Abstract
Rickettsiae, a diverse group of obligately intracellular gram-negative bacteria, include etiologic agents of the spotted fever and typhus groups of diseases. Rocky Mountain spotted fever and boutonneuse fever, due to Rickettsia rickettsii and R. conorii, respectively, are characterized by widespread infection of the vascular endothelium, microvascular injury, and vasculitis. Cultured human endothelial cells (EC) are highly susceptible to infection and respond by altering the expression of adhesion molecules, regulatory cytokines, and the antioxidant enzyme heme oxygenase (HO). In the vasculature, HO regulates the cyclooxygenase (COX) enzymes, among which the inducible isozyme COX-2 facilitates the synthesis of prostaglandins (PGs). Using in vitro and ex vivo models of infection, we demonstrate here that R. rickettsii infection of human EC causes robust induction of COX-2 mRNA and protein expression but has no apparent effect on the constitutive COX-1 isoform. Cells infected with viable rickettsiae consistently displayed significantly increased secretion of 6-keto-PGF1α and PGE2. R. rickettsii-induced COX-2 was sensitive to inhibitors of de novo transcription and the pyridinylimidazole-based compound SB 203580, suggesting that this transcriptional host cell response involves signaling through p38 mitogen-activated protein kinase. PG production by infected cells was abrogated by NS 398 (a selective COX-2 inhibitor) and indomethacin (a pan-COX inhibitor). Immunohistochemical staining of sections of infected umbilical cords and corresponding uninfected controls revealed comparatively more intense and abundant staining for COX-2 in infected endothelia. Induction of the endothelial COX-2 system and the resultant enhanced release of vasoactive PGs may contribute to the regulation of inflammatory responses and vascular permeability changes during spotted fever rickettsioses.
Pathogenic species of the genus Rickettsia include gram-negative bacteria capable of causing mild to severe diseases in humans. Spotted fever group (SFG) organisms, which represent one of the two major groups of rickettsiae, are found globally in their characteristic arthropod vectors and are often classified to reflect the location of their predominant geographic distribution, for example, Rickettsia sibirica and R. australis, or the resultant human disease, such as Rocky Mountain spotted fever and Mediterranean spotted fever, caused by R. rickettsii and R. conorii, respectively (27, 47). Rocky Mountain spotted fever is a life-threatening illness and can be fatal in children, the elderly, and immunodeficient individuals if there is a lack of early detection and timely intervention with suitable antibiotic therapy. Yet another recently realized and alarming epidemiologic aspect of this disease is that tick-to-human transmission can occur not only through the previously identified principal vectors of Dermacentor species, but also through the more commonly distributed brown dog tick, Rhipicephalus sanguineus, suggesting the capability of this obligate intracellular pathogen to adapt to new ecological niches (12, 14).
The endothelial cell lining forms a multifunctional semipermeable barrier at the interface between vascular and perivascular compartments and plays a central role in maintaining tissue homeostasis and establishing communications between circulating blood and underlying tissues via regulation of vascular tone and permeability, interactions with circulating inflammatory cells, and other essential vessel wall functions. Due to the vasculotropic nature of invading rickettsiae, a majority of clinical characteristics of resulting diseases are attributed to disseminated infection of the endothelium, and typical pathological features represent the consequences of damage to the vasculature and endothelial dysfunction (46). In conjunction with case reports of patients suffering from rickettsioses, accumulating evidence from studies performed using cultured human endothelial cells (EC) infected with different species of SFG rickettsiae and animal models of infection clearly implicates a crucial role for oxidative stress and antioxidant defense enzyme systems in the pathophysiology of these infections (15, 16, 31). In this context, it has also been demonstrated that HO-1, the inducible form of the antioxidant and vasoprotective enzyme heme oxygenase (3), responds to R. rickettsii infection in vitro as well as in vivo (17, 30). One of the most critical regulatory functions of HO-1 in the vasculature is to control the activity of the cyclooxygenase (COX) system (21, 24), which is responsible for the generation of a number of vasoactive substances, including prostaglandins (PGs), prostacyclin, and thromboxanes (11).
Of the COX isoforms that have been characterized thoroughly, COX-1 is constitutively expressed in various types of cells, including EC. COX-2, on the other hand, is an inducible isoform sensitive to a number of stimuli, such as mitogens, cytokines, and lipopolysaccharide. Although interactions of typhus group rickettsiae with host EC have been shown to trigger enhanced secretion of PG metabolites (48), the mechanisms underlying these responses remain to be elucidated. The objective of the present study was to test the hypothesis that induction of COX is a major component of transcriptional activation in host cells and likely governs the pattern of synthesis/secretion of prostaglandins in response to infection. To address this issue, we investigated the expression of COX isozymes by host EC after infection with two prototype species of SFG rickettsiae, namely, R. rickettsii and R. conorii. The effects of a broad-spectrum COX inhibitor and selective inhibition of COX-2 on the level of PG secretion by infected cells were also examined. The data presented suggest a significantly increased expression of COX-2 in host EC infected with R. rickettsii or R. conorii and clearly indicate that the elevated secretion of PGs in response to infection is dependent on the enhanced COX-2 activity.
(A portion of this study was presented as an abstract at the 4th International Conference on Rickettsiae and Rickettsial Diseases, Logroño [La Rioja], Spain, 18 to 21 June 2005.)
MATERIALS AND METHODS
Isolation, culture, treatment, and infection of EC.
Human umbilical vein EC, an established model cell type that has been used to investigate in vitro rickettsia-endothelium interactions (39) and a number of different host cell responses (8, 9, 13, 15, 22, 30, 32, 34, 37-42), were isolated from freshly collected umbilical cords by collagenase digestion and then seeded on gelatin (2% [wt/vol])-coated cell culture plates as described previously (33). Primary cultures were allowed to grow to confluence in McCoy's medium supplemented with 20% fetal bovine serum, heparin (100 μg/ml), and endothelial cell growth supplement (50 μg/ml), at which point they were routinely split at a ratio of 1:3. For all experiments, cells at passage 2 were infected with R. rickettsii (Sheila Smith strain) or R. conorii (Malish 7 strain), using approximately 6 × 104 PFU of infective bacteria for every cm2 of culture area. In studies to investigate viability requirements, aliquots of rickettsial stocks containing equivalent numbers of organisms were either subjected to heat treatment or incubated with formaldehyde as reported previously (30). To inhibit de novo transcription or protein synthesis and the activities of COX isozymes, cells were incubated with appropriate specific inhibitors for 30 min prior to infection.
Analysis of COX-1 and COX-2 expression.
