Abstract
The neutralizing antibody response to the protective antigen (PA) component of anthrax toxin elicited by approved anthrax vaccines is an accepted correlate for vaccine-mediated protection against anthrax. We reasoned that a human anti-PA monoclonal antibody (MAb) selected on the basis of superior toxin neutralization activity might provide potent protection against anthrax. The fully human MAb (also referred to as MDX-1303 or Valortim) was chosen from a large panel of anti-PA human MAbs generated using transgenic mice immunized with recombinant PA solely on the basis of in vitro anthrax toxin neutralization. This MAb was effective in prophylactic and postsymptomatic treatment of rabbits exposed to aerosolized anthrax spores, and a single intramuscular injection of 1 mg/kg of body weight fully protected cynomolgus monkeys challenged with aerosolized anthrax spores. Importantly, MAb 1303 defines a novel neutralizing epitope that requires Fc receptor engagement for maximal activity. F(ab′)2 fragments of MAb 1303, which retain equivalent affinity for PA, are 10- to 100-fold less potent in neutralizing anthrax toxin in vitro. Addition of Fc receptor-blocking antibodies also greatly reduced the activity of MAb 1303. Moreover, we found that the neutralizing activity of mouse, rabbit, and human antisera elicited by PA vaccines was effectively abrogated by blocking Fc receptors. Selection of an anti-PA MAb by using a functional assay that is a surrogate for protection has resulted in the identification of a fully human MAb with potent activity in vivo and uncovered a previously unrecognized mechanism of antibody-mediated toxin neutralization that is important for currently used anthrax vaccines.
The intentional use of Bacillus anthracis, the causative agent for anthrax, as a bioterrorism or biowarfare agent constitutes a significant health and economic concern. Vaccines for prophylaxis against anthrax have been developed and have been administered to thousands of people, primarily to members of the armed forces (8, 9). Anthrax vaccines are highly effective (5), but because the current licensure for AVA Biothrax requires a multiple-dose schedule, antibiotic therapy may be recommended for postexposure prophylaxis prior to the completion of the vaccination series.
Efficacy of the approved anthrax vaccine, AVA Biothrax (Bioport Corp., Lansing, MI) or the next generation vaccine based upon recombinant protective antigen (PA) correlates with the development of humoral responses to the PA component of the anthrax toxin (12, 21, 23, 27, 28). During infection, PA (PA83) binds to cell surface receptors and is cleaved by a membrane-bound protease, resulting in a receptor-bound truncated form of PA, PA63, that can oligomerize on the cell surface into a heptameric structure (7). This ring structure forms a high-affinity binding site for the bacterial enzymes lethal factor (LF) and edema factor to form holotoxins that are translocated into the host cell cytoplasm, where they mediate their toxic effects.
The correlation of humoral immunity to PA with protection against anthrax has spawned a number of approaches for developing passive antibody therapies directed at PA. Studies have clearly demonstrated the protection elicited by transfer of sera from PA-immunized animals, and antibody-mediated toxin neutralization appears to be the best in vitro correlate for in vivo potency (11, 23). Neutralizing antibody titers are generally determined using the toxin neutralization assay (TNA), which measures the inhibition of toxin-mediated cell death of susceptible murine macrophage-like cell lines. In the absence of the ability to challenge human subjects, potency of antisera in a standardized TNA has become an accepted surrogate for determining the efficacy of various candidate vaccines.
The multiple steps in the pathway of PA binding, processing, oligomerization, formation of holotoxin, and internalization are each potential therapeutic targets using a monoclonal antibody (MAb). Initial studies on the characterization of anti-PA MAbs suggested that MAbs with potent toxin neutralizing activity functioned by inhibiting PA-receptor interactions or PA interactions with LF or edema factor (13, 15). Subsequently, toxin-neutralizing antibodies have been described that appear to function by inhibiting the cleavage of cell-bound PA to PA63 or assembly of the PA63 heptamer (6, 26). Some MAbs that act by these mechanisms have been shown to protect against lethal challenge with aerosolized spores in animals (18, 20) and support the expectation that passive immunity may be conferred with appropriate single anti-PA MAbs to individuals exposed to B. anthracis. The relative in vivo efficacy of antibodies that function by each of these mechanisms has not been established. Moreover, the importance of each of these mechanisms to the immunity provided by anthrax vaccines is unknown.
In this study, we used an unbiased approach with respect to the precise mechanism for selecting an anti-PA MAb with superior anthrax toxin-neutralizing activity and chose performance in the TNA as the primary criterion. We reasoned that, since the TNA provides the best in vitro correlate for protective potency of polyclonal antisera, that it might also provide a useful approach for identifying potent MAbs and provide additional insights into the basis for in vivo protection by the polyclonal response to vaccines. To generate a MAb with optimum characteristics for human application, we developed fully human MAbs specific for PA by immunization of mice engineered to express human immunoglobulin in response to vaccination. The anti-PA human MAb selected on the basis of superior TNA activity is indeed highly potent in animal models of anthrax infection. This MAb, designated 1303 or Valortim, does not block attachment of PA to the cell surface, and full in vitro protective activity is dependent upon interaction with host cell immunoglobulin (Fc) receptors. Furthermore, we found that this Fc receptor dependence is a dominant feature of the effective polyclonal immune response elicited in humans and animals by PA-based anthrax vaccines.
