Abstract
Polyparasitism is common in the developing world, and interactions that alter disease severity may occur. We previously demonstrated that infection with Schistosoma hematobium was associated with protection against Plasmodium falciparum infection in children who were 4 to 8 years old. In this study, we determined whether underlying helminth infections affected the cytokine responses to acute falciparum malaria. A total of 338 schistosomiasis-positive [Sch(+)] children who were 4 to 14 years old were matched by age, residence, and sex with 338 schistosomiasis-negative [Sch(−)] children and monitored for a malaria transmission season (25 weeks). Serologic cytokine levels were measured at the time of the first clinical malaria episode and in children who did not contract malaria. Elevated background levels of interleukin-6 (IL-6) (37.1 pg/ml versus 10.9 pg/ml [P = 0.04]), IL-4 (27.7 pg/ml versus 6.9 pg/ml [P = 0.02]), IL-10 (18.2 pg/ml versus 7.2 pg/ml [P < 0.001]), and gamma interferon (18.2 pg/ml versus 4.7 pg/ml [P = 0.006]) were noted in Sch(+) children compared to Sch(−) children without malaria. IL-6 and IL-10 levels were elevated in association with acute malaria, but the levels appeared to be blunted in Sch(+) children compared to Sch(−) children who were 4 to 8 years old (for IL-6, 96.2 pg/ml versus 137.2 pg/ml [P = 0.08]; for IL-10, 195.9 pg/ml versus 282.2 pg/ml [P = 0.06]). The level of IL-10 was similarly lower in Sch(+) children than in Sch(−) children who were 9 to 14 years old (91.2 pg/ml versus 141.2 pg/ml [P = 0.03]). IL-4 levels were inversely correlated with the time until the first malaria infection in both the Sch(+) children (P < 0.001) and the Sch(−) children (P < 0.001) who were 4 to 8 years old. We postulate that the Th2-enriched environment induced by schistosomiasis protects against malaria and alters the cytokine milieu during an actual infection.
Polyparasitism is common in tropical areas where helminthic and malaria infections are endemic. The host response to dual infection is poorly understood. Increasing evidence suggests that there are interactions between protozoan and helminthic coinfections, but it is not known whether these interactions benefit or harm the human host (21, 27). Determining whether underlying helminth infections alter the immune response to malaria is critical in light of promising efforts in malaria vaccine development.
We have described an age-specific protective effect of Schistosoma hematobium infection upon the acquisition of uncomplicated Plasmodium falciparum malaria (17). Children who were 4 to 8 years old and who had schistosomiasis had less malaria, and for these children the time to the first clinical episode was longer and the parasitemia during the episode was reduced. Although the mechanisms behind schistosoma-associated protection from malaria are unclear, we postulate that underlying helminthiasis modulates the human immune response, affecting the incidence and severity of concomitant falciparum malaria.
Both malaria and schistosomiasis have stage-specific cytokine production patterns where immunologic balance is critical. We and other workers have demonstrated that malaria is an inflammatory disease in which cytokine levels correlate with disease severity (3, 6, 10, 16). Parasitemia is initially controlled by Th1 cytokines, and there is a gradual shift toward Th2 cytokine predominance (31). Interleukin-4 (IL-4) is critical to memory induction in both the humoral arm (15, 30) and the development of memory CD8+ T-cell responses (2). The role of regulatory T cells and the associated cytokines IL-10 and transforming growth factor β in the host's immune response to malaria has captured interest, as they have recently been associated with increased parasite-induced virulence in humans (32). Furthermore, mice with underlying helminth infection, in contrast to helminth-free mice, appear to generate regulatory T cells in response to murine malaria vaccines (8).
Schistosomes have a dominant Th2-biased cytokine response related to egg production (11). Chronic immune activation due to a helminth infection may cause an altered response to a secondary stimulus that depends upon Th1 cytokine production (13, 23) and may alter T-cell memory responses (9). Increases in the levels of anti-S. hematobium schistosomal egg antigen and 28-kDa glutathione S-transferase immunoglobulin G3 and immunoglobulin E have been detected in coinfected Senegalese children with malaria compared to children without malaria (20, 25). Imbalances in serum proinflammatory cytokine levels have similarly been found in children with both schistosomiasis and malaria compared to children with schistosomiasis alone (7). We examined cytokine production patterns in children with S. hematobium who developed falciparum malaria and compared these children to matched, schistosomiasis-negative children. The results provide further evidence of immunologic alterations that could account for schistosome-induced protection against malaria.
MATERIALS AND METHODS
Study site and parasitologic and clinical evaluation.
