Abstract
In recent years, sphingolipids have emerged as critical molecules in the regulation of microbial pathogenesis. In fungi, the synthesis of complex sphingolipids is important for the regulation of pathogenicity, but the role of sphingolipid degradation in fungal virulence is not known. Here, we isolated and characterized the inositol phosphosphingolipid-phospholipase C1 (ISC1) gene from the fungal pathogen Cryptococcus neoformans and showed that it encodes an enzyme that metabolizes fungal inositol sphingolipids. Isc1 protects C. neoformans from acidic, oxidative, and nitrosative stresses, which are encountered by the fungus in the phagolysosomes of activated macrophages, through a Pma1-dependent mechanism(s). In an immunocompetent mouse model, the C. neoformans Δisc1 mutant strain is almost exclusively found extracellularly and in a hyperencapsulated form, and its dissemination to the brain is remarkably reduced compared to that of control strains. Interestingly, the dissemination of the C. neoformans Δisc1 strain to the brain is promptly restored in these mice when alveolar macrophages are pharmacologically depleted or when infecting an immunodeficient mouse in which macrophages are not efficiently activated. These studies suggest that Isc1 plays a key role in protecting C. neoformans from the intracellular environment of macrophages, whose activation is important for preventing fungal dissemination of the Δisc1 strain to the central nervous system and the development of meningoencephalitis.
Cryptococcus neoformans is a ubiquitous environmental microbe found especially in pigeon droppings and associated with eucalyptus trees (6). It causes cryptococcosis, an infectious disease with a variety of clinical manifestations depending on the status of the immune system (6, 16). Exposure to the microorganism is common (19), but the majority of subjects do not develop clinical disease. In conditions in which the host immune system is compromised, such as human immunodeficiency virus infection, organ transplantation, or cancer malignancy, C. neoformans disseminates from the lung to other organs, especially to the brain, where it causes the most common fungal meningoencephalitis worldwide (6). Which cryptococcal factor(s) or/and host component(s) control this neurotropism is largely unknown.
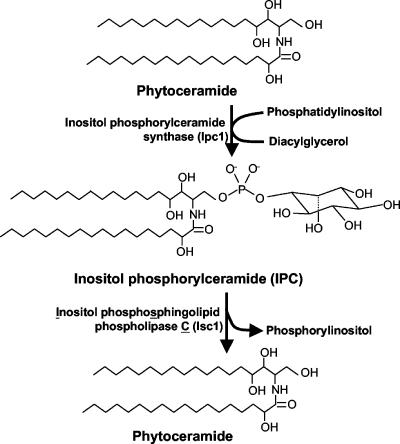
C. neoformans is a facultative intracellular pathogen: in addition to replicating extracellularly, it can also survive and grow intracellularly within the phagolysosome of host phagocytic cells (13, 44). In previous studies, we showed that the synthesis of sphingolipids (Fig. 1) regulates cryptococcal pathogenicity by regulating intracellular and extracellular fungal growth (20, 21, 28-30, 35). However, the role of enzymes that breaks down complex inositol sphingolipids in the regulation of fungal growth and pathogenicity is not known. In this study, we isolated and characterized the inositol phosphosphingolipid-phospholipase C (PLC) gene (ISC1) (Fig. 1) from C. neoformans and found that the Isc1 enzyme promotes the neurotropism of C. neoformans depending on the immune status of the host by protecting the fungus from the hostile intracellular environment of phagocytes.
FIG. 1.
Synthesis and breakdown of inositol sphingolipids. Fungal inositol phosphorylceramide synthase 1 (Ipc1) synthesizes IPC by transferring inositol phosphate from phosphatidylinositol to phytoceramide. From this reaction, a molecule of diacylglycerol is also produced. IPC is then metabolized by inositol phosphosphingolipid phospholipase C1 (Isc1), producing phytoceramide and a molecule of inositol phosphate (or phosphorylinositol).
MATERIALS AND METHODS
Strains and growing media.
C. neoformans var. grubii serotype A wild-type (WT) strain H99; strain M001, an ade2 isogenic derivative of H99; C. neoformans var. neoformans serotype D strain JEC21; Saccharomyces cerevisiae WT (Jk9-3Dα) and Δisc1 strains (38); and the C. neoformans Δisc1 mutant and the reconstituted Δisc1REC strain were used in this study. All strains were routinely grown on yeast extract peptone dextrose (YPD) medium buffered to pH 7.0 with 25 mM HEPES, unless otherwise noted. A synthetic medium, containing 6.7 g/liter of yeast nitrogen base (YNB) without amino acids, 1.3 g/liter amino acid mix lacking adenine, 180 g/liter sorbitol, 20 g/liter glucose, and 20 g/liter agar, was used for selecting the Δisc1 strain obtained by biolistic transformation. The Δisc1REC strain was selected using YPD agar supplemented with 100 μg/ml nourseothricin.
Isolation of the C. neoformans ISC1 gene.
The C. neoformans ISC1 gene was first identified by conducting a BLAST search for the S. cerevisiae ISC1 homolog in the C. neoformans H99 sequencing project (Duke IGSP Center for Applied Genomics and Technology [http://cneo.genetics.duke.edu]). Only one highly similar match was found, and this sequence was used to amplify the ISC1 gene from cDNA of C. neoformans var. grubii serotype A strain H99 using the following primers: ASE-A-5′ (5′-ATG AAC TCA CAA TCT CCC CCT GAA GAC CCT G-3′) and ASE-A-3′ (5′-CTA ACG ATG TAC ATT CTC GTC CAT CTC CAC-3′). The gene was cloned into pCR-TOPO vector (Invitrogen) and sequenced (GenBank accession number DQ487762). Similarly, by conducting a BLAST search for the S. cerevisiae homolog in a C. neoformans var. neoformans database (http://www-Sequence.Stanford.edu/group/C.neoformans), the putative ISC1 gene in C. neoformans serotype D was identified. Two primers, ASE-pro-F (5′-CAG GAT TCT GTA TCG AGT GCG-3′) and ASE-pro-R (5′-CAG TTA AAA GAC CCA CCT CCC ATC-3′), were used to amplify a DNA fragment that was then radiolabeled and used to screen a cDNA library of C. neoformans var. neoformans serotype D strain B3501 to isolate, clone, and sequence the ISC1 gene (GenBank accession number DQ487763). Sequence alignments and homology calculations were performed using MacVector version 7.2.2 (Macintosh).
Disruption and reconstitution of the ISC1 gene.
