Abstract
Haemophilus somnus can cause a devastating fibrinopurulent meningitis with thrombotic vasculitis and encephalitis in cattle. The mechanisms used by H. somnus to migrate from the bloodstream into the central nervous system (CNS) are unknown. In this study, we demonstrate that H. somnus adheres to, but does not invade, bovine brain endothelial cells (BBEC) in vitro. The number of adherent H. somnus was significantly increased by prior activation of the BBEC with tumor necrosis factor alpha (TNF-α). Addition of exogenous glycosaminoglycans significantly reduced H. somnus adherence to resting and TNF-α-activated BBEC. Heparinase digestion of the endothelial cell's glycocalyx or sodium chlorate inhibition of endothelial cell sulfated glycan synthesis significantly reduced the number of adherent H. somnus. In contrast, addition of hyaluronic acid, a nonsulfated glycosaminoglycan, had no inhibitory effect. These findings suggest a critical role for both cellular activation and sulfated glycosaminoglycans in adherence of H. somnus to BBEC. Using heparin-labeled agarose beads, we demonstrated a high-molecular-weight heparin-binding protein expressed by H. somnus. Heparin was also shown to bind H. somnus in a 4°C binding assay. These data suggest that heparin-binding proteins on H. somnus could serve as initial adhesins to sulfated proteoglycans on the endothelial cell surface, thus contributing to the ability of H. somnus to infect the bovine CNS.
Haemophilus somnus, a gram-negative pleomorphic coccobacillus, is capable of causing a wide array of clinical syndromes in cattle, including respiratory disease, arthritis, reproductive failure, and septicemia (2, 8, 25, 28, 47). H. somnus septicemia can result in a devastating acute neurological disease known as thrombotic meningoencephalitis (TME) that is often fatal within 12 to 24 h of clinical onset. TME is characterized by fibrinopurulent meningitis with hemorrhage, abscess formation, and thrombotic vasculitis throughout the central nervous system (CNS) (37). Although the pathogenesis of TME is not well understood, the propensity of H. somnus to cause vasculitis and intravascular thrombosis suggests a critical role for the interactions between the bacterium and endothelial cells in inciting the disease.
The blood-brain barrier is formed by cerebral endothelial cells, surrounded by pericytes and astrocyte foot processes, which actively limit transport of cells, solutes, and macromolecules from the bloodstream into the brain. To gain access to the central nervous system, H. somnus must interact with the highly specialized endothelial cells that comprise the blood-brain barrier. The microvascular endothelial cells of the cerebral cortex are morphologically and functionally distinct from endothelial cells derived from the systemic vascular tree. For example, cerebral microvascular endothelial cells display few plasmalemmal vesicles, are rarely pinocytic, and have intercellular tight junctions (43).
Endothelial cells from the cerebrovasculature have been shown to maintain their unique properties in culture (13, 30). The purpose of this study was to use cultured bovine microvascular endothelial cells to investigate interactions with H. somnus in an in vitro model of the blood-brain barrier. In this study, we demonstrate that H. somnus adheres to bovine brain endothelial cells (BBEC) in a manner that is enhanced by cellular activation and dependent on sulfated proteoglycans on the endothelial cell surface. We infer that similar interactions could play a role in the development of TME.
MATERIALS AND METHODS
Chemicals and media.
RPMI and trypsin were purchased from Cellgro (Kansas City, Mo.). Heparin sodium salt, chondroitin sulfate, RGD peptide A6677 (Arg-Gly-Asp-Ser-Pro-Ala-Ser-Ser-Lys-Pro), sodium chlorate, heparinase III, and hylauronic acid were obtained from Sigma Chemical Co. (St. Louis, Mo.). Brain heart infusion agar, thiamine monophosphate, and yeast extract were purchased from Difco (Detroit, Mich.). Avidin-conjugated agarose beads were purchased from Pierce (Rockford, IL), and biotinylated heparin was obtained from Calbiochem (San Diego, CA).
Endothelial cells.
Simian virus 40-transformed bovine brain endothelial cells were described previously (38). The cells were cultured in RPMI supplemented with 10% fetal bovine serum and passaged by brief enzymatic digestion using 0.1% trypsin EDTA. All experiments were performed on cells prior to passage 50.
Bacteria.
Haemophilus somnus strain 649 was initially isolated from a clinical case of bovine abortion and has been previously described (9). The bacteria were stored as stationary-phase cells, in brain heart infusion broth with 10% glycerol, at −70°C. Prior to each experiment, an aliquot of bacteria was thawed and inoculated at a 1:100 dilution in brain heart infusion broth supplemented with 0.5% yeast extract and 0.01% thiamine monophosphate. The bacteria were then cultured without shaking for 16 h at 37°C and 5% CO2. Prior to inoculation, bacteria were pelleted and resuspended in RPMI with 10% fetal bovine serum (FBS). The number of bacteria present in the inoculum was extrapolated from growth curves performed in our laboratory and confirmed in each experiment by enumeration of CFU on sheep blood agar plates.
Adhesion and invasion studies.
BBEC were cultured overnight at 37°C with 5% CO2 in a 96-well plate at a density of 10,000 cells per well. Each well was then inoculated with approximately 30 bacteria per endothelial cell in RPMI with 10% FBS. At various time points, the wells were washed five times with warm Hank's balanced salt solution (HBSS), and the endothelial cells were lysed to release the adherent bacteria by incubation in 100 μl 0.1% Triton X-100 in H2O for 15 min at 25°C. Control experiments were performed to ensure that the lysis procedure had no negative impact on the survival of H. somnus. The number of CFU in each well was determined by serially diluting the lysates in cold brain heart infusion broth followed by plating on sheep blood agar plates. To enumerate the number of intracellular H. somnus, additional endothelial cell cultures were washed as described above and incubated for an additional 1 h with 10 μg/ml gentamicin to kill any extracellular bacteria. The endothelial cells were then washed five times with HBSS to remove the antibiotic, lysed as described above, and plated on blood agar to enumerate the CFU of intracellular organisms.
Endothelial cell activation.
BBEC were activated by a 1-h incubation with 100 ng/ml recombinant bovine tumor necrosis factor alpha (TNF-α) at 37°C. To evaluate the ability of heparin to modulate endothelial cell activation by TNF-α, in some experiments BBEC were incubated simultaneously with TNF-α and 1 mM heparin. Following activation, cells were washed twice with warm RPMI and assayed for their ability to bind H. somnus as described previously.
Inhibition of adhesion.
Glycosaminoglycans (GAG; heparin, chondroitin sulfate, and hyaluronic acid) were diluted in RPMI and added to the endothelial cells immediately prior to the addition of H. somnus. In later experiments, heparin was added to stationary cultures of H. somnus, incubated for 30 min at 37°C, and then the bacteria were pelleted and washed twice prior to their inoculation into the endothelial cell cultures. Other inhibitors that were examined in this study (A6677, glucose, and maltose) were diluted in RPMI and added to the endothelial cell monolayers immediately before inoculation with H. somnus. The concentrations used were based on previously published studies investigating the adherence of human respiratory pathogens to epithelial cells (24, 41). None of the inhibitors used caused any direct endothelial cell damage, as determined by negligible release of lactate dehydrogenase. Bacterial adhesion assays were then performed as described above.
