Abstract
Bartonella quintana causes trench fever, endocarditis, and the vasculoproliferative disorders bacillary angiomatosis and peliosis hepatis in humans. Little is known about the interaction of this pathogen with host cells. We attempted to elucidate the interaction of B. quintana with human macrophages (THP-1) and epithelial cells (HeLa 229). Remarkably, only B. quintana strain JK-31 induced secretion of vascular endothelial growth factor (VEGF) from THP-1 and HeLa 229 cells upon infection similar to the secretion induced by B. henselae Marseille, whereas other strains (B. quintana 2-D70, B. quintana Toulouse, and B. quintana Munich) did not induce such secretion. Immunofluorescence testing and electron microscopy revealed that the B. quintana strains unable to induce VEGF secretion did not express the variable outer membrane proteins (Vomps) on their surfaces. Surprisingly, the increase in VEGF secretion mediated by B. quintana JK-31 was not paralleled by elevated host cell adherence rates compared with the rates for Vomp-negative B. quintana strains. Our results suggest that the Vomps play a leading role in the angiogenic reprogramming of host cells by B. quintana but not in the adherence to host cells.
Bartonella quintana and Bartonella henselae are reemerging, gram-negative, facultative intracellular bacteria which cause a variety of human diseases. B. henselae is the most common cause of cat scratch disease. In immunocompromised patients (e.g., AIDS patients), both B. henselae and the closely related species B. quintana (1) cause the vasculoproliferative disorders bacillary angiomatosis (BA) and peliosis hepatis (PH) (20). Infections with B. quintana can result in “trench fever,” which is characterized by periodic feverish relapses every fifth day due to intraerythrocytic bacteremia (12). B. quintana was most important during World War I, when more than one million soldiers suffered from this disease. The confirmed transmission vector is the human body louse (33). Today, B. quintana is a well-recognized cause of fever, bacteremia, and endocarditis in human immunodeficiency virus-seronegative, inner-city patients living under poor hygienic conditions, such as the homeless or alcoholics (5, 13, 43). Prolonged periods of intracellular erythrocyte parasitism appear to be a crucial aspect of the pathogenicity of Bartonella spp. (21, 38).
Very little is known about the pathogenicity of B. quintana. It has been shown that Bartonella spp. replicate within endothelial cells in a Bartonella-containing vacuole (6), adhere to endothelial and epithelial cells (3, 9, 17), and invade erythrocytes (36, 39). There is also evidence that B. quintana might interact with human erythroblast cells (37). Contradictory results about the induction of apoptosis by B. quintana have been obtained; while early during B. quintana infection apoptosis of endothelial cells was detectable, this apoptosis was inhibited at later times (26). Recently, variably expressed outer membrane proteins VompA, VompB, VompC, and VompD mediating collagen binding and autoaggregation of B. quintana were identified (44).
Vomps of B. quintana, the highly homologous Bartonella adhesin A (BadA), and the recently identified Bartonella repeat protein A (BrpA) of Bartonella vinsonii (11) belong to the novel class of trimeric autotransporter adhesins (TAAs) (27). These TAAs are believed to form lollipop-shaped trimeric surface structures with a head-stalk-anchor architecture similar to that of the prototypic TAA Yersinia adhesin A (4). The membrane anchor defines this family of adhesins. The stalk domains are fibrous, highly repetitive structures with extremely variable lengths. The head and stalk are composed of a small set of domains, building blocks that are frequently arranged repetitively and probably mediate bacterium-host interactions (27).
In B. henselae, the TAA BadA was shown to represent a major pathogenicity determinant mediating adherence to host cells, binding to fibronectin (Fn), and secretion of angiogenic cytokines from infected host cells (35). Although genes for VompA, VompB, VompC, and VompD were identified in the genome sequence of B. quintana JK-31, only VompA, VompB, and VompC were found to be expressed on the surface (44; J. E. Koehler, personal communication). A spontaneous variant of B. quintana JK-31, B. quintana 2-D70, does not express any Vomp (44).
It was demonstrated previously that infection with B. henselae results in secretion of proangiogenic compounds, such as vascular endothelial growth factor (VEGF) (18, 34). In this study we wanted to analyze the host cell interaction (adhesion, invasion, and VEGF induction) and Fn binding of B. quintana (Vomp+ and Vomp− strains) and B. henselae (BadA+) using THP-1-derived macrophages and HeLa 229 epithelial cells. Our results demonstrate that expression of Vomps correlates with the ability of B. quintana to induce VEGF secretion from infected host cells but not with host cell adhesion or Fn binding (in contrast to BadA of B. henselae). A comparison of the VompA, VompB, and VompC protein sequences of B. quintana with the sequence of BadA of B. henselae revealed a potential Fn-binding domain not present in Vomps which might be responsible for the lack of Fn binding and host cell adherence.
MATERIALS AND METHODS
Bacterial strains.
B. henselae Marseille (10), B. quintana JK-31 (expressing Vomp A, B, and C; kindly provided by J. E. Koehler, San Francisco, CA), B. quintana 2-D70 (VompA− VompB− VompC−; kindly provided by J. E. Koehler, San Francisco, CA), B. quintana Toulouse (Collection de l'Institut Pasteur, Paris, France; kindly provided by A. Sander, Freiburg, Germany), and B. quintana Munich (41) were grown on Columbia blood agar plates supplemented with 5% defibrinated sheep blood (CBA) (Becton Dickinson, Heidelberg, Germany) in a humidified atmosphere at 37°C with 5% CO2. For production of bacterial stock suspensions, bacteria were harvested from agar plates after 5 days of culture, resuspended in Luria-Bertani medium containing 20% glycerol, and stored at −80°C. For infection experiments, bacterial stocks were thawed, washed, and suspended in cell culture medium, and the concentration was adjusted to the appropriate level. The actual inoculum for each experiment was determined by plating serial dilutions of the suspension and calculating the number of CFU.
Cell lines and cell culture.
Human monocytic cell line THP-1 (DSMZ ACC 16; Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Braunschweig, Germany), derived from a 1-year-old boy with acute leukemia, was cultured in RPMI 1640 medium (Biochrom, Berlin, Germany) supplemented with 10% heat-inactivated fetal calf serum (Sigma, Deisenhofen, Germany), 2 mM glutamine, 10 mM HEPES, 10 μg of streptomycin per ml, and 100 U penicillin per ml (Biochrom). Human HeLa 229 cervical epithelial cells (ATCC CCL-2.1; American Type Culture Collection) were maintained in the same way but without addition of HEPES buffer.
Bacterial adhesion and invasion assays.