RNAs from infected EC and corresponding controls for each time point were prepared using the Tri-reagent protocol (Molecular Research Inc., Cincinnati, OH). Semiquantitative PCR analysis was carried out according to previously published procedures, using COX-1- and COX-2-specific primers, with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the internal control (34). For absolute quantitation of changes in mRNA expression levels, 5 to 8 μg of total RNA was subjected to Northern blot analysis as described previously (8), using radioactively labeled cDNA templates designed for specific detection of COX-1 and COX-2 transcripts (Cayman Chemical Co., Ann Arbor, MI). The differences in sample loading among lanes were corrected by stripping and reprobing of the blots with a GAPDH probe (8).
Total protein lysates for immunoblotting were prepared in sodium dodecyl sulfate-containing denaturing buffer supplemented with an inhibitor cocktail to prevent the action of proteases (Sigma, St. Louis, MO). Equal amounts of protein from various experimental samples were subjected to Western blotting, and nitrocellulose membranes were incubated with COX-1- or COX-2-specific antibodies (Cayman Chemical) at a 1:500 dilution in a 5% milk solution. The bands were visualized by a chemiluminescence-based detection approach after incubation with a compatible horseradish peroxidase-linked secondary antibody. The blots were then stripped by a thorough washing with 0.2 N NaOH solution for 30 min at room temperature and were reprobed with a monoclonal antibody against α-tubulin (Accurate Chemical, Westbury, NY).
Measurement of release of COX metabolites.
For PG release assays, culture supernatants were collected and centrifuged at 12,000 × g for 5 min at 4°C to remove any particulates. Aliquots of supernatants were retained in fresh tubes at −80°C for batched analysis of PGI2 and PGE2. The PGE2 and 6-keto-PGF1α (a stable metabolite of PGI2) contents were then estimated by a colorimetry-based competitive one-step enzyme immunoassay (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. All samples were assayed at least in duplicate, and average optical density (OD) readings were used to determine the PG concentration. For each experiment, standard curves (3.2 to 50,000 pg/ml of 6-keto-PGF1α and 39 to 2,500 pg/ml of PGE2) were constructed by plotting OD readings (corrected by subtracting nonspecific binding) and % B/B0 values (the ratio of the standard or sample OD reading to that of maximum binding × 100) against the log10 PG concentration. The sensitivities of the 6-keto-PGF1α and PGE2 assay kits were <1.4 and <13.4 pg/ml, respectively. The accuracy of the assay systems was also tested by adding progressively increasing quantities of the specific PGs to the culture medium and ensuring that the assays were able to quantify the added PGs in a linear fashion. The percent coefficients of variation for intra- and interassay precision for the 6-keto-PGF1α measurement kit were 6.9 and 6.4, respectively, and those for the PGE2 assay were 10.7 and 4.0, respectively. The results were calculated using SOFTmax Pro, version 1.1, which is capable of generating a four-parameter logistic curve fit (Molecular Devices, Sunnyvale, CA), and Microsoft Excel 2002, version SP-2.
Ex vivo model of endothelial infection.
Human umbilical cords collected immediately after Caesarean section deliveries were cut aseptically to an appropriate length, perfused with McCoy's 5a medium containing 1% fetal bovine serum, cannulated, and infected with viable R. rickettsii as described by Courtney et al. (10). About 1-cm-thick segments of both uninfected (control) and infected cords distal to the cannulated ends were obtained at different times by tying of the distal end with suture silk and gentle severing. Cord sections of about 2 to 3 mm in thickness were then fixed in 10% neutral buffered formalin and embedded in paraffin.
Immunohistochemistry and imaging.
Thin tissue sections (5 μm) were mounted onto glass slides, deparaffinized, rehydrated with xylene and a series of ethanol, and immersed in 3% aqueous hydrogen peroxide solution for 30 min to inhibit endogenous peroxidase activity. After being blocked with 5% normal goat serum for 20 min, the sections were incubated with anti-human COX-2 antibody (Cayman Chemical, Ann Arbor, MI) at a 1:400 dilution, followed by detection with the Vectastain ABC-peroxidase system (Vector Laboratories, Burlingame, CA). Immunostaining of HO-1 was carried out by using rabbit anti-HO-1 as the primary antibody (Stressgen, Victoria, British Columbia, Canada), and labeling was done using a universal DakoCytomation LSAB plus peroxidase kit (Dako, Carpinteria, CA). Immunohistochemical staining of von Willebrand factor (vWF) was performed using anti-human vWF and the EPOS peroxidase procedure (Dako). Staining for rickettsiae was conducted using rabbit anti-R. rickettsii antibody (a gift from T. Hackstadt, NIAID Rocky Mountain Laboratories, Hamilton, MT) according to our established protocols (10, 31). All sections were counterstained with hematoxylin. An Olympus Vanox-T microscope with a Diagnostic SPOT camera was used to capture the images, and Image-Pro Plus, version 3.0, was utilized for analysis. Coded slides were subjected to independent pathological evaluation without prior knowledge of the treatment(s), and the intensity of staining was scored on a scale of 0 to 4. Following this evaluation, quantitative image analysis of COX-2 immunohistochemistry was carried out by (i) optimizing the program settings and choice of density filters and (ii) selecting at least four different irregular areas of interest on the vascular endothelium, using the freehand tool feature of Image-Pro Plus. Within the same area, COX-2-positive cells (identified by brown staining) and hematoxylin- and eosin-stained cells with blue nuclei were then counted. The intensity of COX-2 staining for each experimental condition was determined as the ratio of COX-2-positive cells per μm2 to the total number of cells, represented by the sum of brown and blue objects.
Densitometric and statistical analyses.
For quantitative analysis, band intensities were estimated in densitometric units by using an HP Scanjet image scanner at a resolution of 600 dpi in conjunction with ImageQuant software, version 3.3 (Molecular Dynamics, Sunnyvale, CA). Data were calculated as means ± standard errors for at least three independent experiments, and comparisons between the study and control groups were done by Student's t test. P values of ≤0.05 were considered statistically significant.
RESULTS
In vitro infection with spotted fever rickettsiae induces COX-2 but not COX-1 expression.