MATERIALS AND METHODS
Cell lines and reagents.
Anti-PA human reference serum AVR801 was generously supplied by Conrad P. Quinn, Centers for Disease Control and Prevention, Atlanta, GA (24). The mouse macrophage cell line J774A.1 was obtained from the American Type Culture Collection, Manassas, VA. B. anthracis recombinant LF and PA were obtained from List Biological Laboratories (Campbell, CA).
Immunization of transgenic mice and development of hybridomas.
Transgenic mice from HuMab Mouse colony (17), strain HC2/KCo7, were immunized intraperitoneally with 15 μg of purified recombinant PA (83 kDa) in RIBI MPL plus TDM adjuvant system and boosted with 15 μg of proteolytically cleaved (63 kDa) PA twice at 2-week intervals. A final immunization with 10 μg of purified PA63 was given intravenously (i.v.). Additional mice were immunized using purified recombinant PA emulsified in Freund's adjuvant. Three to four days after the final boost, the spleens were harvested and the splenocytes fused with the P3x63Ag8-653 myeloma cells using polyethylene glycol. After fusion, the cells were plated in 96-well plates, and hypoxanthine-aminopterin-thymidine was added to the media for selection of the hybridomas. Cells which grew out of the selection media were screened for immunoglobulin G(κ) [IgG(κ)] production by enzyme-linked immunosorbent assay (ELISA), followed by neutralization activity utilizing the in vitro TNA. Antibodies were purified from the hybridoma supernatant using protein A affinity chromatography.
Production and purification of recombinant 1303.
The 1303 variable light chain and variable heavy chain sequences were obtained by reverse transcription-PCR using RNA extracted from the 1303 hybridoma. The variable light chain and variable heavy chain sequences were then cloned into a mammalian expression vector into which the human Ig(κ) and IgG1 genes had been previously introduced. The fidelity of all cloning steps was verified by DNA sequencing of the entire 1303 heavy chain and light chain coding regions. The heavy chain and light chain sequences were cloned into a single mammalian expression plasmid (p1303) under the control of cytomegalovirus (CMV) promoters and including the neomycin resistance gene for selection.
For expression and purification of the recombinant MAb 1303, the construct was transfected into CHO cells using SuperFect reagent according to the manufacturer's instructions (QIAGEN, Valencia, CA). Stable transfectants were selected by maintaining cells in growth media (α minimal essential medium, 10% dialyzed fetal bovine serum) containing 550 μM G418 (Calbiochem-Novabiochem, San Diego, CA). Subsequently, colonies were isolated using cloning cylinders, and cell lines that produced the highest amounts of MAb were identified by ELISA. MAb was purified from overgrown culture supernatants using a protein A-Sepharose column.
TNA.
An in vitro neutralization assay described previously was modified and used to assess the ability of the MAbs to neutralize anthrax lethal toxin (14). The mouse macrophage cell line J774A.1 was maintained in Dulbecco's modified Eagle medium containing glucose, sodium pyruvate, and GlutaMax and supplemented with 10% heat-inactivated fetal bovine serum. Cells were scraped from confluent flasks and suspended to 6 × 105 to 7 × 105/ml in the media mentioned above, plated in 96-well flat-bottom plates (150 μl per well), and incubated overnight at 37°C in a 5% CO2 humidified incubator. Macrophage serum-free medium containing antibody was preincubated with lethal toxin (400 ng of PA per ml plus 40 ng of LF per ml) for 1 h at 37°C prior to transferring 100 μl to J774A.1 cells. The plates were incubated for 3 h at 37°C, 5% CO2. To assess cell viability, 100 μl of CellTiter-Glo reagent (Promega Corp., Madison, WI) was added to each well after the plate was equilibrated to room temperature for 30 min. The plates were mixed on a plate shaker for 2 min to facilitate cell lysis, and after 10 min, the luminescence signal was recorded on a Wallac 1450 MicroBeta JET liquid scintillation and luminescence counter. Cell viability was calculated from the following formula: (mean luminescence counts per second in sample/mean luminescence counts per second in cells without toxin) × 100%. Experiments to investigate the role of Fc receptor interaction were performed as described, except that rat anti-mouse CD16/CD32 Fc receptor blocking clone 2.4G2 (BD Pharmingen, San Jose, CA) at 100 μg/ml was preincubated with J774A.1 cells for 15 min prior to the addition of anti-PA MAbs and lethal toxin samples.
Cytokine stimulation assay.
Human peripheral blood mononuclear cells (PBMCs) were purified from fresh whole blood by density gradient centrifugation and used for cytokine stimulation experiments as described by Popov et al. (22). Briefly, PBMCs were adjusted to 5 × 106/ml in RPMI containing 10% fetal bovine serum. PBMCs (1 × 106) were incubated overnight at 37°C, 5% CO2 with 10 μg/ml lipopolysaccharide (LPS), 0.5 μg/ml PA, and 0.5 μg/ml LF. MAbs and F(ab′)2 fragments at multiple dilutions were also added to the cultures. The next day, the supernatants were harvested and assayed for the presence of human interleukin-6 (IL-6) using an ELISA kit (Pierce, Rockford, IL).
Protection against inhalational anthrax.
