Abstract
Burkholderia mallei is a gram-negative bacterium which causes the potentially fatal disease glanders in humans; however, there is little information concerning cell-mediated immunity to this pathogen. The role of gamma interferon (IFN-γ) during B. mallei infection was investigated using a disease model in which infected BALB/c mice normally die between 40 and 60 days postinfection. IFN-γ knockout mice infected with B. mallei died within 2 to 3 days after infection, and there was uncontrolled bacterial replication in several organs, demonstrating the essential role of IFN-γ in the innate immune response to this pathogen. Increased levels of IFN-γ, interleukin-6 (IL-6), and monocyte chemoattractant protein 1 were detected in the sera of immunocompetent mice in response to infection, and splenic mRNA expression of IFN-γ, IL-6, IL-12p35, and IL-27 was elevated 24 h postinfection. The effects of IL-18, IL-27, and IL-12 on stimulation of the rapid IFN-γ production were investigated in vitro by analyzing IFN-γ production in the presence of heat-killed B. mallei. IL-12 was essential for IFN-γ production in vitro; IL-18 was also involved in induction of IFN-γ, but IL-27 was not required for IFN-γ production in response to heat-killed B. mallei. The main cellular sources of IFN-γ were identified in vitro as NK cells, CD8+ T cells, and TCRγδ T cells. Our data show that B. mallei is susceptible to cell-mediated immune responses which promote expression of type 1 cytokines. This suggests that development of effective vaccines against glanders should target the production of IFN-γ.
Burkholderia mallei is a gram-negative, nonmotile, aerobic, non-spore-forming bacillus and an obligate animal parasite. It is an intracellular pathogen that can cause the disease glanders in solipeds (horses, donkeys, and mules), humans (2, 18, 35), and experimental animals, such as hamsters (12) and mice (11). B. mallei is currently a potential bioterrorism threat (5) and causes severe disease in humans. Few natural cases of glanders have been reported; the last recorded human case was diagnosed in a U.S. Government laboratory worker following a systemic febrile illness (35). The general acute symptoms include high fever (2) and respiratory distress (35), as well as swollen lymph nodes and chest pain (18, 35). Abscesses in the liver and spleen are characteristic of the disease in both humans (35) and experimental mouse models of infection (unpublished observations).
Following aerosol challenge BALB/c mice develop a disease similar to acute human glanders (23). However, BALB/c mice are relatively resistant to B. mallei following intraperitoneal challenge (24) and develop an acute disease only with doses of ≥107 CFU (11). At lower doses, an infection similar to the infection observed in chronic human glanders develops. This infection is characterized by bacterial colonization of organs, including the spleen, liver, lungs, and blood (11), and survival for several months postinfection with the appearance of chronic foci of infection (24). Pathological changes to the liver and spleen and inflammatory infiltrates are observed early in the infection (11), and abscesses develop within 2 weeks after infection (unpublished observations). B. mallei is a close phylogenetic relative of Burkholderia pseudomallei, the causative agent of human melioidosis, an important public health problem in Southeast Asia and northern Australia (38). The immune response to B. pseudomallei has been well characterized, in contrast to the response to B. mallei, for which there is little information regarding host immune responses. It is not known whether there are parallels in the abilities of these two organisms to stimulate host immune responses.
Control of gamma interferon (IFN-γ) production is crucial for regulating cell-mediated immunity responses to intracellular infection (32). Following infection with the intracellular pathogens B. pseudomallei (16) and Listeria monocytogenes (30), host resistance is mediated by IFN-γ. In other infection models, interleukin-12 (IL-12), IL-18, and the newly discovered cytokine IL-27 are involved in IFN-γ production (31). IL-12, a proinflammatory cytokine released by macrophages during the early stages of infection, has an essential role in inducing production of IFN-γ from T cells and NK cells (14, 31). This cytokine is composed of two subunits (p35 and p40) and has been shown to be important in host defense against a range of intracellular pathogens, including Mycobacterium tuberculosis (10) and Leishmania major (17). In other intracellular infections, including Francisella tularensis infections (9), the p40 subunit but not the p35 subunit is important for protection against infection. IL-18, a proinflammatory cytokine secreted by activated antigen-presenting cells, acts synergistically with IL-12 to induce the production of IFN-γ during infection with a number of pathogens, including B. pseudomallei (16), L. monocytogenes (26), and Mycobacterium leprae (13). IL-18 is also essential in host defense during L. monocytogenes infection through IFN-γ-independent induction of NO and tumor necrosis factor alpha (TNF-α) from macrophages (26). IL-27, produced by antigen-presenting cells, is also involved in IFN-γ production from naïve CD4+ T cells in vitro and acts synergistically with IL-12 (29); however, little is known about its role in induction of IFN-γ in vivo.
The mechanisms of cell-mediated immunity to B. mallei are currently not known. Therefore, our goal was to characterize the patterns of cytokine expression and release during early B. mallei infection in BALB/c mice. A strong IFN-γ response, accompanied by expression of IL-18, IL-12, IL-27, and IL-6, was observed within 24 h after B. mallei infection. IFN-γ was found to be essential in innate host immune responses to B. mallei infection. IL-12 was critical for inducing IFN-γ in response to heat-killed B. mallei in vitro, and IL-18 was involved in this induction. In vitro studies revealed that the main cellular IFN-γ sources are NK cells and CD8+ T cells. The data presented in this paper provide novel, valuable information on protective host immune responses to B. mallei and should assist in the development of an effective vaccine against this pathogen.
MATERIALS AND METHODS
B. mallei.
B. mallei strain ATCC 23344 (American Tissue Culture Collection) was used for all challenge experiments. All work involving live B. mallei was carried out under Advisory Committee on Dangerous Pathogens containment level 3. Bacterial cells were grown in nutrient broth at 37°C for 48 h. Cells were harvested by centrifugation and washed three times in phosphate-buffered saline (PBS) before they were resuspended in 0.1 volume of PBS. The number of bacteria present was calculated by spreading 0.25-ml aliquots of diluted culture onto Congo red plates and incubating the plates at 37°C for 48 h.
