Abstract
Toll-like receptors (TLRs) expressed by the corneal epithelium represent a first line of host defense to microbial keratitis. The current study examined the role of TLR2, TLR4, and TLR9 and the common adaptor molecule myeloid differentiation factor 88 (MyD88) in a Staphylococcus aureus model of corneal inflammation. The corneal epithelia of C57BL/6, TLR2−/−, TLR4−/−, TLR9−/−, and MyD88−/− mice were abraded using a trephine and epithelial brush and were exposed to heat- or UV-inactivated S. aureus clinical strain 8325-4 and other clinical isolates. Corneal thickness and haze were measured by in vivo confocal microscopy, neutrophil recruitment to the corneal stroma was quantified by immunohistochemistry, and cytokine production was measured by enzyme-linked immunosorbent assay. The exposure of corneal epithelium to S. aureus induced neutrophil recruitment to the corneal stroma and increased corneal thickness and haze in control C57BL/6 mice but not in TLR2−/− or MyD88−/− mice. The responses of TLR4−/− and TLR9−/− mice were similar to those of C57BL/6 mice. S. aureus-induced cytokine production by corneal epithelial cells and neutrophils was also significantly reduced in TLR2−/− mice compared with that in C57BL/6 mice. These findings indicate that S. aureus-induced corneal inflammation is mediated by TLR2 and MyD88 in resident epithelial cells and infiltrating neutrophils.
Staphylococcus aureus is a major cause of bacterial keratitis worldwide, especially with the increased incidence of infections by methicillin-resistant S. aureus (4, 5, 29, 30). When S. aureus penetrates the corneal epithelium and the corneal stroma, there is rapid bacterial replication, production of toxins, including hemolytic α-toxin, and severe tissue damage, leading to corneal opacity. S. aureus infection also stimulates extensive neutrophil infiltration to the corneal stroma, and subsequent degranulation and release of cytotoxic mediators further contribute to the pathogenesis of this disease (11, 13, 25, 26, 37).
Our laboratory has focused on the interaction of microbial pathogens in the cornea and on identifying pathways that lead to neutrophil recruitment to the corneal stroma and loss of corneal clarity. We demonstrated that bacterial products activate the Toll-like receptor (TLR) family of pathogen recognition molecules on corneal epithelial cells to produce CXC chemokines, which then facilitate neutrophil recruitment from limbal vessels into the corneal stroma, leading to corneal haze (18). We examined three members of the TLR family, TLR2, which binds lipoproteins, TLR4, which binds bacterial lipopolysaccharide (LPS), and TLR9, which binds unmethlyated CpG-rich DNA, and reported that specific activation of TLR2, TLR4, and TLR9 in the mouse corneal epithelium induces this sequence of events and that corneal inflammation induced by the activation of these TLRs is completely dependent on the common adaptor molecule myeloid differentiation factor 88 (MyD88) (15).
In the current study, we examined the role of TLRs and MyD88 in the host response to UV-inactivated S. aureus, which can adhere to corneal epithelial cells (14) and allowed us to examine the role of TLRs in the absence of toxin production. We found that (i) clinical and laboratory strains of S. aureus induced neutrophil recruitment to the corneal stroma and increased corneal haze and thickness, (ii) corneal inflammation and CXC chemokine production are dependent on TLR2 and MyD88, but not on TLR4 or TLR9, and (iii) S. aureus-induced neutrophil activation is dependent on TLR2. Together, these findings demonstrate a critical role for the TLR2/MyD88 pathway in the host response to S. aureus.
MATERIALS AND METHODS
S. aureus strains and preparation.
S. aureus (strain 8325-4) isolates were incubated in 10 ml of tryptic soy broth (Difco, Detroit, MI) at 37°C overnight and subcultured 1:100 in fresh tryptic soy broth; a log-phase culture was grown to an optical density of 0.3 at 650 nm (approximately 108 CFU/ml), washed three times with phosphate-buffered saline (BioWhittaker), and diluted in phosphate-buffered saline. Clinical strains were obtained from University Hospitals of Cleveland, and the laboratory-derived Wood strain was obtained from Molecular Probes, Inc. Heat-inactivated S. aureus isolates were incubated at 85°C for 15 min. For UV-inactivated S. aureus, we utilized a UV Stratalinker (Stratagene) at a setting of 2400 for 15 min, and bacterial killing by both methods was confirmed by incubating treated bacteria on blood agar plates.
HEK-293 cells.
Human embryonic kidney 293 cells stably transfected with TLR2, TLR3, or TLR4 were obtained from Eicke Latz (University of Massachusetts Medical School, Worchester, MA) and maintained in Dulbecco's modified Eagle's medium containing 4.5 g/liter glucose with l-glutamine (BioWhittaker), 10% low endotoxin-fetal calf serum (Atlanta Biological), and 10 μg/ml Cipro (Cellgro) at 37°C in a humidified atmosphere of 5% CO2. A total of 1 × 105 adherent cells/well were stimulated overnight in duplicate with S. aureus and TLR controls, and TLR activation was determined using an enzyme-linked immunosorbent assay (ELISA) for secreted interleukin-8 (IL-8) (R & D Systems, Minneapolis, MN). Given that the background can be variable among transfected cell lines, data are reported as the ratio of IL-8 in ligand-stimulated cells to that in unstimulated cells (media alone).