Preparation of H. somnus culture filtrates.
Culture filtrate preparation was based on a previously published protocol of Corbeil et al. (10). Briefly, H. somnus was grown overnight in brain heart infusion broth supplemented with 0.5% yeast extract and 0.01% thiamine monophosphate. The bacteria were incubated without shaking for 16 h at 37°C and 5% CO2. The bacterial cells were removed from the suspension by centrifugation at 6,000 × g for 10 min. The supernatant was then passed through a 2-μm filter unit to remove any remaining H. somnus cells and then concentrated using an Amicon ultrafiltration device with a YM filter (50,000 molecular weight cutoff).
Isolation of H. somnus heparin-binding proteins.
Avidin-conjugated agarose beads and biotinylated heparin were used to identify heparin-binding proteins in H. somnus culture filtrates. To label the avidin beads (Pierce, Rockford, IL) with heparin, 25 μl of beads with a binding capacity of 1 to 3 mg/ml heparin was incubated for 1 h at room temperature with 50 μg biotinylated heparin (Calbiochem, San Diego, CA). The beads were then centrifuged for 5 min at 2,000 rpm and washed three times with phosphate-buffered saline (PBS) to remove the unbound heparin. Concentrated culture filtrates or outer membrane preparations (15 μg) were incubated for 30 min at room temperature with both heparin-labeled and unlabeled beads. The beads were then washed five times as previously described. Proteins were removed from the beads by boiling for 5 min in 50 μl of Laemmli loading buffer. An equal volume of each sample was then run for 2 h at 100 V on a 4 to 20% gradient denaturing polyacrylamide gel. The gel was then stained using the Bio-Rad Silver Stain Plus kit (Hercules, CA), according to the manufacturer's instructions.
Heparin-binding assay.
Nunc Maxisorp plates (Nunc/Nalgene, Rochester, NY) were coated overnight at room temperature with 5 mg/ml heparin in PBS (pH 7.4) and then blocked for 1 h at room temperature in 4% bovine serum albumin in PBS. H. somnus was grown for 16 h at 37°C and 5% CO2 in brain heart infusion broth supplemented with 0.5% yeast extract and 0.01% thiamine monophosphate. The bacteria were then pelleted and resuspended in HBSS. A 1-ml aliquot of the resuspension was incubated with 1 μl of 1,000× SYTO BC green fluorescent nucleic acid stain (Invitrogen, Carlsbad, CA) for 15 min at 37°C. The fluorescently labeled H. somnus cells were then pelleted and washed three times in PBS. The final pellet was resuspended in 900 μl of PBS (pH 7.4). Approximately 8 × 106 labeled H. somnus cells (25 μl of the final suspension) were then added in triplicate to 75 μl PBS in uncoated and heparin-coated wells, and the plate was incubated for 1 h at 4°C. The plate was washed four times with PBS, and the fluorescence in each well was measured at 495/530 using a Beckman Coulter DTX880 plate reader.
Statistical analysis.
Data are shown as the mean ± the standard error of the mean (SEM) of at least three independent experiments. In experiments with more than two treatment groups, a one-way analysis of variance was performed, followed by the Bonferroni posttest. In experiments with only a control and a single treatment group, a paired t test was performed. All statistics were performed using the Prism 3 statistical software package. Significance was set at P of <0.05.
RESULTS
H. somnus adheres to bovine brain endothelial cells.
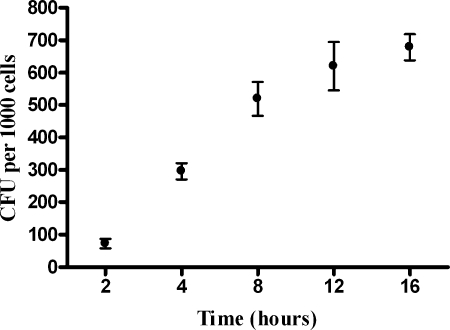
Using our adhesion assay, we demonstrated that H. somnus adherence to BBEC increased during a 16-h incubation period when the cells were incubated at 37°C (Fig. 1). We were unable to detect any significant invasion of BBEC by H. somnus during a 24-h incubation (fewer than 5 CFU per 10,000 endothelial cells) (data not shown). To ensure that we could detect endothelial cell invasion (if it occurred) in our assay, in some experiments we included Listeria monocytogenes as a positive control for invasion of endothelial cells (19, 21). We observed that L. monocytogenes efficiently invaded the BBEC (greater than 400 CFU per 10,000 endothelial cells), while H. somnus invasion was a rare event (<5 CFU) (data not shown). Comparative binding experiments at 37°C and 4°C were also performed. Following a 1-hour incubation of H. somnus with the endothelial cells, the relative number of adherent H. somnus at 4°C was 18% ± 8.3% (mean ± SEM of three independent experiments) of the number of H. somnus adherent to endothelial cells at 37°C.
FIG. 1.
H. somnus adheres to BBEC in a time-dependent manner. BBEC were incubated for 2 to 16 h at 37°C with approximately 30 bacteria per endothelial cell. At each time point, endothelial cells were washed five times with PBS and then lysed to release adherent bacteria. The CFU of adherent bacteria were estimated by plating BBEC lysates on sheep blood agar. Data represent the mean ± SEM of three independent experiments.
Glycosaminoglycans modulate adhesion of H. somnus to BBEC.