For in vitro stimulation assays, bacteria were killed by heat treatment in a water bath at 70°C for 3 h with occasional gentle shaking, and each suspension was checked for sterility by inoculating 5-ml portions of nutrient broth with 0.5-ml aliquots of the suspension ml and incubating the preparations for 7 days at 37°C. Nutrient agar plates were then inoculated with the total broth cultures to check for bacterial growth and incubated for 7 days at 37°C. If no growth occurred on agar plates, inactivation of the bacterial suspension was assumed to have occurred.
Infection of mice with B. mallei.
All mice were housed in a rigid wall isolator in an Advisory Committee on Dangerous Pathogens animal containment level 3 facility. All in vivo work was carried out within the guidelines of the Animal Scientific Procedures Act, 1986. Female BALB/c mice that were 6 to 8 weeks old were purchased from Charles River (Margate, United Kingdom); 8- to 14-week-old female BALB/c IFN-γ knockout (KO), C57BL/6 wild-type, C57BL/6 IFN-γ KO, C57BL/6 IL-12p40 KO, and C57BL/6 IL-12p35 KO mice were bred at the London School of Hygiene and Tropical Medicine (London, United Kingdom). C57BL/6 WSX-1 (IL-27 receptor) KO mice were obtained from the University of Manchester, United Kingdom. BALB/c mice were inoculated intraperitoneally (0.1 ml) with B. mallei strain ATCC 23344 using a 0.5-ml syringe.
Bacteriological analysis of organs.
Organs were dissected and passed through sterile nylon sieves into PBS, and cell suspensions (250 μl) were spread onto Congo red plates and incubated at 37°C for 48 h. Average bacterial counts for each organ were calculated. Blood was removed from mice by cardiac puncture using 0.5-ml insulin syringes following Halothane anesthesia. The blood was placed into O-ring-sealed sarstedt tubes and centrifuged (13,000 rpm, 5 min). The serum was removed and stored at −20°C until a cytokine analysis was performed.
Extraction of RNA from spleens.
Spleens were mashed in Trizol (0.8 ml; Invitrogen, United Kingdom) using micropestles (Eppendorf, United Kingdom) and then stored at −20°C prior to RNA extraction. Following defrosting, chloroform (0.2 ml; Sigma, United Kingdom) was added, and samples were shaken vigorously and incubated at room temperature for 5 min. Samples were centrifuged (12,000 × g, 15 min), each aqueous layer was transferred into a fresh RNase-free Eppendorf tube, and the pellet was discarded. Isopropanol (0.5 ml; Sigma, United Kingdom) was added to the aqueous supernatant, and samples were mixed and incubated at room temperature for 10 min. Samples were centrifuged (12,000 × g, 15 min), and each pellet was washed in 75% molecular biology grade ethanol (Sigma, United Kingdom) and centrifuged (7,000 × g, 5 min). The ethanol was removed, and the RNA pellets were left to air dry and were then dissolved in nuclease-free water (0.1 ml) and stored at −80°C prior to PCR analysis. DNA contamination was removed from RNA samples using a DNA-free kit (Ambion, United Kingdom), and samples were stored at −80°C prior to analysis.
Real-time reverse transcriptase PCR.
RNA samples were reverse transcribed using the GeneAmp 9700 PCR system (Perkin-Elmer, United Kingdom) following treatment with an Omniscript kit (QIAGEN, United Kingdom), random primers, and recombinant RNasin RNase inhibitor (Promega, United Kingdom). cDNA samples were stored at −20°C prior to analysis. Real-time reverse transcriptase PCR (RT-PCR) was performed using a TaqMan 7000 PCR machine (Applied Biosystems, United Kingdom). Expression of murine IFN-γ, IL-12p35, IL-12p40, and IL-6 and the internal 18S RNA control gene was detected using predeveloped assay reagents (Applied Biosystems, United Kingdom). Assay-on-Demand gene expression products (Applied Biosystems, United Kingdom) were used to detect murine IL-18 and the murine IL-27 p28 subunit. TaqMan universal PCR master mixture (Applied Biosystems, United Kingdom) and nuclease-free water (Promega, United Kingdom) were used in all TaqMan PCRs. PCRs were performed in triplicate, and the 18S RNA internal control was included for each sample.
Analysis of RT-PCR data.
Real-time RT-PCR data were analyzed using the ABI PRISM 7000 analysis software (Applied Biosystems, United Kingdom) and the comparative threshold cycle (CT) method. Differences in the amount of transcribed RNA for each sample were accounted for by subtracting the CT value obtained for 18S RNA from the CT value obtained for each cytokine to obtain ΔCT, which allowed actual changes in gene expression to be identified.
In vitro stimulation of murine spleen cells.
Uninfected mice were killed by cervical dislocation, the spleens were removed aseptically, and single-cell suspensions were prepared. Erythrocytes were lysed using red blood cell lysis buffer (Sigma, United Kingdom), the resulting cell suspensions were washed, and the concentrations were adjusted to 5 × 106 cells/ml. The cells were suspended in RPMI 1640 medium (Life Technologies, United Kingdom) supplemented with 10% fetal calf serum, 200 U/ml penicillin, 200 μg/ml streptomycin, and l-glutamine (10 mM) and incubated with 0.1 ml/well of a preparation containing 1 × 106 heat-killed B. mallei cells/ml or a medium control in 96-well flat-bottom cultured plates (5% CO2, 37°C) for 24 h. Anti-IL-18 receptor antibodies (TC30-28E3; Anne O'Garra, National Institute for Medical Research, London, United Kingdom; originally made at DNAX Research Institute, Palo Alto, CA [26]) or isotype-matched control antibodies (Mac 5; immunoglobulin G2b [IgG2b]) were added in vitro to wild-type C57BL/6 spleen suspensions at a dose of 10 μg/ml at the same time that heat-killed B. mallei was added.
For analysis of secreted cytokines, supernatants were removed from wells after 24 h and were stored at −20°C prior to analysis with a cytometric bead array (CBA). For analysis of intracellular IFN-γ by flow cytometry, cytokine secretion in spleen cell cultures stimulated with heat-killed B. mallei or medium was blocked by addition of brefeldin A (0.01 mg/ml) 3 h before cells were harvested for analysis of cytokine production at 21 h after stimulation.
Flow cytometry analysis of intracellular IFN-γ and cell surface marker staining.