Reagents.
Pam3CysK4 was purchased from EMC Microcollections (Tübingen, Germany). Ultrapure LPS (Escherichia coli O111:B4) (TLR4/MD2) and poly(I:C) (TLR3) were purchased from InvivoGen (San Diego, CA) and used at a concentration of 100 ng/ml LPS and Pam3Cys and 125 ng/ml poly(I:C).
Source of mice.
C57BL/6 mice (6 to 8 weeks old) were purchased from the Jackson Laboratory (Bar Harbor, ME). TLR2-deficient, TLR4-deficient, and MyD88-deficient mice were generously provided by Shizuo Akira (Research Institute for Microbial Disease, Osaka University, Osaka, Japan). TLR2−/− and TLR4−/− were fully backcrossed to C57BL/6 mice, and age-matched littermates were used for MyD88−/− mice. All animals were housed under specific pathogen-free conditions in microisolator cages and maintained according to institutional guidelines and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
In vivo model of S. aureus-induced corneal inflammation.
Mice were anesthetized by a intraperitoneal injection of 0.4 ml 2,2,2-tribromoethanol (1.2%). A 1-mm2 area of the central cornea was incised using a sterile trephine (Miltex, Tuttlingen, Germany), and the epithelial layer within that area was abraded using an Algerbrush II (Alger, Lago Vista, TX). Histology showed that the abrasion did not include the stroma (not shown). A 1-μl aliquot containing heat- or UV-inactivated S. aureus or saline was applied to the denuded cornea.
In vivo confocal microscopy.
In vivo analysis of the corneal infiltration was evaluated by a ConfoScan 3 microscope system (Nidek Technologies, Fremont, CA) as described previously (15). Mice were anesthetized and immobilized on a platform, and a transparent gel (GenTeal; Novartis Ophthalmics, Duluth, GA) was used to provide contact between the corneal surface and the 40× objective. Images were captured using NAVIS software (NAVIS; Lucent Technologies, Murray Hill, NJ) and stored as a stack for analysis of corneal thickness and haze. Stromal thickness was defined as the distance between the basal epithelium and corneal endothelium, and stromal haze was defined as stromal thickness times the combined light intensity of each image of the corneal stroma. These values were exported to Microsoft Excel and then to Prism (GraphPad Software, San Diego, CA) to generate a curve, and the area under the curve was calculated and compared with baseline measurements of naïve mouse corneas.
Immunohistochemistry.
Eyes from control and infected mice were enucleated at 24 h posttreatment and snap-frozen in liquid nitrogen, and 5-μm sections from the center of the eye (as determined by noncontiguous iris morphology) were fixed in 4% formaldehyde for 30 min. The slides were washed with 0.05 M Tris buffer (TBS, pH 7.6), and sections were incubated for 2 h with anti-neutrophil antibody NIMP-R14 (1.91 μg/ml) in 1% fetal calf serum-TBS as described previously (15, 18). Sections were washed as described above and incubated with fluorescein isothiocyanate-conjugated rabbit anti-rat antibody (Vector Laboratories, Burlingame, CA) diluted 1:200 in 1% fetal calf serum-TBS for 45 min. After being washed, the slides were mounted in Vectashield (Vector Laboratories, Burlingame, CA). The number of neutrophils in the corneal stroma per section was counted by fluorescence microscopy (magnification, ×40) (Olympus Optical Co. Ltd., Tokyo, Japan).
Measurement of cytokines in the corneas.
To measure cytokine production in the corneal epithelium, corneas were carefully dissected so that there were no attached limbuses, irises, or lenses. The epithelial layer was teased apart from the stroma after 20 min of incubation at 37°C with 20 mM EDTA and placed into RPMI 1640 medium. Samples were disrupted by sonication for 88 s with 40% duty cycle (Vibracell; Sonics and Material, Danbury, CT). Cytokines were measured by ELISA using commercially available antibodies according to the manufacturer's instructions (R & D Systems, Minneapolis, MN).
In vitro neutrophil activation.
Mice were injected with 1 ml of 9% casein at −19 h and −3 h. After euthanasia, cells were recovered from peritoneal lavage, washed, and layered onto sterile 90% Percoll gradient (Pharmacia Biotech, Piscataway, NJ). Cells were then centrifuged at 4°C at an average centrifugal force of 65,000 × g. The neutrophil population (>95%) was recovered from the second layer on the gradient as determined by cytology. Neutrophils were then washed in Hank's balanced salt solution, incubated in Dulbecco's modified Eagle's medium for 2 h at 37°C with 50 ng/ml granulocyte-macrophage colony-stimulating factor, and stimulated for 15 h with S. aureus or TLR ligands. Cytokines in the culture supernatants were measured by ELISA (R & D Systems, Minneapolis, MN), and viability was >95%, as determined by trypan blue exclusion.
Statistics.
Student's t test was used to analyze data (Prism), and statistical significance was defined as a P value of <0.05.