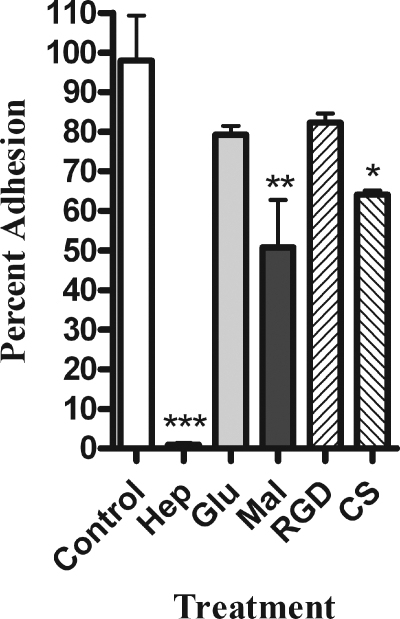
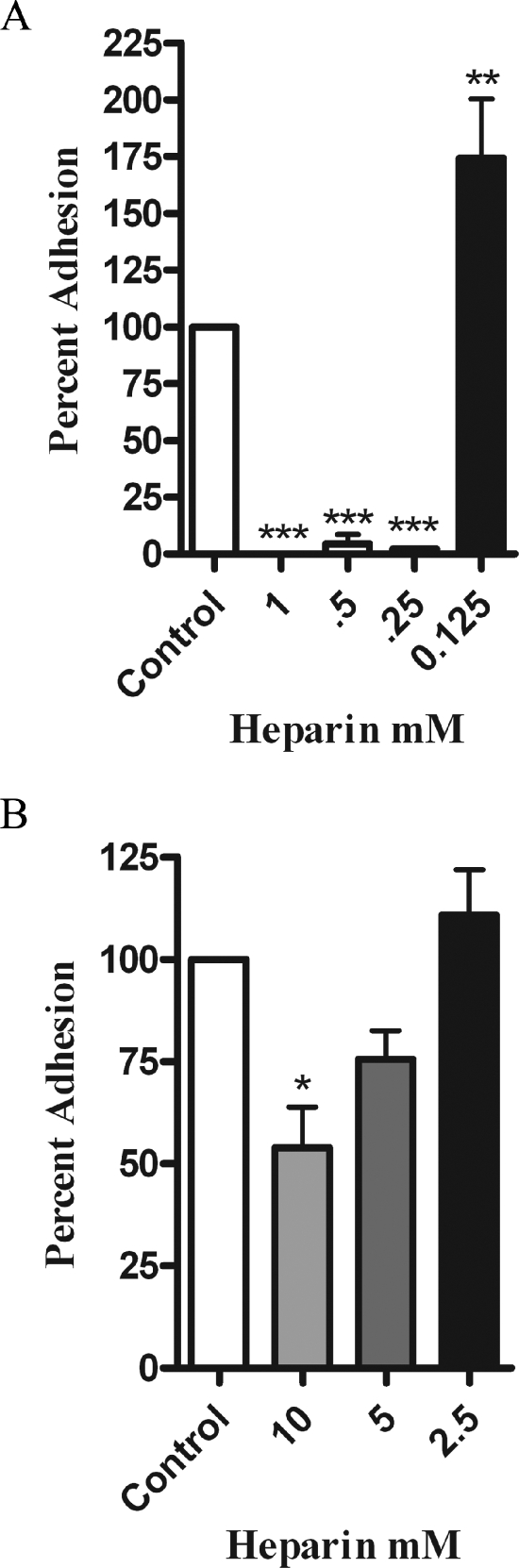
We next used several types of inhibitors to determine what class of adhesins might be involved in the interaction between H. somnus and BBEC. We selected the inhibitors based on previously published work that identified molecules involved in the adhesion of other bacterial pathogens to mammalian cells (6, 14, 41). The greatest level of inhibition was achieved by addition of the glycosaminoglycan heparin (Fig. 2). A second glycosaminoglycan, chondroitin sulfate, also significantly decreased adhesion, although to a lesser degree (Fig. 2). We also evaluated the ability of several monosaccharides to inhibit H. somnus adhesion. High concentrations (50 mM) of the disaccharide maltose reduced adhesion of H. somnus by approximately 50%, while glucose had little effect. Finally, we evaluated the possible role of the β2-integrins. As shown in Fig. 2, we did not detect any significant decrease in H. somnus adhesion to BBEC when we added a peptide containing the RGD sequence (Arg-Gly-Asp-Ser-Pro-Ala-Ser-Ser-Lys-Pro), which is known to block β2-integrin-binding sites. The above findings led us to hypothesize that proteoglycans, specifically those containing the glycosaminoglycan heparan sulfate, are involved in adhesion of H. somnus to BBEC. To further evaluate this hypothesis, we tested the ability of heparin to modulate the interaction of H. somnus with BBEC at both 37 and 4°C. The addition of heparin at concentrations of 0.25 mM or greater reduced adhesion of H. somnus to BBEC by more than 90% in a 4-h incubation period at 37°C (Fig. 3A). Interestingly, lower concentrations (0.125 mM or less) of heparin enhanced, rather than inhibited, the adherence of H. somnus to BBEC. This effect was seen with heparin concentrations as low as 0.01 mM (Fig. 3A and data not shown). Similar enhancement of bacterial adhesion by low concentrations of heparin has been reported by other investigators (7, 49). When the experiment was performed at 4°C, 10 mM heparin was required to achieve approximately a 50% reduction in the level of adhesion, compared to control cells (Fig. 3B). Furthermore, heparin at concentrations ranging from 0.25 to 1 mM was less effective at reducing H. somnus adhesion to BBEC over shorter incubations at 37°C. Heparin (1 mM) reduced H. somnus adherence to nondetectable levels in a 4-h adhesion assay at 37°C but only reduced adhesion to approximately 85% of the control value in a 1-hour adhesion assay (data not shown).
FIG. 2.
Heparin, chondroitin sulfate, and maltose inhibit adherence of H. somnus to BBEC. Adhesion of H. somnus to BBEC was tested in the presence of 1 mM heparin (Hep), 50 mM glucose (Glu), 50 mM maltose (Mal), 1 mM A6677 (RGD peptide), 1 mM chondroitin sulfate (CS), or RPMI as a negative control. Approximately 30 H. somnus CFU were added per endothelial cell. The cells were then incubated for 4 h at 37°C and washed five times with PBS. The endothelial cells were then lysed, and the adherent bacteria were enumerated by plating BBEC lysates on sheep blood agar plates. Data represent the mean ± SEM of three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
FIG. 3.
Heparin inhibits H. somnus adherence to BBEC. The indicated concentrations of heparin were added to BBEC concurrently with H. somnus (30 CFU per endothelial cell) and incubated for 4 h 37°C (A) or 4°C (B). The BBEC were then washed five times with PBS and lysed, and the CFU of adherent bacteria were enumerated by plating the lysates on sheep blood agar plates. Results are expressed as the percentage of CFU recovered from untreated BBEC. Data represent the mean ± SEM of three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Heparin alters H. somnus adherence to resting and TNF-α-activated BBEC.
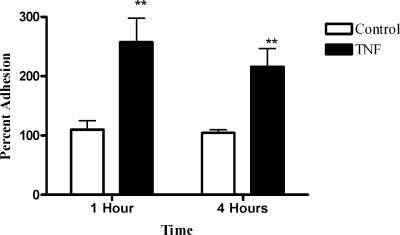
Because heparin inhibited H. somnus adherence to BBEC to a greater extent after a 4-h incubation period, we hypothesized that alterations in endothelial cell activation might occur during the 4-h incubation period that amplified the effect of heparin. Kwiecien et al. (27) previously demonstrated that H. somnus adherence to bovine aortic endothelial cells was significantly increased following activation of the endothelial cells with TNF-α. We found that BBEC secreted >600 pg/ml TNF-α during a 4-h incubation with H. somnus at 37°C (data not shown). We then tested whether BBEC responded to exogenously added TNF-α in a similar manner to the aortic endothelial cells used in the previous study (27). As shown in Fig. 4, a 1-hour preincubation of BBEC with TNF-α (100 ng/ml) more than doubled the number of adherent H. somnus after a 1- or 4-h incubation, compared to control BBEC.
FIG. 4.
Activation of BBEC with TNF-α increases the number of adherent H. somnus. BBEC were activated with 100 ng/nl TNF-α at 37°C for 1 h. Cells were washed, and approximately 30 H. somnus CFU were added per endothelial cell. The cells were incubated for 1 or 4 h at 37°C and then washed five times with PBS. The endothelial cells were lysed, and the adherent bacteria were enumerated by plating BBEC lysates on sheep blood agar plates. Results are displayed as the percentage of CFU adherent to untreated control BBEC. Data represent the mean ± SEM of three independent experiments. **, P < 0.01.