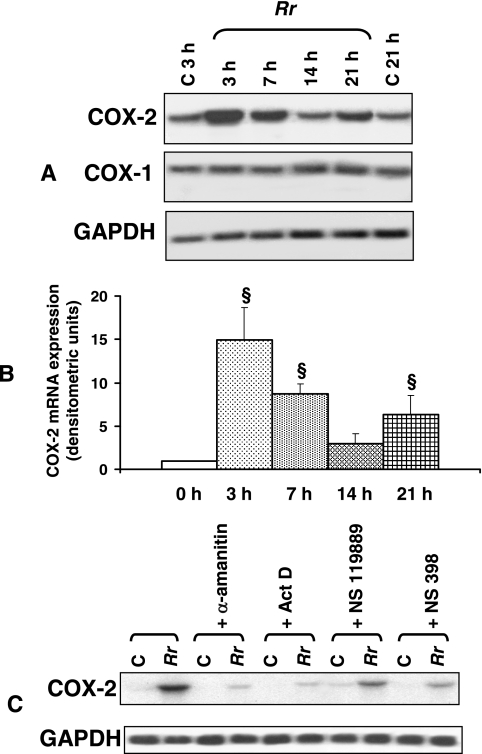
The unstimulated EC used in this study were found to express detectable mRNA levels of the constitutive isoform COX-1 by Northern blot analysis, whereas very minimal or nearly undetectable expression of COX-2 transcripts was seen, as expected. Cells infected with R. rickettsii or R. conorii for 1.5 or 3 h, on the other hand, exhibited dramatically increased expression of COX-2 mRNA, while that of COX-1 mRNA remained relatively unaltered. Semiquantitative reverse transcription-PCR analysis using human COX-1- and COX-2-specific primers, with GAPDH as a housekeeping control, confirmed these results, suggesting a selective induction of COX-2 in infected cells (not shown). Time course studies further revealed that the initial peak of induction at 3 h (15 ± 4 times higher than that in corresponding uninfected controls) and the subsequent return to close to the baseline at 14 h were followed by an apparent secondary response, as evidenced by increased COX-2 expression (7- ± 2-fold) at 21 h postinfection (Fig. 1A and B). Infection in the presence of cell-permeating inhibitors of eukaryotic RNA polymerases (α-amanitin and actinomycin D) and protein arginine and lysine methyltransferases (NSC 119889) resulted in significant reductions, of ≥75% and ≥50%, respectively, in the intensity of R. rickettsii-induced COX-2 expression, suggesting a requirement of de novo transcription and protein synthesis (Fig. 1C). Interestingly, the presence of NS 398, added to specifically inhibit COX-2 activity, was also able to diminish the activation of COX-2 mRNA expression during R. rickettsii infection, implicating the possible involvement of a negative feedback mechanism. Manipulation of host-Rickettsia interactions via treatment of EC with cytochalasin B to inhibit rickettsial uptake (26, 41) or by rendering rickettsiae inactive by heat treatment or incubation with formalin (30, 31) suggested that invasion with viable infectious organisms is essential for triggering the COX-2 response in host EC (data not shown).
FIG. 1.
Time course of R. rickettsii-induced COX-2 expression in endothelial cells. (A) Northern analysis of RNAs isolated from uninfected EC at 3 and 21 h (C, control) and from cells infected with R. rickettsii (Rr) for 3, 7, 14, and 21 h. Blots were probed in succession with 32P-labeled human-specific COX-2, COX-1, and GAPDH (housekeeping gene) cDNA probes, and the results of a typical representative experiment are shown. (B) The steady-state levels of COX-2 mRNA in R. rickettsii-infected EC at different times postinfection were normalized to that of GAPDH and compared with the average values of basal levels in simultaneously cultured cells that were left uninfected. The mean baseline COX-2 expression level in each experiment was assigned a value of 1. The results are presented as means ± standard errors for a minimum of three independent experiments, and statistically significant changes from uninfected controls (0 h) are indicated by the symbol §. (C) Effects of the transcriptional inhibitors α-amanitin (5 μg/ml) and actinomycin D (Act D; 0.25 μg/ml), the protein synthesis blocker NSC 119889 (20 μM), and the selective COX-2 inhibitor NS 398 (50 μM) on R. rickettsii-induced COX-2 mRNA expression. C, uninfected controls; Rr, R. rickettsii-infected EC.
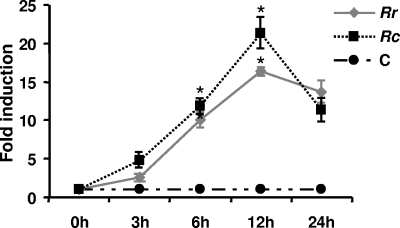
To verify that the induction of endothelial COX-2 mRNA by spotted fever rickettsiae is followed by an increase in steady-state protein levels, lysates from EC infected with R. rickettsii and R. conorii for 3, 6, 12, and 24 h and corresponding uninfected controls were processed for determinations of protein content by Western blotting (data not shown). The time points for this aspect of the study were adjusted to accommodate for intermediate steps in the translation process that may lead to a lag time between the induction of COX-2 mRNA and that of the protein in vascular endothelial cells (6, 35). The detailed densitometric analysis depicted in Fig. 2 revealed that COX-2 protein levels in R. rickettsii- and R. conorii-infected cells were significantly higher than the basal levels at all investigated time points. Indirect immunofluorescence staining of cells infected and fixed on coverslips also suggested a similar pattern of COX-2 activation following infection (not shown).
FIG. 2.
Densitometric data obtained from immunoblot analyses using COX-2-specific antibody and normalized for loading variations by stripping and probing of the blots with a monoclonal anti-tubulin antibody (n ≥ 3) were plotted as mean levels of induction ± standard errors relative to the control (C). Rr, R. rickettsii; Rc, R. conorii. *, P ≤ 0.05.
Enhanced production and release of eicosanoids by Rickettsia-infected EC are dependent on increased COX-2 activity.