RESULTS
S. aureus activates TLR2 in a reporter cell line.
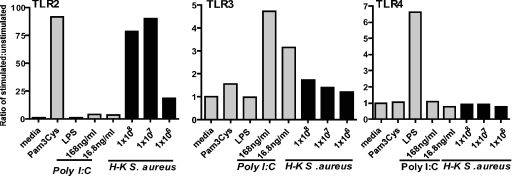
To determine which TLRs are directly activated by S. aureus, human embryonic kidney cells expressing TLR2, TLR3, or TLR4 were incubated with heat-inactivated bacteria overnight, and activation was determined by IL-8 production. As shown in Fig. 1, cells expressing TLR2, -3, and -4 were activated by only specific ligands Pam3Cys, poly(I:C), and LPS, respectively. S. aureus strain 8325-4 stimulated TLR2, but not TLR3 or TLR4, indicating that S. aureus specifically activates this receptor.
FIG. 1.
S. aureus-induced TLR activation in IL-8/TLR reporter cells. A human embryonic kidney (H-K) cell line expressing TLR2, TLR3, or TLR4 was incubated overnight with specific ligand Pam3Cys (TLR2), poly(I:C) (TLR3), or LPS (TLR4) or with S. aureus (number of bacteria/well). IL-8 production was measured by ELISA and presented as a ratio of stimulated to unstimulated cells. Data represent the means of three wells per experimental group. The experiment was repeated three times with similar results.
TLR2 plays a pivotal role in S. aureus-induced corneal inflammation.
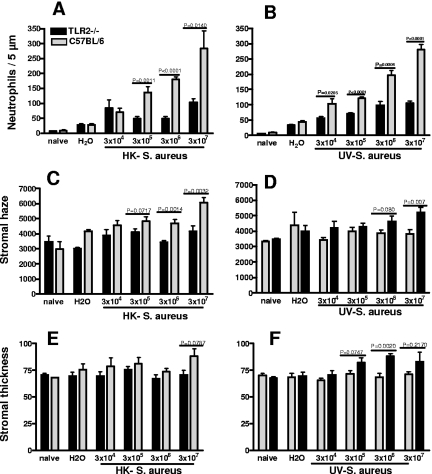
To determine whether TLR2 is important in the development of corneal inflammation in response to inactivated S. aureus, corneas of C57BL/6 and TLR2−/− mice were abraded as described above and heat- or UV-inactivated S. aureus (strain 8325-4) isolates were added topically. After 24 h, the epithelia had healed in all animals, as determined by fluorescein exclusion (data not shown). Neutrophil infiltration to the corneal stroma was examined by immunohistochemistry, and corneal thickness and haze were measured by in vivo confocal microscopy. As shown in Fig. 2A and B, the number of neutrophils in the corneal stroma increased according to the number of bacteria added to the corneal surface. Neutrophil recruitment to the corneas of TLR2−/− mice was significantly lower than that of C57BL/6 mice at each concentration.
FIG. 2.
Corneal inflammation in C57BL/6 and TLR2−/− mice induced by S. aureus strain 8325-4. Corneas of control C57BL/6 mice and TLR2−/− mice were abraded and exposed to either heat- or UV-inactivated S. aureus strain 8325-4. After 24 h, mice were sacrificed and examined by in vivo confocal microscopy to measure corneal thickness and haze. Neutrophils were detected in 5-μm corneal sections by immunohistochemistry and counted from limbus to limbus. (A and B) Neutrophils in C57BL/6 mice and TLR2−/− mice. (C and D) Corneal haze. (E and F) Corneal thickness. Data are the means ± standard errors of the means (SEM) (error bars) of five mice per group. P values are noted. Experiments were repeated with similar results.
Our previous studies demonstrated that neutrophil infiltration is also associated with changes in the corneal structure, which are detected as increased corneal thickness and haze (15). To determine whether TLR2 also has a role in the development of increased corneal haze and thickness, corneas of C57BL/6 and TLR2−/− mice treated as described above were examined by in vivo confocal microscopy using a Nidek ConfoScan. As shown in Fig. 2C to F, corneal thickness and haze values for TLR2−/− mice were significantly lower than those for control mice.