Bandiagara (population, 13,600) is located in Mali, West Africa, and has intense seasonal transmission (July to December) of P. falciparum malaria. The dominant ethnic group is Dogon (81%). Most individuals have daily water exposure, and S. hematobium is endemic to the area, whereas Schistosoma mansoni is scarce. Pilot studies revealed that the prevalence of S. hematobium among local children who were 3 months to 14 years old was 25.4% (91/381) (Lyke, unpublished data). Before the 2002 and 2003 malaria transmission seasons, children who were 4 to 14 years old were enrolled in a study designed to determine whether underlying helminth infection altered the acquisition, incidence, or character of falciparum malaria (17). Each child submitted two or three sequential morning (between 10 a.m. and 12 p.m.) urine samples and two stool samples for examination for parasite detection (via standard filtration and the Kato-Katz method). S. hematobium-positive subjects (n = 338) were matched by age, gender, and residence with children without schistosomiasis (n = 338), cleared of other occult intestinal helminth infections with 400 mg albendazole (a drug to which S. hematobium is not susceptible) given orally, and monitored weekly for 25 weeks (175 days). Age categories were defined as follows: 4, 5, 6, 7 to 8, 9 to 10, 11 to 12, and 13 to 14 years. Residence was defined as one of eight distinct sectors of Bandiagara. Children were excluded from enrollment if they were S. mansoni positive, had evidence of chronic infection, were pregnant, or had evidence of complications related to S. hematobium (gross hematuria or renal insufficiency). Children enrolled in 2002 were not eligible for enrollment in 2003.
Study protocols were reviewed and approved by the University of Bamako's Institutional Review Board, as well as by the University of Maryland Institutional Review Board. Village permission to conduct research was obtained from village chiefs, government officials, and traditional healers prior to initiation of the study. Individual informed consent was obtained from the parent or legal guardian of each child prior to screening and enrollment.
Longitudinal monitoring.
The primary outcome was the first clinical malaria infection. Participants were monitored weekly and were evaluated for symptoms of acute malaria infection. Blood smear analysis and hemoglobin analysis were performed monthly and at the onset of symptoms consistent with malaria. A clinical episode of malaria was defined as P. falciparum parasitemia and an axillary temperature of ≥37.5°C during active surveillance or as parasitemia and symptoms leading to treatment-seeking behavior in the absence of another clear cause during passive surveillance. Symptomatic infections were treated with a 3-day course of chloroquine according to the National Malaria Control Program policy at the time of the study. All schistosomiasis infections were treated with praziquantel (40 mg/kg) after malaria transmission had ceased and 6 to 8 weeks after standing water had evaporated to allow for maturation of immature schistosomula in order to maximize the efficacy of treatment.
Serum collection.
Whole blood (1 ml) was collected in sterile microcentrifuge tubes at the time of the first clinical malaria infection of the transmission season (or at the end of the season for the individuals that remained disease free) and prior to institution of antimalarial therapy. Blood was processed via centrifugation. Serum was preserved at −70°C at the field site and remained frozen until cytokine measurements were performed.
Circulating cytokine measurements.
Serum IL-13 levels were determined by a standard enzyme-linked immunosorbent assay (PeliKine, Amsterdam, The Netherlands). The levels of IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, tumor necrosis factor alpha (TNF-α), and gamma interferon (IFN-γ) were determined utilizing cytometric bead array technology (BD Biosciences, San Diego, CA) and fluorescence detection by flow cytometry, following the manufacturer's recommendations, with modifications. Bead populations that had discrete fluorescent intensities of peridinin chlorophyll protein-Cy5.5 and were coated with cytokine-specific capture antibodies were added to patient sera or standards for each cytokine (0 to 5,000 pg/ml) and phycoerythrin-conjugated anti-human cytokine antibodies. After a 3-day incubation, flow cytometric analysis was performed, the results were analyzed by a single operator, and standard curves were derived from the data for the cytokine standards. The lower limit of detection ranged from 2.5 to 10 pg/ml for the cytokines analyzed by flow cytometry and from 0.5 to 1.3 pg/ml for IL-13. Dilution measurement was performed to accurately determine cytokine levels in samples with levels above the linear portions of the standard curves (typically >5,000 pg/ml).
Statistical analysis.
Pooled analyses of differences in cytokine levels between clinical groups were performed using a two-sided Student's t test for continuous variables with equal variance (SPSS 10.0; SPSS, Chicago, IL) and Mann-Whitney rank sum analysis for populations not normally distributed (SigmaStat 3.0; SigmaStat, Chicago, IL). For the purpose of analyzing differences in cytokine levels between matched pairs, the P value for two-sided statistical significance was set at <0.05. Linear regression analysis was used to assess the relationship between cytokine levels at the time of the first malaria infection and time to first clinical malaria infection, as well as parasitemia/mm3. No adjustments were made for multiple comparisons.
RESULTS
Patients.
Serum for cytokine measurements was available from 607 of the 676 children enrolled in the study (324 children with S. hematobium and 283 children without S. hematobium). Twenty-one children (3.1%) exited the study. Two children were removed from the study because of clinical symptoms (subsequently determined not to be related to underlying schistosomiasis), and 19 children relocated out of the study area during the monitoring period. For volunteers who did not experience a malaria infection a venous blood sample was drawn at the end of the malaria transmission season (98 of 140 volunteers). The results represent the background cytokine levels in helminth-free [Sch(−)] individuals or the response to the underlying schistosoma infection in the schistosomiasis-positive [Sch(+)] group. The clinical characteristics of individuals who contributed sera for cytokine analysis are shown in Table 1. No statistical differences between groups were noted.
TABLE 1.