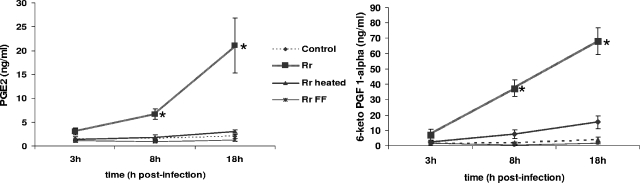
Figure 3 shows time-release profiles for PGE2 and 6-keto-PGF1α (the main hydrolysis product of PGI2) during R. rickettsii infection. A pattern of time-dependent increases in PG release into the culture medium of infected cells was clearly evident compared with the case for control (uninfected) monolayers. Although enhanced secretion of both PGE2 and PGI2 was noticed as early as 3 h after infection (about 2- and 5-fold higher levels of PGE2 and 6-keto-PGF1α, respectively, than those in the corresponding time point controls), the differences at 8 h (4- and 20-fold induction, respectively) and 18 h (10- and 17-fold induction, respectively) postinfection were statistically significant. As shown in Fig. 3, incubation of EC with heat-inactivated or formalin-fixed rickettsiae produced either very minimal or no stimulation of PG synthesis and release (Fig. 3). Exposure of EC to R. conorii also triggered striking increases in the secretion of both of these prostaglandins, with kinetics similar to that in R. rickettsii. Furthermore, the induced release of PGE2 and PGI2 from cells infected with both species of rickettsiae was completely prevented by treatment with NS 398 (a COX-2-specific inhibitor) or indomethacin (a broad-spectrum COX inhibitor), as evidenced by no statistically significant differences in the levels of secretion for uninfected cells treated with 10 μM NS 398 or 50 μM indomethacin alone and cells infected in the presence of inhibitors (data not shown). The concentrations of NS 398 and indomethacin were determined on the basis of their respective 50% inhibitory concentrations of 1.77 ± 0.55 and 24.6 ± 13.0 μM for human COX-2 (5) and the further demonstration that NS 398 effectively inhibits COX-2-dependent synthesis of arachidonic acid metabolites in the concentration range of 1 to 50 μM (23).
FIG. 3.
Enhanced secretion of prostaglandins by R. rickettsii-infected EC. The levels of PGE2 and 6-keto-PGF1α in culture supernatants collected from uninfected controls and infected cells (Rr) at the indicated times were measured by an enzyme-linked immunosorbent assay. Also shown is a comparison of the effects of host cell interactions with viable (Rr) and heat-inactivated (Rr heated) or formalin-fixed (Rr FF) rickettsiae. Data points represent accumulated PG concentrations as means ± standard errors from at least three independent experiments. *, significant increase (P ≤ 0.05) in comparison to the baseline control level.
Signaling through p38 MAP kinase, but not through the ERK module, mediates R. rickettsii-induced COX-2 mRNA expression.
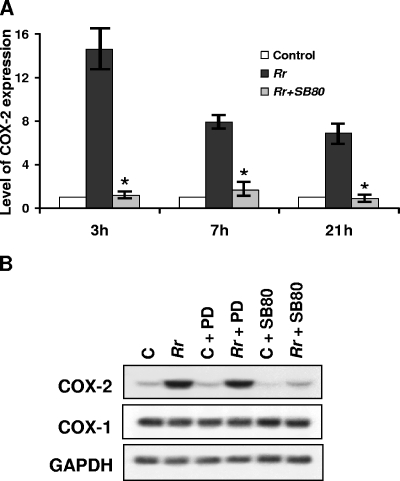
To examine the potential involvement of signal transduction through the p38 mitogen-activated protein (MAP) kinase pathway, which has been shown to be activated during R. rickettsii infection (32), EC were infected in the presence and absence of a p38-specific inhibitor, SB 203580, as well as its inactive structural analog, SB 202474. R. rickettsii-induced COX-2 expression was dramatically attenuated in cells infected in the presence of SB 203580, indicating a dependence on p38 activity (Fig. 4A). Infection in the presence of SB 202474, on the other hand, had no significant effect on the level of COX-2 mRNA, further suggesting that upstream signaling mechanisms responsible for increased COX-2 expression during infection are mediated by the p38 signaling pathway (Fig. 4A and B). Since R. rickettsii infection of EC is unable to induce c-Jun N-terminal protein kinase activation (32), we did not investigate the involvement of this MAP kinase module. Inhibition of extracellular signal-regulated kinase (ERK) signaling by PD 98059 treatment prior to and during infection, however, had no effect on the level of COX-2 expression (Fig. 4B).
FIG. 4.
Inhibition of p38 MAP kinase activity decreases R. rickettsii-induced COX-2 expression. (A) Detailed densitometric analysis of COX-2 expression during R. rickettsii infection (Rr) in the presence and absence of a specific p38 MAP kinase inhibitor, SB 203580 (SB80). The results were calculated as means ± standard errors from three independent observations. *, significant reduction (P ≤ 0.05) in COX-2 mRNA level compared to that in infected cells alone, i.e., in the absence of the p38 inhibitor SB 203580. (B) Comparison of the effects of selective inhibition of ERK and p38 MAP kinase pathways via PD 98059 (PD; 10 μM) and SB 203580 (SB80; 10 μM), respectively, on the COX-2 mRNA level in uninfected (C) and R. rickettsii-infected (Rr) endothelial cells.
High levels of COX-2 expression in an ex vivo model of infection.
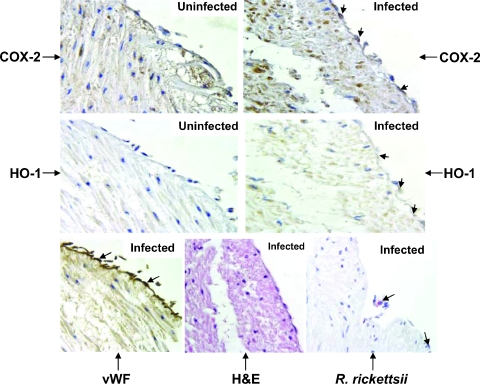
We next determined whether R. rickettsii infection of the intact vascular endothelium lining the human umbilical vein stimulates COX-2 expression in the target cell type, i.e., endothelial cells. Immunohistochemical staining of sections of infected umbilical cords along with uninfected controls revealed that immunostaining for COX-2 in infected endothelia was more intense and more abundant than that in simultaneously processed controls. Because R. rickettsii infection of cultured human EC also induces the expression of HO-1, an isoform of HO, we stained adjacent serial sections for the expression of HO-1. Microscopic examination suggested an enhanced immunoreactivity of EC for HO-1 in infected cords, whereas baseline expression in uninfected endothelium was negligible (Fig. 5). Although endothelial cells were positive for the physiologic marker vWF under all experimental conditions, differences in staining intensity likely reflecting an enhanced release of vWF from Weibel-Palade bodies, as reported earlier for in vitro infection (40), were also clearly evident (Fig. 5 and Table 1). Positive staining for rickettsial antigen was evident in the vasculature of infected cords (Fig. 5), and Rickettsia organisms were seen as discrete, punctate entities (10). Pathological examination and quantification of the relative intensities of COX-2 and HO-1 in control and infected endothelia ascertained a significant enhancement in their immunostaining at different times postinfection (Table 1). Quantitative image analysis also revealed a time-dependent increase in COX-2 positivity in infected endothelia in comparison to that in uninfected controls. The number of COX-2-positive cells in the infected endothelium was increased by 18% at 8 h (0.0050 ± 0.0005 [controls] versus 0.0059 ± 0.0008 per μm2) but was significantly larger at 12 h (0.0050 ± 0.0005 versus 0.0102 ± 0.0009 per μm2; P = 0.005) and 24 h (0.0050 ± 0.0005 versus 0.0107 ± 0.0013 per μm2; P = 0.016) postinfection. Taken together, these results demonstrate that intact human endothelium challenged with invasive R. rickettsii exhibits an inflammatory oxidative stress response characterized by increased expression of COX-2 and HO-1, and they suggest the involvement of these enzymes in determining the host cell response(s) to Rickettsia infection.