A pΔisc1/ADE2 disruption construct was made as follows (Fig. 2): first, an area in the 5′ untranslated region (UTR) upstream of the ATG start codon of the ISC1 gene was amplified by PCR using H99 genomic DNA, prepared as previously described (29), as a template and primers ASE-H3 (5′-CAT AAGCTT GCT CGC AGA ATC GGC AGC TGT TAT-3′) and ASE-R1 (5′-GAA GAATTC GAG ATT GTG AGT TCA TGG TGC G-3′), containing a HinDIII site and an EcoRI site, respectively (underlined). This product was then digested with HinDIII and EcoRI, producing the 5′-UTR fragment (1,037 bp). Second, an area in the 3′ UTR downstream of the ISC1 stop codon was amplified using H99 genomic DNA as a template and primers ASE-R4 (5′-CTT GAATTC CTA TTA TCT CGC AGA GGC C-3′) and ASE-S2 (5′-GAT CCGAGCTCC AGA CTC ATA CGT TTA CTC-3′) containing an EcoRI site and a SacI site, respectively (underlined). This fragment was then digested with EcoRI and SacI, producing the 3′-UTR fragment (1,046 bp). Third, the 5′-UTR fragment was combined with the 3′-UTR fragment, ligated, and cloned into the HindDIII and SacI sites of the SK+ pBluescript vector (Invitrogen). Finally, the EcoRI-restricted ADE2 fragment was inserted into the EcoRI site located between the 5′- and 3′-UTR fragments to form the pΔisc1/ADE2 disruption construct. The pΔisc1/ADE2 construct was transformed into C. neoformans strain M001, using biolistic delivery of DNA (44).
FIG. 2.
Deletion and reconstitution of Isc1 in C. neoformans. (A) Schematic representation of disruption and reconstitution of the ISC1 gene. In the assembly of the Δisc1 construct, the ADE2 selectable marker was flanked on either side by a DNA fragment homologous to the 5′ UTR upstream of the ISC1 ATG start codon and a DNA fragment homologous to the 3′ UTR downstream of the ISC1 stop codon. In the assembly of the Δisc1REC construct, the full-length ISC1 gene from genomic DNA, including 1,000 base pairs upstream of the start codon, was ligated to a nourseothricin resistance cassette (NAT1). Genomic DNA of transformants was digested with HinDIII (H3) and KpnI (K1) and probed with a fragment homologous to the 5′ UTR. (B) Southern analysis of transformants. (C) IPC-PLC activity of the C. neoformans WT, Δisc1, and Δisc1REC strains.
To reconstitute the ISC1gene, a pISC1REC reconstitution plasmid containing the ISC1 gene was made (including 1,000 base pairs upstream from the start codon) along with the nourseothricin resistance cassette NAT1. First, the 5′ UTR and ISC1 gene were amplified from genomic DNA from strain H99 using primers R002S (5′-GCA GAG CTC GCA CTC GGC CAG TAT CTT ATG-3′) and R001A (5′-TCT GAA TTC TGT ACA TTC TCG TCC ATC TCC AC-3′), containing an XhoI site and an EcoRI site, respectively (underlined). This PCR fragment was digested with XhoI and EcoRI, yielding a 2,927 bp fragment which was then ligated into the XhoI and EcoRI sites of the SK+ pBluescript vector. The nourseothricin acetyltransferase gene (NAT1) under the control of H99 actin promoter was released from pCR2.1-TOPO-NAT1 (21) via an XbaI digest, and ligated into the pSK-5′UTR-ISC1 plasmid into the XbaI sites. Biolistic delivery of DNA, selection of transformants, and Southern hybridization analyses were performed as previously described (37, 43).
Preparation of IPC.
Radiolabeled cryptococcal complex inositol sphingolipids were extracted and purified from 5 × 107 cells incubated for 4 h with 25 μCi of myo-[2-3H]inositol (20 Ci/mmol) (American Radiolabeled Chemicals, St. Louis, MO) using a solvent containing ethanol, water, diethyl ether, pyridine, and concentrated ammonia (15:15:5:1:0.018) (38). Extracted lipids were base-hydrolyzed with monomethylamine reagent (10), dried down, dissolved in chloroform-methanol-water (2:2:0.6), and separated on a Whatman K6 60A silica preparative thin-layer chromatograph (500 μm thick; Fisher Scientific) using a solvent mixture containing chloroform, methanol, and 4.2 N ammonium hydroxide (9:7:2). An inositol phosphorylceramide (IPC) standard was prepared essentially as described by Sawai et al. (38). A mutant strain of S. cerevisiae lacking mannose inositol phosphoryl ceremide (MIPC) synthase (Sur1) (2) was labeled with [3H]inositol, and total lipids were extracted, subjected to base hydrolysis, and separated by thin-layer chromatography as previously described for C. neoformans. The MIPC synthase mutant produces a large amount of IPC but little MIPC compared to S. cerevisiae WT JK9-3dα. Nonradiolabeled C26-IPC was custom made by Avanti Polar Lipids, Alabaster, AL.
In vitro activity for IPC-PLC.
Strains were grown in 10 ml YPD for 24 h at 30°C with shaking. Cells were harvested by centrifugation and washed with sterile distilled water, and the pellets were resuspended in lysis buffer (25 mM Tris at pH 7.4, 5 mM EDTA, 1 mM phenylmethylsulfonyl fluoride [Sigma], and 10 μg/ml each chymostatin [Sigma], leupeptin [Sigma], antipain [Sigma], and pepstatin A [Sigma]). Then, acid-washed glass beads (for a volume equal to three-fourths of the cell suspension) were added, and cells were homogenized three times for 45 s using the Bead Beader 8. After centrifugation at 2,500 × g for 10 min at 4°C, supernatant (∼100 μl) was transferred to a sterile 1.5-ml microcentrifuge tube for quantification of proteins by the Bradford method (4). Phospholipase C activity against IPC was performed as previously described (38). Briefly, cell lysates containing 100 μg protein were incubated at 30°C for 30 min in 100 μl of buffer containing 50 mM Tris (pH 7.5), 5 mM MgCl2, 5 mM dithiothreitol, 0.1% Triton X-100, 10 nmol of phosphatidylserine (Avanti Polar Lipids), 2 nmol of unlabeled IPC, and 30,000 dpm of myo-[2-3H]inositol-labeled IPC. After the incubation, 0.8 ml of chloroform, 0.4 ml of methanol, and 0.2 ml of 1% perchloric acid were added (15), and the radioactivity in a portion (300 μl) of the upper (aqueous) phase was measured by liquid scintillation counting. Experiments were repeated at least three times.
Virulence studies in murine models of cryptococcal meningitis.
