony at the inoculation site.
Histopathology.
At 1 week and 4 weeks postinoculation, mice were euthanized and sections of stomach, duodenum, small intestine, large intestine, and cecum were immediately fixed in 10% neutral buffered formalin. Following fixation, tissue samples were embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin-eosin for light microscopic examination. Histopathological scoring was based on a scale described by Berg et al. (6) that ranged from 0 to 4. Briefly, scores were based on the following criteria: grade 0, no change from normal tissue; grade 1, one or a few multifocal mononuclear cell infiltrates with minimal epithelial hyperplasia or mucus depletion; grade 2, lesions more involved or frequent with mild inflammatory infiltrates, hyperplasia, and mucin depletion and rare submucosal inflammation; grade 3, lesions more involved, with inflammation that is moderate and involves the submucosa with occasional crypt abscesses and ulcers; grade 4, lesions involving most of the section with severe inflammation that is sometimes transmural, with crypt abscesses and ulcers but few mucin-containing cells.
Data presentation and statistical analysis.
Colonization-level results are expressed as the mean with error bars denoting the standard error of the mean (SEM). Statistical analysis of colonization results was performed with the unpaired two-sample t test with unequal variances, while analysis of histopathology scores was performed with a nonparametric two-sample Wilcoxon rank-sum (Mann-Whitney) test, using Stata software, version 8.0.
RESULTS
Colonization establishment and persistence in limited flora mice.
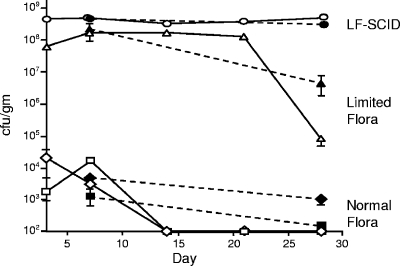
Murine models of Campylobacter infection suffer from inefficient establishment, lack of persistence, high variability, and absence of consistent clinical or pathological findings (8, 10, 27, 52). As shown in Fig. 1, colonization establishment was inconsistent (∼50%) in C3H and BALB/c mice (Charles River) following an intragastric inoculum of 5 × 108 CFU of C. jejuni strain 81-176. Moreover, low levels of C. jejuni were initially recovered from stool and intestinal tissue (103 to 105 CFU/g), with clearance by day 14 p.i. from feces and by day 28 from large intestine tissue in BALB/c mice and shortly thereafter in C3H animals.
FIG. 1.
Time course of GI tract colonization by C. jejuni in wild-type normal flora mice, LF mice, and LF-SCID mice. Groups of 16 normal flora mice of each strain and groups of 19 LF and LF-SCID mice were intragastrically inoculated with approximately 5 × 108 CFU of wild-type strain 81-176. Stool and GI tract tissue were collected and processed as described in Materials and Methods. Fresh stool was collected for all mice at the indicated time points; C. jejuni recovered from stool is denoted by open symbols. Large intestine (cecum and colon) tissue was collected from four to eight mice euthanized at the indicated time points, and C. jejuni recovered is represented by closed symbols. C3H mice are denoted by ovals, triangles, and diamonds; BALB/c mice are denoted by squares. Error bars represent the SEM and may be obscured by symbols denoting the mean value. Pairwise differences in colonization levels between normal flora, LF, and LF-SCID C3H mice at day 7 were statistically significant (defined as P < 0.05, with P values ranging from 0.0015 to 0.040) except for large intestine tissue of LF versus SCID mice (P = 0.095). At day 28, the pairwise differences in colonization were statistically significant (P values ranging from 0.0019 to 0.034) except for tissue and stool of normal flora versus LF mice (P = 0.37 and 0.05, respectively). Statistical analysis of colonization levels was performed with the unpaired two-sample t test with unequal variances.
We reasoned that both the efficiency and extent of colonization could be limited by the complex, normal gastrointestinal flora present in commercially available mice. We therefore took advantage of the availability of C3H mice with a limited, defined enteric flora (LF) consisting primarily of nonpathogenic Clostridial species, Lactobacillus, and Acinetobacter (42). When a 5 × 108 CFU dose of strain 81-176 was orally administered to LF C3H mice, all 16 became colonized. As shown in Fig. 1, at 1 week p.i., colonization levels in feces and large intestine tissue (108 to 109 CFU/g) were 4 to 5 orders of magnitude greater than previously observed in normal flora mice with the same genetic background. Furthermore, animal-to-animal variation in C. jejuni recovery was extremely small among LF C3H mice. By 4 weeks p.i., the yield of C. jejuni began to decrease, dropping to 106 to 107 CFU/g of large intestine and 104 to 105 CFU/g of feces (Fig. 1). However, in comparison to normal flora mice, the extent and duration of colonization in LF mice were remarkably robust.
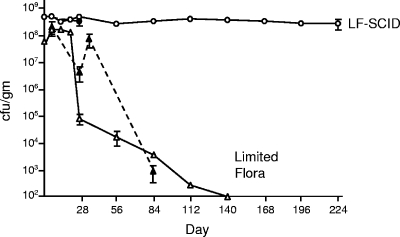
Prolonged persistence in LF-SCID mice.
Although LF mice were efficiently colonized by C. jejuni, infection levels began to decrease 3 to 4 weeks after oral inoculation, as evidenced by drops in CFU recovered from feces and large intestine tissue. These yields continued to decrease over time, and by 20 weeks C. jejuni levels in feces were below the limits of detection in LF mice (Fig. 2). The timing of this decrease and eventual clearance of C. jejuni suggested that the adaptive immune response may play a role. Indeed, sera from mice colonized for 4 to 6 weeks contained antibodies that cross-reacted with C. jejuni whole-cell extracts on Western blots (data not shown). To explore the contribution of adaptive immunity to clearance of C. jejuni colonization in LF mice, we inoculated LF C3H mice homozygous for the SCID mutation with 5 × 108 CFU of strain 81-176. SCID mice are defective in antigen receptor recombination, resulting in a relative absence of mature B and T lymphocytes (16). As expected, all inoculated LF-SCID mice were efficiently colonized by C. jejuni at a high level of ∼109 CFU/g of feces or large intestine tissue, with negligible animal-to-animal variation (Fig. 1 and 2). In striking contrast to the clearance of C. jejuni by immunocompetent LF mice, we observed persistent, high-level infection in all LF-SCID mice, as measured by fecal shedding (Fig. 2). C. jejuni was recovered at a constant, high level that remained remarkably consistent from mouse to mouse (error bars in Fig. 2 are obscured by the symbol denoting the mean value). This persistent infection continued for 8 months p.i., at which time the animals were euthanized.