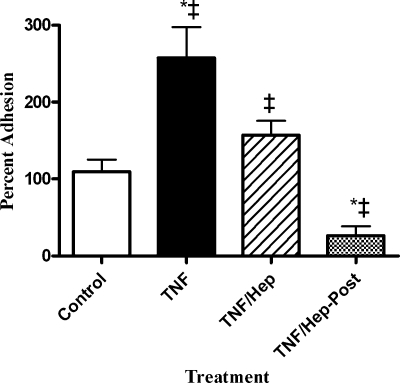
Heparin has been reported to have antiinflammatory properties, possibly due in part to its ability to bind various cytokines (11, 18, 20). To test whether heparin both inhibits TNF-α activation of BBEC and decreases H. somnus adherence to activated BBEC, we added heparin during either the TNF-α activation stage of the experiment or while TNF-α-activated BBEC were incubated with H. somnus. As shown in Fig. 5, preincubation of BBEC with TNF-α and heparin significantly reduced the subsequent number of adherent H. somnus, compared to BBEC preincubated with TNF-α alone. Addition of heparin subsequent to TNF-α activation of the BBEC also dramatically reduced the number of H. somnus adhering to the TNF-α-activated BBEC. These data support the hypothesis that heparin might both decrease cellular activation by TNF-α and interfere with the ability of H. somnus to bind resting or activated BBEC.
FIG. 5.
Heparin decreases the ability of TNF-α to augment adhesion of H. somnus to BBEC. BBEC were activated for 1 h at 37°C with 100 ng/nl TNF-α (TNF). Some cultures were coincubated with 1 mM heparin to determine whether heparin would prevent BBEC activation by TNF-α (TNF/Hep). In other wells, 1 mM heparin was added to BBEC previously activated with TNF-α to determine its ability to block H. somnus adhesion to TNF-α-activated BBEC (TNF/Hep-Post). Untreated cells served as a negative control. In all cases, H. somnus was incubated with BBEC (30 CFU per cell) for 1 at 37°C and then the BBEC were washed five times with PBS. The endothelial cells were lysed, and the CFU of adherent bacteria were enumerated by plating BBEC lysates on sheep blood agar plates. Results are expressed as the percentage of CFU recovered from nonactivated control BBEC. Data represent the mean ± SEM of three independent experiments. *, P < 0.05 compared to untreated control; ‡,P < 0.05 compared to each other.
Enzymatic digestion of heparan sulfate chains decreases H. somnus adhesion to BBEC.
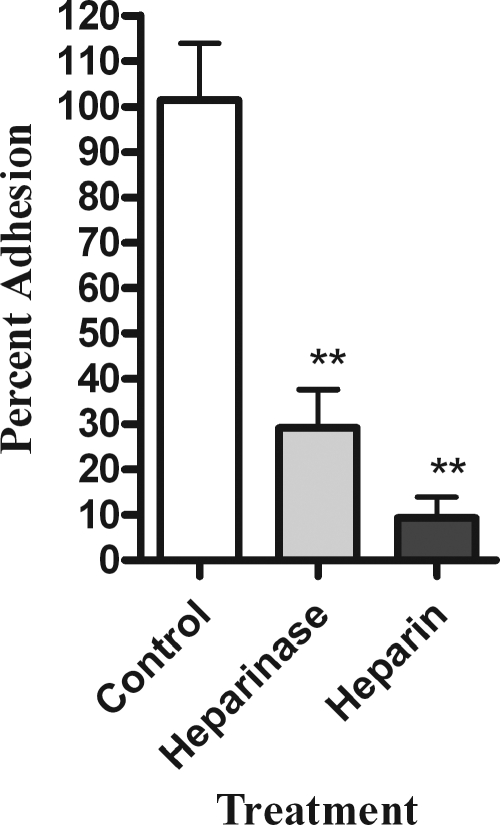
To further evaluate the role of heparan sulfate proteoglycans (HSPG) in H. somnus adhesion to BBEC, we next tested the ability of H. somnus to adhere to BBEC depleted of heparan sulfate chains. Heparinase III was used to enzymatically cleave the 1-4 linkages between the hexosamine and glucuronic acid residues from heparan sulfate molecules on the surface of the BBEC. We found that heparinase III treatment of the endothelial cells decreased the number of adherent H. somnus by more than 70% compared to untreated cells (Fig. 6).
FIG. 6.
Heparinase treatment reduces H. somnus adherence to BBEC. Adhesion of H. somnus to BBEC was evaluated following a 1-h incubation with 1 U/ml heparinase at 37°C to enzymatically digest HSPG. BBEC incubated with 1 mM heparin were included as a control for inhibition of bacterial adhesion. Approximately 30 H. somnus CFU were added per endothelial cell. The cells were then incubated for 4 h at 37°C and washed five times with PBS. The endothelial cells were lysed, and the adherent bacteria were enumerated by plating BBEC lysates on sheep blood agar plates. Data represent the mean ± SEM of three independent experiments. **, P < 0.01.
H. somnus expresses heparin-binding proteins.
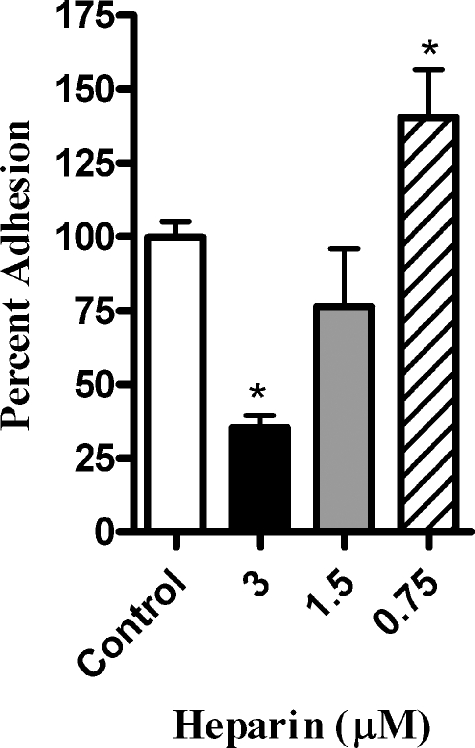
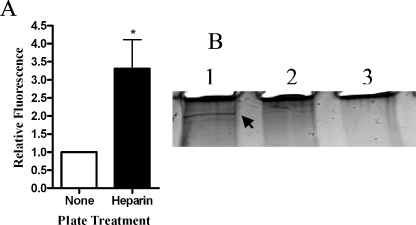
We next asked whether H. somnus produces heparin-binding proteins that interact with the endothelial cell surface. To address this point, we preincubated either the H. somnus cells or BBEC with heparin (0.001 to 2.5 mM) and then removed excess heparin by washing the cells prior to performing adhesion assays. Preincubating H. somnus with heparin at concentrations as low as 0.003 mM significantly reduced the ability of the bacterial cells to adhere to BBEC (Fig. 7 and data not shown). In contrast, preincubation of the BBEC with heparin (up to 1 mM) prior to adding H. somnus did not reduce adherence of the H. somnus to BBEC (data not shown). This finding led us to infer that the ability of exogenous heparin to reduce H. somnus adherence to BBEC was more likely due to a blockade of heparin-binding proteins on the H. somnus surface than a direct effect of heparin on the endothelial cells. To address this possibility further, we tested the ability of H. somnus to interact with heparin in a 4°C binding assay. Fluorescently labeled H. somnus bound with a higher efficiency (greater than a threefold increase in fluorescence) to plastic plates coated with heparin than to uncoated plates (Fig. 8A).