Four- to six-week-old female CBA/J mice (Jackson Laboratory, Bar Harbor, Maine) and four- to six-week-old male/female Tgɛ26 mice (an isogenic mouse line lacking in mature T cells and NK cells [47, 48], available in the Animal Core Facility, Medical University of South Carolina, Charleston, SC) were used. Mice were anesthetized with an intraperitoneal injection of 60 μl of xylazine/ketamine mixture, containing 95 mg of ketamine per kilogram of body weight and 5 mg of xylazine per kilogram of body weight. C. neoformans WT (H99), Δisc1, and Δisc1REC strains were prepared by growing yeast cells from glycerol stocks for 24 h at 30°C in YPD medium. The cells were pelleted, washed thrice, and resuspended in phosphate-buffered saline (PBS; pH 7.4) at a concentration of 2.5 × 107 cells/ml. For survival studies, 10 mice each were infected with 5 × 105 cells (of either the WT, Δisc1, or Δisc1REC strain) in a volume of 20 μl through nasal inhalation. The mice were fed ad libitum and monitored with twice-daily inspections. Mice that appeared moribund or in pain were sacrificed by CO2 inhalation. All animal procedures are approved by the Medical University of South Carolina Institutional Animal Care and Use Committee and follow the guidelines of the American Veterinary Medical Association. Survival data from the mice experiment were analyzed via the Kaplan-Meier method with log rank analysis using the statistical program Prism (Macintosh). A P value of less than 0.05 was considered to be significant.
For studies of fungal burdens in tissue cultures, C. neoformans strains were prepared, and mice were infected as described above. The mice were fed ad libitum and monitored with twice-daily inspections. At selected time points after inoculation, at least three mice for each set of experimental conditions were sacrificed by CO2 inhalation. The lung, brain, kidney, spleen, and liver were removed and homogenized in 10 ml sterile PBS using Stomacher 80 (Lab System; Fisher Scientific, Pittsburgh, Pennsylvania) for 120 s at high speed. Serial dilutions were then plated in duplicate onto YPD agar plates containing 100 μg/ml ampicillin and 100 μg/ml chloramphenicol. Plates were incubated at 30°C for 72 h, yeast colonies were counted, and the number was recorded as the geometric mean of the logarithm of CFU per organ. Statistical analysis was performed using Student's t test, and a P value of less than 0.05 was considered to be significant.
For histological studies, C. neoformans strains were prepared, and mice were infected as described above. At selected time points, mice were sacrificed by CO2 inhalation, and lungs and brains were removed, placed in 10% formalin, and then embedded in paraffin. Sections were stained with periodic acid-Schiff stain to identify the fungal cell wall, with mucicarmine as an additional stain for the identification of C. neoformans capsule, and with hematoxylin and eosin to examine the host inflammatory response. Sections were examined by light microscopy. Yeast cellular and capsular diameters were determined using Adobe Photoshop 9.0 and normalized to the erythrocytes (assumed to have a mean diameter of 7 μm) present in those sections.
Macrophage depletion in murine models of cryptococcosis.
Clodronate-encapsulated liposomes were prepared as previously described (45). Briefly, 86 mg phosphatidylcholine (egg lecithin; Avanti Polar Lipids) and 8 mg cholesterol (Avanti Polar Lipids) were dissolved in 10 ml chloroform. The chloroform was then slowly removed by evaporation, and the remaining phospholipid residue was resuspended in 10 ml 0.6 M clodronate (dichloromethylene-bisphosphonate; Sigma-Aldrich) prepared in sterile deionized water. Control solutions were prepared in a similar way, but the phospholipid residue was resuspended in PBS. The suspensions were kept under N2 gas and shaken on an orbital shaker for 2 h. The suspensions were sonicated in a water bath sonicator for 3 min and then stored overnight at 4°C for the swelling of the liposomes. The suspensions were then washed once with sterile PBS after one centrifugation of 10,000 × g for 15 min and washed twice again with sterile PBS after two subsequent centrifugations of 25,000 × g for 30 min. The final pellet was resuspended in 4 ml sterile PBS and stored under N2 gas until it was used. Liposomal preparations were used within 2 weeks.
To study the in vivo effects of macrophage depletion, CBA/J mice were anesthetized as described above, and 60 μl of clodronate-encapsulated liposomes (or PBS as a control) was given intranasally to each mouse. After 48 h, mice were infected with the C. neoformans WT or Δisc1 strain as described above. To maintain macrophage depletion, mice were anesthetized, and clodronate or PBS was administered weekly. Fungal burdens in tissue culture were assessed as described above.
Intracellular growth of C. neoformans in J774.16 macrophage-like cell line.
J774.16 is a murine reticulum sarcoma macrophage-like cell line that is well characterized (7) and is extensively used as a model for cryptococcal interactions with macrophages (13, 44). J774.16 cells were used up to passage 10, with each passage dilution equal to 1:12. Cells were plated on poly-d-lysine-coated glass-bottomed plates (MatTek Corporation, Ashland, MA) in Dulbecco's minimal essential medium supplemented with 10% fetal calf serum and 10 μg/ml of the anti-GXM monoclonal antibody 18B7 (5) (kindly provided by Arturo Casadevall, Albert Einstein College of Medicine, Bronx, NY) at 37°C with 5% CO2. Macrophages were activated with the addition of 50 units/ml of recombinant murine gamma interferon (IFN-γ; Sigma) and 0.3 μg/ml of lipopolysaccharide (LPS; Sigma). C. neoformans WT, Δisc1, and Δisc1REC strains were grown from glycerol stocks in YPD broth (pH 7.0) for 24 h at 30°C, washed three times in PBS, and added to the J774.16 cells at an effector-to-target ratio of 1:1. After a 2-h incubation, extracellular C. neoformans cells were washed away with three changes of cell culture medium, and fresh medium (containing IFN-γ and LPS for activated macrophages) was added. After the end of a 24-h incubation, medium was removed, and macrophages were lysed by incubating cells with sterile deionized water for 30 min followed by vigorous aspiration. The lysates were vortexed for 20 s to prevent cryptococcal aggregation in the presence of the monoclonal antibody. Lysates were then plated on YPD agar to determine numbers of CFU of viable intracellular fungal cells.
Phagocytic indices were determined as described above, with the exception that after 2 h of coincubation, cells were washed three times with PBS, fixed with ice-cold methanol, and stained with Giemsa (Sigma). The phagocytic indices (defined as the number of internalized yeast cells per 100 macrophages) were determined for each set of experimental conditions. Eight fields per set of conditions were counted and averaged.
In vitro growth studies.
From overnight YPD broth cultures of C. neoformans WT, Δisc1, and Δisc1REC strains, cells were washed twice in sterile deionized water, resuspended, and diluted to a final density of 104 cells/ml in 40 ml fresh YPD broth with 25 mM HEPES at a pH of 7.0 or 4.0. Aliquots of medium were sampled during growth to ensure that the medium pH was stable throughout the experiments. The cultures were incubated at 250 rpm in a shaker incubator at 30°C. Samples were taken from the cultures at various time points (0, 3, 6, 9, 12, 18, 24, 36, 48, 72, and 96 h), diluted with sterile deionized water, and plated onto YPD plates for counting of CFU.