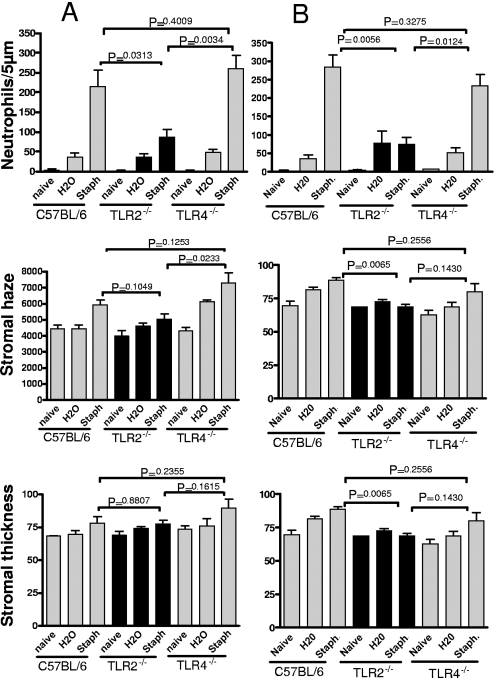
To determine whether the effect of TLR2 was specific for S. aureus strain 8325-4, corneas of C57BL/6, TLR2−/− and TLR4−/− mice were abraded and exposed to 1 × 107 heat-inactivated bacteria of either the Wood strain of S. aureus (Fig. 3A) or a methicillin-resistant hospital isolate (Fig. 3B). As shown in the upper panels of Fig. 3, both strains of S. aureus induced a significant increase in the number of neutrophils in the corneal stroma compared with that in H2O controls in C57BL/6 mice and TLR4−/− mice; however, the number of neutrophils in TLR2−/− corneas was significantly lower. Consistent with this finding, there was no significant difference in stromal thickness and haze between C57BL/6 mice and TLR4−/− mice exposed to either isolate of S. aureus, whereas stromal haze was significantly reduced in TLR2−/− mice compared with that in C57BL/6 mice and TLR4−/− mice. Stromal thickness showed results similar to those of stromal haze, although the difference between C57BL/6 mice and TLR2−/− mice exposed to the Wood strain was not statistically significant.
FIG. 3.
Corneal inflammation induced by S. aureus (Staph) laboratory strain and methicillin-resistant S. aureus clinical isolate. Corneas of C57BL/6, TLR2−/−, and TLR4−/− mice were treated as described in the legend for Fig. 2 and exposed to (A) heat-inactivated Wood strain of S. aureus or (B) a clinical isolate from University Hospitals of Cleveland. Neutrophils, corneal thickness, and haze were measured as described in the text. Data are the means ± SEM (error bars) of five mice per group. The P value was <0.05 for C57BL/6 versus TLR2−/− mice but not C57BL/6 versus TLR4−/− mice. Experiments were repeated twice with similar results.
Taken together with results shown in Fig. 2, these findings clearly demonstrate an essential role for TLR2, but not TLR4, in S. aureus-induced corneal inflammation.
MyD88 is required for the development of S. aureus-induced corneal inflammation.
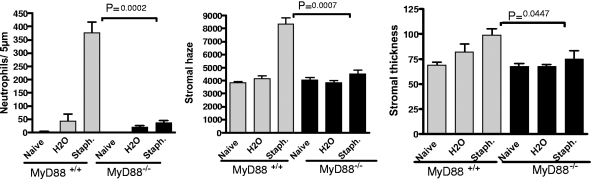
As TLR2 has been shown to signal through the common adaptor molecule MyD88 (15, 32), we next examined whether MyD88 is required for S. aureus-induced corneal inflammation. Corneas of MyD88−/− mice and control littermates were abraded, and 3 × 107 heat-inactivated S. aureus (Wood strain) isolates were added to the corneal surface. Neutrophil infiltration and corneal thickness and haze were measured as described above.
Figure 4 shows that S. aureus-induced neutrophil infiltration and increased thickness and haze were significantly reduced in MyD88−/− mice, indicating that MyD88 has an essential role in S. aureus-induced corneal inflammation.
FIG. 4.
The role of MyD88 in S. aureus (Staph)-induced corneal inflammation. Corneas of control and MyD88−/− mice were treated as described in the legend for Fig. 2 and exposed to heat-inactivated S. aureus Wood strain. Neutrophils, corneal thickness, and haze were measured as described in the text. Data are the means ± SEM (error bars) of five mice per group. The P value was <0.05 for C57BL/6 versus MyD88−/− mice. Similar results were found in repeat experiments.
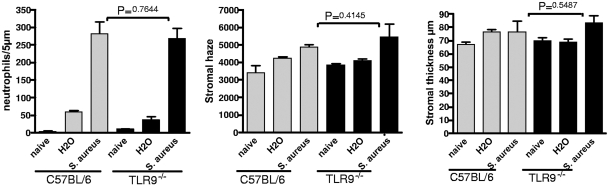
TLR9 is not required for the development of S. aureus-induced corneal inflammation.
TLR9 is the receptor for CpG-rich bacterial DNA, and the activation of this receptor by CpG oligonucleotides can induce corneal inflammation (15). Furthermore, TLR9 can be activated by heat-inactivated bacteria to induce a cellular response (17). To determine whether TLR9 has a role in corneal inflammation induced by inactivated S. aureus, corneas of C57BL/6 and TLR9−/− mice were abraded and exposed to 1 × 107 S. aureus heat-inactivated strain 8325-4, and neutrophil infiltration and corneal thickness and haze were measured as described above. As shown in Fig. 5, neutrophil infiltration and corneal thickness and haze were increased in control C57BL/6 and TLR9−/− mice, with no significant differences between the two groups. These data indicate that TLR9 has no essential role in S. aureus-induced corneal inflammation.
FIG. 5.
The role of TLR9 in S. aureus (Staph)-induced corneal inflammation. Corneas of control and TLR9−/− mice were treated as described in the legend for Fig. 2 and exposed to heat-inactivated S. aureus strain 8325-4. Neutrophils, corneal thickness, and haze were measured as described in the text. Data are the means ± SEM (error bars) of five mice per group. The P value was >0.05 for C57BL/6 versus TLR9−/− mice. A repeat experiment showed similar results.