FIG. 5.
Immunohistochemical detection of COX-2 and HO-1 in R. rickettsii-infected endothelia from intact human umbilical veins. The serial sections of uninfected controls and infected cord specimens were stained with COX-2- and HO-1-specific antibodies. Sections from infected cords were stained for vWF and rickettsial antigen (indicated by large arrows) as described in Materials and Methods. Counterstaining with hematoxylin and eosin (H&E) was also carried out. Positive COX-2 (dark brown) and HO-1 (light brown) staining was predominantly detected in the endothelial cells of infected vasculature and is indicated by small arrows. Positive COX-2 staining in some inflammatory and mesenchymal cells of infected cord specimens was also seen. Magnification, ×400.
TABLE 1.
Scores related to histological and immunohistochemical examination of endothelia infected with R. rickettsii and corresponding control tissues
| Treatmenta | Time (h) | Scoreb
|
||||
|---|---|---|---|---|---|---|
| COX-2 | HO-1 | vWF | Rickettsia stain | Hematoxylin and eosin stain | ||
| Control | 4 | + | − | +++ | − | − |
| 1× | 4 | + | − | ++ | + | − |
| 5× | 4 | ++++ | − | ++ | +++ | − |
| Control | 8 | − | − | +++ | − | − |
| 1× | 8 | ++ | + | ++ | + | − |
| 5× | 8 | ++++ | − | + | +++ | − |
| Control | 12 | + | − | +++ | − | − |
| 1× | 12 | ++++ | + | + | + | − |
| 5× | 12 | ++++ | ++ | ++ | +++ | + |
| Control | 24 | + | + | +++ | − | − |
| 1× | 24 | ++ | ++ | ++ | ++ | − |
| 5× | 24 | ++ | ++ | +++ | ++++ | ++ |
Cords were perfused with medium alone (control) or medium inoculated with either 5 × 105 PFU/ml (1×) or 2.5 × 106 PFU/ml (5×) of R. rickettsii for the indicated times.
−, negative staining; positive staining was rated on a scale of 1 (+ [weakly positive]) to 4 (++++ [strongly positive]).
DISCUSSION
The endothelial lining of the inner surfaces of blood vessels is specifically targeted by pathogenic rickettsiae during human infections. Therefore, the most prominent pathophysiologic effects of these infections include changes in vessel wall permeability leading to fluid imbalance (46, 47), increased leukocyte-endothelium interactions (44), a switch from a nonthrombogenic endothelial surface to a procoagulant and proinflammatory phenotype (13, 37, 42), and the release of powerful vasoactive mediators (9, 22), cumulatively described as generalized vascular inflammation. Earlier studies of in vitro interactions between EC, monocytes, or macrophages and the typhus group species R. prowazekii showed that infected host cells synthesize and/or secrete significantly larger amounts of leukotrienes, platelet activating factor, and prostaglandins (48-50). The present study demonstrates that transcriptional activation of host EC in response to stimulation with the spotted fever group Rickettsia species R. rickettsii and R. conorii involves rapid and robust up-regulation of COX-2 expression and that inhibition of COX-2 activity during infection abrogates the enhanced secretion of prostaglandins. Expression of the constitutive isoform, COX-1, however, remained unaffected at all examined time points. We also identified upstream signaling through p38 MAP kinase as an important determinant of infection-induced COX-2 expression.
COX is the initial rate-limiting enzyme responsible for the oxidative breakdown of arachidonic acid (AA) to PGH2, an intermediary metabolite which is subsequently converted into a series of biologically active PGs in a cell type-specific manner. Several studies have suggested COX-2 as an important factor in gastric carcinoma associated with Helicobacter pylori infection (7, 53), and its roles in the diseases caused by obligate intracellular bacteria of Chlamydia species and pathogenic spirochetes of Borrelia species have begun to be elucidated only recently (1, 18, 28). Although the status of COX isozymes in EC infected with typhus rickettsiae is currently under investigation, evidence derived from the presented data and earlier observations (48, 50) suggest significant increases in the production of the major endothelial prostaglandins PGE2 and PGI2 during infection and implicate COX-2 and its reaction metabolites as having an integral role in the pathophysiology of rickettsial diseases. In this context, comparative studies with highly pathogenic versus avirulent species of SFG rickettsiae or with individual strains with various degrees of virulence, for example, R. rickettsii isolates that have been characterized for their ability to cause oxidative stress in EA.hy 926 endothelial cells (16) or R. prowazekii strains at opposing ends of the virulence spectrum (19), to define potential differences in the intensity and kinetics of COX-2-mediated host responses will yield important new information about virulence determinants and pathogenic mechanisms. While our initial studies suggested that in vitro infections of endothelial cells with the virulent Sheila Smith and avirulent Iowa B strains trigger COX-2 mRNA and protein expression (E. Rydkina and S. K. Sahni, unpublished observations), a detailed investigation of the correlation between the extent of infection, host cell response, and cytopathic potential of different rickettsial species and strains is currently in progress. It follows that PGs are locally acting autocoids with pleiotropic roles in both physiologic and pathologic states and that the regulation of their synthesis and secretion constitutes an important aspect of vascular homeostasis. PGE2 and PGI2 released by EC are vasodilatory PGs and may synergize with other vasoactive mediators to cause increased vascular permeability and edema, which are thought to be the cardinal features of inflammation during rickettsial infections. Among the major organ systems of the host affected are the lungs and brain, manifesting as interstitial pneumonia, acute respiratory distress syndrome, and neurological deficits leading to seizures and coma (44-46). It is essential to keep in mind in this context that treatment of brain microvascular endothelial cells with PGE2 and PGF2α induces significant increases in permeability and changes in cytoskeletal organization (25). Another intriguing possibility, which has yet to be explored in further detail, is that similar to interactions between host cells and viruses or pathogenic bacteria, such as Chlamydia and Salmonella (43, 51, 55), PGs secreted by infected EC may also function to support or augment the growth and replication of intracellular rickettsiae.