Spleen cells harvested following in vitro stimulation were washed and stained with fluorescein isothiocyanate-labeled cell surface markers obtained from Sigma, United Kingdom, including anti-CD4 (H129.19, rat IgG2a), anti-CD8a (53-6.7, rat IgG2a), and anti-TCRαβ (H57-597, hamster Ig), and from Pharmingen, United Kingdom, including anti-TCRγδ (GL3, hamster Ig), anti-pan NK cells (DX5, rat IgM), anti-NK1.1 (PK136, rat IgG2a), anti-CD44 (IM7, rat IgG2b), and CD3-PE-Cy5 (145-2C11, recombinant IgG1). Isotype-matched control antibodies were included in each analysis. Cells were washed, fixed, permeabilized, and incubated with phycoerythrin-labeled anti-IFN-γ (XMG1.2, rat IgG1; Pharmingen). Up to 100,000 events were collected, and viable cells were gated by forward and side scatter and analyzed using a FACScan flow cytometer with CellQuest software (Becton Dickinson, Mountain View, CA) or using a Beckman Coulter EPICS XL flow cytometer with Winlist software.
Cytokine protein analysis.
Cell culture supernatants at containment level 2 were analyzed to determine the presence of cytokines using mouse inflammation CBA kits (Becton Dickinson, United Kingdom) according to the manufacturer's instructions. Sera obtained from infected animals at containment level 3 were analyzed using CBA kits according to the manufacturer's instructions, except that in the final step the pellet of cytokine beads was resuspended in 6% paraformaldehyde (0.5 ml) and refrigerated for 24 h prior to analysis to ensure that the sample was sterile. Samples were analyzed using a FACScan flow cytometer (Becton Dickinson, United Kingdom), and BD CBA software was used for data analysis.
Statistical analysis.
Data obtained during the real-time RT-PCR analysis and the analysis of serum cytokines were nonparametric; therefore, data are presented below as medians ± 99% confidence intervals. A Kruskal-Wallis test was performed with the data, followed by a Mann-Whitney U test. A P value of <0.01 indicated statistical significance in comparisons with uninfected control mice. A one-way analysis of variance was performed in order to compare the responses of wild-type and knockout mice using cytokine data obtained during in vitro stimulation assays with heat-killed B. mallei, and a Student t test was performed with bacterial count data obtained during B. mallei infection in vivo. A P value of <0.05 indicated statistical significance.
RESULTS
Bacterial colonization of the spleen.
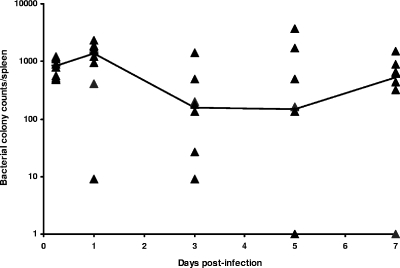
During the first 7 days of infection, BALB/c mice did not die as a result of infection with 1 × 106 CFU B. mallei and showed no ruffling of fur or any other overt signs of disease. The spleens of all mice were colonized uniformly within 5 h after infection. At 24 h postinfection, between 102 and 103 CFU were identified in the spleens of all mice except one mouse, in which 10 CFU was detected. Variability in bacterial counts occurred from days 3 to 7 postinfection, although the median bacterial burden in the spleen remained between 102 and 103 CFU for the first 7 days of infection (Fig. 1).
FIG. 1.
Kinetics of B. mallei colonization of the spleen: number of B. mallei CFU detected in the spleens of infected BALB/c mice following intraperitoneal challenge with 1 × 106 CFU B. mallei during the first 7 days of infection (eight mice/time). The line indicates the median number of colonies identified at each time. The symbols indicate data for individual animals at each time.
Cytokine mRNA expression in the spleen.
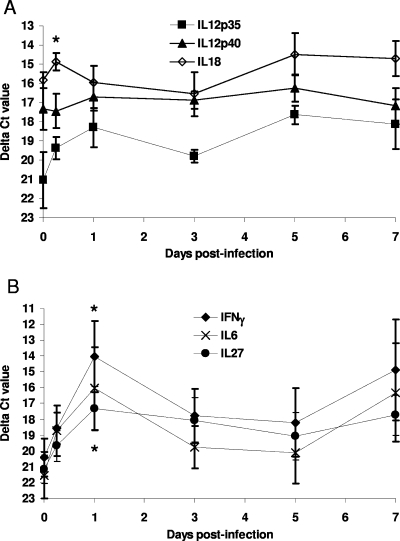
We aimed to determine the type 1 cytokine profiles induced during the early stages of intraperitoneal B. mallei infection (1 × 106 CFU) in the spleen. Cytokine responses were assessed during the first 7 days of infection by examining mRNA expression by real-time RT-PCR. At 5 h postinfection, a twofold increase in IL-18 expression (P < 0.01) was detected in B. mallei-infected animals compared to the expression in naïve animals (Fig. 2A). No significant change in the expression of any other cytokine was observed at this time. By 24 h postinfection, the expression of IFN-γ and IL-27 had increased (P < 0.01) to levels above the control expression levels (49-fold increase for IFN-γ and 14-fold increase for IL-27) (Fig. 2B). A 35-fold increase in IL-6 expression (P = 0.0124) and increased IL-12p35 expression were also detected (Fig. 2A and B). Expression of IL-18 and expression of IL-12p40 were not elevated compared to the expression in uninfected animals at 24 h postinfection (Fig. 2A). Between 3 and 7 days postinfection, the levels of cytokine expression decreased to preinfection levels for IFN-γ, IL-6, and IL-27 and were not significantly higher than the levels of cytokine expression in uninfected controls (Fig. 2A and B).
FIG. 2.
Splenic cytokine mRNA expression in B. mallei-infected animals: median ΔCT values for IL-12p35, IL-12p40, and IL-18 expression (A) and for IFN-γ, IL-27 and IL-6 expression (B) in the spleens of BALB/c mice infected with 1 × 106 CFU B. mallei during the first 7 days after infection. The error bars indicate 99% confidence intervals. The relative change graphs show the median levels of expression compared with the levels of expression in uninfected animals. The asterisks indicate statistically significant increases in cytokine expression compared to the expression in uninfected animals, which were calculated with ΔCT data. The data are representative of the data from two separate experiments.