Rabbit and nonhuman primate (NHP) models of inhalational anthrax were conducted at Battelle Medical Research and Evaluation Facility, West Jefferson, Ohio, in compliance with the Animal Welfare Act. New Zealand White rabbits (10/group) were injected i.v. with MAb 1303 at 1, 24, or 48 h post-muzzle-only aerosol challenge with 100× 50% lethal dose (LD50) of B. anthracis strain Ames spores. Cynomolgus monkeys (6/group) were injected intramuscularly (i.m.) with MAb 1303 at 1 h post-head-only aerosol challenge with 200× LD50 of B. anthracis strain Ames spores. Aqueous suspensions of B. anthracis spores were aerosolized by a collision nebulizer. Inhaled doses were calculated using the concentration of B. anthracis spores in the test aerosol from the plate counts of the impinger and individual plethysmography data. Rabbits were observed for 14 days postexposure, and cynomolgus monkeys were observed for 90 days postexposure. Blood was drawn periodically during the study for evaluation of serum levels of MAb 1303, antitoxin antibodies, bacteremia, and clinical pathology.
Immunoassays for MAb binding to PA.
ELISA microtiter plates were coated with 5 μg/ml mouse anti-PA MAb clone BAP 0105 (Biodesign, Saco, ME). After blocking with phosphate-buffered saline (PBS) containing 5% bovine serum albumin (BSA), full-length (83 kDa) or cleaved (63 kDa) PA (5 μg/ml) was captured to the plates. Unbound PA was removed, and test samples were incubated on the plate for 1 h at 37°C. Unbound antibody was washed from the plate with PBS containing 1% Tween, and the wells were incubated with an alkaline-phosphatase-conjugated goat anti-human IgG (Fc specific) for 1 h at 37°C. The excess conjugated antibody was washed from the wells, and the plate was developed with pNPP substrate (Pierce, Rockford, IL). The optical density was determined at 405 nm using a Molecular Devices SPECTRAmax PLUS384 plate reader.
Western blotting was performed on full-length PA (PA83) and PA fragments generated by trypsin and chymotrypsin digestion after immunoprecipitation with MAb 1303 or isotype control MAb. Mabs were bound to protein A agarose beads (Roche Diagnostics, Indianapolis, IN) for 1 h at room temperature on a tube rotator. The resin was washed with a buffer containing 50 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 0.1% NP-40, 0.25% gelatin, and 0.02% NaN3, pH 7.5, to remove unbound MAb before incubating with the PA preparations for 1 h at room temperature on a tube rotator. The beads were washed with the buffer described above, and the pellet was resuspended in reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer and boiled for 2 min. The proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on a 12.5% Tris-HCl gel (Bio-Rad Laboratories, Hercules, CA) and transferred to an immunoblot polyvinylidene difluoride membrane (Bio-Rad Laboratories, Hercules, CA). The membrane was blocked with SuperBlock blocking buffer in PBS (Pierce, Rockford, IL), and the PA fragments were detected with polyclonal rabbit anti-PA IgG at 1 μg/ml, followed by an incubation with a goat anti-rabbit IgG (Fc specific) alkaline phosphatase conjugate (Jackson ImmunoResearch, West Grove, PA) at a 1:10,000 dilution. The reactive bands were detected with CDP-Star chemiluminescence substrate (Roche Diagnostics, Indianapolis, IN).
Inhibition of PA binding to cells.
Anti-PA MAbs (400 μg/ml) or human anti-PA immune sera (diluted 1:2) were incubated for 20 min at room temperature with fluorescein (FITC)-labeled PA83 (PA-FITC) at 40 μg/ml in PBS containing 0.1% BSA and 0.05% sodium azide. This solution was then added to human PBMCs and incubated for an additional 1 h at room temperature on a plate shaker. Samples were washed twice with PBS containing 0.1% BSA and 0.05% sodium azide to remove unbound antibody and then read immediately on a Becton Dickinson LSR flow cytometer to determine the level of fluorescence of the PA-FITC bound to the monocyte population.
RESULTS
Human anti-PA MAbs selected for potent toxin-neutralizing activity.
The HuMAb mouse was engineered to express human rather than murine Ig and has been successfully used to generate human MAbs to a variety of antigens. Human MAbs derived from these mice are in a variety of clinical trials, have demonstrated low immunogenicity, and persist in circulation for an extended time in humans (16). To generate human MAbs to PA, HuMAb mice were immunized with recombinant PA and the spleen cells were fused with myeloma cells. Hybridomas were initially selected on the basis of human IgG(κ) secretion by ELISA. Supernatants from all of the stable human IgG(κ)-secreting hybridomas were then evaluated for toxin neutralization activity in vitro. Screening of hybridomas from 7 different fusions yielded more than 100 clones with neutralizing activity. A wide range of activity was observed (50% effective dose, ∼1 to 100 nM). One MAb, designated MAb 1303, consistently had the lowest 50% effective dose values and compared favorably to the previously characterized murine anti-PA MAbs (Fig. 1). MAb 1303, generated from immunization containing both PA63 and PA83 and selected solely on potency in the neutralization assay, was further developed for testing in animal models of inhalational anthrax.
FIG. 1.