FIG. 2.
Persistence of GI tract colonization by C. jejuni in LF and LF-SCID mice. Results are for a survey of C. jejuni colonization persistence in groups of four to eight LF or LF-SCID mice, as described for Fig. 1.
C. jejuni ID50 for LF mice.
We hypothesized that an oral inoculum of 5 × 108 CFU of C. jejuni was in excess of the dose needed to colonize the GI tract of LF mice. We therefore determined the 50% infective dose (ID50) for establishment of colonization. Groups of four mice were orally inoculated with progressively higher CFU, and fecal pellets were assayed for C. jejuni at days 3, 7, and 14 p.i. Inocula of 2 × 103 CFU and greater led to efficient colonization by day 3 and recovery of 108 CFU/g of feces. In contrast, 7 days were required to detect bacteria in 4/4 mice inoculated with 200 CFU, while an inoculum of 20 CFU failed to establish colonization in all 4 mice even after 14 days. We therefore estimated the oral ID50 for strain 81-176 in LF mice to be ≤200 CFU.
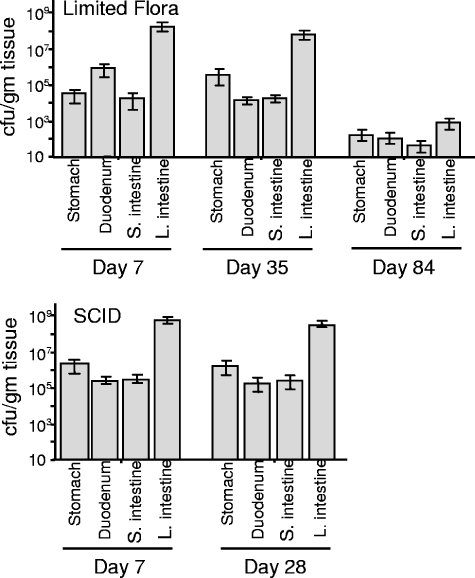
C. jejuni is concentrated in the large intestine and cecum.
C. jejuni was able to successfully colonize the entire GI tract of LF mice following oral inoculation with 5 × 108 CFU (Fig. 3). At day 7 p.i., the cecum and large intestine were consistently colonized with 108 to 109 CFU/g of tissue in both LF and LF-SCID mice. The stomach, duodenum, and small intestine were reproducibly colonized at a lower level, between 104 and 107 CFU/g (Fig. 3). This level of colonization was maintained in the LF-SCID mice, with approximately 3 orders of magnitude greater recovery from the lower intestine than the upper intestine, until the animals were euthanized at 8 months. In contrast, immunocompetent LF mice began to clear C. jejuni from the intestine although consistently maintaining higher levels in the lower intestine tissues. The yield of C. jejuni from fresh fecal samples mirrored recovery from the lower intestine tissues in both LF and LF-SCID mice (Fig. 1 and 2). Bacterial counts in feces therefore serve as a sensitive and easily obtained indicator of colonization levels.
FIG. 3.
Colonization of specific segments of the GI tract in LF and LF-SCID mice. Groups of four to eight mice were euthanized at the indicated day postinoculation, and colonization levels were determined as described in Materials and Methods.
Throughout the experimental infection with C. jejuni, none of the LF or LF-SCID mice displayed any clinical or behavioral signs of disease; no diarrhea or changes in stool consistency were noted. In addition, no mice died during the course of the experiment. Macroscopic inspection of dissected intestinal tissue colonized by C. jejuni failed to reveal any increased erythema, congestion, or friability compared to uninfected controls for both LF and LF-SCID mice.
C. jejuni causes marked inflammation in the stomach, cecum, and large intestine of LF-SCID mice.
Despite the absence of clinical signs of disease or macroscopic changes in the gut of colonized mice, we surveyed the alimentary tract tissue of LF and LF-SCID mice for inflammation and other microscopic changes. Tissue harvested from groups of mice colonized for 7 and 28 days by strain 81-176 along with sections from mock-infected control mice were examined (Table 1; see also Fig. 4 to 6, below).
TABLE 1.
Histopathology scores of gastrointestinal tissues
| Day p.i. and mouse type | Histopathology scorea
|
|||||
|---|---|---|---|---|---|---|
| Stomach | Duodenum | Small intestine | Cecum | Cecum-colon junction | Colon | |
| Day 7 | ||||||
| SCID uninfected | 0, 0 | 0, 0 | 0, 0 | 0, 0 | 0, 0 | 0, 0 |
| SCID infected | 0, 0, 0 | 2, 2, 1 | 2, 2, 1 | 2, 3, 3 | 2, 3, 2 | 2, 2, 2 |
| LF uninfected | 0, 0 | 0, 0 | 0, 0 | 0, 0 | 0, 0 | 0, 0 |
| LF infected | 0, 0, 0 | 0, 0, 0 | 0, 0, 0 | 0, 0, 0 | 0, 0, 0 | 0, 0, 0 |
| Day 28 | ||||||
| SCID uninfected | 0, 0 | 0, 0 | 0, 0 | 0, 0 | 0, 0 | 0, 0 |
| SCID infected | 4, 3, 1, 1, 0 | 1, 1, 1, 2, 1 | 2, 2, 2, 2, 1 | 4, 3, 2, 4, 3 | 4, 4, 1, 3, 2 | 4, 3, 2, 2, 1 |
| LF uninfected | 0, 0 | 0, 0 | 0, 0 | 0, 0 | 0, 0 | 0, 0 |
| LF infected | 0, 0 | 0, 0 | 0, 0 | 1, 0 | 1, 0 | 0, 0 |
Scores (0 to 4) were assigned to tissue sections stained with hematoxylin-eosin, using criteria established by Berg et al. (6) from groups of two to five infected and uninfected mice. At day 28, differences in histopathology scores between SCID infected tissue and each of the experimental mice groups were statistically significant (P < 0.05) except for the stomach. Score differences were not statistically significant at day 7 due to the smaller sample size. The two-sample Wilcoxon rank-sum (Mann-Whitney) test was used for statistical analysis.