At 5 h postinfection, the bacterial numbers were uniform in the six animals investigated (Fig. 1), and this corresponded with uniform cytokine expression at this time (Fig. 2A and B). At 24 h postinfection, a lower level of bacterial colonization of the spleen was associated with lower levels of cytokine expression (IFN-γ, IL-6, IL-12p35, and IL-27), highlighting the relationship between the intensity of proinflammatory cytokine expression and the level of infection.
Serum cytokine levels.
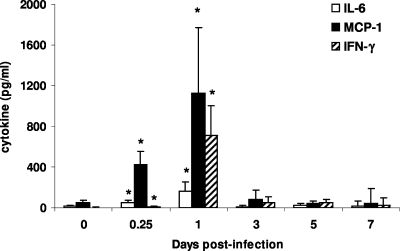
Systemic levels of IFN-γ, IL-6, IL-12p70, TNF-α, and the chemokine macrophage chemoattractant protein 1 (MCP)-1 in the serum were investigated during the first week after infection with 1 × 106 CFU B. mallei. No increase in production of TNF-α or IL-12p70 was detected at any time during the infection (data not shown). Increases (P < 0.01) in serum levels of MCP-1, IL-6, and IFN-γ were observed within 5 h after infection compared with the levels in naïve animals (Fig. 3). At 24 h postinfection, the serum IFN-γ levels were maximal and were higher (P < 0.001) than the levels found in uninfected mice. Serum MCP-1 and IL-6 levels also peaked 24 h postinfection at values higher (P < 0.01) than the values in control animals. Between days 3 and 7 postinfection, no significant increases above the values for naïve mice were detected for any of the cytokines assayed.
FIG. 3.
Median cytokine levels in the sera of B. mallei-infected animals. The serum from BALB/c mice (eight mice per group) infected with 1 × 106 CFU B. mallei or uninfected controls was removed, and the IFN-γ, MCP-1, and IL-6 levels were determined at several times postinfection. The error bars indicate 99% confidence intervals, and the asterisks indicate statistical significance (P < 0.01).
B. mallei infection of IFN-γ knockout and IL-12 knockout mice.
The importance of the IFN-γ peak observed during the initial stages of B. mallei infection in BALB/c mice was investigated. IFN-γ knockout mice with BALB/c and C57BL/6 backgrounds were used to assess the effect of the genetic background on the importance of IFN-γ during B. mallei infection as knockout mice during in vitro studies originated from a C57BL/6 mouse strain. Wild-type BALB/c mice challenged with the both a low dose (1 × 102 CFU) and a high dose (1 × 106 CFU) of B. mallei survived for the duration of the experiment (Fig. 4A). However, most BALB/c IFN-γ KO mice (9 of 10) died 2 days after infection following challenge with a high dose of B. mallei (Fig. 4A). Challenge with a low dose of B. mallei caused six of seven IFN-γ KO BALB/c mice to die within 5 days after infection (Fig. 4A). Wild-type C57BL/6 mice challenged with both the low dose (41 CFU) and the high dose (4.1 × 104 CFU) of B. mallei survived for the duration of the experiment (Fig. 4B). Following challenge with a high dose of B. mallei, all six IFN-γ KO mice with a C57BL/6 background died 2 days postinfection (Fig. 4B), and all six IFN-γ KO C57BL/6 mice challenged with a low dose of B. mallei died within 3 days after infection (Fig. 4B). Analysis of the bacterial burdens in the organs of C57BL/6 mice that received the low dose revealed significantly higher numbers of bacteria, including 106-fold-higher counts in the spleen and liver and 103-fold-higher counts in the lung compared with the counts in infected wild-type mice (Fig. 5). This demonstrated that the early IFN-γ response detected in vivo following B. mallei infection is critical for protection in both BALB/c and C57BL/6 mice.
FIG. 4.
IFN-γ is essential for protection against B. mallei infection. (A) Survival of IFN-γ knockout (IFN-γKO) BALB/c mice challenged with 1 × 106 CFU (high) or 1 × 102 CFU (low) B. mallei and wild-type BALB/c mice challenged with 1 × 106 CFU. (B) Survival of IFN-γ KO C57BL/6 mice and IL-12p40 knockout C57BL/6 mice (IL-12KO) challenged with 4.1 × 104 CFU B. mallei (high) and survival of IFN-γ KO C57BL/6 mice challenged with 41 CFU (low) B. mallei. Wild-type C57BL/6 mice were challenged with both low and high doses.
FIG. 5.
IFN-γ is essential for controlling bacterial replication: number of bacterial CFU isolated from the spleen, liver, and lungs of IFN-γ KO or wild-type C57BL/6 mice 3 days postinfection for mice challenged with a low dose (41 CFU) of B. mallei. The bars indicate the average numbers of CFU per organ, and the error bars indicate standard deviations. Asterisks indicate statistical significance (P < 0.05).
The importance of IL-12 during B. mallei infection was investigated by using IL-12p40 knockout C57BL/6 and wild-type mice infected with a high dose (4.1 × 104 CFU) of B. mallei. IL-12p40 KO mice (n = 6) succumbed to infection between days 6 and 27 after infection. In comparison, wild-type mice did not die as a result of the infection (Fig. 4B).
Cellular sources of IFN-γ induced by B. mallei in vitro.
To determine the number of heat-killed bacteria required for optimal IFN-γ induction by C57BL/6 splenocytes, a dose range study was performed (Fig. 6). After 24 h of incubation intracellular IFN-γ production by splenocytes was assessed using flow cytometry. The optimal dose of heat-killed B. mallei was found to be 1 × 106 CFU (Fig. 6). This contrasted with the optimal dose of heat-killed B. pseudomallei (positive control) (21), which was 5 × 105 CFU (Fig. 6).
FIG. 6.
Dose-dependent induction of IFN-γ produced by C57BL/6 mouse spleen cells in response to different numbers of heat-killed B. mallei and B. pseudomallei cells after 24 h of stimulation in vitro. Splenocytes from three uninfected C57BL/6 mice were incubated with medium alone or with different numbers of heat-killed B. mallei (Bm) and B. pseudomallei (Bps) cells prior to analysis of IFN-γ production by flow cytometry. The y axis indicates the percentage of positively gated cells, and the data shown are representative of the data from three independent experiments.