To quantify bacterial adhesion to and invasion into THP-1 macrophages, cells were seeded in 24-well tissue culture plates at a concentration of 2.5 × 105 cells per well and differentiated for 30 h by incubation with 75 ng of phorbol 12-myristate 13-acetate (PMA) (Sigma) per ml. HeLa 229 cells were seeded in 24-well plates at a concentration of 1 × 105 cells/well on the day before the experiment. Media were removed 2 h before infection and replaced with culture media without antibiotics to allow bacterial growth. Cells were infected at a multiplicity of infection (MOI) of 100, and the bacteria were sedimented onto the cultured cells by centrifugation at 300 × g for 5 min at room temperature. Bacterial adhesion was quantified 30 min after infection. At this time, only a negligible amount of bacteria was present intracellularly (data not shown). For this analysis, cells were washed three times in supplemented RPMI medium, and osmotic lysis was performed to determine the total number of adherent bacteria, as previously described (17). Briefly, 900 μl of sterile water was added, and the cells were passaged 10 times using a 25-gauge needle. Osmotic lysis was overcome by addition of 100 μl of 10× phosphate-buffered saline (PBS) to the cell lysates, and numbers of bacteria were determined by spread plating 10-fold serial dilutions on CBA. Intracellular presence at 1, 4, 24, and 48 h was determined by gentamicin protection assays by addition of gentamicin (100 μg/ml) for 2 h to kill extracellular bacteria, as described previously (17).
Determination of VEGF secretion.
THP-1 macrophages were infected at an MOI of 100 as described above, and HeLa 229 cells were infected at an MOI of 250. VEGF secretion after Bartonella infection was determined without antibiotics to allow bacterial growth and without fetal calf serum to avoid nonspecific VEGF secretion. Supernatants were obtained after 24 h (THP-1) or 48 h (HeLa 229), which turned out to be the optimal times, as reported previously (15, 18, 34, 35). Collected supernatants were centrifuged to remove insoluble particles (4,000 × g, 10 min, 4°C) and frozen at −20°C. The VEGF concentration was determined using a human VEGF165 enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer's instructions (Quantikine; R&D Systems, Wiesbaden, Germany).
Transmission electron microscopy.
Transmission electron microscopy (TEM) was performed as previously described (35). Briefly, Bartonella spp. cell pellets were fixed at 4°C for 24 h in Karnovsky's reagent. Postfixation was based on 1% osmium tetroxide containing 0.05 M potassium ferrocyanide for 2 h. After cells were embedded in glycide ether, the blocks were cut using an ultramicrotome (Ultracut; Reichert, Vienna, Austria). Ultrathin sections (60 nm) were stained (Ultrastainer; LKB, Sweden) with 12% uranyl acetate for 3 min at 22°C and with lead citrate for 1 min at 20°C. Bacterial morphology and infected macrophages were analyzed using a Zeiss EM 109 transmission electron microscope (Zeiss, Oberkochen, Germany) operating at 80 kV.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting.
Bartonella spp. were resuspended in sodium dodecyl sulfate sample buffer and heated at 98°C for 3 min. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed in 12% gels. For immunoblotting, proteins were transferred onto nitrocellulose membranes (Schleicher and Schuell, Dassel, Germany). Blots were blocked for 1 h in 5% skim milk powder in 25 mM Tris (pH 7.5)-0.15 M NaCl-0.05% Tween 20 (Sigma) and incubated with BadA- and Fn-directed antibodies (35) overnight. For detection, a horseradish peroxidase-conjugated secondary antibody was used, and signals were visualized either with 3,3′-diaminobenzidine tetrahydrochloride (Sigma) or by chemiluminescence (ECL; Amersham).
Immunostaining and confocal laser scanning microscopy.
Bacteria were grown on CBA plates for 5 days, resuspended in PBS, dried on glass slides, and fixed in 3.75% PBS-buffered paraformaldehyde. Immunostaining of B. quintana was performed as described previously (17) using BadA-directed rabbit antibodies. Fluorescein isothiocyanate-conjugated secondary antibodies were purchased from Dianova. For control reasons, bacteria were also stained with 4′,6′-diamidino-2-phenylindole (DAPI) (10 μg/ml dissolved in PBS). Cellular fluorescence was evaluated using a Leica DM IRE 2 confocal laser scanning microscope. Two different fluorochromes representing the green (fluorescein isothiocyanate) and blue (DAPI) channels were detected. Images were digitally processed with Photoshop 6.0 (Adobe Systems).
Protein sequence analysis.
Sequence similarity searches were performed using the programs BLAST and PSI-BLAST with the nonredundant and microbial genome databases at the NCBI (www.ncbi.nlm.nih.gov). Sequence alignments were constructed with MACAW (42), and coiled coil segments were predicted using the program COILS (28). Other secondary structure predictions were made with PSIPRED (30). The Vomp sequences were aligned with the sequence of BadA (35) using a hidden Markov model database that identifies known domains of trimeric autotransporter adhesins (P. Szczesny and A. Lupas, unpublished).
Statistical analysis.
Experiments were performed at least three times, and comparable results were obtained. Data obtained from representative experiments are shown below. A paired Student's t test was used to analyze differences between mean values of experimental and control groups. A P value of <0.05 was considered statistically significant.
RESULTS
Vomp expression by B. quintana is crucial for induction of VEGF secretion from THP-1 and HeLa 229 cells.
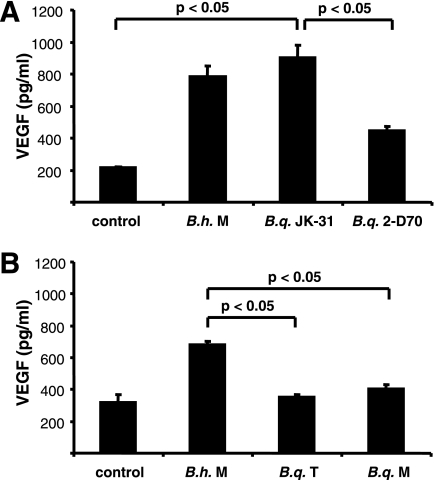
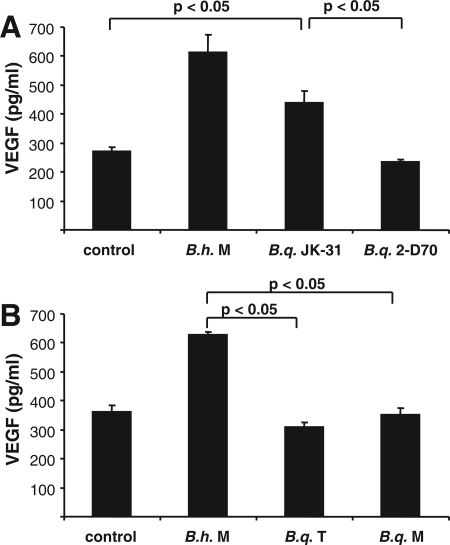
As it has been shown previously that a B. henselae infection results in secretion of proangiogenic VEGF likely involved in the induction of angioproliferative disorders (BA and PH) in humans (15, 18, 34), we wanted to determine whether such cytokine secretion was also detectable when host cells (epithelial cells and macrophages) were infected with B. quintana. For this purpose, THP-1 macrophages were infected with B. henselae Marseille, B. quintana JK-31 (Vomp+), B. quintana 2-D70 (Vomp−), B. quintana Toulouse, and B. quintana Munich, and supernatants were obtained 24 h after infection and analyzed by ELISA. The data revealed that only strains expressing TAAs (B. henselae Marseille expressing BadA and B. quintana JK-31 expressing VompA, VompB, and VompC) induced substantially increased VEGF secretion from host cells, in contrast to cells infected with B. quintana 2-D70 (Vomp−), B. quintana Toulouse, or B. quintana Munich (Fig. 1). Very similar results were obtained using HeLa 229 cells (Fig. 2); in this case, only B. henselae Marseille and B. quintana JK-31 triggered the secretion of VEGF 48 h after infection, whereas all other strains did not induce secretion of this proangiogenic cytokine. These data show that the previously described proangiogenic gene program induced by B. henselae Marseille (15) can be observed when cells are infected with the Vomp-expressing B. quintana JK-31 strain but not when cells are infected with B. quintana 2-D70, B. quintana Toulouse, or B. quintana Munich, suggesting strongly that Vomps play a critical role in the activation of an angiogenic host cell response.