Demographic characteristics at the time of enrollment of S. haematobium-positive participants and age-, gender-, and residence-matched S. haematobium-negative participants who contributed serum for cytokine measurement
| Group | n | No. who werea:
|
Mean age (yr) | No. (%) of females | Hemo globin concn (g/dl) | No. (%) from residential sector:
|
No. (%) with intestinal infectionb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 to 8 yr old | 9 to 14 yr old | I | II | III | IV | V | VI | VII | VIII | ||||||
| S. haematobium negative | 283 | 141 (6.33) | 142 (11.2) | 8.76 | 146 (51.6) | 12.3 | 24 (8.5) | 23 (8.1) | 121 (42.8) | 39 (13.8) | 7 (2.5) | 10 (3.5) | 42 (14.8) | 17 (6.0) | 25 (8.8) |
| S. haematobium positive | 324 | 163 (6.38) | 161 (11.2) | 8.77 | 166 (51.2) | 12.2 | 30 (9.3) | 29 (9.0) | 130 (40.1) | 46 (14.2) | 18 (5.6) | 10 (3.1) | 44 (13.6) | 17 (5.2) | 27 (8.3) |
The numbers in parentheses are mean ages (in years).
Intestinal infection refers to the presence of Necator americanus, Ascaris lumbricoides, Hymenolepsis nana, and Enterobius vermicularis at the time of enrollment.
Differences in cytokine levels between matched groups.
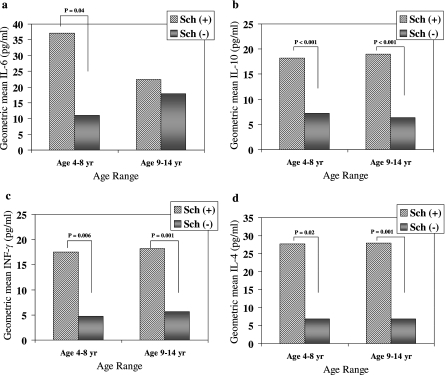
Background IL-4, IL-6, IL-10, and IFN-γ cytokine levels were elevated in Sch(+) children compared to Sch(−) controls (Fig. 1). No differences in the IL-1β, IL-2, IL-5, IL-8, IL-12p70, IL-13, or TNF-α levels were noted during the malaria-free state. Results were stratified by age groups in which clinical differences had previously been noted (i.e., 4 to 8 years versus 9 to 14 years). Increased geometric mean cytokine levels (referred to as cytokine levels below) were observed in S. hematobium-infected children who were 4 to 8 years old (for IL-4, 27.7 pg/ml versus 6.9 pg/ml [P = 0.02]; for IL-6, 37.1 pg/ml versus 11.0 pg/ml [P = 0.04]; for IL-10, 18.2 pg/ml versus 7.2 pg/ml [P < 0.001]; and for IFN-γ, 17.6 pg/ml versus 4.7 pg/ml [P = 0.006]) and in children who were 9 to 14 years old (nearly identical findings with the exception of IL-6) (Fig. 1a to d).
FIG. 1.
Background geometric mean values for the cytokines IL-6 (a), IL-10 (b), IFN-γ (c), and IL-4 (d) in S. hematobium-positive [Sch(+)] and S. hematobium-negative [Sch(−)] individuals, stratified by age group. Increased cytokine levels were found in Sch(+) children (n = 34) compared to Sch(−) children (n = 12) who were 4 to 8 years old (a to d). Cytokine levels were also higher in Sch(+) children (n = 37) than in Sch(−) children (n = 19) who were 9 to 14 years old (b to d). No alterations in IL-1β, IL-2, IL-5, IL-8, IL-12p70, IL-13, or TNF-α levels were found. A Mann-Whitney rank sum analysis was performed with nonnormally distributed data.
Serum cytokine levels were measured at the time of acute malaria infection to evaluate our hypothesis that underlying S. hematobium infection affects the host cytokine response during the early stages of falciparum infection (Table 2 and Fig. 2). Analysis of the 4- to 14-year-old volunteers revealed lower levels of IL-10 in Sch(+) individuals than in matched Sch(−) children (134.3 pg/ml versus 202.8 pg/ml [P = 0.008]). Small but statistically significant elevations in IL-5 levels were observed in Sch(+) individuals compared to Sch(−) children.
TABLE 2.
Serologic cytokine levels during the first clinical malaria episode of the transmission season for Malian children who were 4 to 14 years old with S. haematobium matched by age, sex, and residence with children without S. haematobium
| Cytokine | Geometric mean concn (range) (pg/ml)
|
P valuea | |
|---|---|---|---|
| S. haematobium-positive children (n = 253) | S. haematobium-negative children (n = 252) | ||
| IL-1β | 17.5 (2.5-1,086) | 17.8 (2.5-584) | 0.88 |
| IL-2 | 3.9 (2.0-76) | 3.5 (2.5-28) | 0.07 |
| IL-4 | 16.1 (5.0-181) | 15.7 (4.2-553) | 0.77 |
| IL-5 | 3.1 (2.5-315) | 2.59 (2.5-229) | 0.001 |
| IL-6 | 86.7 (2.5-23,440) | 105.3 (2.5-5,984) | 0.18 |
| IL-8 | 147.8 (5.9-26,106) | 126.2 (4.5-17,439) | 0.35 |
| IL-10 | 134.3 (4.9-12,480) | 202.8 (2.5-20,762) | 0.008 |
| IL-12p70 | 12.3 (2.3-1,742) | 14.3 (1.6-868) | 0.35 |
| IL-13 | 1.73 (0.5-117) | 1.60 (0.5-108) | 0.63 |
| TNF-α | 5.6 (2.5-263) | 4.6 (2.5-159) | 0.05 |
| IFN-γ | 6.9 (2.5-126) | 7.27 (2.5-233) | 0.69 |
Boldface indicates a significant difference as determined by the Mann-Whitney rank sum method with the level of significance set at a P value of <0.05.