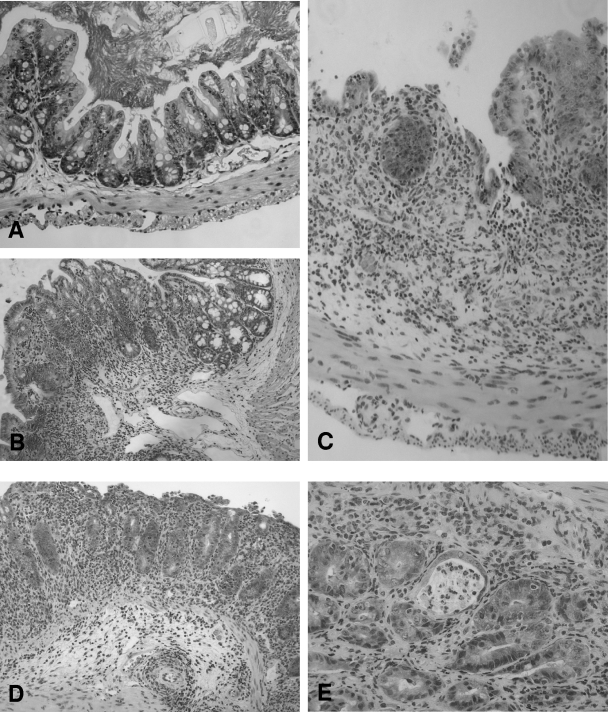
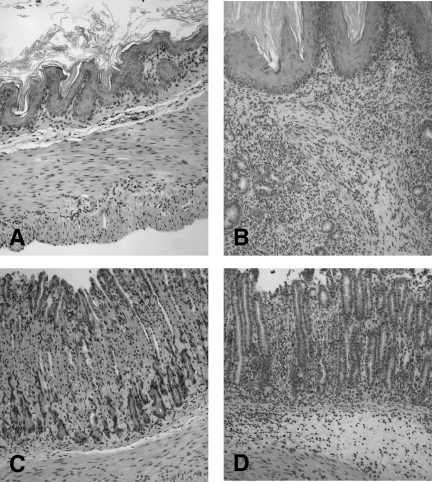
FIG. 4.
Histopathology of large intestine tissue from LF and LF-SCID mice 28 days postinoculation. (A) Only mild inflammation was detected in the lamina propria of LF mice colonized by C. jejuni, with preservation of the normal tissue architecture. (B to E) Severe inflammation was evident in the mucosa and submucosa of the cecum and colon tissue of similarly colonized SCID mice, with marked inflammatory infiltrate, including ulceration (B), epithelial hyperplasia and loss of goblet cells (C), and edema and architectural distortion (D). (E) Cryptitis was also frequently appreciated. Magnification, ×200.
FIG. 6.
Histopathology of stomach tissue from LF and LF-SCID mice 28 days postinoculation. The stomachs of immunocompetent LF mice colonized for 28 days had minimal inflammatory infiltrate and normal tissue architecture in both the aglandular (A) and glandular (C) regions. In comparison, colonized LF-SCID mice demonstrated an intense inflammatory infiltrate in both the aglandular (B) and glandular (D) zones. Magnification: ×100 (A to C); ×150 (D). Marked edema and gland abscesses were appreciated in panel D.
At day 7 p.i., immunocompetent LF mice showed no inflammation of tissue from the upper or lower GI tract compared to control mice. At day 28 p.i., mild inflammation of the lamina propria, limited to the cecum and cecal-colonic junction, was observed (Fig. 4A). Despite moderate to high levels of colonization by C. jejuni, the stomach, duodenum, small intestine, and colon did not demonstrate an inflammatory response compared to tissues from uninfected control mice.
In striking contrast, severe areas of inflammation were observed in LF-SCID mice following just 7 days of colonization. Cecal inflammation was most severe, with inflammatory cells in the lamina propria and submucosa, edema, and focal ulcerations with hemorrhage (Fig. 5). The duodenum, small intestine, and colon exhibited milder inflammation restricted to the mucosa, while the cellular infiltrate of the cecal-colonic junction extended into the submucosa as well. Interestingly, inflammation was absent in the stomach at day 7 p.i. However, after 28 days of colonization by C. jejuni, all portions of the stomach in a subset of mice developed marked inflammation of the mucosa and submucosa that was often transmural, with accompanying gland abscesses (Fig. 6B and D). The upper intestines were only mildly inflamed, whereas lower intestinal tissue at 28 days p.i. displayed intense inflammation (Fig. 4B to E). Mucosal, submucosal, and often muscularis spaces were infiltrated with inflammatory cells; cryptitis and epithelial cell hyperplasia were evident, as was marked mucosal edema. Multiple small ulcerated foci, often transmural, were noted as well. The inflammatory infiltrate in LF-SCID gut tissue consisted primarily of granulocytes (Fig. 5D) with some mononuclear cells and leaky oligoclonal lymphocytes (11). It is noteworthy that in spite of the marked inflammation of the lower GI tract of LF-SCID mice colonized with C. jejuni, none displayed any signs of clinical illness or diarrhea.
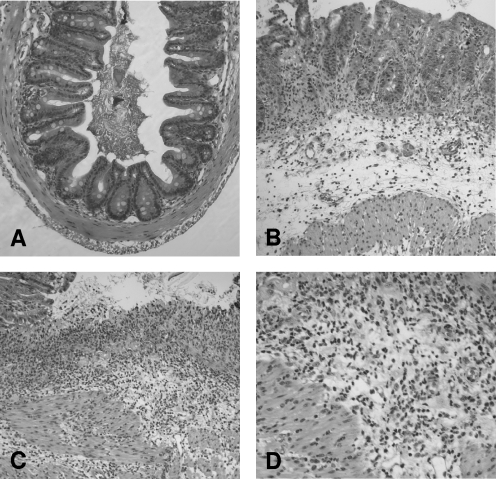
FIG. 5.