FIG. 1.
VEGF secretion of THP-1 macrophages upon cocultivation with B. henselae Marseille (B.h. M), B. quintana JK-31 (Vomp+) (B.q. JK-31), and B. quintana 2-D70 (Vomp−) (B.q. 2-D70) (A) and B. henselae Marseille (B.h. M), B. quintana Toulouse (B.q. T), and B. quintana Munich (B.q. M) (B). THP-1 cells were seeded in 24-well plates, differentiated with PMA, and infected at an MOI of 100. The VEGF levels in cell culture supernatants were determined by ELISA after 24 h of coculture. Control cells were not infected. Significant differences are indicated.
FIG. 2.
VEGF secretion of HeLa 229 cells upon cocultivation with B. henselae Marseille (B.h. M), B. quintana JK-31 (Vomp+) (B.q. JK-31), and B. quintana 2-D70 (Vomp−) (B.q. 2-D70) (A) and B. henselae Marseille (B.h. M), B. quintana Toulouse (B.q. T), and B. quintana Munich (B.q. M) (B). Cells were seeded in 24-well plates and infected at an MOI of 250. The VEGF levels in cell culture supernatants were determined by ELISA after 48 h of coculture. Control cells were not infected. Significant differences are indicated.
B. quintana exhibits highly reduced adhesion to host cells compared with the adhesion of B. henselae.
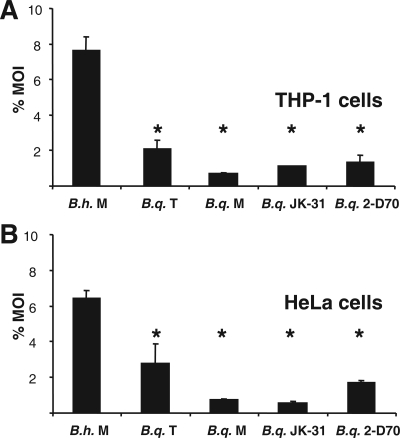
It has been stated previously that secretion of VEGF might be directly correlated to host cell adherence (18, 35). In order to investigate the mechanisms underlying the induction of VEGF secretion during B. quintana infection, we analyzed the host cell adherence of B. quintana. THP-1 macrophages and HeLa 229 cells were infected with B. henselae Marseille, B. quintana JK-31 (Vomp+), B. quintana 2-D70 (Vomp−), B. quintana Toulouse, and B. quintana Munich. Host cell adherence was determined 30 min after infection. Adherence to THP-1 macrophages was greatest for B. henselae Marseille (7.67% ± 0.72% of the inoculum), whereas, surprisingly, the adherence of all B. quintana strains to THP-1 macrophages was significantly less (B. quintana JK-31 [Vomp+], 1.11% ± 0.02%; B. quintana 2-D70 [Vomp−], 1.37% ± 0.36%; B. quintana Toulouse, 2.14% ± 0.46%; B. quintana Munich, 0.72% ± 0.05%) (Fig. 3A), independent of Vomp expression (see below). Similar results were obtained when HeLa 229 cells were infected (B. henselae Marseille, 6.44% ± 0.43%; B. quintana JK-31 [Vomp+], 0.58% ± 0.08%; B. quintana 2-D70 [Vomp−], 1.73% ± 0.10%; B. quintana Toulouse, 2.81% ± 1.04%; B. quintana Munich, 0.75% ± 0.06%) (Fig. 3B). Therefore, we conclude that the expression of VompA, VompB, and VompC of B. quintana JK-31 correlates with induction of VEGF secretion but not with increased bacterial adhesion to host cells.
FIG. 3.
Adherence of B. henselae Marseille (B.h. M), B. quintana Toulouse (B.q. T), B. quintana Munich (B.q. M), B. quintana JK-31 (Vomp+) (B.q. JK-31), and B. quintana 2-D70 (Vomp−) (B.q. 2-D70) to THP-1 macrophages (A) and HeLa 229 cells (B). THP-1 cells were differentiated by treatment with PMA. Cells were infected at an MOI of 100, and adherence was determined 30 min after infection. An asterisk indicates that a value is significantly different from the value obtained for B. henselae Marseille (P < 0.05).
Invasion of THP-1 macrophages by B. quintana compared with invasion by B. henselae.