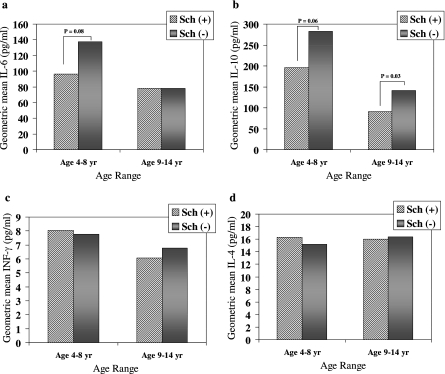
FIG. 2.
Geometric mean values for the cytokines IL-6 (a), IL-10 (b), IFN-γ (c), and IL-4 (d) in children who were infected with P. falciparum only [Sch(−)] or were coinfected with S. hematobium [Sch(+)], stratified by age group. Increased IL-6 and IL-10 cytokine levels were found with acute malaria compared to the background levels, but the levels appeared to be blunted in Sch(+) children (n = 129) compared to Sch(−) children (n = 129) who were 4 to 8 years old (a and b). Lower IL-10 was measured in Sch(+) children (n = 124) compared to Sch(−) children (n = 123) who were 9 to 14 years old (b). No alterations in IL-1β, IL-2, IL-5, IL-8, IL-12p70, IL-13, or TNF-α were found. A Mann-Whitney rank sum analysis was performed with nonnormally distributed data.
Cytokine levels during the acute malaria event were stratified by age and examined (Fig. 2 and Table 3). IL-6 and IL-10 levels were elevated in association with acute malaria, but the levels appeared to be blunted in Sch(+) children compared to Sch(−) children who were 4 to 8 years old (for IL-6, 96.2 pg/ml versus 137.2 pg/ml [P = 0.08]; for IL-10, 195.9 pg/ml versus 282.2 pg/ml [P = 0.06]). The level of reduced IL-10 was similarly lower in Sch(+) children than in Sch(−) children who were 9 to 14 years old (91.2 pg/ml versus 141.2 pg/ml [P = 0.03]). Whereas elevated background levels of IFN-γ and IL-4 were found in the age-stratified Sch(+) groups, the cytokine levels at the time of the first clinical malaria infection were lower than the background levels (P < 0.001 for both cytokines). This effect was not seen in the Sch(−) group, where the absolute levels of IL-4 were higher than the background levels, while IFN-γ levels were similar. When we controlled for age (within the stratified 4- to 8-year-old and 9- to 14-year-old subgroups), no correlation was found between cytokines and the mean parasite density during the clinical malaria episode. No age-associated differences in IL-1β, IL-2, IL-5, IL-8, IL-12p70, IL-13, or TNF-α were observed during the malaria episode. The ratio of anti-inflammatory cytokines to proinflammatory cytokines was examined. A significant difference in the geometric mean IL-10/TNF-α ratio was observed at the time of acute malaria between Sch(+) and Sch(−) children who were 9 to 14 years old (33.2 pg/ml versus 14.7 pg/ml [P = 0.006]), while a trend was observed for children who were 4 to 8 years old (56.3 pg/ml versus 38.6 pg/ml [P = 0.164]). No differences in IL-10/IFN-γ ratios were found between Sch(+) and Sch(−) children in either age group.
TABLE 3.
Serum geometric mean IL-4, IL-6, IL-10, and IFN-γ cytokine levels detected at the time of an acute malaria event in age-stratified Malian children with S. haematobium matched by age, sex, and residence with children without S. haematobium compared to background cytokine levels detected in individuals without malaria
| Cytokine | Group | Age range (yr) | Geometric mean concn (range) (pg/ml)
|
P valuea | |
|---|---|---|---|---|---|
| Children with no malaria | Children with acute malaria | ||||
| IL-4 | Sch(+) | 4-8 | 27.7 (5.0-346.2) (n = 34) | 16.3 (5.0-73.3) (n = 129) | 0.007 |
| 9-14 | 27.9 (5.0-77.3) (n = 37) | 16 (5.0-73.6) (n = 123) | 0.008 | ||
| Sch(−) | 4-8 | 6.9 (5.0-37.0) (n = 12) | 15.2 (5.0-110.1) (n = 129) | 0.06 | |
| 9-14 | 6.8 (5.0-19.0) (n = 19) | 16.4 (5.0-352.5) (n = 124) | <0.001 | ||
| IL-6 | Sch(+) | 4-8 | 37.1 (2.5-339.5) | 95.0 (2.5-5,040) | <0.001 |
| 9-14 | 22.4 (2.5-520.3) | 78.1 (2.5-2,807.7) | <0.001 | ||
| Sch(−) | 4-8 | 22.5 (2.5-81.8) | 137.2 (2.5-3,504.3) | <0.001 | |
| 9-14 | 17.9 (2.5-125.8) | 78.9 (2.5-5,984.2) | <0.001 | ||
| IL-10 | Sch(+) | 4-8 | 18.2 (2.5-60.9) | 195.9 (5.4-2,863.6) | <0.001 |
| 9-14 | 19.0 (2.5-64.3) | 91.2 (6.0-4,999) | <0.001 | ||
| Sch(−) | 4-8 | 7.22 (2.5-99.3) | 282.2 (5.1-7,800) | <0.001 | |
| 9-14 | 6.4 (2.5-32.7) | 141.2 (2.5-5,830.7) | <0.001 | ||
| IFN-γ | Sch(+) | 4-8 | 17.6 (2.5-88.0) | 8.0 (2.5-126.3) | <0.001 |
| 9-14 | 18.2 (5.0-187.2) | 8.0 (2.5-93.1) | <0.001 | ||
| Sch(−) | 4-8 | 4.7 (2.5-13.8) | 7.8 (2.5-73.8) | 0.61 | |
| 9-14 | 5.7 (2.5-32.4) | 6.8 (2.5-232.7) | 0.70 | ||
Boldface indicates that there is a significant difference as determined by the Mann-Whitney rank sum method between pooled geometric mean background and acute malaria cytokine levels stratified by age with significance set at a P value of <0.05.