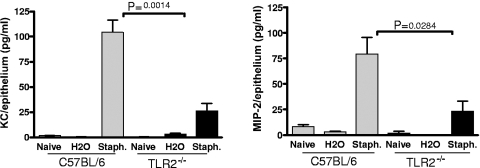
TLR2-dependent S. aureus-induced chemokine production by corneal epithelial cells.
Previous studies showed that CXC chemokines CXCL1/KC and CXCL2/macrophage inflammatory protein 2 (MIP-2) contribute to neutrophil recruitment to the corneal stroma (8, 18). To determine whether S. aureus stimulates chemokine production and to determine the role of TLR2, corneas of C57BL/6 and TLR2−/− mice were abraded and exposed to 3 × 107 S. aureus. After 5 h, which is prior to neutrophil infiltration, corneas were dissected and corneal epithelium was separated from the stroma after incubation with EDTA. The epithelial layer was sonicated, and chemokines were measured by ELISA. As shown in Fig. 6, corneas of C57BL/6 mice produced CXCL1/KC and CXCL2/MIP-2 in response to S. aureus stimulation; however, chemokine production in corneas of TLR2−/− mice was significantly reduced compared with that in control corneas, indicating an essential role for TLR2 in S. aureus-induced chemokine production by corneal epithelial cells.
FIG. 6.
S. aureus (Staph.)-induced chemokine production by corneal epithelial cells. Corneas of control and TLR2−/− mice were treated as described in the legend for Fig. 2 and exposed to UV-inactivated S. aureus strain 8325-4. After 5 h, corneas were dissected and epithelia were removed and sonicated. CXCL1/KC and CXCL2/MIP-2 in the supernatants were measured by ELISA. The P value was <0.05 for C57BL/6 versus TLR2−/− mice for both chemokines. A repeat experiment showed similar results. Error bars indicate SEM.
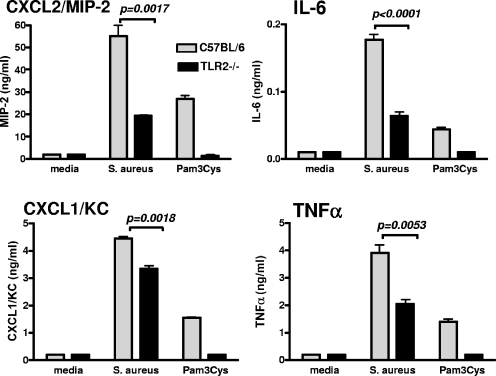
TLR2-dependent S. aureus-induced cytokine production by neutrophils.
Activation of TLR2 on human neutrophils induces cytokine production and modulates the expression of neutrophil surface molecules (10, 27). As neutrophils recruited to the corneal stroma are likely to be activated by S. aureus, we examined the role of TLR2 in the neutrophil response to S. aureus. A highly enriched (>98%) population of neutrophils was isolated from peritoneal lavage cells from C57BL/6 and TLR2−/− mice and incubated with S. aureus. IL-6, tumor necrosis factor alpha, CXCL1/KC, and CXCL2/MIP-2 were measured by ELISA. As shown in Fig. 7, S. aureus induced production of each of these cytokines by C57BL/6 neutrophils, whereas cytokine production by TLR2−/− neutrophils was significantly different from that by C57BL/6 neutrophils, although unlike the synthetic TLR2 ligand, Pam3Cys was not ablated. These data indicate that TLR2 is an important, though not exclusive, mediator of S. aureus-induced neutrophil activation.
FIG. 7.
S. aureus-induced cytokine production by peritoneal neutrophils. A highly purified population of neutrophils from C57BL/6 and TLR2−/− mice was incubated overnight with 1 × 108 S. aureus or the TLR2 ligand Pam3Cys, and cytokine production was measured by ELISA. Values are means ± SEM (error bars) of three replicate wells. Similar results were found in a repeat experiment. TNF-α, tumor necrosis factor alpha.
DISCUSSION
S. aureus is a common commensal of normal individuals, where it is detected on the skin and periocular tissue. Proximity to the cornea is likely to be via the tear film, where bacteria are exposed to antimicrobial components, such as β-defensins, lysozymes, and phospholipases. S. aureus is readily killed by tear components, especially phospholipase A2 (24), but bacterial products can stimulate a local inflammatory response (15, 18, 28). In a murine model of S. aureus keratitis, a topical application of 1 × 108 bacteria to scarred corneas caused keratitis in A/J and BALB/c, though not C57BL/6, mouse strains as measured by slit lamp examination (7). In the model described here, we used C57BL/6 mice as all the knockout gene mice are on this background. Consistent with the previous study (7), we saw no changes by slit lamp examination (data not shown); however, when we examined early subclinical responses using in vivo confocal microscopy, we found consistent increases in stromal thickness and haze in response to S. aureus that were dependent on functional TLR2 and MyD88.