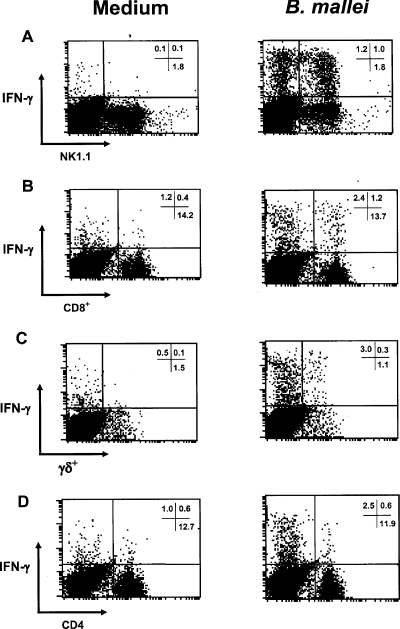
To identify the cellular source of IFN-γ generated in response to B. mallei, splenocytes from uninfected C57BL/6 mice were stimulated with a predetermined optimal concentration of heat-killed bacteria (1 × 106 cells/ml) for 24 h (data not shown), and the intracellular IFN-γ was analyzed by flow cytometry. The predominant cell population involved in IFN-γ production was identified as NK1.1+ cells (Fig. 7A). IFN-γ was also detected in several T-cell subsets. The T-cell subsets responsible for IFN-γ production were CD8+ T cells and TCRγδ T cells (Fig. 7B and C). No increase in IFN-γ production was detected in the CD4+ T-cell population compared with the production in controls that received only medium (Fig. 7D).
FIG. 7.
Cellular sources of IFN-γ following in vitro stimulation with heat-killed B. mallei. Splenocytes from uninfected C57BL/6 mice were incubated with medium alone or with 1 × 106 heat-killed B. mallei cells/ml for 24 h prior to analysis by flow cytometry. The plots show intracellular IFN-γ in NK1.1+ cells (A), CD8+ T cells (B), TCRγδ T cells (C), and CD4+ T cells (D). The numbers in quadrants are the percentages of positive gated cells, and the data shown are representative of the data from three independent experiments.
Role of cytokines in B. mallei-induced IFN-γ production in vitro.
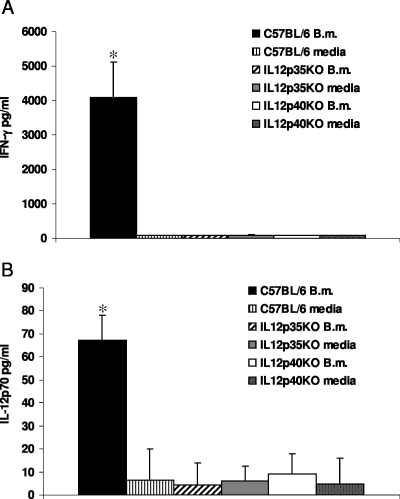
The role of the cytokines IL-12p35, IL-12p40, IL-18, and IL-27 in inducing IFN-γ production was investigated using either neutralizing monoclonal antibodies or KO mice. Stimulation of splenic cultures from IL-12p35 KO or IL-12p40 KO mice with heat-killed B. mallei did not induce an IFN-γ response compared with the response of wild-type C57BL/6 mice, in which 3 to 5 ng/ml of IFN-γ was detected in cell culture supernatants (Fig. 8A). In wild-type cultures stimulated with heat-killed B. mallei, IL-12p70 was detected; this cytokine was absent in IL-12p35 KO and IL-12p40 KO spleen cultures (Fig. 8B). Intracellular analysis of IFN-γ in NK and T-cell populations from IL-12p35 KO and IL-12p40 KO mice confirmed the absence of an IFN-γ response, although IFN-γ was detected in both types of cells in wild-type mice. In contrast, spleen cells from IL-12p35 KO and IL-12p40 KO mice responded like spleen cells from wild-type mice in terms of IFN-γ production in response to direct addition of a combination of IL-12 and IL-18 in the absence of bacteria (data not shown).
FIG. 8.
IL-12 is essential for B. mallei-induced IFN-γ production in vitro: IFN-γ and IL-12p70 production in response to heat-killed B. mallei in wild-type C57BL/6 mice, IL-12p35 KO mice, and IL-12p40 KO mice in vitro. The data are the mean amounts of cytokines detected in the supernatants of splenic cell cultures from individual C57BL/6 mice, IL-12p35 KO mice, or IL-12p40 KO mice stimulated in the presence of heat-killed B. mallei (B.m.) or medium. (A) IFN-γ. (B) IL-12p70. The error bars indicate standard deviations. The data are representative of the data from two experiments performed with groups of four mice.
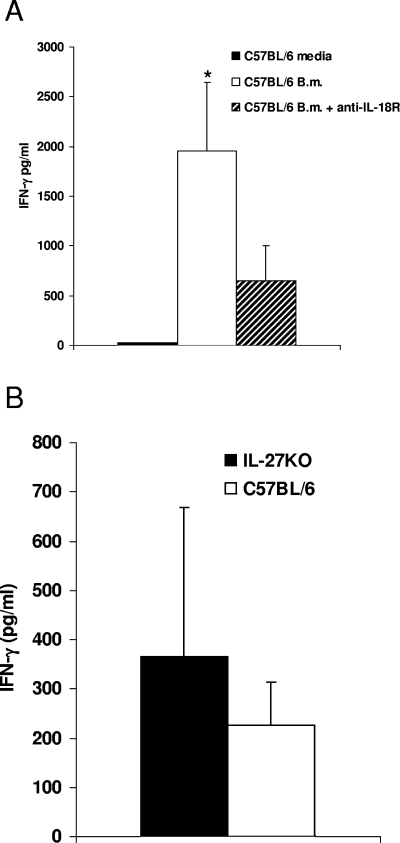
Following neutralization of the IL-18 receptor in spleen cells from wild-type mice, a threefold reduction in IFN-γ production compared with the production in wild-type mice (without antibody) was observed (P < 0.05) (Fig. 9A). There were no significant differences in IFN-γ secretion between cell culture supernatants from WSX-1 (IL-27 receptor) KO mice and cell culture supernatants from control mice following stimulation with heat-killed B. mallei (Fig. 9B). These data demonstrated that IL-12 is essential for IFN-γ production in vitro in response to heat-killed B. mallei and that IL-18 is involved, but IL-27 does not play a role, in induction of IFN-γ responses in vitro.