Histopathology of large intestine tissue from LF-SCID mice 7 days postinoculation. (A) Control cecum tissue from an uninfected SCID mouse revealed normal crypt architecture with only a small number of inflammatory cells in the lamina propria. Magnification, ×100. (B to D) In contrast, severe inflammation of the mucosa and submucosa in the cecum and colon was observed in LF-SCID mice 7 days after inoculation with C. jejuni strain 81-176, with architectural distortion, hyperplasia, and edema evident (B). (C) An ulcerated region of the mucosa revealed an intense inflammatory infiltrate with accompanying hemorrhage. Magnification in panels B and C, ×100. (D) A ×200 magnification confirmed that the infiltrate was composed primarily of neutrophils, although mononuclear cells were also present.
Motility and chemotaxis mutants fail to colonize LF mice.
The challenge facing colonizing campylobacters to avoid removal by intestinal flow and to navigate through the gut mucus layer imposes a requirement for motility and chemotaxis for efficient establishment in the GI tract. Nonmotile or aflagellated C. jejuni mutants failed to efficiently colonize the intestinal tracts in experimental infections of suckling mice (33), chicks (23, 24, 34, 48), and humans (9). In addition, actively motile yet nonchemotactic mutants did not colonize the suckling mouse gut except at very high inocula (45). C. jejuni with defined mutations in the cheY locus or in a gene encoding a putative methyl-accepting chemotaxis protein also poorly colonized ferrets (50) and chicks (23). Thus, motility and chemotaxis represent two virulence determinants consistently shown to be important for effective colonization by C. jejuni in multiple animal models.
To validate our LF murine colonization model, we measured colonization phenotypes of mutants defective in motility or chemotaxis. Insertion-deletion mutations were generated in three open reading frames with proposed functions in motility or chemotaxis in C. jejuni strain 81-176, based on the annotated genome sequence of strain NCTC 11168 (37) and earlier mutational analyses (12, 22-24). In each mutant strain, a portion of the protein-coding sequence was replaced with aphA-3 (encoding kanamycin resistance) by double-crossover homologous recombination. We mutated a CheAWY orthologue, which is a hybrid-fusion polypeptide consisting of an N-terminal histidine kinase domain followed by a CheW docking domain and a CheY response regulator motif. cheAWY::aphA-3 has all three domains deleted and was expected to be chemotaxis deficient. motB::aphA-3 has a lesion in the proposed flagellar motor protein, and fliI::aphA-3 is disrupted in the putative flagellum-specific ATP synthase; both mutants are expected to be nonmotile. To verify the chemotaxis-deficient or nonmotile phenotypes of these mutants, we inoculated each into brucella broth plates containing 0.4% agar. Following overnight growth at 42°C under microaerophilic conditions, none of the mutants was able to swarm compared to the wild-type parent strain 81-176. cheAWY::aphA-3 formed a very small halo of growth beyond the inoculation site, whereas both motB::aphA-3 and fliI::aphA-3 exhibited no migration and grew in a compact colony localized to the point of inoculation (data not shown). The observations are consistent with the prediction that the cheAWY mutation abolishes chemotaxis while retaining motility and that the motB and fliI mutations are nonmotile. Mutations in all three open reading frames generated by random transposon mutagenesis also demonstrated motility defects (12, 22-24).
To measure colonization, we orally inoculated groups of 6-week-old mice with approximately 5 × 102 CFU of wild-type strains 81-176 or NCTC 11168 or mutants cheAWY::aphA-3, motB::aphA-3, or fliI::aphA-3. Both wild-type strains colonized the lower GI tract at high levels, as demonstrated by recovery from tissue homogenates or stool. In striking contrast, analysis of feces collected at days 4, 7, and 28 p.i. as well as gut tissue harvested at days 7 and 28 demonstrated the complete absence of colonization in all mice inoculated by the chemotaxis-deficient or nonmotile strains (Fig. 7). Although these models show unusually efficient colonization establishment and persistence when inoculated with wild-type strains, the absolute requirements for motility and chemotaxis identified in earlier animal infection studies are retained.
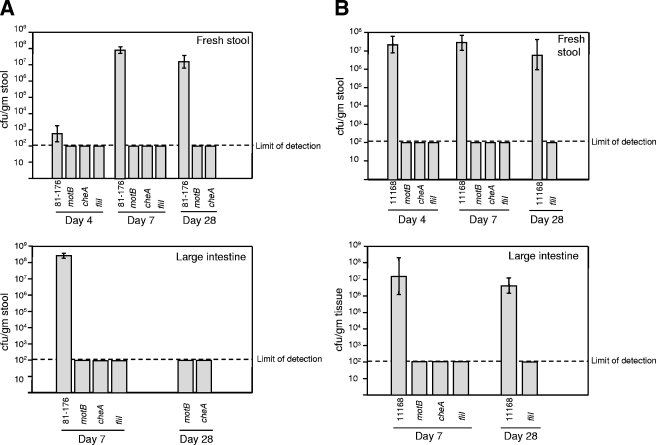
FIG. 7.
C. jejuni mutants defective in motility or chemotaxis are unable to colonize the GI tract of LF mice. Insertion-deletion mutations were made in motB, cheAWY, and fliI in both the 81-176 (A) and 11168 (B) backgrounds. Groups of four to eight mice were inoculated with 102 to 103 CFU of wild-type or mutant C. jejuni, and stool and tissue samples were collected at the indicated days p.i., with half the group sacrificed at day 7 to assess intestinal colonization. In striking contrast to both wild-type strains, all three mutants failed to colonize the LF C3H mice as demonstrated by analysis of fresh stool and large intestine tissue. Error bars represent the SEM. The limit of detection is 100 CFU/g of stool or tissue. Colonization-level differences between wild-type 81-176 and each of the mutants at day 7 for both stool and large intestine tissue were statistically significant (P values ranging from 0.0014 to 0.0467). For later time points and for analysis in the 11168 background, statistical significance was not achieved despite a lack of colonization by chemotaxis and motility mutants (within the limits of detection), due to smaller sample sizes. Statistical analysis of colonization levels was performed with the unpaired two-sample t test with unequal variances.