Although several protein kinases with unique specificities for serine or threonine residues are expressed in human vascular EC, only selective isoforms of protein kinase C and p38 MAP kinase have so far been established to play a crucial role in the regulation of cellular functions (32, 34). Our results suggesting attenuation of R. rickettsii-induced COX-2 expression by a specific inhibitor of p38 MAP kinase (SB 203580) extend our recent findings that signaling through this particular MAP kinase module promotes changes in the level of chemokine expression/secretion and demonstrate that p38 activation may also act as an important and selective regulator of PG synthesis. Whereas a decline in COX-2 transcription between 7 and 14 h postinfection correlates well with the kinetics of p38 phosphorylation, more instability due to multiple copies of Shaw-Kamen pentamer AUUUA repeats in the 3′-untranslated region may also contribute to the disappearance of COX-2 mRNA (2, 36). The possibility that the regulation of COX-2 expression during Rickettsia infection of endothelial cells involves posttranscriptional control mechanisms, a precedent of which was reported earlier for plasminogen activator inhibitor 1 expression in R. rickettsii-infected host cells (38), is the subject of further detailed investigation in our laboratory. Furthermore, higher levels of protein in cells infected for 6 and 12 h likely reflect the time delay between promoter activation, leading to enhanced transcription early during the infection, and subsequent synthesis of bioactive protein, as reported to occur during endothelial cell responses to hypoxia (35), activated protein C (6), and other physiological mediators (54). The transcription factor NF-κB has also been implicated variously in COX-2 stimulation by several factors, but neither PD98059 (ERK inhibitor) nor SB 203580 affects Rickettsia-induced activation of NF-κB (32). Selective regulation of p38 kinase may therefore offer an alternative mechanism for controlling rapid host cell responses to infection.
At least 11 species of rickettsiae have been reported to contain a pld gene in their genomes, and the phospholipase D (PLD) enzymes of R. conorii and R. prowazekii have been confirmed to have enzymatic activity and likely serve as virulence factors (29). More recently, rickettsial escape from the phagosomal vesicle has also been attributed to PLD activity (52). Since membrane-bound phospholipids are cleaved by phospholipases and host cells exposed to large numbers of R. prowazekii secrete considerable amounts of lysophospholipids and free fatty acids (20), it is reasonable to suspect that rickettsial phospholipase may also be involved in the provision of AA, the substrate for COX-2. The activation of host cell phospholipases in response to infection also remains a likely possibility because cytosolic PLA2 not only is an important source of AA but is also capable of activating secretory PLA2 in certain situations (4).
Using in vitro and ex vivo models of infection, this study demonstrates for the first time that SFG rickettsiae stimulate the expression of COX-2 in vascular endothelial cells. This host cell response requires interaction with viable rickettsiae and is associated with a marked increase in the production of prostanoids that may contribute to and explain certain characteristic pathological and clinical features of rickettsial diseases, such as pain, inflammation, fever, and vasculitis. Moreover, selective inhibition of Rickettsia-induced p38 kinase abolishes the activation of COX-2. Further studies are currently being undertaken to elucidate whether the products of COX-2 action could be responsible for supporting rickettsial replication and/or triggering infection-accelerated vascular inflammation.
Acknowledgments
We thank Loel C. Turpin and Semion Kiriakidi for excellent technical assistance and Linda Callahan and David Pasternack of the URMC Pathology/Morphology Imaging core facility for assistance with all imaging procedures.
This research was supported in part by USPHS grants AI 40689 and HL 30616 from the National Institutes of Health.
Editor: J. B. Bliska
REFERENCES
- 1.Anguita, J., S. Samanta, S. K. Ananthanarayanan, B. Revilla, G. P. Geba, S. W. Barthold, and E. Fikrig. 2002. Cyclooxygenase 2 activity modulates the severity of murine Lyme arthritis. FEMS Immunol. Med. Microbiol. 34:187-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appleby, S. B., A. Ristimaki, K. Neilson, K. Narko, and T. Hla. 1994. Structure of the human cyclo-oxygenase-2 gene. Biochem. J. 302:723-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bach, F. H. 2005. Heme oxygenase-1: a therapeutic amplification funnel. FASEB J. 19:1216-1219. [DOI] [PubMed] [Google Scholar]
- 4.Balsinde, J., M. A. Balboa, and E. A. Dennis. 1998. Functional coupling between secretory phospholipase A2 and cyclooxygenase-2 and its regulation by cytosolic group IV phospholipase A2. Proc. Natl. Acad. Sci. USA 95:7951-7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnett, J., J. Chow, D. Ives, M. Chiou, R. Mackenzie, E. Osen, B. Nguyen, S. Tsing, C. Bach, J. Freire, H. Chan, E. Sigal, and C. Ramesha. 1994. Purification, characterization and selective inhibition of human prostaglandin G/H synthase 1 and 2 expressed in the baculovirus system. Biochim. Biophys. Acta 1209:130-139. [DOI] [PubMed] [Google Scholar]
- 6.Brueckmann, M., S. Horn, S. Lang, K. Fukudome, A. S. Nahrup, U. Hoffmann, J. J. Kaden, M. Borggrefe, K. K. Haase, and G. Huhle. 2005. Recombinant human activated protein C upregulates cyclooxygenase-2 expression in endothelial cells via binding to endothelial cell protein C receptor and activation of protease-activated receptor-1. Thromb. Haemost. 93:743-750. [DOI] [PubMed] [Google Scholar]
- 7.Chan, F. K. 2003. COX-2 inhibition, Helicobacter pylori infection and the risk of gastrointestinal complications. Curr. Pharm. Des. 9:2213-2219. [DOI] [PubMed] [Google Scholar]