Oxidative and nitrosative stress studies.
Resistance to hydrogen peroxide was tested on YPD agar plates with 25 mM HEPES (pH 7.0 or pH 4.0) supplemented with 5 mM H2O2. Nitrosative stress was tested on YNB agar plates with 25 mM succinate acid (pH 4.0) with 0.1 mM NaNO2. At this pH, NaNO2 spontaneously decomposes to nitric oxide (NO) (31). C. neoformans strains were grown for 24 h in YPD broth (pH 7.0) and washed twice with sterile deionized water, the density was adjusted to 107 cells/ml, and 10-fold serial dilutions were made. Five microliters of each dilution was spotted onto the solid medium and grown at 30°C.
Growth inhibition by ebselen.
Experiments to determine MICs and minimal fungicidal concentrations were performed by the broth microdilution method according to the recommendations of the National Committee for Clinical Laboratory Standards (33). C. neoformans WT, Δisc1, and Δisc1REC strains were grown in YPD (pH 7.0) for 24 h at 30°C. Cells were washed twice with sterile deionized water, and the density was adjusted to a final concentration of 2.5 × 103 cells/ml in YNB with 25 mM HEPES at a pH of 7.0 or 4.0. Ebselen was obtained from Sigma. The MIC was defined as the lowest drug concentration in which the optical density at 490 nm is ≤80% of that produced by the growth control well, as measured by a VERSAmax microtiter plate reader (Molecular Devices). Aliquots (100 μl) from wells with growth inhibition were plated onto YPD agar plates. The lowest concentration that yielded three or fewer colonies was recorded as the minimum fungicidal concentration.
Nucleotide sequence acession numbers.
The C. neoformans var. grubii serotype A strain H99 and C. neoformans var. neoformans serotype D strain B3501 ISC1 cDNAs were sequenced, and the sequences have been deposited in the GenBank database under accession numbers DQ487762 and DQ487763, respectively.
RESULTS
Disruption of the ISC1 gene completely abolishes IPC-PLC activity.
The C. neoformans ISC1 gene was isolated and sequenced as described in Materials and Methods. To investigate the role of Isc1 in the pathobiology of C. neoformans, we created a mutant (Δisc1) strain by deletion of the ISC1 gene from C. neoformans var. grubii strain H99. A plasmid in which the ADE2 selectable marker was flanked by upstream and downstream regions of the ISC1 open reading frame was constructed and biolistically transformed into strain M001, an ade2 isogenic derivative of WT strain H99. Homologous recombinants were screened by Southern hybridization using a fragment of the 5′ UTR as a probe (Fig. 2A and B). To fulfill the molecular Koch's postulates, we also constructed a reconstituted strain in which the ISC1 gene was reintroduced into the Δisc1 strain (Δisc1REC), using a nourseothricin resistance cassette (NAT1) as a selectable marker (Fig. 2A and B). The deletion of the ISC1 gene in the Δisc1 strain and its reintroduction into the Δisc1REC strain was confirmed by Southern hybridization using a fragment of the ISC1 gene as a probe (data not shown).
To elucidate whether the putative ISC1 has in vitro PLC activity against complex yeast inositol sphingolipids, crude cell lysates were incubated with radiolabeled IPC, and the release of radioactive inositol phosphate was measured. As shown in Fig. 2C, disruption of the ISC1 locus completely abolished IPC-PLC activity. Phospholipase C activity against the mannosylated complex sphingolipids MIPC and M(IP)2C was also undetectable in the Δisc1 strain (data not shown). Reintroduction of the ISC1 gene into the Δisc1REC strain completely restored IPC-PLC activity, suggesting that ISC1 is the only gene encoding PLC activity against inositol sphingolipids in C. neoformans. The enzymatic characterization of Isc1 showed that the cryptococcal IPC-PLC activity was dependent on magnesium and phosphatidylserine as cofactors and had optimal activity at a neutral pH (data not shown), similar to that of S. cerevisiae Isc1 (38).
Loss of ISC1 increases the survival of infected immunocompetent mice.
We next investigated whether the loss of IPC-PLC activity would affect the pathogenicity of C. neoformans. Immunocompetent CBA/J mice were infected intranasally with 5 × 105 cryptococcal (WT, Δisc1, or Δisc1REC) cells, and their morbidity and mortality were observed. As shown in Fig. 3A, mice infected with the WT strain survived an average of 24.9 ± 3.2 days. Interestingly, whereas 70% of the mice infected with the Δisc1 strain survived as long as the WT-infected mice (24.7 ± 2.8 days), 30% of the mice survived 81 days postinfection. After 81 days, these mice were sacrificed, and their lungs and brain tissue were collected to assess cryptococcal burdens. Surprisingly, there was no detectable C. neoformans in these organs, suggesting that these mice had successfully cleared the infection. In these cases, the Δisc1 strain may have adapted poorly to the host environment, and the host immune system was eventually able to succeed. In the remaining mice, the Δisc1 cells may have adapted in a way to avoid clearance from the host, including production of a large polysaccharide capsule. On the other hand, mice infected with the Δisc1REC strain had 100% mortality, with an average survival of 23.0 ± 1.7 days. These results suggest that ISC1 may contribute to the virulence of C. neoformans.
FIG. 3.
Isc1 is required for the efficient dissemination of C. neoformans to the brain. (A) Survival of immunocompetent (CBA/J) mice after intranasal infection with the WT, Δisc1, or Δisc1REC strain. WT-infected mice survived 24.9 ± 3.2 days. Mice infected with the Δisc1REC strain survived 23.0 ± 1.7 days. Thirty percent of the Δisc1 strain-infected mice survived to day 81 postinfection. (B) Fungal burdens in tissue cultures of immunocompetent mice at days 8, 12, and 24 postinfection after intranasal infection with the WT, Δisc1, or Δisc1REC strain. Results are expressed as numbers of CFU per organ on a logarithmic scale. There were lower numbers of detectable cryptococcal cells in the brains of Δisc1 strain-infected mice compared to the controls at days 12 and 24 (*, P < 0.05).