FIG. 7.
Pretreatment of H. somnus with heparin reduces its adherence to BBEC. H. somnus was incubated with heparin at 0.75 to 3 μM for 30 min at 37°C. Bacterial cells were pelleted, washed, and added to cultures of BBEC (30 H. somnus CFU per endothelial cell). The BBEC were then incubated for 4 h at 37°C, washed five times with PBS, and lysed. The number of adherent bacteria was enumerated by plating BBEC lysates on sheep blood agar plates. Data represent the mean ± SEM of three independent experiments. *, P < 0.05.
FIG. 8.
H. somus binds to heparin. (A) SYTO-BC Green-labeled H. somnus cells were added to heparin-coated plates incubated for 1 h at 4°C. Uncoated wells were used as a negative control. The plates were washed four times with PBS, and the fluorescence in each well was measured at 495/530 nm. Data are expressed as the ratio of fluorescence units relative to uncoated wells (mean ± SEM of five independent experiments). (B) Heparin-labeled beads were incubated with H. somnus culture filtrates (10 μg) for 1 h at 22°C. Beads that lacked heparin were included as a negative control. The beads were then washed five times with PBS, and the proteins were eluted by boiling in sample loading buffer. The eluted proteins were separated on a 4 to 20% polyacrylamide gel and visualized by silver staining. Lane 1, H. somnus filtrate incubated with heparin-coated beads; lane 2, H. somnus filtrate incubated with control beads; lane 3, heparin-coated bead control. The arrow in lane A indicates a 128-kDa putative heparin-binding protein. Results shown are for one representative experiment from three that were performed.
We next investigated the ability of H. somnus culture filtrate proteins (which include fibrillar network proteins) to bind biotinylated heparin attached to avidin-conjugated agarose beads. As shown in Fig. 8B, a high-molecular-weight protein of H. somnus (estimated molecular mass of approximately 128 kDa) bound to heparin-labeled beads but not to beads without heparin.
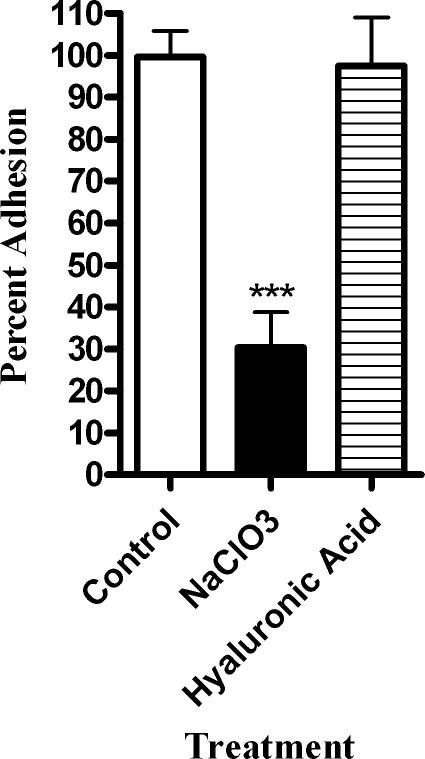
To further evaluate the role of glycosaminoglycan sulfation in H. somnus adherence, BBEC were incubated overnight with 50 μM sodium chlorate, which has been shown to reversibly inhibit the sulfation of cellular proteoglycans (23). The cells were then washed and inoculated with H. somnus. This treatment reduced the adhesion of H. somnus to the BBEC by more than 60% compared to control cells. In contrast, addition of 1 mM hyaluronic acid, a nonsulfated glycosaminoglycan, did not alter H. somnus adhesion (Fig. 9). These data suggest that sulfation of glycosaminoglycans is an important factor in the ability of endothelial cells to bind H. somnus.
FIG. 9.
H. somnus adherence to BBEC is reduced by pretreating BBEC with NaClO3. BBEC were incubated overnight with 50 mM NaClO3 to reduce GAG sulfation or coincubated with 1 mM hyaluronic acid (a nonsulfated GAG). Approximately 30 H. somnus CFU were added per endothelial cell. The cells were then incubated for 4 h at 37°C and washed five times with PBS. The endothelial cells were lysed, and the adherent bacteria were enumerated by plating BBEC lysates on sheep blood agar plates. Data represent the mean ± SEM of three independent experiments. ***, P < 0.001.
DISCUSSION
H. somnus can cause a devastating meningitis and encephalitis (TME) in cattle. Brain microvascular endothelial cells form the cellular backbone of the blood-brain barrier and normally serve to protect the CNS from pathogenic microorganisms in the bloodstream. Because H. somnus-induced neurological disease (TME) is generally preceded by septicemia, the interaction between H. somnus and the highly specialized brain microvascular endothelial cell is potentially a vital step in the progression of TME. This in vitro study is the first detailed investigation of H. somnus adherence to endothelial cells over time. Our findings identify the mechanisms involved in the interaction between H. somnus and microvascular endothelial cells of neurological origin.
We found that adherence of H. somnus to brain endothelial cells increased over time and was further increased by pretreatment of the BBEC with TNF-α. Adding greater numbers of H. somnus per BBEC failed to increase the number of adherent organisms recovered (data not shown). This observation, in conjunction with the relatively slow growth of H. somnus in RPMI 1640 medium (approximately 1 log10 CFU in 4 h at 37°C) suggests that changes that occur in the BBEC during the 4-h incubation might be responsible for the greater number of adherent H. somnus. In addition to production of cytokines (such as TNF-α), factors secreted by H. somnus (e.g., outer membrane blebs) could play a role in the activation of nearby endothelial cells and lead to an increase in H. somnus adhesion to BBEC over time.
Although this is the first study to investigate adhesion of H. somnus to endothelial cells over time, previously published studies demonstrated a role for cellular activation of large vessel endothelial cells in the adherence of H. somnus. Kwiecien et al. (27) demonstrated greater adherence of H. somnus to bovine aortic endothelial cells following treatment with TNF-α. In that study, TNF-α concentrations and duration of preincubation were varied, but the adhesion of H. somnus to resting and TNF-α-activated endothelial cells was investigated at only one time point (1.5 h). Therefore, we do not know if adherence of H. somnus to large vessel endothelial cells increased over time as it did for BBEC in the current study. Despite the differences in methodologies, the two studies clearly indicate a consistent trend of increased adherence of H. somnus to activated endothelial cells. The ability of TNF-α to increase the adherence of H. somnus to endothelial cells could play a role in the progression of TME. Perhaps cytokine activation of the endothelial cells during H. somnus septicemia promotes the ability of H. somnus to interact with BBEC lining the blood-brain barrier. These findings may help explain why TME has not yet been reported independent of septicemia, which frequently triggers a cytokine storm.