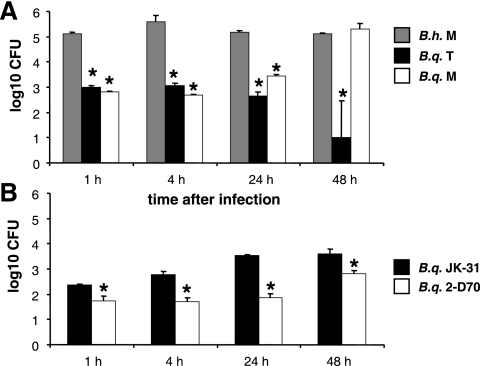
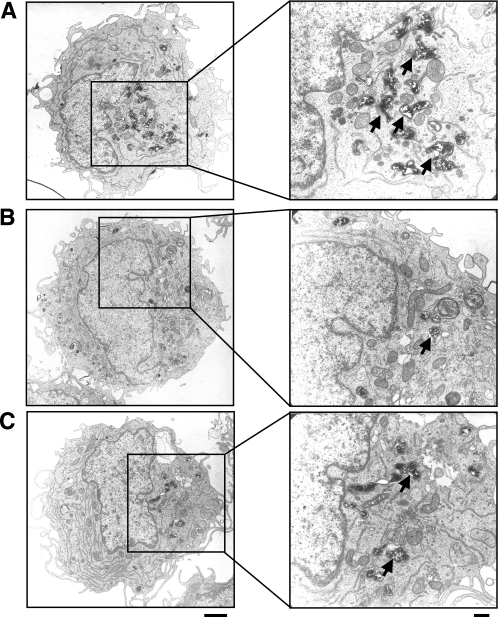
To analyze whether differences in cytokine production might be linked to the intracellular presence of bacteria, invasion of host cells by Bartonella spp. was investigated using THP-1 macrophages. To do this, the amounts of intracellular B. henselae Marseille, B. quintana JK-31 (Vomp+), B. quintana 2-D70 (Vomp−), B. quintana Toulouse, and B. quintana Munich were determined 1, 4, 24, and 48 h after infection using gentamicin protection assays. After 1 h, invasion was greatest for B. henselae Marseille, and the level of invasion was approximately 200-fold higher than the levels of invasion by B. quintana Toulouse and B. quintana Munich (Fig. 4A). The amount of intracellular B. henselae Marseille increased up to 4 h postinfection and then decreased up to 48 h. In contrast, little B. quintana Toulouse was recovered from THP-1 macrophages over a 48-h period. With B. quintana Munich there was a delayed increase in the number of intracellular bacteria up to 48 h, and then the number decreased at later times (data not shown). The amounts of intracellular B. quintana JK-31 (Vomp+) and B. quintana 2-D70 (Vomp−) were in the same range as the amounts of B. quintana Toulouse and B. quintana Munich. However, the invasion rate for the Vomp-expressing strain B. quintana JK-31 was significantly higher than the invasion rate for the Vomp− 2-D70 isolate (Fig. 4B). The results of TEM performed 24 h postinfection were consistent with intracellular survival data at this time (Fig. 5). In the infected macrophages, B. quintana strain Toulouse and Munich and B. henselae cells were located in small, perinuclear, Bartonella-containing vacuoles. Most remarkably, the host cell morphology appeared to be unaffected after bacterial infection. From these data we conclude that B. quintana is taken up in a vacuolic compartment in THP-1 macrophages, similar to the uptake of B. henselae; however, the host cell invasion rates of B. quintana are obviously lower than those of B. henselae.
FIG. 4.
Invasion of (1 h) and intracellular presence in (4, 24, and 48 h) THP-1 macrophages infected with B. henselae Marseille (B.h. M), B. quintana Toulouse (B.q. T), and B. quintana Munich (B.q. M) (A) and with B. quintana JK-31 (Vomp+) (B.q. JK-31) and B. quintana 2-D70 (Vomp−) (B.q. 2-D70) (B). THP-1 cells were seeded in 24-well plates, differentiated with PMA, and infected at an MOI of 100. Invasion and intracellular presence were determined by gentamicin protection assays. An asterisk indicates that a value is significantly different from the value obtained for B. henselae Marseille (A) or B. quintana JK-31 (B) (P < 0.05).
FIG. 5.
TEM of THP-1 macrophages infected (MOI, 100) for 24 h with B. henselae Marseille (A), B. quintana Toulouse (B), or B. quintana Munich (C). Bacteria are in membrane-bound Bartonella-containing vacuoles (arrows). Scale bars, 2 μm (left) and 0.5 μm (right).
Strain-specific Vomp expression of B. quintana analyzed by TEM, immunofluorescence, and Western blotting.
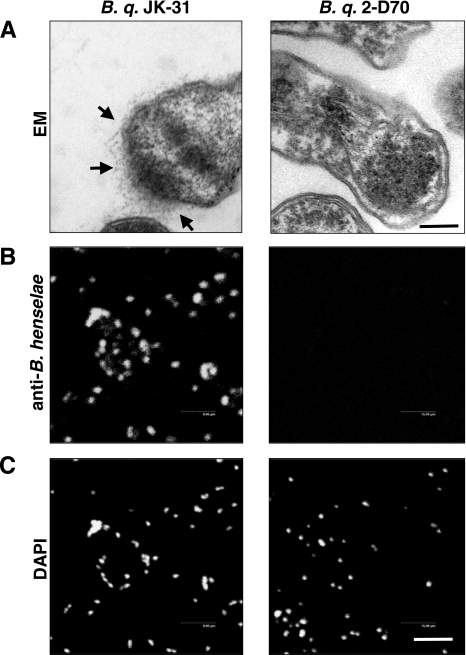
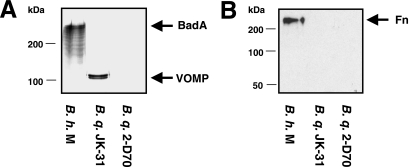
Next, we investigated the Vomp expression of several B. quintana isolates, including B. quintana JK-31 (Vomp+), B. quintana 2-D70 (Vomp−), B. quintana Toulouse, and B. quintana Munich. For this purpose, TEM was performed with CBA-grown B. quintana, and the results revealed that Vomps were detectable on B. quintana JK-31 but not on B. quintana 2-D70 (Fig. 6A). The results of TEM were consistent with those of an immunofluorescence analysis using BadA-directed antibodies, which revealed bright green fluorescence on the bacterial surface (Fig. 6B). Western blot analysis of Vomp expression using BadA-directed antibodies also produced consistent results, including a protein band at ∼100 kDa for B. quintana JK-31 consistent with the size of Vomps not present in B. quintana 2-D70 (44) (Fig. 7A). Surprisingly, the Vomp-expressing B. quintana JK-31 strain did not bind Fn (present in the CBA on which the strains were grown), whereas bacterium-bound Fn was detected on BadA-expressing B. henselae Marseille, as reported previously (35) (Fig. 7B). Therefore, we conclude that VompA, VompB, and VompC of B. quintana do not bind Fn, in contrast to BadA of B. henselae.
FIG. 6.
Analysis of Vomp expression by TEM and immunofluorescence. (A) Hairy structures (arrows) on the surface of B. quintana (B. q.) JK-31 (B. q. JK-31) representing Vomps which are not expressed on B. quintana 2-D70 (B. q. 2-D70). Scale bar, 0.25 μm. (B) Immunofluorescence test of Vomp expression using BadA-directed antibodies. (C) Bacteria stained with DAPI (control). Scale bar, 8 μm.
FIG. 7.