DISCUSSION
Progress toward understanding Campylobacter enteritis, both in terms of the pathogenic mechanisms employed and the host responses to colonization, has been hampered by the paucity of high-resolution experimental systems for studying infection in vivo (52). An optimal model for characterizing Campylobacter infection should be readily available and reasonable in cost, easy to maintain and handle, well studied biologically, and able to reproduce the pathology associated with human infection. Even in the absence of overt clinical enteritis or intestinal pathology, tractable and reproducible models to study colonization—an essential step in pathogenesis and initiation of a host response—are of critical importance. The significant natural reservoir of asymptomatic colonization across a range of host animals, including the worldwide poultry population, and in humans underlies the importance of a detailed, mechanistic understanding of this process.
With the exception of a single report (39), oral inoculation of newborn chickens leads to efficient colonization of intestinal contents (4, 24, 44) without clinical signs or intestinal pathology, as would be expected in a commensal association. Young weanling ferrets and nonhuman primates also develop mild intestinal symptoms, but they are expensive, limited in availability, and difficult to handle (5, 15, 32). Similar factors limit the use of gnotobiotic canine pups and newborn piglets as infection models. In contrast, laboratory mice overcome many of these limitations and offer the advantage of an immune system that is well characterized and has been extensively manipulated using transgenic and knockout technologies. However, in virtually all studies, oral inoculation of wild-type mice results in sporadic intestinal colonization and an absence of intestinal disease. Interestingly, published experimental infections of immunocompromised mice demonstrate more efficient colonization and sporadic intestinal pathology (20, 25, 51, 53, 54).
In this study, we demonstrated that orally inoculated C3H mice with a defined, limited flora are efficiently colonized at high levels compared to C3H mice with a normally complex enteric flora. At 4 weeks p.i., the yield of C. jejuni from stool and large intestine tissue began to drop and by 4 to 5 months p.i. was below the limit of detection. In contrast, LF-SCID mice remained persistently colonized at a high and uniform level until they were euthanized nearly a year after inoculation with C. jejuni, thus demonstrating the critical role of adaptive immunity in clearing C. jejuni infection from the gut. In support of this idea, preliminary experiments indicate that adoptive transfer of splenocytes harvested from LF C3H mice colonized by C. jejuni into LF-SCID mice does not detectibly affect the establishment of gut infection by C. jejuni. In contrast, persistence of C. jejuni in the gut was dramatically decreased, with clearance kinetics that resembled the time course seen in immunocompetent LF mice (data not shown). While C. jejuni was recovered throughout the intestinal tract and stomach in both LF and LF-SCID mice, the colon and cecum were consistently colonized at much higher levels. Despite such extensive colonization, none of the infected mice displayed any signs of clinical disease or diarrhea. Nevertheless, the LF-SCID mice had severe inflammation of the cecum and colon at days 7 and 28 p.i. and marked inflammation of the stomach in a subset of mice by day 28. In striking contrast, immunocompetent LF mice manifested minimal or no inflammation of either the upper or lower alimentary tract tissues.
Discrepant results using mice as an experimental model have appeared in the literature over the past 20 years and are likely due to the use of different Campylobacter strains and inoculum sizes to infect diverse murine strains with varied gut flora complexity and immune repertoires. While some groups reported efficient colonization of normal flora mice by high oral doses of Campylobacter (8, 10, 25, 51), our results more closely reflect studies showing robust colonization only in a gut environment with decreased microbial diversity, as in germfree (27, 53, 54), neonatal (19), or antibiotic-treated mice (18). Colonization persistence has been less thoroughly tracked in these reports, although continued excretion of Campylobacter from both normal and immunocompromised mice for up to 2 months was shown (10, 20, 25). Of note, both athymic and euthymic germfree mice monoassociated with Campylobacter since birth were persistently colonized for over 7 months, suggesting that neonatal exposure tolerizes the adaptive immune response. Finally, clinical disease or GI inflammation has been observed only sporadically (25), transiently (54), or unexpectedly (gastritis without intestinal inflammation) (51) when using clinical isolates in immunocompromised mice.
To develop a reproducible and widely applicable murine model to study Campylobacter infection, we chose to use C. jejuni strain 81-176 because it is a well-characterized and readily available laboratory strain originally derived from a human clinical isolate. 81-176 has continued to produce intestinal disease in recent human infection trials (38). We initially inoculated commercially supplied BALB/c and C3H mice strains. Our inability to efficiently colonize these mice suggested that the normal complex gut microflora was a critical innate defense to infection that may confer “bacterial interference” (7) by occupying potential environmental niches needed for Campylobacter to establish colonization, competing for critical nutrients, and/or generating by-products that attenuate its competitive fitness in the gut. Campylobacter infection studies with germfree mice demonstrated high levels of colonization throughout the GI tract (27, 53), especially when compared to conventional mice and even mice pretreated with antibiotics (18, 27). Moreover, introduction of a normal fecal microflora to germfree mice monoassociated with C. jejuni led to the eventual clearance of C. jejuni (53). Successful experimental colonization by C. jejuni of neonatal animals (19), including 1-day-old chicks (4, 44), may be explained by an underpopulated gut environment, perhaps coupled with an immature immune system. Due to a lack of antigenic stimulation by indigenous microflora, germfree mice have attenuated inflammatory and immune responses as well as gut architecture and general physiologic differences compared to conventional mice (7, 17).
With these considerations in mind, we used C3H mice with a limited, defined gut flora. Despite an indigenous, albeit limited, flora, recent characterization of the immune status of C57BL/6 mice with similar flora from the same facility demonstrated an impaired innate B-cell repertoire manifested by fewer marginal zone and B-1a B cells and a decreased antibody response (B. Wei and J. Braun, unpublished data). Moreover, these mice were distinguished by a deficiency in absolute numbers of naïve splenic CD4+ and CD8+ T cells and a state of T-cell receptor hyperresponsiveness in the CD4+ population (26). Hence, both decreased bacterial competition and attenuated host response may contribute to efficient and robust colonization of C3H mice with limited gut flora (Fig. 1). The relative contribution of each factor may prove difficult to precisely define given the complex interplay among the environmental microbiota, immune development or maturity, and innate and adaptive host responses. Nevertheless, our results provide several early clues regarding the relationships governing gut colonization, infection clearance, and host responses to infection.