Neutralization of lethal toxin activity by anti-PA MAbs. Various concentrations of purified human (1303) and murine (1G3, 14B7) MAbs were premixed with anthrax lethal toxin (400 ng PA and 40 ng LF per ml) before addition to J774A.1 macrophages. Viability was determined after 3 h of incubation. Values represent the means of results from duplicate wells of a representative experiment.
MAb 1303 protects against inhalational anthrax.
The prophylactic activity of MAb 1303 was evaluated in a rabbit inhalational model of anthrax infection. The initial study using MAb 1303 purified from supernatants of the hybridoma culture demonstrated significant protection from an aerosol challenge of anthrax spores (average dose, 200× LD50). Nine of 10 animals receiving a total dose of 17 mg/kg of body weight survived, and 8 of 10 animals receiving 9 mg/kg survived. All 10 animals in the saline control group died within 5 days. The MAb 1303 was subsequently engineered into a human IgG expression vector and produced from transfected CHO cells for higher productivity. In a second efficacy study, lower doses of MAb 1303 were used and found to be equally effective in protecting rabbits against inhalational anthrax (Fig. 2A). The lowest dose tested (1 mg/kg) injected 1 h and 3 days post-anthrax exposure was sufficient to protect 9 of 10 animals. Surviving animals were free of pathology or other evidence of infection at 14 days postexposure.
FIG. 2.
Efficacy of MAb 1303 in animal models of inhalation anthrax. Various doses of MAb 1303 were administered to rabbits (A) or cynomolgus monkeys (B) following aerosol challenge with B. anthracis Ames spores. (A) Results are from 2 studies consisting of groups of 10 rabbits injected i.v. with MAb 1303 at the indicated times. (B) Groups of 6 monkeys were injected i.m. with MAb 1303 approximately 1 h after aerosol challenge. The challenge dose ranged from 100 to 300 LD50 in these studies. d, day.
The activity of MAb 1303 against inhalational anthrax was confirmed in an animal model using cynomolgus macaques. Animals were challenged with anthrax spores (200× LD50) and injected i.m. with a single dose of 1 mg/kg or 10 mg/kg of MAb 1303 1 h post-anthrax exposure. Both dose levels resulted in complete protection from severe clinical symptoms and death for the duration of the 90-day observation period (Fig. 2B). Pharmacokinetic analysis of MAb 1303 in macaques suggests that serum concentrations between 3 to 10 μg/ml during the first 2 weeks of exposure were sufficient to mediate protective immunity (data not shown).
Therapeutic activity of MAb 1303 against inhalational anthrax.
In addition to prophylaxis, the antitoxin activity of anti-PA MAbs may be therapeutically useful after symptoms have emerged. To model this, MAb 1303 was administered to rabbits 24 or 48 h after aerosol exposure to B. anthracis spores. At 24 h postexposure, the animals were not overtly symptomatic; however, 1 of 10 animals had slightly elevated serum enzyme levels and all animals had significant decreases in peripheral blood neutrophil counts. Eight of the nine animals treated at 24 h survived the infection; therefore, no decrease in the antibody's efficacy was noted compared to prophylactic treatment.
In the 48-h-postexposure group, 3 of 10 animals died prior to receiving the MAb. Three of the seven animals treated with MAb at 48 h died within 24 h of the first treatment (2 to 3 days post-exposure to anthrax), and one animal died 3 days after treatment (5 days postexposure). The remaining three animals survived until study termination (43% survival). Clinical signs of anthrax infection were apparent at the time of treatment in six of the seven animals. Four of these animals exhibited decreased appetite, and two animals had decreased activity at 48 h post exposure. In addition, elevated serum enzyme levels were apparent in five of the seven animals. In two of these animals, alanine transaminase levels were dramatically increased (>1,500 U/liter), indicating that significant hepatoxicity was present prior to MAb 1303 administration, and these two animals died within 24 h of treatment. One of the three surviving animals also had elevated aspartate transaminase and alanine transaminase levels at the time of administration of the MAb. Combined, these data indicate that treatment with MAb 1303 can decrease mortality rates in overtly symptomatic animals as well as in animals that show laboratory signs consistent with infection. Thus, the premise of selecting an anti-PA MAb based on superiority in the in vitro toxin neutralization assay indeed resulted in a highly effective MAb against inhalational anthrax.
Functional characterization of MAb 1303.
The specificity and mechanism of action of MAb 1303 was further investigated using in vitro experiments. MAb 1303 recognized both full-length PA (PA83) and PA63 by ELISA (Fig. 3). Immunoprecipitation of PA83 and its cleavage products obtained by trypsin and/or chymotrypsin treatment further identified the PA47 fragment to contain the MAb 1303 epitope (Fig. 3c). This fragment is largely composed of domain IV of PA and includes the receptor binding region. However, MAb 1303 does not competitively bind the epitope of MAb 14B7 (data not shown) that blocks receptor binding, 14B7 (15), and is unable to block FITC-labeled PA from binding to cells (Fig. 4, top). In addition, as receptor-blocking antibodies can also neutralize in vitro preformed lethal toxin (PA-63)7 plus LF by preventing binding to cells, MAb 1303 is unable to neutralize in vitro preformed lethal toxin complexes (Fig. 5). Thus, MAb 1303 represents an antibody with specificity for the carboxy-terminal region of PA that does not interfere with receptor binding or LF-PA interactions but neutralizes lethal toxin cytotoxicity and infection with B. anthracis spores.