We next examined the fungal burdens in the lungs and brains of the mice during the course of the infection. Mice were infected intranasally with one of the three strains and sacrificed at 8, 12, or 24 days postinfection. Their lungs and brains were harvested and examined for cryptococcal CFU. Whereas the fungal burdens in lung tissue of mice infected with the three strains were similar, the fungal burden of the Δisc1 mutant in brain tissue was significantly lower than those of the control strains (Fig. 3B). By day 12 postinfection, the WT or Δisc1REC strain already had disseminated to the brain, whereas there were no detectable cryptococcal cells in the brains of Δisc1 strain-infected mice. Likewise, at day 24 postinfection, there was significantly lower dissemination of the Δisc1 strain to the brain compared to control strains (P < 0.05). Very interestingly, tissue cultures of liver, kidney, and spleen showed no significant differences in fungal burden among the tested strains (data not shown). These results suggest that cryptococcal pneumonia or liver, spleen, or kidney failure, and not meningoencephalitis, could be the cause of death of the Δisc1 strain-infected immunocompetent CBA/J mice. The presence of the Δisc1 strain in the lungs of mice early (<24 days) but not late (>80 days) in infection suggests that some mice were able to successfully clear the Δisc1 strain from their lungs.
Next, we investigated whether this virulence phenotype of the Δisc1 mutant would also be present in an immunodeficient animal model. For this experiment, we used the isogenic Tgɛ26, deficient in T and NK cells (47, 48). In this model, the average survival of the WT-infected mice was 15.7 ± 1.0 days and the average survival of the Δisc1 strain-infected mice was 19.4 ± 2.0 days, and interestingly, there was no significant difference in fungal burden in brain tissue between WT- and Δisc1 strain-infected mice (data not shown), suggesting that the Δisc1 strain does infect and replicate in the brain tissue at the same rate of WT cells when the host immune system is severely compromised. Taken together, these results suggest that Isc1 is not important for C. neoformans to escape the lung but rather for it to enter the brain when the host is immunocompetent.
Then, the lung inflammatory response of mice infected with the WT or the Δisc1 strain was examined at day 12 postinfection. As shown in Fig. 4A and C, lung tissue infected with WT cells shows an intense inflammation, with extensive remodeling of the alveolar spaces and heavy inflammatory infiltrates. In contrast, Δisc1 strain-infected mice showed little sign of pulmonary inflammation, with preservation of the alveolar architecture in spite of having a pulmonary fungal burden similar to that of WT-infected mice (Fig. 4B and D). Closer examination (Fig. 4E; see Fig. S1 in the supplementary material) revealed that WT cells were found in both the extracellular environment (77.4% ± 11.2%) and as well as inside intracellular compartments (alveolar macrophages) (22.6% ± 11.2%). Surprisingly, Δisc1 cells (Fig. 4F; see Fig. S1 in the supplementary material) appeared almost exclusively in the extracellular compartment (99.0% ± 1.3%). Importantly, whereas there was no difference in body and capsule size between intra- and extracellular C. neoformans WT cells at the time of observation, the Δisc1 strain was found to be larger and more heavily encapsulated (the difference between encapsulated and unencapsulated yeast cells) compared to WT cells (Fig. 5). This trend was only observed during the infection of immunocompetent CBA/J-infected mice and not during the infection of the immunocompromised Tgɛ26 mice (Fig. 5). Under in vitro capsule-inducing conditions or during infection, yeast cell and capsule sizes were similar for the WT and Δisc1 mutant strains. This suggests that the Δisc1 strain is expressing a larger capsule in response to the pulmonary environment of the immunocompetent mouse. Thus, it appears that deletion of ISC1 limits C. neoformans to behave only as an extracellular pathogen in the presence of a competent host immune system.
FIG. 4.
Histological differences in pulmonary inflammation caused by WT and Δisc1 strains. (A and C) Lungs of WT-infected immunocompetent mice show intense inflammation (arrows) by day 12 postinfection with destruction of the lung alveolar architecture. Sections were stained with hematoxylin and eosin (A) and mucicarmine (C). Bars, 200 μm. (B and D) Lungs of Δisc1 strain-infected mice show little sign of inflammatory infiltrate and little damage to pulmonary tissue. Sections were stained with hematoxylin and eosin (B) and mucicarmine (D). Bars, 200 μm. (E) In WT-infected mice, cryptococcal cells (stained with mucicarmine) are found in both the extracellular and intracellular compartments (arrows). Bar, 50 μm. (F) In Δisc1 strain-infected mice, cryptococcal cells (stained with mucicarmine) are found exclusively extracellularly and appear more heavily encapsulated than WT cells. Bar, 50 μm. (Inset) A giant C. neoformans Δisc1 cell. Bar, 50 μm. (G and H) In infected Tgɛ26 mice, WT (G) or Δisc1 (H) cells (stained with mucicarmine) are found intracellularly (arrows) and extracellularly. Bars, 20 μm.
FIG. 5.
Loss of Isc1 results in larger encapsulated yeast cells during pulmonary infection of immunocompetent mice. Cryptococcal cell (“Yeast” and “Yeast with capsule”) sizes were measured from histological pulmonary samples. Individual sizes are plotted as dots, and the mean size is depicted as a horizontal bar. The mean cell and capsular sizes of the Δisc1 strain were found to be significantly larger than those of the WT for the immunocompetent mouse (top) ( , P < 0.001) but not for the immunocompromised mouse (bottom).
, P < 0.001) but not for the immunocompromised mouse (bottom).
ISC1 protects against the antifungal properties of activated macrophages.
The observation that the Δisc1 strain was only found in the extracellular compartments of lungs of immunocompetent mice led us to wonder whether this strain would survive and replicate within the phagolysosomes of activated macrophages. We first investigated whether ISC1 protects C. neoformans from being phagocytosed by macrophages using the J774.16 macrophage-like cell line. C. neoformans WT, Δisc1, or Δisc1REC strains were incubated with J774.16 cells and, after 2 h, phagocytic indices were measured. No differences in the phagocytic indices among the three strains were observed (data not shown), suggesting that ISC1 does not regulate the internalization of C. neoformans by macrophages.
We then investigated whether ISC1 protects C. neoformans from the antimicrobial effects of the phagolysosomes of J774.16 cells. C. neoformans WT, Δisc1, or Δisc1REC strains were incubated with J774.16 cells, and LPS and IFN-γ were added to activate macrophage cells by stimulating the oxidative burst and nitric oxide production. After 2 h, the cells were gently washed to remove external C. neoformans cells, fresh medium was added, and the cells were incubated for an additional 22 h. As shown in Fig. 6, upon activation of the macrophages, the intracellular survival of the Δisc1 strain was significantly lower than that of the WT or Δisc1REC strains (P < 0.05). C. neoformans survival was also tested in unactivated macrophages (without the addition of LPS and IFN-γ) where survival of the Δisc1 strain was found to be lower than that of the control strains, although this trend was not significant. These results suggest that the presence of Isc1 does indeed protect C. neoformans from the antifungal properties of an activated macrophage.
FIG. 6.