Association of cytokine and time to clinical malaria infection.
Univariate linear regression models of subjects who developed malaria revealed that the IL-4 levels were inversely correlated with time until first infection in both the Sch(+) (r = 0.275, P < 0.001) and Sch(−) (r = 0.349, P < 0.001) 4- to 8-year-old subgroups; i.e., the greater the time to malaria infection, the lower the levels of IL-4 at the time of infection in the subjects (data not shown). IL-4 levels did not correlate with age within age-stratified groups. A direct linear correlation between IFN-γ and IL-4 levels in the Sch(+) 4- to 8-year-old group (r = 0.724, P < 0.001) [which was not seen in the Sch(−) group or the 9- to 14-year-old group] suggested that the levels of IFN-γ and IL-4 are interdependent in this age group. No relationship was observed between time to malaria infection and the levels of IL-6 and IL-10 measured during the malaria episode.
Dose-dependent effect of egg production.
Sch(+) children were categorized as individuals who excreted low to moderate numbers of eggs (1 to 49 eggs/10 ml urine) or individuals who excreted high numbers of eggs (>50 eggs/10 ml urine) with intercohort analysis and compared to Sch(−) children. No differences in cytokine production were found between the individuals who excreted low to moderate numbers of eggs and the individuals who excreted high numbers of eggs for either the 4- to 8-year-old or 9- to 14-year-old children (data not shown).
DISCUSSION
In Malian children the serologic cytokine milieu at baseline and at the time of an acute falciparum malaria infection appears to be altered by the presence of an underlying schistosomiasis infection. At baseline, the Th2 (IL-4, IL-6, and IL-10) and IFN-γ cytokine levels were elevated in Sch(+) children. An expected robust inflammatory response to erythrocytic falciparum infection was also observed in this study, but when we controlled for age and parasitemia, both IL-6 and IL-10 levels in children who were 4 to 8 years old and IL-10 levels in children who were 9 to 14 years old appeared to be blunted in the presence of S. hematobium compared to the levels in schistosomiasis-negative controls. IL-4 levels measured at the time of an acute malaria infection inversely correlated with the time to first malaria infection; i.e., the longer the time to the malaria episode, the lower the levels of IL-4 at the time of infection in the volunteers. Although the significance of this phenomenon is unclear, we postulate that the Th2-enriched environment elicited by chronic schistosomiasis protects against acquisition of a secondary infection (i.e., malaria). Moreover, it is reasonable to speculate that in individuals who do sustain a malaria infection, the resultant cytokine response is altered by this polarizing Th2 environment.
Concomitant infections have been shown to alter the host immunologic response. Mice with ova-producing S. mansoni infections have impaired lesional healing and an altered Th1/Th2 balance after Leishmania major infection (14), an enhanced ability to expel Trichuris muris via Th2 cytokine-dependent mechanisms (5), and an altered immune response to Plasmodium spp. (12, 35). An underlying nematode infection impairs protective immunity to murine erythrocytic malaria via mechanisms involving transforming growth factor β and IL-10, whereas eradication of the nematode infection restores protective immunity (28). Furthermore, the immunologic response to murine malaria vaccination is blunted by the presence of an underlying nematode infection (29). Elevated levels of plasma IFN-γ and TNF-RII were found in Senegalese children who were 7 to 15 years old and were coinfected with S. hematobium and P. falciparum compared to children with malaria alone (7). Rather than increases in the levels of proinflammatory cytokines in coinfected children, we observed a blunting of Th2 cytokine production, representing an altered immune response to the malaria insult. Low IL-10/TNF-α ratios have been described in association with anemia and cerebral malaria complications and suggest that there is a loss of the counterregulatory anti-inflammatory function of IL-10 (18, 22). We did observe a reduction in the IL-10/TNF-α cytokine ratio (but not in the IL-10/IFN-γ cytokine ratio) for Sch(+) children compared with Sch(−) children who were 9 to 14 years old, suggesting that there was a loss of anti-inflammatory regulation. However, this does not appear to be the operative mechanism underlying the clinical protection against malaria afforded the younger 4- to 8-year-old Sch(+) children. Moreover, our results have to be evaluated in the context that serum cytokine levels may not represent cytokine levels in the cellular microenvironment. Cellular immunologic studies are necessary to further delineate the mechanisms behind helminth-induced protection against malarial disease.