While a limited gut flora allows efficient colonization establishment by C. jejuni, infection levels begin to drop 4 weeks after initial inoculation, and colonization was ultimately undetectable after approximately 4 months. The importance of acquired immunity in clearing an established C. jejuni infection was convincingly shown by prolonged, persistent colonization at high levels in isogenic LF-SCID C3H mice with the same limited gut flora but lacking functional B and T lymphocytes (Fig. 2). This observation is consistent with persistent C. jejuni colonization in C.B-17-SCID-Beige and nude mice (25, 53, 54). Although persistence was also noted in euthymic monoassociated mice, their immune system may have been “tolerized” to C. jejuni by perinatal colonization within their colony. Severe and prolonged Campylobacter infections of patients with hypogammaglobulinemia or AIDS highlight the contributions of both humoral and cell-mediated immunity in the control of human infection (28, 43, 46). Preliminary results showing the elimination of an established infection in LF-SCID mice following adoptive transfer of splenocytes from immunocompetent LF mice suggests that splenocytes contain the immune cell repertoire needed to mediate clearance (data not shown). The relative contributions of B-cell and CD4+ and CD8+ T-cell populations, as well as the need for their preexposure to C. jejuni, can be further assessed using our models.
While the adaptive immune response mediates clearance of colonizing C. jejuni from the mouse gut, it is not directly responsible for the marked inflammation and tissue pathology seen in the large intestine and stomach of infected LF-SCID mice. Rather, the inflammation first noted at day 7 p.i. in the cecum and colon, and in the stomach by day 28 p.i., appeared to reflect an acute, innate immune response likely mediated by neutrophils, macrophages, and NK cells. The composition of the inflammatory infiltrate in the mucosa and submucosa was primarily granulocytic, with additional mononuclear cells likely composed of leaky oligoclonal lymphocytes. The severe inflammation we observed in asymptomatic LF-SCID mice, which was absent in immunocompetent LF mice, mirrors the chronic atrophic gastritis associated with C. fetus colonization of outbred ICR mice (51); however, no intestinal pathology was detected in this model despite persistent colonization. In contrast, mild to moderate large intestine inflammation and moderately severe gastritis and duodenitis were observed with C. jejuni 81-176 colonization of NF-κB-deficient (p50−/− p65+/−) C57BL/129 mice (20). Although approximately 10% of C.B-17 SCID-Beige mice colonized by clinical isolates of C. jejuni developed sporadic diarrhea, colonic inflammation was relatively mild compared to our LF-SCID mice (Fig. 4) (25). Variations in results from these murine models reflect differences in genetic background and immune competency in the infected mice and in the Campylobacter species and strains studied. Taken together, our observations and the results from other laboratories indicate that mice with a compromised immune repertoire are more prone to gut inflammation than immunocompetent mice.
What actually drives this inflammatory reaction in LF-SCID mice remains undefined. While the colonizing Campylobacter undoubtedly triggers the response, it is unlikely to directly cause the tissue damage or sustain the acute response via pathogen-derived toxins or free radicals or by processes such as epithelial invasion or architectural distortion. C. jejuni was recovered at high levels, and within an order of magnitude of each other, from stomach and large intestine tissue at both time points in LF-SCID and immunocompetent LF mice (Fig. 3). Given that their only baseline difference is functional B and T cells, we hypothesize that these lymphocytes directly or indirectly suppress acute inflammation. Alternatively, the SCID mutation may lead to secondary effects that include a generalized hyperreactive innate inflammatory response.
Besides their future promise for the study of host responses and for developing vaccines, our murine models can identify Campylobacter genes important for gut colonization by targeted or random approaches. The low ID50 (≤200 CFU of strain 81-176) in our LF mouse models provides a highly sensitive means to detect phenotypes that decrease the efficiency of colonization establishment, and the prolonged duration of colonization in immunocompetent mice, and especially in SCID mice, will allow sensitive assessments of persistence. As a demonstration of their utility, we established that mutations in genes that abolish motility, chemotaxis, or flagellar assembly led to a complete absence of detectable colonization in LF mice (Fig. 7), despite administration of inocula approximately fivefold above the ID50. These colonization profiles were consistent with, or even more attenuated than, results with other animal infection models (24, 33, 34, 45, 48, 50). Interestingly, at high inocula, all three mutants were detectable at varied levels from the stool (102 to 106 CFU/g) but were not recovered from large intestine tissue (data not shown). Clearly, motility and chemotaxis are critical for the colonizing C. jejuni to penetrate the intestinal mucus layer and possibly adhere to or even invade the intestinal epithelium (36). Mutants may thus remain on the superficial mucus and be susceptible to expulsion by intestinal flow and thus recovered in stool yet remain undetected in association with intestinal tissue. Our murine models have also measured more subtle colonization attenuation in mutants of a two-component signal transduction system (31), indicating a high degree of sensitivity that can be further magnified using colonization competition assays with wild-type C. jejuni.
Acknowledgments
We thank Peggy Cotter (University of California, Santa Barbara) for helpful discussion in developing the murine infection models, Nora Rozengurt for mouse pathology interpretation, and members of the J. F. Miller lab for helpful input on the manuscript.
C.C. was supported by NIH grant KO8 DK61547 and a Foundation for Digestive Health and Nutrition Training Award.