Isc1 promotes the survival of C. neoformans against the antifungal effects of activated macrophages. WT, Δisc1, and Δisc1REC strains were cocultured for 24 h with J774.16 cells. Macrophages were activated with the addition of IFN-γ and lipopolysaccharide. In each condition, percent survival was normalized to that for the WT. The Δisc1 strain shows a statistically significant decrease of survival inside activated macrophages compared to that for the WT or Δisc1REC strain ( , P < 0.05).
, P < 0.05).
Depletion of alveolar macrophages increases the Δisc1 strain's dissemination to the central nervous system.
Previous studies have suggested that strains of C. neoformans that express larger polysaccharide capsules may have difficulty transversing the blood-brain barrier and establishing infection in the central nervous system (8, 24). Perhaps by expressing a larger polysaccharide capsule to avoid the internalization by activated macrophages, the Δisc1 strain is hindering its own ability to disseminate to the brain in an immunocompetent host, such as the CBA/J mouse. Thus, we hypothesize that activated macrophages pressure the Δisc1 strain to produce a large antiphagocytic capsule with a consequent decrease of neurotropism, and that the Δisc1 strain would readily disseminate to the brain in this mouse model if macrophages would be depleted. To test this hypothesis, we depleted alveolar macrophages in the CBA/J immunocompetent mice using liposome-encased clodronate (45) and monitored the fungal burden in tissue culture. This method has been used to elucidate the role of macrophages in fighting pathogens in vivo, including Mycobacterium tuberculosis, Pseudomonas aeruginosa, and C. neoformans (9, 17, 26, 39). Thus, an intranasal administration of liposome-encased clodronate, which suppresses the number of alveolar macrophage by ∼60% compared to PBS-treated controls, was administrated weekly (data not shown). Depletion of alveolar macrophages in the CBA/J immunocompetent mouse model did not significantly change lung or brain fungal burdens of the C. neoformans WT strain compared to in the control-treated mice, whereas it significantly facilitated the dissemination of the Δisc1 strain to the brain (Fig. 7). These results support a role of alveolar macrophages in limiting dissemination of the Δisc1 strain to the brain in CBA/J mice. Interestingly, among the mice infected with the Δisc1 strain, the drug-treated group showed fungal burdens in kidneys, liver, spleen, and blood that were similar to those observed in the control-treated group (data not shown), suggesting that the dissemination defect is limited only to the brain and not to other organs.
FIG. 7.
Depletion of alveolar macrophages facilitates dissemination of the Δisc1 strain to the brain. Depletion of alveolar macrophages had no effect on the fungal burdens in the lungs or brain in immunocompetent mice infected with the WT strain. In contrast, depletion of alveolar macrophages resulted in significantly increased fungal burdens in the brains of mice infected with the Δisc1 strain compared to those of control-treated mice ( , P < 0.05).
, P < 0.05).
The fungal burden results obtained with the WT strain are different from the results obtained by Shao et al., who observed that depletion of alveolar macrophages in mice lowered pulmonary fungal burdens, whereas depletion of alveolar macrophages in rats (which are naturally more resistant to cryptococcal infection) exacerbated the fungal load (39). However, our immunocompetent mouse line, CBA/J, is naturally more resistant to cryptococcal infection than other strains (3), and this strain may in fact represent an intermediate level of resistance between rats and other mice. In addition, it should be noted that we used a 50-fold-higher inoculum than Shao et al., which might mask the more subtle differences in pulmonary fungal burdens that were observed in their studies.
The presence of Isc1 protects C. neoformans against stresses associated with an activated phagolysosome in vitro.
We next examined the potential mechanism by which Isc1 protects C. neoformans against macrophage killing. Upon phagocytosis, a microbe is subjected to acidic, oxidative, and nitrosative stresses. Thus, we tested whether these stresses would affect growth of the C. neoformans Δisc1 strain in vitro. We found that under acidic conditions, the Δisc1 strain shows a significantly longer Δisc1 cells are significantly hypersensitive to ebselen compared to control cells under acidic but not neutral conditions (P < 0.05), supporting the hypothesis that Isc1 may promote Pma1 function under acidic stress conditions.
DISCUSSION
This study illustrates that the gene ISC1, which encodes an enzyme that hydrolyzes inositol sphingolipids, enhances C. neoformans survival in macrophages and is critical for controlling the dissemination of the microorganism to the brain. The histological observation that, differently from what observed in the immunodeficient mouse model, the Δisc1 strain is only found extracellularly in an immunocompetent mouse model also provides evidence that Isc1 is an important mediator in the interaction of C. neoformans with host alveolar macrophages, depending on the status of the immune system.
The role of macrophages in cryptococcal infection is a complex one. Alveolar macrophages can play a role in limiting cryptococcal pathogenesis; they are the first line of defense against the inhaled pathogen, and they can ingest and kill C. neoformans independently of other immune cells (49). However, it is also clear that macrophages represent an Achilles heel for some hosts during cryptococcosis because C. neoformans can survive and replicate within their phagolysosomes (13, 44). Our studies show that Isc1 is important for C. neoformans to survive intracellularly. In addition, our results suggest that when C. neoformans is pressured to exist extracellularly, it becomes hyperencapsulated. The hyperencapsulation could be due either to selection pressure by the host's alveolar macrophages resulting in phagocytosis and killing of C. neoformans with a smaller capsule or to a C. neoformans response to a factor(s) produced by the host in the presence of macrophages. The fact that no difference in capsule size between intra- and extracellular C. neoformans WT cells was found at the time of observation rather supports the hypothesis that the Δisc1 mutant is hyperencapsulated and extracellular in response to an active host stimulus. The importance of the presence of active macrophages for inducing this hyperencapsulation of the Δisc1 strain is further supported by the observations that in conditions in which macrophages are not active (Tgɛ26 mice) or are depleted (clodronate treatment of CBA/J mice), the hyperencapsulation is no longer observed, and dissemination to the brain is promptly restored.
The hyperencapsulation of the Δisc1 strain is a productive response for the pathogen because the capsule is negatively charged, complement depleting, and poorly antigenic (34). In addition, shed capsular components limit inflammation by inhibiting leukocyte migration and by stimulating expression of the anti-inflammatory cytokine(s) (12, 46). This would allow the pathogen to replicate extracellularly with minimal interference by the host immune system. Indeed, histological analysis of Δisc1 strain-infected lungs of immunocompetent mice show very little pulmonary inflammation, although the pulmonary fungal load is similar to that of control-infected mice. These observations underscore the fact that C. neoformans has an array of pathogenic strategies by which it can readily adapt to suit the uniqueness of the host.