FIG. 9.
IL-18, but not IL-27, is involved in B. mallei-induced IFN-γ production in vitro: IFN-γ production in response to heat-killed B. mallei in WSX-1 (IL27RKO mice) and wild-type C57BL/6 mice with or without IL-18 receptor neutralizing antibodies in vitro. (A) Mean IFN-γ production in the supernatants of splenic cell cultures from C57BL/6 mice (n = 3) with (+ anti-IL-18R) or without IL-18 receptor neutralizing antibodies. (B) Individual IFN-γ production in the supernatants of WSX-1 mice (n = 5) stimulated in the presence of heat-killed B. mallei (B.m.) or medium. The error bars indicate standard deviations, and the asterisk indicates statistical significance (P < 0.05).
DISCUSSION
Previous studies have shown that B. mallei causes a chronic disease in BALB/c mice, in which there is colonization of several organs and inflammatory infiltration of macrophages and neutrophils into tissues (11). The cytokine response to B. mallei and its relevance in host defense, however, are not currently understood. We investigated the role of IFN-γ in defense against B. mallei infection in mice and determined the presence of known IFN-γ-inducing cytokines in a chronic model of infection characterized by early colonization of the spleen.
Our data show that B. mallei induces a strong, early IFN-γ response in vivo in BALB/c mice, both in the spleen and systemically, as indicated by the levels in the serum. The level of serum IFN-γ protein was elevated 5 h postinfection and peaked 24 h postinfection simultaneously with a peak in IFN-γ mRNA expression in the spleen. This IFN-γ response is essential for protection against B. mallei infection since disruption of the IFN-γ gene rendered both BALB/c and C57BL/6 mice highly susceptible to B. mallei infection, with death occurring 2 to 3 days postinfection. This increased susceptibility was associated with rapid expansion of bacterial populations in the spleen, liver, and lungs, demonstrating that IFN-γ has an essential role in controlling bacterial growth. Thus, B. mallei joins a number of other intracellular pathogens, including the closely related organism B. pseudomallei (16) and L. monocytogenes (30), for which a rapid, innate IFN-γ response is essential for resistance to infection.
The importance of IL-12 was assessed in IL-12p40 KO mice, and IL-12p40 was found to play an important role in in vivo protection against B. mallei infection. An increase in IL-12p35 expression was also observed throughout infection with B. mallei. Both subunits of IL-12 were found to be critical for IFN-γ production in vitro in response to heat-killed B. mallei. The fact that IFN-γ could not be produced in the absence of either subunit suggests that IL-12 but not IL-23 is essential for IFN-γ production (20). Increases in IL-18 expression 5 h postinfection suggested that IL-18 may also be involved in induction of IFN-γ responses. We demonstrated that IL-18 is involved in (but not essential for) induction of the IFN-γ in response to B. mallei in vitro, suggesting that the IFN-γ response may be partially due to synergy between IL-18 and IL-12. IL-18 is important in host defense against infection by a number of intracellular pathogens, including B. pseudomallei (16, 21), Yersinia enterocolitica (4), and L. major (27), because it induces IFN-γ, in synergy with IL-12, from T cells (28) and NK cells (19).
Our intracellular IFN-γ analysis showed that NK1.1+ cells, CD8+ T cells, and TCRγδ T cells provide most of the IFN-γ in response to B. mallei. NK1.1+ cells are essential in the host defense against a number of intracellular pathogens, including L. major (34) and B. pseudomallei (16, 22), because they produce IFN-γ. CD8+ T cells, the predominant T-cell population involved in innate production of IFN-γ in response to B. mallei, also produce IFN-γ during infection with other pathogens, including B. pseudomallei (22). Our data suggest that both NK cells and CD8 T cells are important in the generation of cell-mediated immunity to B. mallei and that IL-12 acts on both NK cells and T cells to induce the IFN-γ in response to heat-killed B. mallei.
The high levels of IL-27 and IL-6 expression detected in the spleen during the first 24 h of infection also indicated that these cytokines are potential inducers of IFN-γ. IL-27 is involved in IFN-γ production during L. major infection in mice (39) but is not involved in IFN-γ production during infection with Toxoplasma gondii (36). Our findings demonstrated that IL-27 is not involved in IFN-γ production in vitro in response to B. mallei, suggesting that IL-27 may have an independent role in Th1-mediated immunity during B. mallei infection. A number of studies have implicated IL-6 in the development of protective immunity by induction of IFN-γ production during infection with the intracellular bacteria M. tuberculosis (33) and L. monocytogenes (8). However, the role of IL-6 in induction of IFN-γ in response to B. mallei is currently not known.
IL-6 is able to induce MCP-1 production during bacterial infection (3) and may be responsible for the high levels of MCP-1 in the host's circulation during B. mallei infection. MCP-1 is a chemokine involved in the chemoattraction of macrophages to sites of infection (7) and is important in protective immune responses during bacterial infection (15). Macrophages are the main cell type activated by IFN-γ and are essential for bacterial clearance during innate immune responses. The high levels of MCP-1 identified during the first 24 h of B. mallei infection implicate this chemokine in the promotion of macrophage responses for bacterial killing as macrophage influx into the spleen peaks 24 h postinfection (data not shown). MCP-1 is also involved in the recruitment of memory T cells and NK cells (6), suggesting that MCP-1 may be a key link between IFN-γ production and macrophage activation.
The initial burst of IFN-γ (and potentially other cytokines) during the first 24 h after infection seems to be sufficient to control proliferation of bacterial colonies during the first week of infection, but it is not able to clear the bacteria. The down-regulation of the IFN-γ response 3 days postinfection may be caused by an immune evasive mechanism of B. mallei. IL-6 has been implicated in suppression of IFN-γ responses during infection with M. tuberculosis (25) and is, therefore, a potential candidate for suppression of this response.