An IL-4-rich environment may polarize the cytokine response toward Th2 predominance (4). Th2 cytokine production appears to be critical for the development of immunity to falciparum malaria. IL-4 levels have been shown to correlate with age, development of antibody to malaria antigen, and malaria protection and are believed to play a role in parasite clearance (1, 15, 30, 33). CD4+-derived IL-4 has been shown to be critical in the development of memory CD8+ T-cell responses to malaria liver stage antigens (2). We found that background IL-4 levels were elevated in children with schistosomiasis. Interestingly, the same children had been monitored for 175 days without acquiring malaria, suggesting that IL-4 has a role in mediating protection against malaria. Our paradoxical observation of an inverse correlation between IL-4 levels and time to first malaria infection seems to be at odds with this finding. Further conclusions concerning the role of IL-4 in mediating protection against malaria cannot be made without documenting IL-4 levels prior to malaria infection in individual children and prospectively monitoring the children to determine malaria acquisition. Further clinical and cellular studies are needed to address this question.
The finding that the IFN-γ level was elevated at baseline in the Sch(+) group was surprising in that enhanced Th2 cytokine production but not Th1 cytokine production is expected in response to worm ovipositing. Ordinarily, extrinsic Th2 cytokine stimulation would polarize CD8+ T cells to secrete Th2 cytokines (26). This paradoxical finding has also been obtained for mice immunized against S. mansoni, where CD8+ T-cell production of IFN-γ and IL-10 was found to be driven by IL-4 derived from CD4+ T cells (24). In schistosomiasis, it is thought that CD8+ cells (demonstrated to be critical in the granulomatous response to egg deposition) (34) may secrete IFN-γ in response to ova-induced IL-4 production in order to down-regulate Th2 production and therefore host immunopathology. We observed a direct correlation between IFN-γ and IL-4 in Sch(+) children who were 4 to 8 years old, contradicting findings reported in other malaria studies performed in the absence of schistosomiasis (19). To our knowledge, these are the first human data demonstrating this phenomenon.
Although there is no a priori reason to believe that serum cytokine levels are different at the beginning and at the end of the transmission season, our conclusions were limited by our inability to measure cytokine levels in all enrolled children prior to the onset of malaria transmission; instead, we relied on cytokine levels measured in individuals who did not succumb to malaria infection as representative background levels. The effect of other concomitant infections is unknown. The baseline prevalence of intestinal infections before albendazole therapy was similar at the time of enrollment. The prevalence of adult human immunodeficiency virus is quite low in the region (1 to 2%), and filarial infection (i.e., Loa loa, Onchocerca volvulus, or Wuchereria bancrofti) was undetectable in pilot studies conducted with 120 area children. Every attempt was made to enroll healthy individuals, but we cannot exclude the possibility that occult infections altered cytokine measurements.
In summary, these studies revealed that the polarized Th2-enriched environment induced by underlying schistosomiasis modulates the human immune response, possibly affecting the incidence and severity of concomitant falciparum malaria. Our results demonstrated that there were elevated background Th2-biased cytokine levels, as well as elevated IFN-γ levels, in children with schistosomiasis compared to uninfected children and suggested that IL-4 and IL-10 may play significant roles in modulating the host response to malaria infection. Additional studies at a cellular level to investigate the cytokine responses to specific schistosomal and malarial antigens are critical to further discern the immunomodulatory role of schistosomes in the presence of secondary antigen stimuli. This research may have a direct impact on the implementation of malaria vaccination in the developing world.
Acknowledgments
We acknowledge the dedication of the Bandiagara Malaria Project staff and the populace of Bandiagara, Mali, who have been so supportive of research efforts and so in need of the benefits of malaria vaccines.
This work was supported by the U.S. National Institutes of Health through contract N01-AI-85346 and career development award K23-AI-49203 from the National Institute of Allergy and Infectious Diseases and by training grant D43TW001589 from the Fogarty International Center.