We are hypothesizing that in expressing a larger polysaccharide capsule, the Δisc1 strain is compromising its ability to infect the central nervous system. There are several independent lines of evidence which support our hypothesis: first, C. neoformans can infect the brain within 1 h after an intravenous inoculum and the capsule size at 1 h postinfection is significantly smaller than the capsule size at 6 or 24 h postinfection (8); second, capsule sizes of C. neoformans inside the brain are normally much smaller than those found in the lungs (36); third, C. neoformans can attach and pass through endothelial cells in a process similar to phagocytosis, but this process is inhibited by the presence of capsule (23); fourth, C. neoformans expressing a large capsule is particularly virulent in a mouse model, but has difficulty disseminating to the brain, in contrast to an isogenic strain with a smaller capsule (24). Altogether, these observations suggest that C. neoformans needs a small capsule in order to efficiently transverse the blood-brain barrier and establish infection in the brain. Our studies not only corroborate previous observations but add new insights into the mechanisms of cryptococcal neurotropism and how it is regulated. Our results show that in immunocompetent mice the hyperencapsulated Δisc1 cells poorly disseminate to the brain but not to other organs, such as liver, kidney, and spleen, in which they are found in the same amounts as WT cells. Importantly, dissemination of Δisc1 cells to the brain is completely restored in conditions in which alveolar macrophages are depleted or in immunodeficient mice, corroborating what was observed in rats, where depletion of alveolar macrophages (which are efficient in controlling intracellular C. neoformans) triggered cryptococcal dissemination to the brain (39). Thus, we hypothesize that activated macrophages, which can effectively kill the C. neoformans Δisc1 strain, pressures the mutant to mount antiphagocytic defenses, and in doing so, limits its ability to infect the brain.
Isc1 regulates intracellular survival through the protection against acidic, oxidative, and nitrosative stresses of the phagolysosome. A possible explanation for the Δisc1 strain's sensitivity to acidic stress could be the altered function of the proton pump Pma1, which is critical for maintaining the intracellular pH of C. neoformans within the physiological ranges necessary for optimal enzymatic activities and crucial metabolic processes (22). Indeed, Pma1's physiological importance is reflected by the inviability of Pma1 deletion mutants of S. cerevisiae or C. neoformans (1, 41). Several reports have shed light on the role that sphingolipids have on the correct oligomerization and localization of Pma1 during its synthesis and transport to the plasma membrane (reviewed in reference 14). A recent report has shown that phytoceramide containing a very-long-chain fatty acid (C26) is required for the association of newly synthesized Pma1 into detergent-resistant microdomains in the endoplasmic reticulum, which appears to be a requirement for the proper trafficking of Pma1 (18). In S. cerevisiae mutants lacking C26-phytoceramide, Pma1 is synthesized and transported to the plasma membrane, but it is then rapidly internalized and targeted to the yeast vacuole, where it is degraded (18). Interestingly, mass spectrometry measurements show that the level of C26-phytoceramide is significantly lower in the C. neoformans Δisc1 strain than in the WT or Δisc1REC strain (manuscript in preparation). It is possible that Isc1 regulates free C26-phytoceramide levels by hydrolyzing specific pools of C26-IPC. If C26-phytoceramide also regulates Pma1 stability in C. neoformans (currently under investigation), then a mislocalized Pma1 would account for the increased sensitivity of the Δisc1 strain to acidic stresses. Indeed, an S. cerevisiae Pma1 mutant (with about 50% of ATPase activity) has a phenotype similar to that of the C. neoformans Δisc1 strain under acidic stresses, including a longer adaptation time (reflected by a prolonged lag phase) with eventual recovery of growth (22). Decreased expression of Pma1 on the plasma membrane could explain the increased sensitivity of the Δisc1 strain to the Pma1 inhibitor ebselen.
In conclusion, this study highlights that cryptococcosis may have different clinical presentations and outcomes depending on the host's immune responses and the pathogen's ability to survive intracellularly. By controlling the intracellular survival of the fungus, Isc1 favors the adaptation of C. neoformans to this hostile environment by protecting it from acidic, oxidative, and nitrosative stresses through a Pma1-dependent mechanism. The shifting of the Δisc1 strain to the extracellular environment promotes hyperencapsulation, and the mutant still causes the death of the host but through a mechanism(s) different from those observed with control strains. A model for the role of Isc1 during the infection is illustrated in Fig. 9. Further investigations into how Isc1 regulates these effects will provide new insights into the mechanisms of pathogenicity of this, and perhaps other, intracellular fungal pathogens.
FIG. 9.
Model for the role of Isc1 during the C. neoformans-host interaction. Upon inhalation of WT cryptococci, the host macrophages ingest the yeast cells and subject them to antimicrobial effectors in the phagolysosome, including acidic, oxidative, and nitrosative stresses. Being resistant to these stresses, WT remains hidden from other host immune effectors and replicates intracellularly, eventually lysing the macrophages and disseminating to the brain through the blood-brain barrier. In contrast, loss of Isc1 (Δ) results in a strain that is not resistant to the antimicrobial effectors of activated macrophages. Therefore, Δisc1 cells, once engulfed, are killed by macrophages, and the Δisc1 cell population is pressured to produce a larger polysaccharide capsule as an antiphagocytic defense. Whereas this adaptation aids in the survival of the mutant strain in the lungs, it may also inhibit the yeast's transcellular migration through the endothelial barrier of cerebral capillaries, thus preventing fungal infection of the brain.
Supplementary Material
FIG. 8.
Isc1 protects C. neoformans from stresses associated with an activated phagolysosome. (A) The Δisc1 strain shows a significantly longer lag time in growth under acidic conditions (pH 4.0) ( , P < 0.05). (B) There are no differences among WT, Δisc1, and Δisc1REC strains in their ability to grow under unstressed conditions (rich medium buffered to pH 7.0). (C) Serial dilutions of WT, Δisc1, and Δisc1REC strains on solid neutral medium (pH 7.0) supplemented with 5 mM H2O2. At 48 h, the Δisc1 strain shows a delay in growth in the presence of hydrogen peroxide, but it recovers by 96 h. (D) Serial dilutions of WT, Δisc1, and Δisc1REC strains on solid acidic medium (pH 4.0) supplemented with 5 mM H2O2. Growth of the Δisc1 strains is completely inhibited in the presence of acidic and oxidative stresses. There is no recovery by 96 h. (E) Serial dilutions of WT, Δisc1, and Δisc1REC strains on solid acidic medium (pH 4.0) supplemented with 0.1 mM NaNO2. At this pH, NaNO2 spontaneously decomposes to NO. In the presence of NO and low pH, the growth of the Δisc1 strain is completely inhibited despite prolonged incubation.