Analysis of Vomp and BadA expression and fibronectin binding of B. henselae Marseille (B.h. M), B. quintana JK-31(Vomp+) (B.q. JK-31), and B. quintana 2-D70 (Vomp−) (B.q. 2-D70). Western blots of whole-cell lysates were incubated with BadA- or Fn-directed antibodies. (A) BadA-directed antibodies detected BadA in B. henselae Marseille and Vomp in B. quintana JK-31 but not in B. quintana 2-D70 (Vomp−). (B) Fibronectin binding was detected in B. henselae Marseille but not in B. quintana JK-31 or 2-D70.
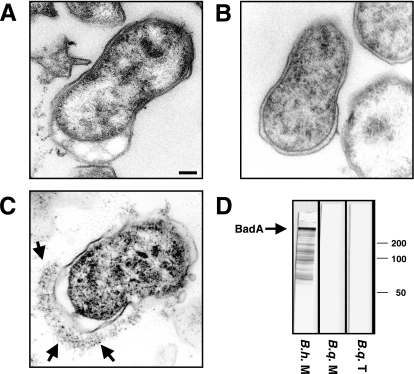
There was also no Vomp expression in B. quintana Toulouse and B. quintana Munich, whereas expression of the TAA BadA was detected in B. henselae Marseille (Fig. 8A to C). In addition, analysis of TAA expression by Western blotting using BadA-directed antibodies (Fig. 8D) or immunofluorescence (data not shown) did not reveal Vomp expression in B. quintana Toulouse or B. quintana Munich.
FIG. 8.
TEM of B. quintana Toulouse (A), B. quintana Munich (B), and B. henselae Marseille (C). BadA is expressed on the surface of B. henselae Marseille (arrows), whereas no Vomp expression is detectable on the surface of either B. quintana Toulouse or B. quintana Munich. Scale bar, 0.15 μm. (D) Analysis of BadA expression by Western blotting using BadA-directed antibodies (whole-cell lysates of B. quintana Toulouse [B.q. T], B. quintana Munich [B.q. M], and B. henselae Marseille [B.h. M]). Note the reactivity of BadA-directed antibodies with B. henselae Marseille but not with B. quintana Toulouse or B. quintana Munich.
Protein sequence analysis revealed a unique BadA domain of B. henselae not expressed in VompA, VompB, and VompC of B. quintana.
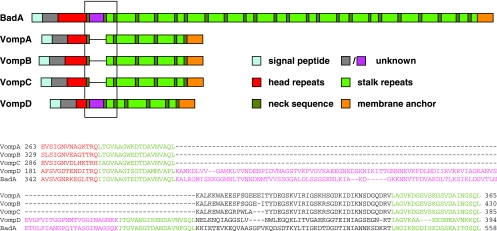
It has been shown previously that the genome of B. quintana JK-31 harbors the four vomp genes (vompA, vompB, vompC, and vompD) and that only vompA, vompB, and vompC are expressed (44; Koehler, personal communication). Collagen binding and autoagglutination are known functions of VompA, VompB, and VompC (44); however, no data elucidating the Vomp-dependent interaction of B. quintana with host cells have been published previously. We wanted to analyze whether some of the frequently repetitive protein domains in BadA of B. henselae are missing in the surface-expressed VompA, VompB, and VompC of B. quintana. Protein sequence analysis revealed that VompA, VompB, and VompC and BadA are constructed in very similar ways: all the Vomps and BadA consist of head repeats, repetitive neck and stalk domains, and a membrane anchor (Fig. 9), although the number of neck-stalk repeats is significantly lower in the Vomp family (five repeats) than in BadA (24 repeats). Surprisingly, a domain between the head region and the repetitive neck-stalk region in VompD (starting at amino acid 214) (Fig. 9) exhibits significant homology to a domain of BadA (amino acid 375) (Fig. 9) and is missing in VompA, VompB, and VompC. Because VompD is not expressed in B. quintana JK-31 (44) and because this domain is missing in B. quintana VompA, VompB, and VompC, we assume that it might be responsible only for fibronectin binding in BadA of B. henselae.
FIG. 9.
Repetitive structure of B. henselae BadA and B. quintana JK-31 VompA, VompB, VompC, and VompD. VompA, VompB, VompC, and VompD differ from BadA in the number of head and stalk repeats. Furthermore, a small domain between the head and stalk, framed by two neck sequences, is present only in BadA and VompD (pink). As only VompA, VompB, and VompC are expressed on the surface of B. quintana JK-31 (44) and B. quintana does not bind fibronectin (Fig. 8), this domain might harbor the fibronectin-binding site. The GenBank accession numbers for the proteins shown are as follows: BadA gi:50082485, AAT69970; BadA gi:50082486, AAT69971; VompA gi:51949816, AAU14841; VompB gi:51949817, AAU14842; VompC gi:51949818, AAU14843; and VompD gi:51949815, AAU14840.
DISCUSSION
B. quintana is a reemerging human pathogen which is transmitted by human body lice and causes trench fever, bacteremia, and endocarditis (29). The ability to induce endothelial proliferations is a common feature of B. quintana, B. henselae, and B. bacilliformis (2). Mechanisms underlying vasculoproliferations in B. henselae infections (e.g., direct stimulation of endothelial proliferation [8], inhibition of endothelial cell apoptosis [19, 40], activation of hypoxia-inducible factor 1, and subsequent secretion of VEGF [15, 18]) have been demonstrated previously. However, little is known about the pathogenicity of B. quintana.
Macrophages appear to play an important role in the pathogenesis of Bartonella-induced vasculoproliferations. BA lesions are typically infiltrated by activated monocytes and macrophages (24). B. henselae causes secretion of VEGF (23, 34) and inhibition of apoptosis in infected macrophages or monocytes (16). One of the recently discussed pathogenicity strategies of B. henselae was designated the two-step paracrine VEGF-loop model. According to this hypothetical model, endothelial proliferations underlying the vasculoproliferative disorders BA and PH are the result of vasculoproliferative compounds secreted by Bartonella sp.-infected host cells (14, 15). It is known that B. henselae is able to adhere to and to invade human and murine macrophages, thereby inducing secretion of VEGF (23, 34). Additionally, inhibition of apoptosis in monocytic cells leads to prolonged VEGF secretion, which might account for the vasculoproliferations that occur in BA or PH (16). Recently, it was demonstrated that expression of the TAA BadA is crucial for induction of VEGF secretion by B. henselae (35). As it was shown previously that B. quintana is a genomic derivative of B. henselae (1) and that B. quintana also expresses TAAs (so-called “Vomps”) on the surface which are highly homologous to BadA of B. henselae (27, 44), it might be suggested that the two pathogens share pathogenicity strategies.