Our data begin to characterize the host immune response to B. mallei infection, adding to the very limited number of studies in which cell-mediated immune responses to B. mallei infection were investigated. There is currently no vaccine against B. mallei, and, as the host relies on IFN-γ-mediated immunity for control of infection, this may be useful in the development of vaccines and vaccination strategies against this potentially lethal agent. This possibility is supported by recent studies suggesting that Th1-like responses provide limited protection against B. mallei infection in BALB/c mice (1) and that type 1 cytokines, including IL-12 and IFN-γ, are associated with the CpG-mediated protection of low-dose aerosol B. mallei infection (37). It is, therefore, likely that successful intervention strategies against B. mallei will exploit the cell-mediated immune responses responsible for control of the early stages of infection observed in this study.
Acknowledgments
We thank Bob Gwyther, Dstl, Porton Down, United Kingdom, for performing statistical analysis of data and Helena Helmby (London School of Hygiene and Tropical Medicine, London, United Kingdom) for her generous gift of IL-12p35 and IL-12p40 KO mice.
Editor: J. B. Bliska
REFERENCES
- 1.Amemiya, K., J. L. Meyers, S. R. Trevino, T. C. Chanh, S. L. Norris, and D. M. Waag. 2006. Interleukin-12 induces Th1-like response to Burkholderia mallei and limited protection in BALB/c mice. Vaccine 24:1413-1420. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein, J. M., and E. R. Carling. 1909. Human glanders: with a study of six cases and a discussion of the methods of diagnosis. Br. Med. J. 1:319-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biswas, P., F. Delfanti, M. Bernasconi, M. Mengozzi, N. Cota, A. Polentarutti, A. Mantovani, S. Lazzarin, S. Sozzani, and G. Poli. 1998. Interleukin-6 induces monocyte chemotactic protein-1 in peripheral blood mononuclear cells and in the U937 cell line. Blood 91:258-265. [PubMed] [Google Scholar]
- 4.Bohn, E., A. Sing, R. Zumbihl, C. Bielfeldt, H. Okamura, M. Kurimoto, J. Heeseman, and I. B. Autenrieth. 1998. IL-18 (IFN-γ-inducing factor) regulates early cytokine production in, and promotes resolution of, bacterial infection in mice. J. Immunol. 160:299-307. [PubMed] [Google Scholar]
- 5.Bossi, P., A. Tegnell, A. Baka, F. Van Loock, J. Hendriks, A. Werner, H. Maidhof, and G. Gouvras. 2004. Bichat guidelines for the clinical management of glanders and melioidosis and bioterrorism-related glanders and melioidosis. Eurosurveillance 9:1-6. [DOI] [PubMed] [Google Scholar]
- 6.Carr, M. W., S. J. Roth, E. Luther, S. S. Rose, and T. A. Springer. 1994. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc. Natl. Acad. Sci. USA 91:3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chae, P., M. Im, F. Gibson, Y. Jiang, and D. T. Graves. 2002. Mice lacking monocyte chemoattractant protein 1 have enhanced susceptibility to an interstitial polymicrobial infection due to impaired monocyte recruitment. Infect. Immun. 70:3164-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalyrymple, S. A., L. A. Lucian, R. Slattery, T. McNeil, D. M. Aud, S. Fuchino, F. Lee, and R. Murray. 1995. Interleukin-6-deficient mice are highly susceptible to Listeria monocytogenes infection: correlation with inefficient neutrophilia. Infect. Immun. 63:2262-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elkins, K. I., A. Cooper, S. M. Colombini, S. C. Cowley, and T. L. Kieffer. 2002. In vivo clearance of an intracellular bacterium, Francisella tularensis LVS, is dependent on the p40 subunit of interleukin-12 (IL-12) but not on IL-12p70. Infect. Immun. 70:1936-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flesch, I. E. A., J. Hess, S. Huang, M. Aguet, J. Rothe, H. Bluethmann, and S. H. E. Kaufman. 1995. Early interleukin 12 production by macrophages in response to mycobacterial infection depends on interferon γ and tumour necrosis factor α. J. Exp. Med. 181:1615-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fritz, D. L., P. Vogel, D. R. Brown, D. Deshazer, and D. M. Waag. 2000. Mouse model of sublethal and lethal intraperitoneal glanders (Burkholderia mallei). Vet. Pathol. 37:626-636. [DOI] [PubMed] [Google Scholar]
- 12.Fritz, D. L., P. Vogel, D. R. Brown, and D. M. Waag. 1999. The hamster model of intraperitoneal Burkholderia mallei (glanders). Vet. Pathol. 36:276-291. [DOI] [PubMed] [Google Scholar]
- 13.Garcia, V. E., K. Uyemura, P. A. Sieling, M. T. Ochoa, C. T. Morita, H. Okamura, M. Kurimoto, T. H. Rea, and R. L. Modlin. 1999. IL-18 promotes type I cytokine production from NK cells and T cells in human intracellular infection. J. Immunol. 162:6114-6121. [PubMed] [Google Scholar]
- 14.Gazzinelli, R. T., M. Wysocka, S. Hayashi, E. Y. Denkers, S. Hieny, P. Caspar, G. Trinchieri, and A. Sher. 1994. Parasite-induced IL-12 stimulates early IFN-γ synthesis and resistance during acute infection with Toxoplasma gondii. J. Immunol. 153:2533-2543. [PubMed] [Google Scholar]
- 15.Guleria, I., and J. W. Pollard. 2001. Aberrant macrophage and neutrophil population dynamics and impaired Th1 response to Listeria monocytogenes in colony-stimulating factor 1-deficient mice. Infect. Immun. 69:1795-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haque, A., A. Easton, D. Smith, A. O'Garra, N. van Rooijen, G. Lertmemongkolchai, R. W. Titball, and G. J. Bancroft. 2006. The role of T cells in innate and adaptive immunity against murine Burkholderia pseudomallei infection. J. Infect. Dis. 193:370-379. [DOI] [PubMed] [Google Scholar]