None of the authors has commercial interests that pose a conflict of interest relevant to the results reported in this paper.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Al Yaman, F., B. Genton, J. Taraika, R. Anders, and M. P. Alpers. 1997. Association between cellular response (IL-4) to RESA/Pf155 and protection from clinical malaria among Papua New Guinean children living in a malaria endemic area. Parasite Immunol. 19:249-254. [DOI] [PubMed] [Google Scholar]
- 2.Carvalho, L. H., G. Sano, J. C. Hafalla, A. Morrot, M. A. Curotto de Lafaille, and F. Zavala. 2002. IL-4-secreting CD4+ T cells are crucial to the development of CD8+ T-cell responses against malaria liver stages. Nat. Med. 8:166-170. [DOI] [PubMed] [Google Scholar]
- 3.Clark, I. A., W. B. Cowden, G. A. Butcher, and N. H. Hunt. 1987. Possible roles of tumor necrosis factor in the pathology of malaria. Am. J. Pathol. 129:192-199. [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper, P. J., M. E. Chico, C. Sandoval, I. Espinel, A. Guevara, M. W. Kennedy, J. F. Urban, Jr., G. E. Griffin, and T. B. Nutman. 2000. Human infection with Ascaris lumbricoides is associated with a polarized cytokine response. J. Infect. Dis. 182:1207-1213. [DOI] [PubMed] [Google Scholar]
- 5.Curry, A. J., K. J. Else, F. Jones, A. Bancroft, R. K. Grencis, and D. W. Dunne. 1995. Evidence that cytokine-mediated immune interactions induced by Schistosoma mansoni alter disease outcome in mice concurrently infected with Trichuris muris. J. Exp. Med. 181:769-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Day, N. P., T. T. Hien, T. Schollaardt, P. P. Loc, L. V. Chuong, T. T. Chau, N. T. Mai, N. H. Phu, D. X. Sinh, N. J. White, and M. Ho. 1999. The prognostic and pathophysiologic role of pro- and antiinflammatory cytokines in severe malaria. J. Infect. Dis. 180:1288-1297. [DOI] [PubMed] [Google Scholar]
- 7.Diallo, T. O., F. Remoue, A. M. Schacht, N. Charrier, J. P. Dompnier, S. Pillet, O. Garraud, A. A. N′Diaye, A. Capron, M. Capron, and G. Riveau. 2004. Schistosomiasis co-infection in humans influences inflammatory markers in uncomplicated Plasmodium falciparum malaria. Parasite Immunol. 26:365-369. [DOI] [PubMed] [Google Scholar]
- 8.Druilhe, P., J. P. Sauzet, K. Sylla, and C. Roussilhon. 2006. Worms can alter T cell responses and induce regulatory T cells to experimental malaria vaccines. Vaccine 24:4902-4904. [DOI] [PubMed] [Google Scholar]
- 9.Elrefaei, M., N. El Sheikh, K. Kamal, and H. Cao. 2003. HCV-specific CD27− CD28− memory T cells are depleted in hepatitis C virus and Schistosoma mansoni co-infection. Immunology 110:513-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grau, G. E., P. F. Piguet, P. Vassalli, and P. H. Lambert. 1989. Tumor-necrosis factor and other cytokines in cerebral malaria: experimental and clinical data. Immunol. Rev. 112:49-70. [DOI] [PubMed] [Google Scholar]
- 11.Grzych, J. M., E. Pearce, A. Cheever, Z. A. Caulada, P. Caspar, S. Heiny, F. Lewis, and A. Sher. 1991. Egg deposition is the major stimulus for the production of Th2 cytokines in murine schistosomiasis mansoni. J. Immunol. 146:1322-1327. [PubMed] [Google Scholar]
- 12.Helmby, H., M. Kullberg, and M. Troye-Blomberg. 1998. Altered immune responses in mice with concomitant Schistosoma mansoni and Plasmodium chabaudi infections. Infect. Immun. 66:5167-5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kullberg, M. C., E. J. Pearce, S. E. Hieny, A. Sher, and J. A. Berzofsky. 1992. Infection with Schistosoma mansoni alters Th1/Th2 cytokine responses to a non-parasite antigen. J. Immunol. 148:3264-3270. [PubMed] [Google Scholar]
- 14.La Flamme, A. C., P. Scott, and E. J. Pearce. 2002. Schistosomiasis delays lesion resolution during Leishmania major infection by impairing parasite killing by macrophages. Parasite Immunol. 24:339-345. [DOI] [PubMed] [Google Scholar]
- 15.Luoni, G., F. Verra, B. Arca, B. S. Sirima, M. Troye-Blomberg, M. Coluzzi, D. Kwiatkowski, and D. Modiano. 2001. Antimalarial antibody levels and IL4 polymorphism in the Fulani of West Africa. Genes Immun. 2:411-414. [DOI] [PubMed] [Google Scholar]
- 16.Lyke, K. E., R. Burges, Y. Cissoko, L. Sangare, M. Dao, I. Diarra, A. Kone, R. Harley, C. V. Plowe, O. K. Doumbo, and M. B. Sztein. 2004. Serum levels of the proinflammatory cytokines interleukin-1 beta (IL-1beta), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect. Immun. 72:5630-5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyke, K. E., A. Dicko, A. Dabo, L. Sangare, A. Kone, D. Coulibaly, A. Guindo, K. Traore, M. Daou, I. Diarra, M. B. Sztein, C. V. Plowe, and O. K. Doumbo. 2005. Association of Schistosoma haematobium infection with protection against acute Plasmodium falciparum malaria in Malian children. Am. J. Trop. Med. Hyg. 73:1124-1130. [PMC free article] [PubMed] [Google Scholar]