Our observation that H. somnus adheres to, but does not invade, BBEC is consistent with previous reports of endothelial cells from large vessels (i.e., pulmonary artery and aortic endothelial cells) (39, 42). In the present study, we did not detect appreciable invasion of BBEC by H. somnus (less than 5 CFU recovered from lysates of 10,000 endothelial cells) in a 24-h incubation period (data not shown). This lack of BBEC invasion contrasts with other bacteria capable of causing meningitis. For example, Neisseria meningitidis, Escherichia coli K1, group B streptococcus, Listeria monocytogenes, Citrobacter freundii, and Streptococcus suis serotype 2 have all been reported to invade brain endothelial cells (4, 19, 21, 33, 45). We included L. monocytogenes in some experiments to verify our ability to detect an invasive pathogen in our BBEC assay. It is important to note that the number of H. somnus added and the incubation times used in all our adhesion assays did not cause generalized cytotoxicity to the BBEC, as determined by negligible release of lactate dehydrogenase (data not shown).
The lack of BBEC invasion by H. somnus is somewhat unusual for a neurological pathogen and heightens the importance of understanding the events that occur once H. somnus adheres to the BBEC. Our observations contrast with those of Thompson et al. (42), who reported H. somnus cells within phagosomes in bovine aortic endothelial cells from carotid artery explants. However, because brain endothelial microvascular cells do not undergo endocytosis and are rarely pinocytic (13), it is not surprising that we did not find intracellular organisms in BBEC. However, we cannot exclude the possibility that our culture conditions were not conducive to the expression of some factor by the BBEC, or the bacterium, that is required for endothelial cell invasion (3). Because H. somnus does not appear to invade the brain endothelium, identifying molecules of either bacterial or host origin that influence the initial adherence of H. somnus to endothelial cells, or the activation of endothelial cells in a manner that augments H. somnus adherence (e.g., TNF-α), might delineate the pathogenesis of this important agent.
The role of proteoglycans in bacterial adherence to mammalian cells is an area of active investigation. Proteoglycans have been shown to be a cellular receptor for numerous bacterial species, including the related human pathogens Haemophilus influenzae and Haemophilus ducreyi (12, 17, 34). Proteoglycans, which consist of a protein core covalently linked to glycosaminoglycan chains, are highly hydrophilic, heterogenous, and ubiquitous on cellular surfaces (26). Proteoglycans on bovine endothelial cells most commonly incorporate either heparan sulfate or chondroitin sulfate into their glycosaminoglycan chains (35). Heparan sulfate proteoglycans (HSPG) have been shown to be involved in the adherence of a growing list of bacterial and viral pathogens to eukaryotic cells (5, 12, 16, 46, 48). Chondroitin sulfate has also been shown to be involved, to a lesser degree, in attachment of several bacterial pathogens (including Haemophilus influenzae) to mammalian cells (1, 5, 34). Some bacterial pathogens have been reported to utilize multiple subclasses of proteoglycans to adhere to eukaryotic cells (5, 29, 34). In our experiments, adherence of H. somnus to BBEC was greatly reduced in the presence of exogenous heparin, while chondroitin sulfate was less able to inhibit H. somnus adherence.
The strong inhibition of H. somnus adherence to BBEC by the addition of exogenous heparin suggests there are important interactions between H. somnus and heparan sulfate proteoglycans on the BBEC surface. Although structurally similar to the heparan sulfate expressed on cell surfaces, it is important to note that heparin has a higher number of sulfate groups that in turn give it a high-density negative charge. This negative charge will, in turn, interact with any protein containing a region of highly positive charges (31). Thus, heparin is a somewhat nonspecific inhibitor of highly charged physical interactions. However, the decrease in H. somnus adherence to BBEC when the endothelial cells were treated with either heparinase, to enzymatically remove sulfate chains from HSPG, or sodium chlorate, to inhibit the synthesis of all sulfated glycans, supports the hypothesis that sulfated proteoglycans play a role in adherence of H. somnus to BBEC.
Our observation that adhesion was inhibited by treating the bacteria, but not the endothelial cells, with heparin suggests that H. somnus expresses heparin-binding proteins on its surface. The observation that heparin also binds H. somnus at 4°C further supports the presence of heparin-binding proteins on the surface of H. somnus. This hypothesis was further strengthened by our demonstration of a high-mass (128-kDa) heparin-binding protein in H. somnus. Tagawa et al. (40) recently reported the presence of a heparin-binding domain in the high-molecular-mass immunoglobulin-binding protein IbpA (128 kDa) that is secreted by H. somnus. These authors also demonstrated decreased adherence of H. somnus to bovine pulmonary artery endothelial cells, after the addition of exogenous heparin (40), that was similar to what we observed with BBEC in the present report. Further work is needed to determine whether the putative heparin-binding domain in IbpA facilitates the observed adherence of H. somnus to BBEC.
The ability of heparin to modulate H. somnus adherence to BBEC might be more complex than simply inhibition of interactions between the BBEC and a bacterial heparin-binding protein. Heparin binds a number of cytokines and has been reported to decrease the inflammatory response of a variety of cell types, including endothelial cells (11, 18, 20). Besides directly blocking H. somnus adherence to BBEC, heparin pretreatment of BBEC also reduced the stimulatory effect of TNF-α on H. somnus binding. These data suggest that heparin could play an antiinflammatory role in our system. Perhaps in our longer-duration experiments, heparin abrogated paracrine activation of BBEC by cytokines that were released during the 4-h incubation with H. somnus.
The unexpected increase in H. somnus adherence when lower concentrations (0.125 mM or less) of heparin were added directly to the BBEC might be explained in several ways. One possibility is that the BBEC in our culture system are deficient in cellular HSPG, and addition of low concentrations of exogenous glycosaminoglycans (GAG) (i.e., heparin) results in increased synthesis (32), or sulfation (22), of endothelial cell GAG. The BBEC cell surface would then present additional docking sites (i.e., GAG) for H. somnus. A second possibility relates to the reported ability of heparin to enhance biofilm formation by Staphylococcus aureus (36). However, we consider this an unlikely possibility, because 4 h is not sufficient time for H. somnus to form a mature biofilm. A third possibility is that, at low concentrations, heparin acts as a bridging molecule between the bacterial cells and the endothelial cell. Precedence for this possibility is provided by Leishmania donovani promastigotes, which use heparin as a bridging molecule to adhere to peritoneal macrophages (7). While the lack of effect seen with pretreatment of the BBEC with heparin makes this explanation difficult to accept, the same adhesion-enhancing effect was observed when H. somnus was pretreated with low concentrations of heparin.