FIG. 3.
Binding of MAb 1303 to PA. MAb 1303 was tested for binding to PA83 (A) and PA63 (B) by ELISA. Recombinant PA was captured to microtiter plates with murine MAb and exposed to various concentrations of MAb 1303 or isotype control antibody. MAb binding was detected with an anti-human IgG-specific probe. PA83 was subjected to trypsin (T) and/or chymotrypsin (C) digestion followed by immunoprecipitation with MAb 1303 or isotype control IgG (C). PA and PA fragments were visualized by Western blotting with a rabbit anti-PA polyclonal antibody probe. OD, optical density.
FIG. 4.
MAb 1303 does not block PA binding to cells. Flow cytometry of PA-FITC binding to human monocytes in the presence or absence of anti-PA antibodies was analyzed. Anti-PA antibodies (14B7, 10 μg/ml; 1303, 10 μg/ml; AVR801, 1:100) were preincubated with PA-FITC for 20 min prior to incubation with monocytes for 1 h at ambient temperature. After washing, the cell-associated fluorescence was determined. The shaded histogram represents cells not exposed to PA-FITC.
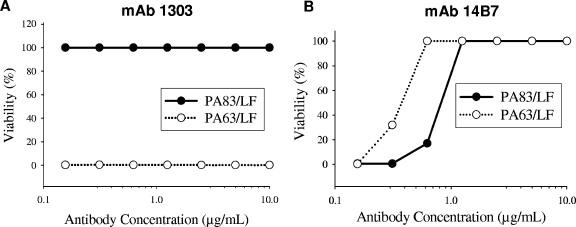
FIG. 5.
Lethal toxin formed in vitro is not neutralized by MAb 1303. The standard TNA was performed with either PA83 or PA63 (heptamer) and lethal factor preincubated with various concentrations of MAb 1303 (A) or MAb 14B7 (B). Viability of the cells was determined after 3 h. Values represent the means of results from duplicate wells of a representative experiment.
These results suggested that the in vitro neutralization activity of MAb 1303 was mediated by interference with toxin assembly at the cell surface. In fact, antibodies that block the proteolytic processing or heptamerization of PA have been recently described (6, 26). We hypothesized that the function of MAb 1303 should be retained by the F(ab′)2 fragment of the antibody. Surprisingly, removal of the Fc portion of MAb 1303 resulted in a nearly 2-log reduction in activity (Fig. 6). Moreover, addition of an anti-Fc receptor MAb that blocks Ig binding (25), similarly reduced the neutralizing capacity of the MAb 1303, independently demonstrating the requirement of Fc receptor interactions for the full potency of MAb 1303 (Fig. 6). The anti-Fc receptor antibody had no effect on the neutralizing activity of the receptor-blocking MAb 14B7 (data not shown).
FIG. 6.
Fc receptors cooperate in the toxin neutralization activity by MAb 1303. The TNA activities of MAb 1303, MAb 1303 F(ab′)2 fragments, and MAb 1303 in the presence of an Fc receptor-blocking antibody (2.4G2, 20 μg/ml) were determined using J774A.1 cells. Values represent the means of results from duplicate wells of a representative experiment. α-FcγRII/III, anti-FcγRII/III.
To explore whether the observation of Fc receptor dependence was a characteristic of the particular cellular TNA employed in these experiments, we examined the activity of MAb 1303 and MAb 1303 F(ab′)2 in another relevant assay for anthrax toxin-mediated toxicity. We adapted an assay described by Popov et al., who measured suppression of cytokine secretion by human PBMC stimulated by LPS in the presence of anthrax lethal toxin (22). In these assays, MAb 1303 was again much more potent than F(ab)2 fragments of MAb 1303 in restoring the ability of the human PBMC to produce inflammatory cytokines (IL-6) in response to LPS (Fig. 7). Similar effects were observed with isolated cultured human monocytes or dendritic cells (data not shown). These data demonstrate that the Fc receptor-dependent mechanism of toxin neutralization by MAb 1303 is applicable to multiple relevant assay systems for anthrax toxin-mediated toxicity.
FIG. 7.
MAb 1303 abrogates anthrax toxin-mediated inhibition of cytokine induction in human cells. Human PBMCs were exposed to LPS (1 μg/ml) in the presence of lethal toxin with or without anti-PA antibodies. The level of IL-6 in culture supernatant after overnight incubation was determined by ELISA. Values represent the means of results from duplicate wells of a representative experiment.
Fc receptor-dependent neutralization activity of anthrax vaccine-induced antibodies.
An Fc receptor-dependent toxin neutralization activity had not been previously described, and thus, we investigated whether this characteristic was true of antibodies that are generated in response to protective anthrax vaccines. AVR801 is a CDC human reference antiserum prepared from pooled antisera of AVA-immunized individuals and contains 109.4 mg/ml anti-PA IgG (24). The TNA activity of AVR801 was effectively abrogated by the addition of the blocking anti-Fc receptor MAb (Fig. 8b). Similar results were observed with immune serum collected from mice and rabbits vaccinated with recombinant PA83 (Fig. 8a). These data suggest that Fc receptor-dependent neutralization is a common characteristic of polyclonal antibodies generated by PA-containing anthrax vaccines. Interestingly, under the conditions of our experiments, AVR801 was also unable to interfere with PA binding to cells (Fig. 4, lower panel). This suggests that an alternative mechanism to blocking of PA binding to cell receptors, which is dependent on Fc receptors, is important for the toxin neutralizing activity of immune sera.