- 8.Clifton, D. R., E. Rydkina, R. S. Freeman, and S. K. Sahni. 2005. NF-κB activation during Rickettsia rickettsii infection of endothelial cells involves the activation of catalytic IκB kinases IKKα and IKKβ and phosphorylation-proteolysis of the inhibitor protein IκBα. Infect. Immun. 73:155-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clifton, D. R., E. Rydkina, H. Huyck, G. Pryhuber, R. S. Freeman, D. J. Silverman, and S. K. Sahni. 2005. Expression and secretion of chemotactic cytokines IL-8 and MCP-1 by human endothelial cells after Rickettsia rickettsii infection: regulation by nuclear transcription factor NF-κB. Int. J. Med. Microbiol. 295:267-278. [DOI] [PubMed] [Google Scholar]
- 10.Courtney, M. A., P. J. Haidaris, V. J. Marder, and L. A. Sporn. 1996. Tissue factor mRNA expression in the endothelium of an intact umbilical vein. Blood 87:174-179. [PubMed] [Google Scholar]
- 11.Davidge, S. T. 2001. Prostaglandin H synthase and vascular function. Circ. Res. 89:650-660. [DOI] [PubMed] [Google Scholar]
- 12.Demma, L. J., M. S. Traeger, W. L. Nicholson, C. D. Paddock, D. M. Blau, M. E. Eremeeva, G. A. Dasch, M. L. Levin, J. Singleton, Jr., S. R. Zaki, J. E. Cheek, D. L. Swerdlow, and J. H. McQuiston. 2005. Rocky Mountain spotted fever from an unexpected tick vector in Arizona. N. Engl. J. Med. 353:587-594. [DOI] [PubMed] [Google Scholar]
- 13.Drancourt, M., M. C. Alessi, P. Y. Levy, I. Juhan-Vague, and D. Raoult. 1990. Secretion of tissue-type plasminogen activator and plasminogen activator inhibitor by Rickettsia conorii- and Rickettsia rickettsii-infected cultured endothelial cells. Infect. Immun. 58:2459-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dumler, J. S., and D. H. Walker. 2005. Rocky Mountain spotted fever—changing ecology and persisting virulence. N. Engl. J. Med. 353:551-553. [DOI] [PubMed] [Google Scholar]
- 15.Eremeeva, M. E., G. A. Dasch, and D. J. Silverman. 2000. Interaction of rickettsiae with eukaryotic cells. Adhesion, entry, intracellular growth, and host cell responses. Subcell. Biochem. 33:479-516. [PubMed] [Google Scholar]
- 16.Eremeeva, M. E., G. A. Dasch, and D. J. Silverman. 2001. Quantitative analyses of variations in the injury of endothelial cells elicited by 11 isolates of Rickettsia rickettsii. Clin. Diagn. Lab. Immunol. 8:788-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eremeeva, M. E., Z. Liang, C. Paddock, S. Zaki, J. G. Vandenbergh, G. A. Dasch, and D. J. Silverman. 2003. Rickettsia rickettsii infection in the pine vole, Microtus pinetorum: kinetics of infection and quantitation of antioxidant enzyme gene expression by RT-PCR. Ann. N. Y. Acad. Sci. 990:468-473. [DOI] [PubMed] [Google Scholar]
- 18.Fukuda, E. Y., S. P. Lad, D. P. Mikolon, M. Iacobelli-Martinez, and E. Li. 2005. Activation of lipid metabolism contributes to interleukin-8 production during Chlamydia trachomatis infection of cervical epithelial cells. Infect. Immun. 73:4017-4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ge, H., Y.-Y. E. Chuang, S. Zhao, M. Tong, M.-H. Tsai, J. J. Temenak, A. L. Richards, and W.-M. Ching. 2004. Comparative genomics of Rickettsia prowazekii Madrid E and Breinl strains. J. Bacteriol. 186:556-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghannoum, M. A. 2000. Potential role of phospholipases in virulence and fungal pathogenesis. Clin. Microbiol. Rev. 13:122-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haider, A., R. Olszanecki, R. Gryglewski, M. L. Schwartzman, E. Lianos, A. Kappas, A. Nasjletti, and N. G. Abraham. 2002. Regulation of cyclooxygenase by the heme-heme oxygenase system in microvessel endothelial cells. J. Pharmacol. Exp. Ther. 300:188-194. [DOI] [PubMed] [Google Scholar]
- 22.Kaplanski, G., N. Teysseire, C. Farnarier, S. Kaplanski, J. C. Lissitzky, J. M. Durand, J. Soubeyrand, C. A. Dinarello, and P. Bongrand. 1995. IL-6 and IL-8 production from cultured human endothelial cells stimulated by infection with Rickettsia conorii via a cell-associated IL-1 alpha-dependent pathway. J. Clin. Investig. 96:2839-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kundu, N., Q. Yang, R. Dorsey, and A. M. Fulton. 2001. Increased cyclooxygenase-2 (cox-2) expression and activity in a murine model of metastatic breast cancer. Int. J. Cancer 93:681-686. [DOI] [PubMed] [Google Scholar]
- 24.Kushida, T., G. Li Volti, S. Quan, A. Goodman, and N. G. Abraham. 2002. Role of human heme oxygenase-1 in attenuating TNF-α-mediated inflammation injury in endothelial cells. J. Cell. Biochem. 87:377-385. [DOI] [PubMed] [Google Scholar]
- 25.Mark, K. S., W. J. Trickler, and D. W. Miller. 2001. Tumor necrosis factor-α induces cyclooxygenase-2 expression and prostaglandin release in brain microvessel endothelial cells. J. Pharmacol. Exp. Ther. 297:1051-1058. [PubMed] [Google Scholar]
- 26.Martinez, J. J., and P. Cossart. 2004. Early signaling events involved in the entry of Rickettsia conorii into mammalian cells. J. Cell Sci. 117:5097-5106. [DOI] [PubMed] [Google Scholar]
- 27.Parola, P., C. D. Paddock, and D. Raoult. 2005. Tick-borne rickettsioses around the world: emerging diseases challenging old concepts. Clin. Microbiol. Rev. 18:719-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasley, A., I. Marriott, C. R. Halberstadt, K. L. Bost, and J. Anguita. 2004. Substance P augments Borrelia burgdorferi-induced prostaglandin E2 production by murine microglia. J. Immunol. 172:5707-5713. [DOI] [PubMed] [Google Scholar]
- 29.Renesto, P., P. Dehoux, E. Gouin, L. Touqui, P. Cossart, and D. Raoult. 2003. Identification and characterization of a phospholipase D-superfamily gene in rickettsiae. J. Infect. Dis. 188:1276-1283. [DOI] [PubMed] [Google Scholar]
- 30.Rydkina, E., A. Sahni, D. J. Silverman, and S. K. Sahni. 2002. Rickettsia rickettsii infection of cultured human endothelial cells induces heme oxygenase 1 expression. Infect. Immun. 70:4045-4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rydkina, E., S. K. Sahni, L. A. Santucci, L. C. Turpin, R. B. Baggs, and D. J. Silverman. 2004. Selective modulation of antioxidant enzyme activities in host tissues during Rickettsia conorii infection. Microb. Pathog. 36:293-301. [DOI] [PubMed] [Google Scholar]