- 18.May, J., B. Lell, A. J. Luty, C. G. Meyer, and P. G. Kremsner. 2000. Plasma interleukin-10:tumor necrosis factor (TNF)-alpha ratio is associated with TNF promoter variants and predicts malarial complications. J. Infect. Dis. 182:1570-1573. [DOI] [PubMed] [Google Scholar]
- 19.Mshana, R. N., J. Boulandi, N. M. Mshana, J. Mayombo, and G. Mendome. 1991. Cytokines in the pathogenesis of malaria: levels of IL-I beta, IL-4, IL-6, TNF-alpha and IFN-gamma in plasma of healthy individuals and malaria patients in a holoendemic area. J. Clin. Lab. Immunol. 34:131-139. [PubMed] [Google Scholar]
- 20.Mutapi, F., P. D. Ndhlovu, P. Hagan, and M. E. Woolhouse. 2000. Anti-schistosome antibody responses in children coinfected with malaria. Parasite Immunol. 22:207-209. [DOI] [PubMed] [Google Scholar]
- 21.Nacher, M., F. Gay, P. Singhasivanon, S. Krudsood, S. Treeprasertsuk, D. Mazier, I. Vouldoukis, and S. Looareesuwan. 2000. Ascaris lumbricoides infection is associated with protection from cerebral malaria. Parasite Immunol. 22:107-113. [DOI] [PubMed] [Google Scholar]
- 22.Othoro, C., A. A. Lal, B. Nahlen, D. Koech, A. S. Orago, and V. Udhayakumar. 1999. A low interleukin-10 tumor necrosis factor-alpha ratio is associated with malaria anemia in children residing in a holoendemic malaria region in western Kenya. J. Infect. Dis. 179:279-282. [DOI] [PubMed] [Google Scholar]
- 23.Pearce, E. J., P. Caspar, J. M. Grzych, F. A. Lewis, and A. Sher. 1991. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J. Exp. Med. 173:159-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pedras-Vasconcelos, J. A., and E. J. Pearce. 1996. Type 1 CD8+ T cell responses during infection with the helminth Schistosoma mansoni. J. Immunol. 157:3046-3053. [PubMed] [Google Scholar]
- 25.Remoue, F., T. O. Diallo, V. Angeli, M. Herve, D. De Clercq, A. M. Schacht, N. Charrier, M. Capron, J. Vercruysse, A. Ly, A. Capron, and G. Riveau. 2003. Malaria co-infection in children influences antibody response to schistosome antigens and inflammatory markers associated with morbidity. Trans. R. Soc. Trop. Med. Hyg. 97:361-364. [DOI] [PubMed] [Google Scholar]
- 26.Sad, S., R. Marcotte, and T. R. Mosmann. 1995. Cytokine-induced differentiation of precursor mouse CD8+ T cells into cytotoxic CD8+ T cells secreting Th1 or Th2 cytokines. Immunity 2:271-279. [DOI] [PubMed] [Google Scholar]
- 27.Spiegel, A., A. Tall, G. Raphenon, J. F. Trape, and P. Druilhe. 2003. Increased frequency of malaria attacks in subjects co-infected by intestinal worms and Plasmodium falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 97:198-199. [DOI] [PubMed] [Google Scholar]
- 28.Su, Z., M. Segura, K. Morgan, J. C. Loredo-Osti, and M. M. Stevenson. 2005. Impairment of protective immunity to blood-stage malaria by concurrent nematode infection. Infect. Immun. 73:3531-3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su, Z., M. Segura, and M. M. Stevenson. 2006. Reduced protective efficacy of a blood-stage malaria vaccine by concurrent nematode infection. Infect. Immun. 74:2138-2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Troye-Blomberg, M., E. M. Riley, L. Kabilan, M. Holmberg, H. Perlmann, U. Andersson, C. H. Heusser, and P. Perlmann. 1990. Production by activated human T cells of interleukin 4 but not interferon-gamma is associated with elevated levels of serum antibodies to activating malaria antigens. Proc. Natl. Acad. Sci. USA 87:5484-5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von der, W. T., N. Honarvar, and J. Langhorne. 1996. Gene-targeted mice lacking B cells are unable to eliminate a blood stage malaria infection. J. Immunol. 156:2510-2516. [PubMed] [Google Scholar]
- 32.Walther, M., J. E. Tongren, L. Andrews, D. Korbel, E. King, H. Fletcher, R. F. Andersen, P. Bejon, F. Thompson, S. J. Dunachie, F. Edele, J. B. De Souza, R. E. Sinden, S. C. Gilbert, E. M. Riley, and A. V. Hill. 2005. Upregulation of TGF-beta, FOXP3, and CD4+ CD25+ regulatory T cells correlates with more rapid parasite growth in human malaria infection. Immunity 23:287-296. [DOI] [PubMed] [Google Scholar]
- 33.Winkler, S., M. Willheim, K. Baier, D. Schmid, A. Aichelburg, W. Graninger, and P. G. Kremsner. 1999. Frequency of cytokine-producing T cells in patients of different age groups with Plasmodium falciparum malaria. J. Infect. Dis. 179:209-216. [DOI] [PubMed] [Google Scholar]
- 34.Wynn, T. A., and A. W. Cheever. 1995. Cytokine regulation of granuloma formation in schistosomiasis. Curr. Opin. Immunol. 7:505-511. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida, A., H. Maruyama, T. Kumagai, T. Amano, F. Kobayashi, M. Zhang, K. Himeno, and N. Ohta. 2000. Schistosoma mansoni infection cancels the susceptibility to Plasmodium chabaudi through induction of type 1 immune responses in A/J mice. Int. Immunol. 12:1117-1125. [DOI] [PubMed] [Google Scholar]