In summary, this in vitro study provides new insight into cellular events that might occur during the pathogenesis of TME. Bacteria that invade the CNS following septicemia must have a mechanism to breach the blood-brain barrier. The lack of direct endothelial cell invasion by H. somnus suggests that adherence of these bacterial cells to brain endothelial cells is a critical step in the ability of H. somnus to cause neurological disease. Adherence to the endothelium could allow the bacteria to elicit a localized inflammatory response, including the production of vasoactive cytokines and reactive nitrogen and oxygen intermediates, that weakens the blood-brain barrier (15, 44). The production of TNF-α and other inflammatory cytokines by activated endothelial cells, or nearby leukocytes during septicemia, could amplify H. somnus adhesion to the brain endothelium and play a role in the progression of the disease.
Editor: J. T. Barbieri
REFERENCES
- 1.Almeida, R. A., W. Fang, and S. P. Oliver. 1999. Adherence and internalization of Streptococcus uberis to bovine mammary epithelial cells are mediated by host cell proteoglycans. FEMS Microbiol. Lett. 177:313-317. [DOI] [PubMed] [Google Scholar]
- 2.Ames, T. R. 1987. Neurologic disease caused by Haemophilus somnus. Vet. Clin. North Am. Food Anim. Pract. 3:61-73. [DOI] [PubMed] [Google Scholar]
- 3.Badger, J. L., and K. S. Kim. 1998. Environmental growth conditions influence the ability of Escherichia coli K1 to invade brain microvascular endothelial cells and confer serum resistance. Infect. Immun. 66:5692-5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badger, J. L., C. A. Wass, and K. S. Kim. 2000. Identification of Escherichia coli K1 genes contributing to human brain microvascular endothelial cell invasion by differential fluorescence induction. Mol. Microbiol. 36:174-182. [DOI] [PubMed] [Google Scholar]
- 5.Baldassarri, L., L. Bertuccini, R. Creti, P. Filippini, M. G. Ammendolia, S. Koch, J. Huebner, and G. Orefici. 2005. Glycosaminoglycans mediate invasion and survival of Enterococcus faecalis into macrophages. J. Infect. Dis. 191:1253-1262. [DOI] [PubMed] [Google Scholar]
- 6.Boyle, E. C., and B. B. Finlay. 2003. Bacterial pathogenesis: exploiting cellular adherence. Curr. Opin. Cell Biol. 15:633-639. [DOI] [PubMed] [Google Scholar]
- 7.Butcher, B., L. Sklar, L. Seamer, and R. Glew. 1992. Heparin enhances the interaction of infective Leishmania donovani promastigotes with mouse peritoneal macrophages. A fluorescence flow cytometric analysis. J. Immunol. 148:2879-2886. [PubMed] [Google Scholar]
- 8.Chladek, D. W. 1975. Bovine abortion associated with Haemophilus somnus. Am. J. Vet. Res. 36:1041. [PubMed] [Google Scholar]
- 9.Corbeil, L. B., J. E. Arthur, P. R. Widders, J. W. Smith, and A. F. Barbet. 1987. Antigenic specificity of convalescent serum from cattle with Haemophilus somnus-induced experimental abortion. Infect. Immun. 55:1381-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corbeil, L. B., F. D. Bastida-Corcuera, and T. J. Beveridge. 1997. Haemophilus somnus immunoglobulin binding proteins and surface fibrils. Infect. Immun. 65:4250-4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cripps, J. G., F. A. Crespo, P. Romanovskis, A. F. Spatola, and R. Fernandez-Botran. 2005. Modulation of acute inflammation by targeting glycosaminoglycan-cytokine interactions. Int. Immunopharmacol. 5:1622-1632. [DOI] [PubMed] [Google Scholar]
- 12.de Vries, F. P., R. Cole, J. Dankert, M. Frosch, and J. P. van Putten. 1998. Neisseria meningitidis producing the Opc adhesin binds epithelial cell proteoglycan receptors. Mol. Microbiol. 27:1203-1212. [DOI] [PubMed] [Google Scholar]
- 13.Dorovini-Zis, K., R. Prameya, and P. D. Bowman. 1991. Culture and characterization of microvascular endothelial cells derived from human brain. Lab. Investig. 64:425-436. [PubMed] [Google Scholar]
- 14.Eberhard, T., and M. Ullberg. 2002. Interaction of vitronectin with Haemophilus influenzae. FEMS Immunol. Med. Microbiol. 34:215-219. [DOI] [PubMed] [Google Scholar]
- 15.Fiala, M., D. J. Looney, M. Stins, D. D. Way, L. Zhang, X. Gan, F. Chiappelli, E. S. Schweitzer, P. Shapshak, M. Weinand, M. C. Graves, M. Witte, and K. S. Kim. 1997. TNF-alpha opens a paracellular route for HIV-1 invasion across the blood-brain barrier. Mol. Med. 3:553-564. [PMC free article] [PubMed] [Google Scholar]
- 16.Fleckenstein, J. M., J. T. Holland, and D. L. Hasty. 2002. Interaction of an outer membrane protein of enterotoxigenic Escherichia coli with cell surface heparan sulfate proteoglycans. Infect. Immun. 70:1530-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frisk, A., and T. Lagergard. 1998. Characterization of mechanisms involved in adherence of Haemophilus ducreyi to eukaryotic cells. APMIS 106:539-546. [PubMed] [Google Scholar]
- 18.Gori, A. M., M. Attanasio, A. Gazzini, L. Rossi, L. Lucarini, S. Miletti, J. Chini, M. Manoni, R. Abbate, and G. F. Gensini. 2004. Cytokine gene expression and production by human LPS-stimulated mononuclear cells are inhibited by sulfated heparin-like semi-synthetic derivatives. J. Thromb. Haemost. 2:1657-1662. [DOI] [PubMed] [Google Scholar]
- 19.Greiffenberg, L., W. Goebel, K. S. Kim, I. Weiglein, A. Bubert, F. Engelbrecht, M. Stins, and M. Kuhn. 1998. Interaction of Listeria monocytogenes with human brain microvascular endothelial cells: InlB-dependent invasion, long-term intracellular growth, and spread from macrophages to endothelial cells. Infect. Immun. 66:5260-5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hochart, H., P. Vincent Jenkins, O. P. Smith, and B. White. 2006. Low-molecular weight and unfractionated heparins induce a downregulation of inflammation: decreased levels of proinflammatory cytokines and nuclear factor-κB in LPS-stimulated human monocytes. Br. J. Haematol. 133:62-67. [DOI] [PubMed] [Google Scholar]
- 21.Huang, S. H., M. F. Stins, and K. S. Kim. 2000. Bacterial penetration across the blood-brain barrier during the development of neonatal meningitis. Microbes Infect. 2:1237-1244. [DOI] [PubMed] [Google Scholar]