FIG. 8.
Neutralizing activities of animal and human immune sera are dependent on Fc receptor interactions. The TNA activities of immune sera from mice or rabbits immunized with recombinant PA vaccine were assessed in the presence or absence of an Fc receptor-blocking antibody, 2.4G2 (A). Similarly, the human reference serum (AVR-801) was tested for TNA activity with or without MAb 2.4G2 (100 μg/ml) (B). Values represent the means of results from duplicate wells of a representative experiment. α-PA, anti-PA.
DISCUSSION
In this study, we used the cell-based anthrax toxin neutralization assay as the sole method to select a human anti-PA MAb with potential clinical utility, as opposed to other approaches based on antibody affinity or inhibition of receptor binding. As predicted from previous studies correlating potent toxin neutralization activity with protection in animals, MAb 1303 was effective in the prevention of anthrax lethality in rabbits and NHPs despite the aerosolized administration of greater than 100 times the lethal dose of virulent spores. To our knowledge, the lowest dose tested of MAb 1303 (1 mg/kg) is the lowest dose of an anti-PA MAb to show 100% protection in the NHP model. Our unexpected observation was the Fc receptor dependence of toxin neutralization for this anti-PA antibody.
Unlike previously described anti-PA MAbs, MAb 1303 activity was only partially mediated by the direct attachment of the antibody binding domains. Experiments using MAb 1303 F(ab′)2 fragments and Fc receptor-blocking antibodies, in two different assay systems, definitively demonstrated the requirement for Fc receptor binding by the Fc domain of MAb 1303 for full toxin-neutralizing potency. In the absence of Fc receptor interaction, MAb 1303 was 1 to 2 logs less effective than under conditions that promote Fc receptor interactions. Our results may suggest that immune complexes consisting of cell-bound PA, MAb 1303, and Fc receptors are formed at the cell surface, resulting in interference with the subsequent oligomerization of PA into viable toxin complexes and their translocation. Consistent with this hypothesis, MAb 1303 failed to neutralize preformed toxin complexes but could significantly prevent cell death up to 20 min after the addition of PA and LF subunits to J774 cells (data not shown).
Although an Fc receptor dependence of anthrax toxin neutralization was not demonstrated with prior antibodies, we examined whether this feature was unique to MAb 1303. Interestingly, a similar Fc-dependent characteristic was found with the second most potent human MAb generated in our studies as well as with the well-characterized, neutralizing anti-PA MAb 1G3 (data not shown) (15). Furthermore, we demonstrated that the toxin-neutralizing activity of (pooled) human and animal polyclonal immune sera was also substantially abrogated in the presence of an anti-Fc receptor MAb. Fc receptor blockade had no inhibitory effect on the toxin neutralization of an anti-PA MAb that functions by blocking PA binding to cell receptors, consistent with the expectation that such activity would only be active on circulating PA, not after binding to the cell surface. Consistent with the TNA results, we were unable to demonstrate that the polyclonal immune sera contained significant blocking activity for PA binding to cell receptors, suggesting that the natural predominant mechanism of anthrax toxin neutralization by antibodies elicited by anthrax vaccines do not function by inhibiting initial PA binding but via other epitopes that permit an Fc receptor-mediated interaction on the cell surface.
The precise mechanism by which Fc receptor interaction with anti-PA MAbs results in enhanced activity remains to be established. MAb binding to receptor-bound PA and Fc receptors may result in immune complex formation at the cell surface that are endocytosed into degradation pathways mediated by the Fc receptor, or simply interfere with the proper toxin assembly. Recent studies by Abrami et al. demonstrated that the cellular internalization of the anthrax toxin involves a relatively slow clustering of the anthrax toxin receptor (ATR) into lipid rafts (2). The investigators also showed that palmitoylation prevents ATR from association with lipid rafts and premature internalization (1), and only heptameric PA63 (not monomeric PA83) can cluster the ATR into lipid rafts. Thus, endocytosis of the ATR-bound monomeric PA remains slow until the processing and oligomerization of PA63 occurs. Presumably, this would allow time for MAb 1303 to bind ATR-PA83 and ATR-PA63 (before heptamerization) complexes and simultaneously engage Fc receptors with the Fc portion of the MAb. Such interactions may result in premature internalization of the ATR-PA complex or prevent the appropriate migration to lipid rafts for toxin assembly and endocytosis. The fact that the in vitro toxin neutralization assay requires significantly less MAb 1303 than PA83 (Fig. 1) suggests that one molecule of 1303 may be interacting with multiple PA molecules or that a single immune complex may be sufficient to interfere with heptamer formation/function. We are currently investigating how these mechanisms may be contributing to the neutralizing effect of MAb 1303 and other anti-PA antibodies.
Interestingly, Mohamed et al. found that certain anti-PA MAbs could enhance the cytotoxicity mediated by lethal toxin in an Fc receptor-dependent manner (19). Since our selection was for antibodies that mediate potent neutralization of lethal toxin, it is unknown whether human MAbs may also mediate the enhancing effect described with some murine anti-PA MAbs. Alternatively, it may be the epitope specificity that determines the nature of the Fc receptor interaction and its outcome on toxin activity.