- 17.Heinzel, F. P., and R. M. Rerko. 1999. Cure of progressive murine leishmaniasis: interleukin 4 dominance is abolished by transient CD4+ T cell depletion and T helper cell type 1-selective cytokine therapy. J. Exp. Med. 189:1895-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howe, C., and W. R. Miller. 1947. Human glanders: report of six cases. Ann. Intern. Med. 26:93-115. [DOI] [PubMed] [Google Scholar]
- 19.Kawakami, K., Y. Koguchi, M. H. Qureshi, A. Miyazato, S. Yara, Y. Kinjo, Y. Iwakura, K. Takeda, S. Akira, M. Kurimoto, and A. Saito. 2000. IL-18 contributes to host resistance against infection with Cryptococcus neoformans in mice with defective IL-12 synthesis through induction of IFN-γ production by NK cells. J. Immunol. 165:941-947. [DOI] [PubMed] [Google Scholar]
- 20.Lankford, C. S. R., and D. M. Frucht. 2003. A unique role for IL-23 in promoting cellular immunity. J. Leukoc. Biol. 73:49-56. [DOI] [PubMed] [Google Scholar]
- 21.Lauw, F. N., A. J. Simpson, J. M. Prins, M. D. Smith, M. Kurimoto, S. J. van Deventer, P. Speelman, W. Chaoagul, N. J. White, and T. van der Poll. 1999. Elevated plasma concentrations of interferon (IFN)-gamma and the IFN-gamma-inducing cytokines interleukin (IL)-18, IL-12, and IL-15 in severe melioidosis. J. Infect. Dis. 180:1878-1885. [DOI] [PubMed] [Google Scholar]
- 22.Lertmemongkolchai, G., G. Cai, C. A. Hunter, and G. J. Bancroft. 2001. Bystander activation of CD8+ T cells contributes to the rapid production of IFN-γ in response to bacterial pathogens. J. Immunol. 166:1097-1105. [DOI] [PubMed] [Google Scholar]
- 23.Lever, M. S., M. Nelson, P. I. Ireland, A. J. Stagg, R. J. Beedham, G. A. Hall, G. Knight, and R. W. Titball. 2003. Experimental aerogenic Burkholderia mallei (glanders) infection in the BALB/c mouse. J. Med. Microbiol. 52:1109-1115. [DOI] [PubMed] [Google Scholar]
- 24.Miller, W. R., L. Pannell, and M. S. Ingalls. 1948. Experimental chemotherapy in glanders and melioidosis. Am. J. Hyg. 47:205-213. [DOI] [PubMed] [Google Scholar]
- 25.Nagabhushanam, V., A. Solache, L.-M. Ting, C. J. Escaron, J. Y. Zhang, and J. D. Ernst. 2003. Innate inhibition of adaptive immunity: Mycobacterium tuberculosis-induced IL-6 inhibits macrophage responses to IFN-γ. J. Immunol. 171:4750-4757. [DOI] [PubMed] [Google Scholar]
- 26.Neighbors, M., X. Xiuling, F. J. Barrat, S. R. Ruuls, T. Churakova, R. Debets, J. F. Bazan, R. A. Kastelein, J. S. Abrams, and A. O'Garra. 2001. A critical role for interleukin 18 in primary and memory effector responses to Listeria monocytogenes that extends beyond its effects on interferon gamma production. J. Exp. Med. 194:343-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohkusu, K., T. Yoshimoto, K. Takeda, T. Ogura, S.-I. Kashiwamura, Y. Iwakura, S. Akira, H. Okamura, and K. Nakanishi. 2000. Potentiality of interleukin-18 as a useful reagent for treatment and prevention of Leishmania major infection. Infect. Immun. 68:2449-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okamura, H., H. Tsutsui, T. Komatsu, M. Yutsudo, A. Kakura, T. Tanimoto, K. Torigoe, T. Okura, Y. Nukada, K. Hattori, K. Akita, M. Namba, F. Tanabe, K. Kobishi, S. Fukuda, and M. Kurimoto. 1995. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature 378:88-91. [DOI] [PubMed] [Google Scholar]
- 29.Pflanz, S., J. C. Timans, J. Cheung, R. Rosales, H. Kanzler, J. Gilbert, L. Hibbert, et al. 2002. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity 16:779-790. [DOI] [PubMed] [Google Scholar]
- 30.Portnoy, D. A., R. D. Schreiber, P. Connelly, and L. G. Tilney. 1989. Gamma interferon limits access of Listeria monocytogenes to the macrophage cytoplasm. J. Exp. Med. 170:2141-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson, D. S., and A. O'Garra. 2002. Further checkpoints in Th1 development. Immunity 16:755-758. [DOI] [PubMed] [Google Scholar]
- 32.Santanirand, P., V. S. Harley, D. A. B. Dance, B. S. Drasar, and G. J. Bancroft. 1999. Obligatory role of gamma interferon for host survival in a murine model of infection with Burkholderia pseudomallei. Infect. Immun. 67:3593-3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saunders, B. M., A. A. Frank, I. M. Orme, and A. M. Cooper. 2000. Interleukin-6 induces early gamma interferon production in the infected lung but is not required for generation of specific immunity to Mycobacterium tuberculosis infection. Infect. Immun. 68:3322-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scharton, T. M., and P. Scott. 1993. Natural killer cells are a source of interferon gamma that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J. Exp. Med. 178:567-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srinivasan, A., C. N. Kraus, D. Deshazer, P. M. Becker, J. D. Dick, L. Spacek, J. G. Bartlett, W. R. Byrne, and D. L. Thomas. 2001. Glanders in a military research microbiologist. N. Engl. J. Med. 345:256-258. [DOI] [PubMed] [Google Scholar]
- 36.Villarino, A., L. Hibbert, L. Lieberman, E. Wilson, T. Mak, H. Yoshida, R. A. Kastelein, C. Saris, and C. A. Hunter. 2003. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity 19:645-655. [DOI] [PubMed] [Google Scholar]
- 37.Waag, D. M., M. J. McCluskie, N. Zhang, and A. M. Krieg. 2006. A CpG oligonucleotide can protect mice from a low aerosol challenge dose of Burkholderia mallei. Infect. Immun. 74:1944-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White, N. J. 2003. Melioidosis. Lancet 361:343-354. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida, H., S. Hamano, G. Senaldi, T. Covey, R. Faggioni, S. Mu, M. Xia, A. C. Wakeham, H. Nishina, and J. Potter. 2001. WSX-1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity 15:569-578. [DOI] [PubMed] [Google Scholar]