- 32.Rydkina, E., D. J. Silverman, and S. K. Sahni. 2005. Activation of p38 stress-activated protein kinase during Rickettsia rickettsii infection of human endothelial cells: role in the induction of chemokine response. Cell. Microbiol. 7:1519-1530. [DOI] [PubMed] [Google Scholar]
- 33.Sahni, S. K., D. J. Van Antwerp, M. E. Eremeeva, D. J. Silverman, V. J. Marder, and L. A. Sporn. 1998. Proteasome-independent activation of nuclear factor κB in cytoplasmic extracts from human endothelial cells by Rickettsia rickettsii. Infect. Immun. 66:1827-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sahni, S. K., L. C. Turpin, T. L. Brown, and L. A. Sporn. 1999. Involvement of protein kinase C in Rickettsia rickettsii-induced transcriptional activation of the host endothelial cell. Infect. Immun. 67:6418-6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmedtje, J. F., Jr., Y.-S. Ji, W.-L. Liu, R. N. DuBois, and M. S. Runge. 1997. Hypoxia induces cyclooxygenase-2 via the NF-κB p65 transcription factor in human vascular endothelial cells. J. Biol. Chem. 272:601-608. [DOI] [PubMed] [Google Scholar]
- 36.Shaw, G., and R. Kamen. 1986. A conserved AU sequence from the 3′ untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell 46:659-667. [DOI] [PubMed] [Google Scholar]
- 37.Shi, R. J., P. J. Simpson-Haidaris, N. B. Lerner, V. J. Marder, D. J. Silverman, and L. A. Sporn. 1998. Transcriptional regulation of endothelial cell tissue factor expression during Rickettsia rickettsii infection: involvement of the transcription factor NF-κB. Infect. Immun. 66:1070-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi, R. J., P. J. Simpson-Haidaris, V. J. Marder, D. J. Silverman, and L. A. Sporn. 2000. Post-transcriptional regulation of endothelial cell plasminogen activator inhibitor-1 expression during Rickettsia rickettsii infection. Microb. Pathog. 28:127-133. [DOI] [PubMed] [Google Scholar]
- 39.Silverman, D. J. 1984. Rickettsia rickettsii-induced cellular injury of human vascular endothelium in vitro. Infect. Immun. 44:545-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sporn, L. A., R. J. Shi, S. O. Lawrence, D. J. Silverman, and V. J. Marder. 1991. Rickettsia rickettsii infection of cultured endothelial cells induces release of large von Willebrand factor multimers from Weibel-Palade bodies. Blood 78:2595-2602. [PubMed] [Google Scholar]
- 41.Sporn, L. A., S. K. Sahni, N. B. Lerner, V. J. Marder, D. J. Silverman, L. C. Turpin, and A. L. Schwab. 1997. Rickettsia rickettsii infection of cultured human endothelial cells induces NF-κB activation. Infect. Immun. 65:2786-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teysseire, N., D. Arnoux, F. George, J. Sampol, and D. Raoult. 1992. von Willebrand factor release and thrombomodulin and tissue factor expression in Rickettsia conorii-infected endothelial cells. Infect. Immun. 60:4388-4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uchiya, K., and T. Nikai. 2004. Salmonella enterica serovar Typhimurium infection induces cyclooxygenase 2 expression in macrophages: involvement of Salmonella pathogenicity island 2. Infect. Immun. 72:6860-6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valbuena, G., W. Bradford, and D. H. Walker. 2003. Expression analysis of the T-cell-targeting chemokines CXCL9 and CXCL10 in mice and humans with endothelial infections caused by rickettsiae of the spotted fever group. Am. J. Pathol. 163:1357-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valbuena, G., and D. H. Walker. 2004. Effect of blocking the CXCL9/10-CXCR3 chemokine system in the outcome of endothelial-target rickettsial infections. Am. J. Trop. Med. Hyg. 71:393-399. [PubMed] [Google Scholar]
- 46.Walker, D. H., G. A. Valbuena, and J. P. Olano. 2003. Pathogenic mechanisms of diseases caused by Rickettsia. Ann. N. Y. Acad. Sci. 990:1-11. [DOI] [PubMed] [Google Scholar]
- 47.Walker, D. H. 2004. Ricketts creates rickettsiology, the study of vector-borne obligately intracellular bacteria. J. Infect. Dis. 189:938-955. [DOI] [PubMed] [Google Scholar]
- 48.Walker, T. S., J. S. Brown, C. S. Hoover, and D. A. Morgan. 1990. Endothelial prostaglandin secretion: effects of typhus rickettsiae. J. Infect. Dis. 162:1136-1144. [DOI] [PubMed] [Google Scholar]
- 49.Walker, T. S., M. W. Dersch, and W. E. White. 1991. Effects of typhus rickettsiae on peritoneal and alveolar macrophages: rickettsiae stimulate leukotriene and prostaglandin secretion. J. Infect. Dis. 163:568-573. [DOI] [PubMed] [Google Scholar]
- 50.Walker, T. S., and G. E. Mellott. 1993. Rickettsial stimulation of endothelial platelet-activating factor synthesis. Infect. Immun. 61:2024-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waris, G., and A. Siddiqui. 2005. Hepatitis C virus stimulates the expression of cyclooxygenase-2 via oxidative stress: role of prostaglandin E2 in RNA replication. J. Virol. 79:9725-9734. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52.Whitworth, T., V. L. Popov, X. J. Yu, D. H. Walker, and D. H. Bouyer. 2005. Expression of the Rickettsia prowazekii pld or tlyC gene in Salmonella enterica serovar Typhimurium mediates phagosomal escape. Infect. Immun. 73:6668-6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu, C. Y., C. J. Wang, C. C. Tseng, H. P. Chen, M. S. Wu, J. T. Lin, H. Inoue, and G. H. Chen. 2005. Helicobacter pylori promote gastric cancer cells invasion through a NF-κB and COX-2-mediated pathway. World J. Gastroenterol. 11:3197-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu, G., A. P. Mannam, J. Wu, S. Kirbis, J. L. Shie, C. Chen, R. J. Laham, F. W. Sellke, and J. Li. 2003. Hypoxia induces myocyte-dependent COX-2 regulation in endothelial cells: role of VEGF. Am. J. Physiol. Heart Circ. Physiol. 285:H2420-H2429. [DOI] [PubMed] [Google Scholar]
- 55.Yoneda, H., K. Miura, H. Matsushima, K. Sugi, T. Murakami, K. Ouchi, K. Yamashita, H. Itoh, T. Nakazawa, M. Suzuki, and M. Shirai. 2003. Aspirin inhibits Chlamydia pneumoniae-induced NF-κB activation, cyclo-oxygenase-2 expression and prostaglandin E2 synthesis and attenuates chlamydial growth. J. Med. Microbiol. 52:409-415. [DOI] [PubMed] [Google Scholar]