In this study we wanted to elucidate the course of a B. quintana infection of human macrophages and epithelial cells. The most salient findings of our study are as follows: (i) infection of host cells with B. quintana JK-31, but not infection of host cells with B. quintana 2-D70, B. quintana Toulouse, or B. quintana Munich, results in strongly increased VEGF secretion; (ii) compared with B. henselae, all B. quintana strains (JK-31, 2-D70, Toulouse, and Munich) exhibit remarkably reduced host cell adherence and invasion and are unable to bind Fn; (iii) B. quintana resides intracellularly in a vacuolic compartment; and (iv) Vomps are expressed on the surface of B. quintana JK-31 but not on the surfaces of the B. quintana 2-D70, B. quintana Toulouse, and B. quintana Munich strains used in this study.
It has been shown that so-called “pili” are important for the host cell adherence of B. henselae (3, 18). Accordingly, B. quintana lacking expression of these “pili” exhibited significantly reduced adherence to and invasion of HEp-2 cells compared with “piliated” B. henselae (4). We recently demonstrated that in B. henselae these “pili” are represented by the TAA Bartonella adhesin A (BadA) mediating adherence to endothelial cells and extracellular matrix proteins (35). Moreover, expression of BadA is crucial for inducing secretion of VEGF. Sequence analysis of the vomp genes of B. quintana (44) revealed that the Vomp proteins are TAAs that are highly homologous to BadA of B. henselae (27) and to BrpA of B. vinsonii (11). Therefore, it is highly likely that the structures originally designated “pili” in Bartonella spp. (3) are represented in B. quintana by Vomps. In fact, our electron microscopic investigations revealed that Vomps are expressed on the surface of B. quintana JK-31 but not on the surfaces of B. quintana 2-D70 and the B. quintana Toulouse and B. quintana Munich strains used in this study. Consistent results were obtained by either immunofluorescence or Western blotting using BadA-directed antibodies.
The proangiogenic host cell response upon infection with B. henselae seems to play a crucial role in the induction of the vasculoproliferative disorders BA and PH as a B. henselae infection results in activation of hypoxia-inducible factor 1, the key transcriptional regulator involved in angiogenesis and the subsequent secretion of proangiogenic cytokines (e.g., VEGF) in monocytes, macrophages, and epithelial cells (15, 16, 18, 34). We demonstrated that Bartonella infections resulted in significantly increased VEGF secretion by macrophages and HeLa 229 cells only when these cells were infected with TAA-expressing Bartonella strains (B. quintana JK-31 and B. henselae Marseille). Strains lacking Vomp expression (B. quintana 2-D70, B. quintana Toulouse, and B. quintana Munich) were significantly less able to induce VEGF secretion (Fig. 1 and 2); similar effects were described for BadA-negative B. henselae (35).
It was shown previously that induction of VEGF secretion is a process mediated by host cell-adherent B. henselae (35). Surprisingly, this relationship is not as clear in B. quintana infections. In particular, the levels of adherence of all B. quintana strains (including the B. quintana JK-31 strain expressing VompA, VompB, and VompC) to host cells were significantly less than the levels of adherence of B. henselae Marseille. However, why the Vomp-expressing B. quintana JK-31 strain leads to strong induction of VEGF secretion (similar to the induction obtained with B. henselae) remains unclear. A possible explanation is that the adhesion to host cells via Vomps is a transient phenomenon in B. quintana JK-31 and that, in contrast, the adhesion is stronger and more long-lasting in B. henselae infections. Moreover, the possibility that other pathogenicity factors of Bartonella spp. (e.g., the VirB type 4 secretion system important for inhibition of apoptosis and induction of interleukin-8 secretion [40]) are involved in triggering VEGF secretion cannot be ruled out.
Although B. quintana clearly exhibited lower rates of invasion (slightly higher for B. quintana JK-31 Vomp+ than for B. quintana 2-D70 Vomp−), TEM revealed that both B. henselae and B. quintana reside intracellularly in macrophages in a perinuclearly located vacuolic compartment (Bartonella-containing vacuoles) previously described for endothelial cells and murine macrophages (6, 16, 23, 32). Additionally, our observations have consistently shown that in contrast to B. quintana Munich, neither B. quintana Toulouse nor B. henselae Marseille produce a productive infection in human macrophages (Fig. 4). Further experiments elucidating the genes of B. quintana responsible for intracellular persistence and replication are needed.
Unfortunately, we cannot explain at this stage definitively why B. quintana Toulouse and B. quintana Munich lack Vomp expression. It has been reported that extensive passaging of B. quintana JK-31 (passaged for 70 days, resulting in the 2-D70 isolate) resulted in the loss of Vomp expression, and similar observations were made with B. henselae, in which extensive passaging also resulted in the loss of BadA expression (3, 18, 22). We showed that for one B. henselae strain the lack of BadA expression correlates with a 8.5-kb deletion in the badA gene cluster (35). Similarly, deletion of vompA and vompB in the vomp gene cluster of B. quintana 2-D70 has been described previously (44). For this reason it might be speculated that at least the B. quintana Toulouse and Munich strains used in our laboratory had undergone several passages on solid media, presumably before they were frozen, resulting in the loss of Vomp expression by recombination events likely to be responsible for the less virulent in vitro phenotype. Therefore, the strongly diminished VEGF levels might be reflected by the absence of Vomp-mediated host cell interactions.
Protein sequence analysis of the four Vomps and BadA revealed that these TAAs (27) are highly homologous proteins (Fig. 9). Remarkably, BadA and VompD have a very special domain following the head domain. As this is the only protein domain that is present in BadA but not in the Vomps expressed by B. quintana JK-31 (VompA, VompB, and VompC), it might be speculated that this domain is directly involved in Fn binding. In this context it must be mentioned that VompD is not expressed in B. quintana JK-31 (44; Koehler, personal communication). Given that B. henselae binds to host cells via BadA to β1-integrins on the surface of host cells (35), this domain might also be involved in mediating host cell adherence by Fn bridging. It will be interesting to analyze the functions of this Vomp domain in more detail and to find conditions under which VompD is expressed.
It is noteworthy that work on the pathogenicity of B. quintana has been performed using bacteria after variable, unstated numbers of passages (25) and sometimes high numbers of passages (>100 passages [31]) not tested for Vomp expression. Because several B. quintana isolates (2-D70, Toulouse, and Munich) do not express Vomps, we recommend that Vomp expression should be evaluated first when infection experiments are performed with B. quintana. This is also important in terms of interpreting serological immunofluorescence test results obtained with B. quintana (7) as the homologous TAA BadA was shown to be an immunodominant surface protein of B. henselae (35).
Our results represent the first systematic study to analyze the interaction of B. quintana strains with host cells and show that Vomps mediate the induction of an angiogenic host cell phenotype but not Fn binding or host cell adhesion. Improving genetic manipulation techniques and the recent availability of the genome sequence of B. quintana (1) should help in analyzing the role of Vomps and other pathogenicity factors that underlie the host cell interactions of this pathogen.