The absolute relevance of the Fc receptor interaction to the in vivo efficacy of anti-PA antibodies remains to be understood. MAb 1303 does protect against anthrax toxin cytotoxicity in the absence of Fc receptor interactions (albeit at significantly higher concentrations); thus, in vivo protection cannot be unambiguously attributed to the Fc-enhanced neutralization activity. Although some cells that are targets for the anthrax toxin in vivo do not express Fc receptors, many cells of the immune system which are critical to the innate response to pathogens have abundant expression of Fc receptors. In particular, dendritic cells have been implicated as a critical cell in the initial response to B. anthracis infection (3, 4). Therefore, it is reasonable to consider the Fc receptor interactions of anti-PA MAbs to be important to their efficacy in vivo.
In addition to defining a novel mechanism of action for anti-PA antibodies, our studies with delayed administration of MAb 1303 to infected and symptomatic animals suggests this MAb may be effective in a therapeutic setting. Since antibiotics have limited efficacy after exposure, development of additional therapeutic options for symptomatic individuals is of high importance. A recent report has suggested that passive immunity can be effectively combined with antibiotics resulting in greater therapeutic efficacy (10, 20). Therapeutic modeling in animals is challenging with anthrax because of the lack of consistent clinical symptoms prior to death, which makes it difficult to separate postexposure prophylaxis from therapeutic intervention. Further studies with this novel antibody in a therapeutic setting, alone and in combination with antibiotics, will be important to establish the utility of this MAb in the defense against anthrax.
Acknowledgments
This work was partially funded by the National Institutes of Health (grant no. 5 U01 AI061314-02).
We thank Maria Valkova-Valchanova, Alahari Arunakumari, Karen Frey, and Xi-Tao Wang (Medarex, Inc.) for helpful technical contributions, Conrad P. Quinn (Centers for Disease Control and Prevention, Atlanta, GA) for providing the human reference plasma, and Battelle Memorial Institute (West Jefferson, Ohio) for scientific expertise in conducting inhalational anthrax exposure models.
This research program is a part of the Institute for Security Technology Studies, supported under award number 2000-DT-CX-K001 from the U.S. Department of Homeland Security, Science and Technology Directorate.
Points of view in this document are those of the author(s) and do not necessarily represent the official position of the U.S. Department of Homeland Security or the Science and Technology Directorate.
Editor: A. D. O'Brien
REFERENCES
- 1.Abrami, L., S. H. Leppla, and F. G. van der Goot. 2006. Receptor palmitoylation and ubiquitination regulate anthrax toxin endocytosis. J. Cell Biol. 172:309-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abrami, L., S. Liu, P. Cosson, S. H. Leppla, and F. G. van der Goot. 2003. Anthrax toxin triggers endocytosis of its receptor via a lipid raft-mediated clathrin-dependent process. J. Cell Biol. 160:321-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agrawal, A., J. Lingappa, S. H. Leppla, S. Agrawal, A. Jabbar, C. Quinn, and B. Pulendran. 2003. Impairment of dendritic cells and adaptive immunity by anthrax lethal toxin. Nature 424:329-334. [DOI] [PubMed] [Google Scholar]
- 4.Alileche, A., E. R. Serfass, S. M. Muehlbauer, S. A. Porcelli, and J. Brojatsch. 2005. Anthrax lethal toxin-mediated killing of human and murine dendritic cells impairs the adaptive immune response. PLoS Pathog. 1:e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brachman, P. S., H. Gold, S. A. Plotkin, F. R. Fekety, M. Werrin, and N. R. Ingraham. 1962. Field evaluation of an anthrax vaccine. Am. J. Public Health 52:632-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brossier, F., M. Levy, A. Landier, P. Lafaye, and M. Mock. 2004. Functional analysis of Bacillus anthracis protective antigen by using neutralizing monoclonal antibodies. Infect. Immun. 72:6313-6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collier, R. J., and J. A. Young. 2003. Anthrax toxin. Annu. Rev. Cell Dev. Biol. 19:45-70. [DOI] [PubMed] [Google Scholar]
- 8.Friedlander, A. M., S. L. Welkos, and B. E. Ivins. 2002. Anthrax vaccines. Curr. Top. Microbiol. Immunol. 271:33-60. [DOI] [PubMed] [Google Scholar]
- 9.Grabenstein, J. D. 2003. Anthrax vaccine: a review. Immunol. Allergy Clin. N. Am. 23:713-730. [DOI] [PubMed] [Google Scholar]
- 10.Karginov, V. A., T. M. Robinson, J. Riemenschneider, B. Golding, M. Kennedy, J. Shiloach, and K. Alibek. 2004. Treatment of anthrax infection with combination of ciprofloxacin and antibodies to protective antigen of Bacillus anthracis. FEMS Immunol. Med. Microbiol. 40:71-74. [DOI] [PubMed] [Google Scholar]