In systemic S. aureus infection, TLR2 has an important role both in bacterial clearance and in the host response, as TLR2−/− mice are more susceptible to systemic infection (33) and cardiac dysfunction (19) than are control mice. In the current study, we found that as few as 3 × 104 inactivated bacteria stimulated neutrophil infiltration and development of corneal haze compared to that with trauma control, and neutrophil recruitment increased with exposure to higher numbers of bacteria. Although bacteria were inactivated using either UV irradiation or heat rather than phospholipase A2, our findings were consistent with regards to TLR usage: using three bacterial isolates, including strain 8325-4, and two methods of bacterial inactivation, we found that the development of keratitis was dependent on TLR2 and MyD88 and independent of TLR4 and TLR9. Although we cannot eliminate the possibility that TLR9 might contribute in the absence of TLR2, it is clear that TLR2 is the dominant receptor. The TLR2 ligand on S. aureus was shown to be lipoprotein rather than peptidoglycan or lipoteichoic acid, as TLR2 activity is reduced after lipase treatment (9, 34). Proteinase K treatment of S. aureus extracts did not affect TLR2 activity (9), which is consistent with results from the current study in which protein denaturation by heat treatment had no effect on S. aureus-induced TLR2 responses.
The common adaptor molecule MyD88 is a key mediator of TLRs and IL-1 and IL-18 (32, 36). In the current study, we showed that it is also important in TLR2 signaling in the cornea (15). Although IL-1R rather than TLR2 is important in a mouse model of S. aureus dermatitis (23), the current study showed that MyD88 and TLR2 are essential for keratitis induced by inactivated S. aureus. Future studies will determine whether secondary signaling through IL-1R/MyD88 also occurs with live S. aureus.
In addition to providing new findings in the host response to S. aureus, the current study further confirms the presence and functionality of TLR2 and MyD88 in the cornea. We and others have reported the presence and potential roles of TLR2, TLR3, TLR4, TLR5, and TLR9 in the cornea, although the findings between murine models and in human corneal epithelial cells are not always in agreement (2). TLR2, which is clearly functional in the murine model, can be activated by S. aureus in corneal epithelial cells (21). TLR3 and TLR5, which are activated by double-stranded RNA and bacterial flagellin, respectively, are also present and functional in human corneal epithelial cells (16, 22, 38) Although TLR9 has not been described in human corneal epithelial cells, TLR9 activation in the mouse cornea induces keratitis (15) and TLR9 short interfering RNA reduces the severity of Pseudomonas aeruginosa keratitis (12). In contrast, there are conflicting reports of the role of TLR4 in the cornea; two reports indicate it is nonfunctional due to either an intracellular location or the absence of costimulatory molecules (1, 35), whereas another report shows that TLR4 is functional (31). The underlying difference for this discrepancy is not clear, but LPS can induce keratitis in murine and rabbit models of epithelial abrasion (15, 18, 28).
We predict that the initial role of TLR2 and MyD88 in S. aureus keratitis is activating basal and intermediate corneal epithelial cells to produce chemotactic and proinflammatory cytokines that mediate neutrophil recruitment to the corneal stroma. As neutrophils express most TLRs (6, 10), a second role for this pathway would therefore be S. aureus-induced activation of neutrophils and further production of CXC chemokines in the corneal stroma. Results from the current study demonstrate that S. aureus-stimulated neutrophils produce high levels of CXC chemokines in a TLR2/MyD88-dependent manner. Activated neutrophils would also degranulate and release cytotoxic mediators, such as nitric oxide and myeloperoxidase, which cause tissue damage and loss of corneal clarity (3, 18).
In summary, although infection with live S. aureus also involves toxin production, neutrophil infiltration is a common feature of the pathogenesis of S. aureus keratitis and culture-negative keratitis, and the findings presented in the current study demonstrate important roles for TLR2 and MyD88 in CXC chemokine production and neutrophil recruitment to the cornea.
Acknowledgments
This research was funded by NIH grant EY14362 (E.P.) and by Bausch and Lomb, Inc., K08 AI054652 (A.G.H.), with additional support from EY11373, the Research to Prevent Blindness Foundation, and the Ohio Lions Eye Research Foundation.