Editor: V. J. DiRita
REFERENCES
- 1.Allos, B. M. 1997. Association between Campylobacter infection and Guillain-Barre syndrome. J. Infect. Dis. 176(Suppl. 2):S125-S128. [DOI] [PubMed] [Google Scholar]
- 2.Altekruse, S. F., N. J. Stern, P. I. Fields, and D. L. Swerdlow. 1999. Campylobacter jejuni—an emerging foodborne pathogen. Emerg. Infect. Dis. 5:28-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babakhani, F. K., G. A. Bradley, and L. A. Joens. 1993. Newborn piglet model for campylobacteriosis. Infect. Immun. 61:3466-3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beery, J. T., M. B. Hugdahl, and M. P. Doyle. 1988. Colonization of gastrointestinal tracts of chicks by Campylobacter jejuni. Appl. Environ. Microbiol. 54:2365-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell, J. A., and D. D. Manning. 1991. Evaluation of Campylobacter jejuni colonization of the domestic ferret intestine as a model of proliferative colitis. Am. J. Vet. Res. 52:826-832. [PubMed] [Google Scholar]
- 6.Berg, D. J., N. Davidson, R. Kuhn, W. Muller, S. Menon, G. Holland, L. Thompson-Snipes, M. W. Leach, and D. Rennick. 1996. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4+ TH1-like responses. J. Clin. Investig. 98:1010-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berg, R. D. 1996. The indigenous gastrointestinal microflora. Trends Microbiol. 4:430-435. [DOI] [PubMed] [Google Scholar]
- 8.Berndtson, E., M. L. Danielsson-Tham, and A. Engvall. 1994. Experimental colonization of mice with Campylobacter jejuni. Vet. Microbiol. 41:183-188. [DOI] [PubMed] [Google Scholar]
- 9.Black, R. E., M. M. Levine, M. L. Clements, T. P. Hughes, and M. J. Blaser. 1988. Experimental Campylobacter jejuni infection in humans. J. Infect. Dis. 157:472-479. [DOI] [PubMed] [Google Scholar]
- 10.Blaser, M. J., D. J. Duncan, G. H. Warren, and W. L. Wang. 1983. Experimental Campylobacter jejuni infection of adult mice. Infect. Immun. 39:908-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosma, M. J., and A. M. Carroll. 1991. The SCID mouse mutant: definition, characterization, and potential uses. Annu. Rev. Immunol. 9:323-350. [DOI] [PubMed] [Google Scholar]
- 12.Colegio, O. R., T. J. T. Griffin, N. D. Grindley, and J. E. Galan. 2001. In vitro transposition system for efficient generation of random mutants of Campylobacter jejuni. J. Bacteriol. 183:2384-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colgan, T., J. R. Lambert, A. Newman, and S. C. Luk. 1980. Campylobacter jejuni enterocolitis. A clinicopathologic study. Arch. Pathol. Lab. Med. 104:571-574. [PubMed] [Google Scholar]
- 14.Damani, N. N., C. A. Humphrey, and B. Bell. 1993. Haemolytic anaemia in Campylobacter enteritis. J. Infect. 26:109-110. [DOI] [PubMed] [Google Scholar]
- 15.Dolg, P., R. Yao, D. H. Burr, P. Guerry, and T. J. Trust. 1996. An environmentally regulated pilus-like appendage involved in Campylobacter pathogenesis. Mol. Microbiol. 20:885-894. [DOI] [PubMed] [Google Scholar]
- 16.Dorshkind, K., G. M. Keller, R. A. Phillips, R. G. Miller, G. C. Bosma, M. O'Toole, and M. J. Bosma. 1984. Functional status of cells from lymphoid and myeloid tissues in mice with severe combined immunodeficiency disease. J. Immunol. 132:1804-1808. [PubMed] [Google Scholar]
- 17.Falk, P. G., L. V. Hooper, T. Midtvedt, and J. I. Gordon. 1998. Creating and maintaining the gastrointestinal ecosystem: what we know and need to know from gnotobiology. Microbiol. Mol. Biol. Rev. 62:1157-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Field, L. H., J. L. Underwood, and L. J. Berry. 1984. The role of gut flora and animal passage in the colonisation of adult mice with Campylobacter jejuni. J. Med. Microbiol. 17:59-66. [DOI] [PubMed] [Google Scholar]
- 19.Field, L. H., J. L. Underwood, L. M. Pope, and L. J. Berry. 1981. Intestinal colonization of neonatal animals by Campylobacter fetus subsp. jejuni. Infect. Immun. 33:884-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox, J. G., A. B. Rogers, M. T. Whary, Z. Ge, N. S. Taylor, S. Xu, B. H. Horwitz, and S. E. Erdman. 2004. Gastroenteritis in NF-κB-deficient mice is produced with wild-type Camplyobacter jejuni but not with C. jejuni lacking cytolethal distending toxin despite persistent colonization with both strains. Infect. Immun. 72:1116-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedman, C. R., J. Neimann, H. C. Wegener, and R. V. Tauxe. 2000. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations, p. 121-138. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed., vol. 1. ASM Press, Washington, D.C. [Google Scholar]
- 22.Golden, N. J., and D. W. Acheson. 2002. Identification of motility and autoagglutination Campylobacter jejuni mutants by random transposon mutagenesis. Infect. Immun. 70:1761-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hendrixson, D. R., B. J. Akerley, and V. J. Dirita. 2001. Transposon mutagenesis of Campylobacter jejuni identifies a bipartite energy taxis system required for motility. Mol. Microbiol. 40:214-224. [DOI] [PubMed] [Google Scholar]
- 24.Hendrixson, D. R., and V. J. DiRita. 2004. Identification of Campylobacter jejuni genes involved in commensal colonization of the chick gastrointestinal tract. Mol. Microbiol. 52:471-484. [DOI] [PubMed] [Google Scholar]
- 25.Hodgson, A. E., B. W. McBride, M. J. Hudson, G. Hall, and S. A. Leach. 1998. Experimental campylobacter infection and diarrhoea in immunodeficient mice. J. Med. Microbiol. 47:799-809. [DOI] [PubMed] [Google Scholar]
- 26.Huang, T., B. Wei, P. Velazquez, J. Borneman, and J. Braun. 2005. Commensal microbiota alter the abundance and TCR responsiveness of splenic naive CD4+ T lymphocytes. Clin. Immunol. 117:221-230. [DOI] [PubMed] [Google Scholar]
- 27.Jesudason, M. V., D. J. Hentges, and P. Pongpech. 1989. Colonization of mice by Campylobacter jejuni. Infect. Immun. 57:2279-2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson, R. J., C. Nolan, S. P. Wang, W. R. Shelton, and M. J. Blaser. 1984. Persistent Campylobacter jejuni infection in an immunocompromised patient. Ann. Intern. Med. 100:832-834. [DOI] [PubMed] [Google Scholar]