- 22.Humphries, D., C. Silbert, and J. Silbert. 1986. Glycosaminoglycan production by bovine aortic endothelial cells cultured in sulfate-depleted medium J. Biol. Chem. 261:9122-9127. [PubMed] [Google Scholar]
- 23.Humphries, D. E., and J. E. Silbert. 1988. Chlorate: a reversible inhibitor of proteoglycan sulfation. Biochem. Biophys. Res. Commun. 154:365-371. [DOI] [PubMed] [Google Scholar]
- 24.Isaacs, R. D. 1994. Borrelia burgdorferi bind to epithelial cell proteoglycans. J. Clin. Investig. 93:809-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janzen, E. D. 1989. The Haemophilus somnus disease complex (hemophilosis): a review. Can. Vet. J. 30:816-822. [PMC free article] [PubMed] [Google Scholar]
- 26.Kjellen, L., and U. Lindahl. 1991. Proteoglycans: structures and interactions. Annu. Rev. Biochem. 60:443-475. [DOI] [PubMed] [Google Scholar]
- 27.Kwiecien, J. M., P. B. Little, and M. A. Hayes. 1994. Adherence of Haemophilus somnus to tumor necrosis factor-alpha-stimulated bovine endothelial cells in culture. Can. J. Vet. Res. 58:211-219. [PMC free article] [PubMed] [Google Scholar]
- 28.Kwiecien, J. M., and P. B. Little. 1992. Isolation of pathogenic strains of Haemophilus somnus from the female bovine reproductive tract. Can. J. Vet. Res. 56:127-134. [PMC free article] [PubMed] [Google Scholar]
- 29.Leong, J. M., H. Wang, L. Magoun, J. A. Field, P. E. Morrissey, D. Robbins, J. B. Tatro, J. Coburn, and N. Parveen. 1998. Different classes of proteoglycans contribute to the attachment of Borrelia burgdorferi to cultured endothelial and brain cells. Infect. Immun. 66:994-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacLean, A. G., M. S. Orandle, X. Alvarez, K. C. Williams, and A. A. Lackner. 2001. Rhesus macaque brain microvessel endothelial cells behave in a manner phenotypically distinct from umbilical vein endothelial cells. J. Neuroimmunol. 118:223-232. [DOI] [PubMed] [Google Scholar]
- 31.Mulloy, B. 2005. The specificity of interactions between proteins and sulfated polysaccharides. An. Acad. Bras Cienc. 77:651-664. [DOI] [PubMed] [Google Scholar]
- 32.Nader, H. B., V. Buonassisi, P. Colburn, and C. P. Dietrich. 1989. Heparin stimulates the synthesis and modifies the sulfation pattern of heparan sulfate proteoglycan from endothelial cells. J. Cell Physiol. 140:305-310. [DOI] [PubMed] [Google Scholar]
- 33.Nizet, V., K. S. Kim, M. Stins, M. Jonas, E. Y. Chi, D. Nguyen, and C. E. Rubens. 1997. Invasion of brain microvascular endothelial cells by group B streptococci. Infect. Immun. 65:5074-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noel, G. J., D. C. Love, and D. M. Mosser. 1994. High-molecular-weight proteins of nontypeable Haemophilus influenzae mediate bacterial adhesion to cellular proteoglycans. Infect. Immun. 62:4028-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oohira, A., T. N. Wight, and P. Bornstein. 1983. Sulfated proteoglycans synthesized by vascular endothelial cells in culture. J. Biol. Chem. 258:2014-2021. [PubMed] [Google Scholar]
- 36.Shanks, R. M., N. P. Donegan, M. L. Graber, S. E. Buckingham, M. E. Zegans, A. L. Cheung, and G. A. O'Toole. 2005. Heparin stimulates Staphylococcus aureus biofilm formation. Infect. Immun. 73:4596-4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stephens, L. R., P. B. Little, B. N. Wilkie, and D. A. Barnum. 1981. Infectious thromboembolic meningoencephalitis in cattle: a review. J. Am. Vet. Med. Assoc. 178:378-383. [PubMed] [Google Scholar]
- 38.Stins, M. F., N. V. Prasadarao, J. Zhou, M. Arditi, and K. S. Kim. 1997. Bovine brain microvascular endothelial cells transfected with SV40-large T antigen: development of an immortalized cell line to study pathophysiology of CNS disease. In Vitro Cell Dev. Biol. Anim. 33:243-247. [DOI] [PubMed] [Google Scholar]
- 39.Sylte, M. J., L. B. Corbeil, T. J. Inzana, and C. J. Czuprynski. 2001. Haemophilus somnus induces apoptosis in bovine endothelial cells in vitro. Infect. Immun. 69:1650-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tagawa, Y., J. D. Sanders, I. Uchida, F. D. Bastida-Corcuera, K. Kawashima, and L. B. Corbeil. 2005. Genetic and functional analysis of Haemophilus somnus high molecular weight-immunoglobulin binding proteins. Microb. Pathog. 39:159-170. [DOI] [PubMed] [Google Scholar]
- 41.Thomas, R., and T. Brooks. 2004. Common oligosaccharide moieties inhibit the adherence of typical and atypical respiratory pathogens. J. Med. Microbiol. 53:833-840. [DOI] [PubMed] [Google Scholar]
- 42.Thompson, K. G., and P. B. Little. 1981. Effect of Haemophilus somnus on bovine endothelial cell in organ culture. Am. J. Vet. Res. 42:748-754. [PubMed] [Google Scholar]
- 43.Townsend, G. C., and W. M. Scheld. 1995. In vitro models of the blood-brain barrier to study bacterial meningitis. Trends Microbiol. 3:441-445. [DOI] [PubMed] [Google Scholar]
- 44.Vadeboncoeur, N., M. Segura, D. Al-Numani, G. Vanier, and M. Gottschalk. 2003. Pro-inflammatory cytokine and chemokine release by human brain microvascular endothelial cells stimulated by Streptococcus suis serotype 2. FEMS Immunol. Med. Microbiol. 35:49-58. [DOI] [PubMed] [Google Scholar]
- 45.Vanier, G., M. Segura, P. Friedl, S. Lacouture, and M. Gottschalk. 2004. Invasion of porcine brain microvascular endothelial cells by Streptococcus suis serotype 2. Infect. Immun. 72:1441-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vlasak, M., I. Goesler, and D. Blaas. 2005. Human rhinovirus type 89 variants use heparan sulfate proteoglycan for cell attachment. J. Virol. 79:5963-5970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waldhalm, D. G., R. F. Hall, W. A. Meinershagen, C. S. Card, and F. W. Frank. 1974. Haemophilus somnus infection in the cow as a possible contributing factor to weak calf syndrome: isolation and animal inoculation studies. Am. J. Vet. Res. 35:1401-1403. [PubMed] [Google Scholar]
- 48.Wuppermann, F. N., J. H. Hegemann, and C. A. Jantos. 2001. Heparan sulfate-like glycosaminoglycan is a cellular receptor for Chlamydia pneumoniae. J. Infect. Dis. 184:181-187. [DOI] [PubMed] [Google Scholar]
- 49.Zhang, J. P., and R. S. Stephens. 1992. Mechanism of C. trachomatis attachment to eukaryotic host cells. Cell 69:861-869. [DOI] [PubMed] [Google Scholar]