We are grateful to Shizuo Akira for TLR and MyD88 gene knockout mice and Richard O'Callaghan for providing S. aureus strain 8325.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Blais, D. R., S. G. Vascotto, M. Griffith, and I. Altosaar. 2005. LBP and CD14 secreted in tears by the lacrimal glands modulate the LPS response of corneal epithelial cells. Investig. Ophthalmol. Vis. Sci. 46:4235-4244. [DOI] [PubMed] [Google Scholar]
- 2.Chang, J. H., P. J. McCluskey, and D. Wakefield. 2006. Toll-like receptors in ocular immunity and the immunopathogenesis of inflammatory eye disease. Br. J. Ophthalmol. 90:103-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chusid, M. J., D. B. Nelson, and L. A. Meyer. 1986. The role of the polymorphonuclear leukocyte in the induction of corneal edema. Investig. Ophthalmol. Vis. Sci. 27:1466-1469. [PubMed] [Google Scholar]
- 4.Dajcs, J. J., E. B. Hume, J. M. Moreau, A. R. Caballero, B. M. Cannon, and R. J. O'Callaghan. 2000. Lysostaphin treatment of methicillin-resistant Staphylococcus aureus keratitis in the rabbit. Investig. Ophthalmol. Vis. Sci. 41:1432-1437. [PubMed] [Google Scholar]
- 5.Forster, W., K. Becker, D. Hungermann, and H. Busse. 2002. Methicillin-resistant Staphylococcus aureus keratitis after excimer laser photorefractive keratectomy1. J. Cataract Refract. Surg. 28:722-724. [DOI] [PubMed] [Google Scholar]
- 6.Gillette-Ferguson, I., A. G. Hise, Y. Sun, E. Diaconu, H. F. McGarry, M. J. Taylor, and E. Pearlman. 2006. Wolbachia- and Onchocerca volvulus-induced keratitis (river blindness) is dependent on myeloid differentiation factor 88. Infect. Immun. 74:2442-2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Girgis, D. O., G. D. Sloop, J. M. Reed, and R. J. O'Callaghan. 2003. A new topical model of Staphylococcus corneal infection in the mouse. Investig. Ophthalmol. Vis. Sci. 44:1591-1597. [DOI] [PubMed] [Google Scholar]
- 8.Hall, L. R., E. Diaconu, R. Patel, and E. Pearlman. 2001. CXC chemokine receptor 2 but not C-C chemokine receptor 1 expression is essential for neutrophil recruitment to the cornea in helminth-mediated keratitis (river blindness). J. Immunol. 166:4035-4041. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto, M., K. Tawaratsumida, H. Kariya, K. Aoyama, T. Tamura, and Y. Suda. 2006. Lipoprotein is a predominant Toll-like receptor 2 ligand in Staphylococcus aureus cell wall components. Int. Immunol. 18:355-362. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi, F., T. K. Means, and A. D. Luster. 2003. Toll-like receptors stimulate human neutrophil function. Blood 102:2660-2669. [DOI] [PubMed] [Google Scholar]
- 11.Holden, B. A., M. K. Reddy, P. R. Sankaridurg, R. Buddi, S. Sharma, M. D. Willcox, D. F. Sweeney, and G. N. Rao. 1999. Contact lens-induced peripheral ulcers with extended wear of disposable hydrogel lenses: histopathologic observations on the nature and type of corneal infiltrate. Cornea 18:538-543. [PubMed] [Google Scholar]
- 12.Huang, X., R. P. Barrett, S. A. McClellan, and L. D. Hazlett. 2005. Silencing Toll-like receptor-9 in Pseudomonas aeruginosa keratitis. Investig. Ophthalmol. Vis. Sci. 46:4209-4216. [DOI] [PubMed] [Google Scholar]
- 13.Jalbert, I., M. D. Willcox, and D. F. Sweeney. 2000. Isolation of Staphylococcus aureus from a contact lens at the time of a contact lens-induced peripheral ulcer: case report. Cornea 19:116-120. [DOI] [PubMed] [Google Scholar]
- 14.Jett, B. D., and M. S. Gilmore. 2002. Internalization of Staphylococcus aureus by human corneal epithelial cells: role of bacterial fibronectin-binding protein and host cell factors. Infect. Immun. 70:4697-4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson, A. C., F. P. Heinzel, E. Diaconu, Y. Sun, A. G. Hise, D. Golenbock, J. H. Lass, and E. Pearlman. 2005. Activation of Toll-like receptor (TLR)2, TLR4, and TLR9 in the mammalian cornea induces MyD88-dependent corneal inflammation. Investig. Ophthalmol. Vis. Sci. 46:589-595. [DOI] [PubMed] [Google Scholar]
- 16.Kadowaki, N. 2005. Triggering of TLR3 by polyI:C in human corneal epithelial cells to induce inflammatory cytokines. Biochem. Biophys. Res. Commun. 331:285-294. [DOI] [PubMed] [Google Scholar]
- 17.Kalis, C., M. Gumenscheimer, N. Freudenberg, S. Tchaptchet, G. Fejer, A. Heit, S. Akira, C. Galanos, and M. A. Freudenberg. 2005. Requirement for TLR9 in the immunomodulatory activity of Propionibacterium acnes. J. Immunol. 174:4295-4300. [DOI] [PubMed] [Google Scholar]
- 18.Khatri, S., J. H. Lass, F. P. Heinzel, W. M. Petroll, J. Gomez, E. Diaconu, C. M. Kalsow, and E. Pearlman. 2002. Regulation of endotoxin-induced keratitis by PECAM-1, MIP-2, and Toll-like receptor 4. Investig. Ophthalmol. Vis. Sci. 43:2278-2284. [PubMed] [Google Scholar]
- 19.Knuefermann, P., Y. Sakata, J. S. Baker, C. H. Huang, K. Sekiguchi, H. S. Hardarson, O. Takeuchi, S. Akira, and J. G. Vallejo. 2004. Toll-like receptor 2 mediates Staphylococcus aureus-induced myocardial dysfunction and cytokine production in the heart. Circulation 110:3693-3698. [DOI] [PubMed] [Google Scholar]
- 20.Reference deleted.