Acknowledgments
We thank Andrea Schäfer, Diana Neumann, and Birgit Fehrenbacher for excellent technical assistance, Anna Sander and Jane E. Koehler for providing B. quintana strains, and Pierre Kyme for critical reading of the manuscript.
This work was supported by grants from the Deutsche Forschungsgemeinschaft and from the University of Tübingen (IZKF-Programm) to V.A.J.K.
Editor: J. L. Flynn
REFERENCES
- 1.Alsmark, C. M., A. C. Frank, E. O. Karlberg, B. A. Legault, D. H. Ardell, B. Canback, A. S. Eriksson, A. K. Naslund, S. A. Handley, M. Huvet, S. B. La, M. Holmberg, and S. G. Andersson. 2004. The louse-borne human pathogen Bartonella quintana is a genomic derivative of the zoonotic agent Bartonella henselae. Proc. Natl. Acad. Sci. USA 101:9716-9721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, B. E., and M. A. Neuman. 1997. Bartonella spp. as emerging human pathogens. Clin. Microbiol. Rev. 10:203-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batterman, H. J., J. A. Peek, J. S. Loutit, S. Falkow, and L. S. Tompkins. 1995. Bartonella henselae and Bartonella quintana adherence to and entry into cultured human epithelial cells. Infect. Immun. 63:4553-4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolin, I., L. Norlander, and H. Wolf-Watz. 1982. Temperature-inducible outer membrane protein of Yersinia pseudotuberculosis and Yersinia enterocolitica is associated with the virulence plasmid. Infect. Immun. 37:506-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brouqui, P., B. LaScola, V. Roux, and D. Raoult. 1999. Chronic Bartonella quintana bacteremia in homeless patients. N. Engl. J. Med. 340:184-189. [DOI] [PubMed] [Google Scholar]
- 6.Brouqui, P., and D. Raoult. 1996. Bartonella quintana invades and multiplies within endothelial cells in vitro and in vivo and forms intracellular blebs. Res. Microbiol. 147:719-731. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. 1999. Serodiagnosis of emerging infectious diseases: Bartonella and Ehrlichia infections (course manual). Centers for Disease Control and Prevention, Atlanta, Ga.
- 8.Conley, T., L. Slater, and K. Hamilton. 1994. Rochalimaea species stimulate human endothelial cell proliferation and migration in vitro. J. Lab. Clin. Med. 124:521-528. [PubMed] [Google Scholar]
- 9.Dehio, C., M. Meyer, J. Berger, H. Schwarz, and C. Lanz. 1997. Interaction of Bartonella henselae with endothelial cells results in bacterial aggregation on the cell surface and the subsequent engulfment and internalisation of the bacterial aggregate by a unique structure, the invasome. J. Cell Sci. 110:2141-2154. [DOI] [PubMed] [Google Scholar]
- 10.Drancourt, M., R. Birtles, G. Chaumentin, F. Vandenesch, J. Etienne, and D. Raoult. 1996. New serotype of Bartonella henselae in endocarditis and cat-scratch disease. Lancet 347:441-443. [DOI] [PubMed] [Google Scholar]
- 11.Gilmore, R. D., Jr., T. M. Bellville, S. L. Sviat, and M. Frace. 2005. The Bartonella vinsonii subsp. arupensis immunodominant surface antigen BrpA gene, encoding a 382-kilodalton protein composed of repetitive sequences, is a member of a multigene family conserved among Bartonella species. Infect. Immun. 73:3128-3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greub, G., and D. Raoult. 2002. Bartonella: new explanations for old diseases. J. Med. Microbiol. 51:915-923. [DOI] [PubMed] [Google Scholar]
- 13.Jackson, L. A., D. H. Spach, D. A. Kippen, N. K. Sugg, R. L. Regnery, M. H. Sayers, and W. E. Stamm. 1996. Seroprevalence to Bartonella quintana among patients at a community clinic in downtown Seattle. J. Infect. Dis. 173:1023-1026. [DOI] [PubMed] [Google Scholar]
- 14.Kempf, V. A., N. Hitziger, T. Riess, and I. B. Autenrieth. 2002. Do plant and human pathogens have a common pathogenicity strategy? Trends Microbiol. 10:269-275. [DOI] [PubMed] [Google Scholar]
- 15.Kempf, V. A., M. Lebiedziejewski, K. Alitalo, J. H. Wälzlein, U. Ehehalt, J. Fiebig, S. Huber, B. Schütt, C. A. Sander, S. Müller, G. Grassl, A. S. Yazdi, B. Brehm, and I. B. Autenrieth. 2005. Activation of hypoxia-inducible factor-1 in bacillary angiomatosis: evidence for a role of hypoxia-inducible factor-1 in bacterial infections. Circulation 111:1054-1062. [DOI] [PubMed] [Google Scholar]
- 16.Kempf, V. A., A. Schairer, D. Neumann, G. A. Grassl, K. Lauber, M. Lebiedziejewski, M. Schaller, P. Kyme, S. Wesselborg, and I. B. Autenrieth. 2005. Bartonella henselae inhibits apoptosis in Mono Mac 6 cells. Cell. Microbiol. 7:91-104. [DOI] [PubMed] [Google Scholar]
- 17.Kempf, V. A., M. Schaller, S. Behrendt, B. Volkmann, M. Aepfelbacher, I. Cakman, and I. B. Autenrieth. 2000. Interaction of Bartonella henselae with endothelial cells results in rapid bacterial rRNA synthesis and replication. Cell. Microbiol. 2:431-441. [DOI] [PubMed] [Google Scholar]
- 18.Kempf, V. A., B. Volkmann, M. Schaller, C. A. Sander, K. Alitalo, T. Riess, and I. B. Autenrieth. 2001. Evidence of a leading role for VEGF in Bartonella henselae-induced endothelial cell proliferations. Cell. Microbiol. 3:623-632. [DOI] [PubMed] [Google Scholar]
- 19.Kirby, J. E., and D. M. Nekorchuk. 2002. Bartonella-associated endothelial proliferation depends on inhibition of apoptosis. Proc. Natl. Acad. Sci. USA 99:4656-4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koehler, J. E., F. D. Quinn, T. G. Berger, P. E. LeBoit, and J. W. Tappero. 1992. Isolation of Rochalimaea species from cutaneous and osseous lesions of bacillary angiomatosis. N. Engl. J. Med. 327:1625-1631. [DOI] [PubMed] [Google Scholar]
- 21.Kordick, D. L., and E. B. Breitschwerdt. 1995. Intraerythrocytic presence of Bartonella henselae. J. Clin. Microbiol. 33:1655-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kyme, P., B. Dillon, and J. Iredell. 2003. Phase variation in Bartonella henselae. Microbiology 149:621-629. [DOI] [PubMed] [Google Scholar]