- 11.Little, S. F., B. E. Ivins, P. F. Fellows, and A. M. Friedlander. 1997. Passive protection by polyclonal antibodies against Bacillus anthracis infection in guinea pigs. Infect. Immun. 65:5171-5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Little, S. F., B. E. Ivins, P. F. Fellows, M. L. Pitt, S. L. Norris, and G. P. Andrews. 2004. Defining a serological correlate of protection in rabbits for a recombinant anthrax vaccine. Vaccine 22:422-430. [DOI] [PubMed] [Google Scholar]
- 13.Little, S. F., S. H. Leppla, and E. Cora. 1988. Production and characterization of monoclonal antibodies to the protective antigen component of Bacillus anthracis toxin. Infect. Immun. 56:1807-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Little, S. F., S. H. Leppla, and A. M. Friedlander. 1990. Production and characterization of monoclonal antibodies against the lethal factor component of Bacillus anthracis lethal toxin. Infect. Immun. 58:1606-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Little, S. F., J. M. Novak, J. R. Lowe, S. H. Leppla, Y. Singh, K. R. Klimpel, B. C. Lidgerding, and A. M. Friedlander. 1996. Characterization of lethal factor binding and cell receptor binding domains of protective antigen of Bacillus anthracis using monoclonal antibodies. Microbiology 142(Pt 3):707-715. [DOI] [PubMed] [Google Scholar]
- 16.Lonberg, N. 2005. Human antibodies from transgenic animals. Nat. Biotechnol. 23:1117-1125. [DOI] [PubMed] [Google Scholar]
- 17.Lonberg, N., L. D. Taylor, F. A. Harding, M. Trounstine, K. M. Higgins, S. R. Schramm, C. C. Kuo, R. Mashayekh, K. Wymore, J. G. McCabe, et al. 1994. Antigen-specific human antibodies from mice comprising four distinct genetic modifications. Nature 368:856-859. [DOI] [PubMed] [Google Scholar]
- 18.Mohamed, N., M. Clagett, J. Li, S. Jones, S. Pincus, G. D'Alia, L. Nardone, M. Babin, G. Spitalny, and L. Casey. 2005. A high-affinity monoclonal antibody to anthrax protective antigen passively protects rabbits before and after aerosolized Bacillus anthracis spore challenge. Infect. Immun. 73:795-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohamed, N., J. Li, C. S. Ferreira, S. F. Little, A. M. Friedlander, G. L. Spitalny, and L. S. Casey. 2004. Enhancement of anthrax lethal toxin cytotoxicity: a subset of monoclonal antibodies against protective antigen increases lethal toxin-mediated killing of murine macrophages. Infect. Immun. 72:3276-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peterson, J. W., J. E. Comer, D. M. Noffsinger, A. Wenglikowski, K. G. Walberg, B. M. Chatuev, A. K. Chopra, L. R. Stanberry, A. S. Kang, W. W. Scholz, and J. Sircar. 2006. Human monoclonal anti-protective antigen antibody completely protects rabbits and is synergistic with ciprofloxacin in protecting mice and guinea pigs against inhalation anthrax. Infect. Immun. 74:1016-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pitt, M. L., S. Little, B. E. Ivins, P. Fellows, J. Boles, J. Barth, J. Hewetson, and A. M. Friedlander. 1999. In vitro correlate of immunity in an animal model of inhalational anthrax. J. Appl. Microbiol. 87:304. [DOI] [PubMed] [Google Scholar]
- 22.Popov, S. G., R. Villasmil, J. Bernardi, E. Grene, J. Cardwell, T. Popova, A. Wu, D. Alibek, C. Bailey, and K. Alibek. 2002. Effect of Bacillus anthracis lethal toxin on human peripheral blood mononuclear cells. FEBS Lett. 527:211-215. [DOI] [PubMed] [Google Scholar]
- 23.Reuveny, S., M. D. White, Y. Y. Adar, Y. Kafri, Z. Altboum, Y. Gozes, D. Kobiler, A. Shafferman, and B. Velan. 2001. Search for correlates of protective immunity conferred by anthrax vaccine. Infect. Immun. 69:2888-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Semenova, V. A., E. Steward-Clark, K. L. Stamey, T. H. Taylor, Jr., D. S. Schmidt, S. K. Martin, N. Marano, and C. P. Quinn. 2004. Mass value assignment of total and subclass immunoglobulin G in a human standard anthrax reference serum. Clin. Diagn. Lab. Immunol. 11:919-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Unkeless, J. C. 1979. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J. Exp. Med. 150:580-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang, F., P. Ruther, I. Jiang, R. Sawada-Hirai, S. M. Sun, R. Nedellec, P. R. Morrow, and A. S. Kang. 2004. Human monoclonal antibodies that neutralize anthrax toxin by inhibiting heptamer assembly. Hum. Antibodies 13:105-110. [PubMed] [Google Scholar]
- 27.Weiss, S., D. Kobiler, H. Levy, H. Marcus, A. Pass, N. Rothschild, and Z. Altboum. 2006. Immunological correlates for protection against intranasal challenge of Bacillus anthracis spores conferred by a protective antigen-based vaccine in rabbits. Infect. Immun. 74:394-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williamson, E. D., I. Hodgson, N. J. Walker, A. W. Topping, M. G. Duchars, J. M. Mott, J. Estep, C. Lebutt, H. C. Flick-Smith, H. E. Jones, H. Li, and C. P. Quinn. 2005. Immunogenicity of recombinant protective antigen and efficacy against aerosol challenge with anthrax. Infect. Immun. 73:5978-5987. [DOI] [PMC free article] [PubMed] [Google Scholar]