- 29.Korlath, J. A., M. T. Osterholm, L. A. Judy, J. C. Forfang, and R. A. Robinson. 1985. A point-source outbreak of campylobacteriosis associated with consumption of raw milk. J. Infect. Dis. 152:592-596. [DOI] [PubMed] [Google Scholar]
- 30.Labigne-Roussel, A., P. Courcoux, and L. Tompkins. 1988. Gene disruption and replacement as a feasible approach for mutagenesis of Campylobacter jejuni. J. Bacteriol. 170:1704-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacKichan, J. K., E. C. Gaynor, C. Chang, S. Cawthraw, D. G. Newell, J. F. Miller, and S. Falkow. 2004. The Campylobacter jejuni dccRS two-component system is required for optimal in vivo colonization but is dispensable for in vitro growth. Mol. Microbiol. 54:1269-1286. [DOI] [PubMed] [Google Scholar]
- 32.Manning, D. D., and J. A. Bell. 1990. Derivation of gnotobiotic ferrets: perinatal diet and hand-rearing requirements. Lab. Anim. Sci. 40:51-55. [PubMed] [Google Scholar]
- 33.Morooka, T., A. Umeda, and K. Amako. 1985. Motility as an intestinal colonization factor for Campylobacter jejuni. J. Gen. Microbiol. 131:1973-1980. [DOI] [PubMed] [Google Scholar]
- 34.Nachamkin, I., X. H. Yang, and N. J. Stern. 1993. Role of Campylobacter jejuni flagella as colonization factors for three-day-old chicks: analysis with flagellar mutants. Appl. Environ. Microbiol. 59:1269-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oberhelman, R. A., and D. N. Taylor. 2000. Campylobacter infections in developing countries, p. 139-154. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed., vol. 1. ASM Press, Washington, D.C. [Google Scholar]
- 36.Ottemann, K. M., and J. F. Miller. 1997. Roles for motility in bacterial-host interactions. Mol. Microbiol. 24:1109-1117. [DOI] [PubMed] [Google Scholar]
- 37.Parkhill, J., B. W. Wren, K. Mungall, J. M. Ketley, C. Churcher, D. Basham, T. Chillingworth, R. M. Davies, T. Feltwell, S. Holroyd, K. Jagels, A. V. Karlyshev, S. Moule, M. J. Pallen, C. W. Penn, M. A. Quail, M. A. Rajandream, K. M. Rutherford, A. H. van Vliet, S. Whitehead, and B. G. Barrell. 2000. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403:665-668. [DOI] [PubMed] [Google Scholar]
- 38.Prendergast, M. M., D. R. Tribble, S. Baqar, D. A. Scott, J. A. Ferris, R. I. Walker, and A. P. Moran. 2004. In vivo phase variation and serologic response to lipooligosaccharide of Campylobacter jejuni in experimental human infection. Infect. Immun. 72:916-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruiz-Palacios, G. M., E. Escamilla, and N. Torres. 1981. Experimental Campylobacter diarrhea in chickens. Infect. Immun. 34:250-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Russell, R. G., M. J. Blaser, J. I. Sarmiento, and J. Fox. 1989. Experimental Campylobacter jejuni infection in Macaca nemestrina. Infect. Immun. 57:1438-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scupham, A. J., L. L. Presley, B. Wei, E. Bent, N. Griffith, M. McPherson, F. Zhu, O. Oluwadara, N. Rao, J. Braun, and J. Borneman. 2006. Abundant and diverse fungal microbiota in the murine intestine. Appl. Environ Microbiol. 72:793-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scupham, A. J., B. Wei, J. Braun, and J. Borneman. 2003. Identification of intestinal fungal and bacterial microflora in a Crohn's disease mouse model using array-based oligonucleotide fingerprinting of rRNA genes. Gastroenterology 124:A477. [Google Scholar]
- 43.Sorvillo, F. J., L. E. Lieb, and S. H. Waterman. 1991. Incidence of campylobacteriosis among patients with AIDS in Los Angeles County. J. Acquir. Immune Defic. Syndr. 4:598-602. [PubMed] [Google Scholar]
- 44.Stern, N. J., J. S. Bailey, L. C. Blankenship, N. A. Cox, and F. McHan. 1988. Colonization characteristics of Campylobacter jejuni in chick ceca. Avian Dis. 32:330-334. [PubMed] [Google Scholar]
- 45.Takata, T., S. Fujimoto, and K. Amako. 1992. Isolation of nonchemotactic mutants of Campylobacter jejuni and their colonization of the mouse intestinal tract. Infect. Immun. 60:3596-3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tee, W., A. Mijch, E. Wright, and A. Yung. 1995. Emergence of multidrug resistance in Campylobacter jejuni isolates from three patients infected with human immunodeficiency virus. Clin. Infect. Dis. 21:634-638. [DOI] [PubMed] [Google Scholar]
- 47.Tsang, R. S. 2002. The relationship of Campylobacter jejuni infection and the development of Guillain-Barre syndrome. Curr. Opin. Infect. Dis. 15:221-228. [DOI] [PubMed] [Google Scholar]
- 48.Wassenaar, T. M., B. A. van der Zeijst, R. Ayling, and D. G. Newell. 1993. Colonization of chicks by motility mutants of Campylobacter jejuni demonstrates the importance of flagellin A expression. J. Gen. Microbiol. 139:1171-1175. [DOI] [PubMed] [Google Scholar]
- 49.Yao, R., R. A. Alm, T. J. Trust, and P. Guerry. 1993. Construction of new Campylobacter cloning vectors and a new mutational cat cassette. Gene 130:127-130. [DOI] [PubMed] [Google Scholar]
- 50.Yao, R., D. H. Burr, and P. Guerry. 1997. CheY-mediated modulation of Campylobacter jejuni virulence. Mol. Microbiol. 23:1021-1031. [DOI] [PubMed] [Google Scholar]
- 51.Young, V. B., C. A. Dangler, J. G. Fox, and D. B. Schauer. 2000. Chronic atrophic gastritis in SCID mice experimentally infected with Campylobacter fetus. Infect. Immun. 68:2110-2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Young, V. B., D. B. Schauer, and J. G. Fox. 2000. Animal models of Campylobacter infection, p. 287-302. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed., vol. 1. ASM Press, Washington, D.C. [Google Scholar]
- 53.Yrios, J. W., and E. Balish. 1986. Colonization and infection of athymic and euthymic germfree mice by Campylobacter jejuni and Campylobacter fetus subsp. fetus. Infect. Immun. 53:378-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yrios, J. W., and E. Balish. 1986. Pathogenesis of Campylobacter spp. in athymic and euthymic germfree mice. Infect. Immun. 53:384-392. [DOI] [PMC free article] [PubMed] [Google Scholar]