- 21.Kumar, A., J. Zhang, and F. S. Yu. 2006. Toll-like receptor 2-mediated expression of beta-defensin-2 in human corneal epithelial cells. Microbes Infect. 8:380-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar, A., J. Zhang, and F. S. Yu. 2006. Toll-like receptor 3 agonist poly(I:C)-induced antiviral response in human corneal epithelial cells. Immunology 117:11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller, L. S., R. M. O'Connell, M. A. Gutierrez, E. M. Pietras, A. Shahangian, C. E. Gross, A. Thirumala, A. L. Cheung, G. Cheng, and R. L. Modlin. 2006. MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity 24:79-91. [DOI] [PubMed] [Google Scholar]
- 24.Moreau, J. M., D. O. Girgis, E. B. Hume, J. J. Dajcs, M. S. Austin, and R. J. O'Callaghan. 2001. Phospholipase A2 in rabbit tears: a host defense against Staphylococcus aureus. Investig. Ophthalmol. Vis. Sci. 42:2347-2354. [PubMed] [Google Scholar]
- 25.O'Callaghan, R. J., M. C. Callegan, J. M. Moreau, L. C. Green, T. J. Foster, O. M. Hartford, L. S. Engel, and J. M. Hill. 1997. Specific roles of alpha-toxin and beta-toxin during Staphylococcus aureus corneal infection. Infect. Immun. 65:1571-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Callaghan, R. J., D. O. Girgis, J. J. Dajcs, and G. D. Sloop. 2003. Host defense against bacterial keratitis. Ocul. Immunol. Inflamm. 11:171-181. [DOI] [PubMed] [Google Scholar]
- 27.Sabroe, I., L. R. Prince, E. C. Jones, M. J. Horsburgh, S. J. Foster, S. N. Vogel, S. K. Dower, and M. K. Whyte. 2003. Selective roles for Toll-like receptor (TLR)2 and TLR4 in the regulation of neutrophil activation and life span. J. Immunol. 170:5268-5275. [DOI] [PubMed] [Google Scholar]
- 28.Schultz, C. L., D. W. Morck, S. G. McKay, M. E. Olson, and A. Buret. 1997. Lipopolysaccharide induced acute red eye and corneal ulcers. Exp. Eye Res. 64:3-9. [DOI] [PubMed] [Google Scholar]
- 29.Shanmuganathan, V. A., M. Armstrong, A. Buller, and A. B. Tullo. 2005. External ocular infections due to methicillin-resistant Staphylococcus aureus (MRSA). Eye 19:284-291. [DOI] [PubMed] [Google Scholar]
- 30.Solomon, R., E. D. Donnenfeld, H. D. Perry, and S. Biser. 2003. Bilateral methicillin-resistant Staphylococcus aureus keratitis in a medical resident following an uneventful bilateral photorefractive keratectomy. Eye Contact Lens 29:187-189. [DOI] [PubMed] [Google Scholar]
- 31.Song, P. I., T. A. Abraham, Y. Park, A. S. Zivony, B. Harten, H. F. Edelhauser, S. L. Ward, C. A. Armstrong, and J. C. Ansel. 2001. The expression of functional LPS receptor proteins CD14 and Toll-like receptor 4 in human corneal cells. Investig. Ophthalmol. Vis. Sci. 42:2867-2877. [PubMed] [Google Scholar]
- 32.Takeda, K., and S. Akira. 2004. TLR signaling pathways. Semin. Immunol. 16:3-9. [DOI] [PubMed] [Google Scholar]
- 33.Takeuchi, O., K. Hoshino, and S. Akira. 2000. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J. Immunol. 165:5392-5396. [DOI] [PubMed] [Google Scholar]
- 34.Travassos, L. H., S. E. Girardin, D. J. Philpott, D. Blanot, M. A. Nahori, C. Werts, and I. G. Boneca. 2004. Toll-like receptor 2-dependent bacterial sensing does not occur via peptidoglycan recognition. EMBO Rep. 5:1000-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ueta, M., T. Nochi, M. H. Jang, E. J. Park, O. Igarashi, A. Hino, S. Kawasaki, T. Shikina, T. Hiroi, S. Kinoshita, and H. Kiyono. 2004. Intracellularly expressed TLR2s and TLR4s contribution to an immunosilent environment at the ocular mucosal epithelium. J. Immunol. 173:3337-3347. [DOI] [PubMed] [Google Scholar]
- 36.Vogel, S. N., K. A. Fitzgerald, and M. J. Fenton. 2003. TLRs: differential adapter utilization by toll-like receptors mediates TLR-specific patterns of gene expression. Mol. Interv. 3:466-477. [DOI] [PubMed] [Google Scholar]
- 37.Wu, P., F. Stapleton, and M. D. Willcox. 2003. The causes of and cures for contact lens-induced peripheral ulcer. Eye Contact Lens 29:S63-S66, S83-S84, S192-S194. [DOI] [PubMed] [Google Scholar]
- 38.Zhang, J., K. Xu, B. Ambati, and F. S. Yu. 2003. Toll-like receptor 5-mediated corneal epithelial inflammatory responses to Pseudomonas aeruginosa flagellin. Investig. Ophthalmol. Vis. Sci. 44:4247-4254. [DOI] [PubMed] [Google Scholar]