- 23.Kyme, P., A. Haas, M. Schaller, A. Peschel, J. Iredell, and V. A. Kempf. 2005. Unusual trafficking pattern of Bartonella henselae-containing vacuoles in macrophages and endothelial cells. Cell. Microbiol. 7:1019-1034. [DOI] [PubMed] [Google Scholar]
- 24.LeBoit, P. E., T. G. Berger, B. M. Egbert, J. H. Beckstead, T. S. Yen, and M. H. Stoler. 1989. Bacillary angiomatosis. The histopathology and differential diagnosis of a pseudoneoplastic infection in patients with human immunodeficiency virus disease. Am. J. Surg. Pathol. 13:909-920. [PubMed] [Google Scholar]
- 25.Liberto, M. C., G. Matera, A. G. Lamberti, G. S. Barreca, D. Foca, A. Quirino, M. R. Soria, and A. Foca. 2004. Bartonella quintana-induced apoptosis inhibition of human endothelial cells is associated with p38 and SAPK/JNK modulation and with stimulation of mitosis. Diagn. Microbiol. Infect. Dis. 50:159-166. [DOI] [PubMed] [Google Scholar]
- 26.Liberto, M. C., G. Matera, A. G. Lamberti, G. S. Barreca, A. Quirino, and A. Foca. 2003. In vitro Bartonella quintana infection modulates the programmed cell death and inflammatory reaction of endothelial cells. Diagn. Microbiol. Infect. Dis. 45:107-115. [DOI] [PubMed] [Google Scholar]
- 27.Linke, D., T. Riess, I. B. Autenrieth, A. Lupas, and V. A. Kempf. 2006. Trimeric autotransporter adhesins: variable structure, common function. Trends Microbiol. 14:264-270. [DOI] [PubMed]
- 28.Lupas, A., M. Van Dyke, and J. Stock. 1991. Predicting coiled coils from protein sequences. Science 252:1162-1164. [DOI] [PubMed] [Google Scholar]
- 29.Maurin, M., and D. Raoult. 1996. Bartonella (Rochalimaea) quintana infections. Clin. Microbiol. Rev. 9:273-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGuffin, L. J., K. Bryson, and D. T. Jones. 2000. The PSIPRED protein structure prediction server. Bioinformatics 16:404-405. [DOI] [PubMed] [Google Scholar]
- 31.Meghari, S., J. M. Rolain, G. E. Grau, E. Platt, L. Barrassi, J. L. Mege, and D. Raoult. 2006. Antiangiogenic effect of erythromycin: an in vitro model of Bartonella quintana infection. J. Infect. Dis. 193:380-386. [DOI] [PubMed] [Google Scholar]
- 32.Musso, T., R. Badolato, D. Ravarino, S. Stornello, P. Panzanelli, C. Merlino, D. Savoia, R. Cavallo, A. N. Ponzi, and M. Zucca. 2001. Interaction of Bartonella henselae with the murine macrophage cell line J774: infection and proinflammatory response. Infect. Immun. 69:5974-5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raoult, D., and V. Roux. 1999. The body louse as a vector of reemerging human diseases. Clin. Infect. Dis. 29:888-911. [DOI] [PubMed] [Google Scholar]
- 34.Resto-Ruiz, S. I., M. Schmiederer, D. Sweger, C. Newton, T. W. Klein, H. Friedman, and B. E. Anderson. 2002. Induction of a potential paracrine angiogenic loop between human THP-1 macrophages and human microvascular endothelial cells during Bartonella henselae infection. Infect. Immun. 70:4564-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riess, T., S. G. Andersson, A. Lupas, M. Schaller, A. Schäfer, P. Kyme, J. Martin, J. H. Wälzlein, U. Ehehalt, H. Lindroos, M. Schirle, A. Nordheim, I. B. Autenrieth, and V. A. Kempf. 2004. Bartonella adhesin A mediates a proangiogenic host cell response. J. Exp. Med. 200:1267-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rolain, J. M., D. Arnoux, D. Parzy, J. Sampol, and D. Raoult. 2003. Experimental infection of human erythrocytes from alcoholic patients with Bartonella quintana. Ann. N. Y. Acad. Sci. 990:605-611. [DOI] [PubMed] [Google Scholar]
- 37.Rolain, J. M., C. Foucault, P. Brouqui, and D. Raoult. 2003. Erythroblast cells as a target for Bartonella quintana in homeless people. Ann. N. Y. Acad. Sci. 990:485-487. [DOI] [PubMed] [Google Scholar]
- 38.Rolain, J. M., C. Foucault, R. Guieu, S. B. La, P. Brouqui, and D. Raoult. 2002. Bartonella quintana in human erythrocytes. Lancet 360:226-228. [DOI] [PubMed] [Google Scholar]
- 39.Rolain, J. M., M. Maurin, M. N. Mallet, D. Parzy, and D. Raoult. 2003. Culture and antibiotic susceptibility of Bartonella quintana in human erythrocytes. Antimicrob. Agents Chemother. 47:614-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmid, M. C., R. Schülein, M. Dehio, G. Denecker, I. Carena, and C. Dehio. 2004. The VirB type IV secretion system of Bartonella henselae mediates invasion, proinflammatory activation and antiapoptotic protection of endothelial cells. Mol. Microbiol. 52:81-92. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt, H. U., T. Kaliebe, J. Poppinger, C. Bühler, and A. Sander. 1996. Isolation of Bartonella quintana from an HIV-positive patient with bacillary angiomatosis. Eur. J. Clin. Microbiol. Infect. Dis. 15:736-741. [DOI] [PubMed] [Google Scholar]
- 42.Schuler, G. D., S. F. Altschul, and D. J. Lipman. 1991. A workbench for multiple alignment construction and analysis. Proteins 9:180-190. [DOI] [PubMed] [Google Scholar]
- 43.Spach, D. H., A. S. Kanter, M. J. Dougherty, A. M. Larson, M. B. Coyle, D. J. Brenner, B. Swaminathan, G. M. Matar, D. F. Welch, R. K. Root, and W. E. Stamm. 1995. Bartonella (Rochalimaea) quintana bacteremia in inner-city patients with chronic alcoholism. N. Engl. J. Med. 332:424-428. [DOI] [PubMed] [Google Scholar]
- 44.Zhang, P., B. B. Chomel, M. K. Schau, J. S. Goo, S. Droz, K. L. Kelminson, S. S. George, N. W. Lerche, and J. E. Koehler. 2004. A family of variably expressed outer-membrane proteins (Vomp) mediates adhesion and autoaggregation in Bartonella quintana. Proc. Natl. Acad. Sci. USA 101:13630-13635. [DOI] [PMC free article] [PubMed] [Google Scholar]