, P < 0.05). (B) There are no differences among WT, Δisc1, and Δisc1REC strains in their ability to grow under unstressed conditions (rich medium buffered to pH 7.0). (C) Serial dilutions of WT, Δisc1, and Δisc1REC strains on solid neutral medium (pH 7.0) supplemented with 5 mM H2O2. At 48 h, the Δisc1 strain shows a delay in growth in the presence of hydrogen peroxide, but it recovers by 96 h. (D) Serial dilutions of WT, Δisc1, and Δisc1REC strains on solid acidic medium (pH 4.0) supplemented with 5 mM H2O2. Growth of the Δisc1 strains is completely inhibited in the presence of acidic and oxidative stresses. There is no recovery by 96 h. (E) Serial dilutions of WT, Δisc1, and Δisc1REC strains on solid acidic medium (pH 4.0) supplemented with 0.1 mM NaNO2. At this pH, NaNO2 spontaneously decomposes to NO. In the presence of NO and low pH, the growth of the Δisc1 strain is completely inhibited despite prolonged incubation.
TABLE 1.
Loss of ISC1 sensitizes C. neoformans to the PMA1 inhibitor ebselena
| Strain | MFC (μM) of ebselen at:
|
|
|---|---|---|
| pH 7.0 | pH 4.0 | |
| WT | 3.12 | 2.48 |
| Δisc1 | 3.12 | 0.78* |
| Δisc1REC | 3.12 | 3.12 |
Ebselen is fungicidal to the WT, Δisc1, and Δisc1REC strains at a minimum fungicidal concentration (MFC) of 3.125 μM at a neutral pH. In contrast to WT and Δisc1REC strains, however, the Δisc1 strain was killed by a significantly lower concentration of ebselen under acidic conditions (*, P < 0.05).
Acknowledgments
We thank Lena Obeid, Caroline Westwater, Hiroko Hama, Edward Balish, and Yusuf Hannun for constructive comments. We appreciate the work of Jennifer Henry in the preparation of radiolabeled IPC.
This work was supported in part by the Burroughs Wellcome Fund, the National Institutes of Health (grant AI56168 to M.D.P.), and the Centers of Biomedical Research Excellence Program of the National Center for Research Resources (grant RR17677, Project 2 to M.D.P. and Project 6 to C.L.). Animal studies were partially supported by the National Institutes of Health through grant number C06 RR015455 from the Extramural Research Facilities Program of the National Center for Research Resources. J.M.S. was supported in part by a Medical Scientist Training Grant (GM08716) from the National Institutes of Health. M.D.P. is a Burroughs Wellcome New Investigator in Pathogenesis of Infectious Diseases.
Editor: A. Casadevall
Footnotes
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Ambesi, A., M. Miranda, V. V. Petrov, and C. W. Slayman. 2000. Biogenesis and function of the yeast plasma-membrane H(+)-ATPase. J. Exp. Biol. 203:155-160. [DOI] [PubMed] [Google Scholar]
- 2.Beeler, T. J., D. Fu, J. Rivera, E. Monaghan, K. Gable, and T. M. Dunn. 1997. SUR1 (CSG1/BCL21), a gene necessary for growth of Saccharomyces cerevisiae in the presence of high Ca2+ concentrations at 37 degrees C, is required for mannosylation of inositolphosphorylceramide. Mol. Gen. Genet. 255:570-579. [DOI] [PubMed] [Google Scholar]
- 3.Blackstock, R., and J. W. Murphy. 2004. Age-related resistance of C57BL/6 mice to Cryptococcus neoformans is dependent on maturation of NKT cells. Infect. Immun. 72:5175-5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Casadevall, A., W. Cleare, M. Feldmesser, A. Glatman-Freedman, D. L. Goldman, T. R. Kozel, N. Lendvai, J. Mukherjee, L. A. Pirofski, J. Rivera, A. L. Rosas, M. D. Scharff, P. Valadon, K. Westin, and Z. Zhong. 1998. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob. Agents Chemother. 42:1437-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casadevall, A., and J. R. Perfect. 1998. Cryptococcus neoformans, p. 381-405. ASM Press, Washington, D.C.
- 7.Chan, J., Y. Xing, R. S. Magliozzo, and B. R. Bloom. 1992. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J. Exp. Med. 175:1111-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charlier, C., F. Chretien, M. Baudrimont, E. Mordelet, O. Lortholary, and F. Dromer. 2005. Capsule structure changes associated with Cryptococcus neoformans crossing of the blood-brain barrier. Am. J. Pathol. 166:421-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung, D. O., K. Halsey, and D. P. Speert. 2000. Role of pulmonary alveolar macrophages in defense of the lung against Pseudomonas aeruginosa. Infect. Immun. 68:4585-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarke, N. G., and R. M. Dawson. 1981. Alkaline O leads to N-transacylation. A new method for the quantitative deacylation of phospholipids. Biochem. J. 195:301-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Jesus-Berrios, M., L. Liu, J. C. Nussbaum, G. M. Cox, J. S. Stamler, and J. Heitman. 2003. Enzymes that counteract nitrosative stress promote fungal virulence. Curr. Biol. 13:1963-1968. [DOI] [PubMed] [Google Scholar]
- 12.Ellerbroek, P. M., A. M. Walenkamp, A. I. Hoepelman, and F. E. Coenjaerts. 2004. Effects of the capsular polysaccharides of Cryptococcus neoformans on phagocyte migration and inflammatory mediators. Curr. Med. Chem. 11:253-266. [DOI] [PubMed] [Google Scholar]
- 13.Feldmesser, M., Y. Kress, P. Novikoff, and A. Casadevall. 2000. Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect. Immun. 68:4225-4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferreira, T., A. B. Mason, and C. W. Slayman. 2001. The yeast Pma1 proton pump: a model for understanding the biogenesis of plasma membrane proteins. J. Biol. Chem. 276:29613-29616. [DOI] [PubMed] [Google Scholar]
- 15.Folch, J., M. Lees, and G. H. Sloane Stanley. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226:497-509. [PubMed] [Google Scholar]
- 16.Fraser, J. A., S. S. Giles, E. C. Wenink, S. G. Geunes-Boyer, J. R. Wright, S. Diezmann, A. Allen, J. E. Stajich, F. S. Dietrich, J. R. Perfect, and J. Heitman. 2005. Same-sex mating and the origin of the Vancouver Island Cryptococcus gattii outbreak. Nature 437:1360-1364. [DOI] [PubMed] [Google Scholar]
- 17.Fujimoto, J., J. P. Wiener-Kronish, S. Hashimoto, and T. Sawa. 2002. Effects of Cl2MDP-encapsulating liposomes in a murine model of Pseudomonas aeruginosa-induced sepsis. J. Liposome Res. 12:239-257. [DOI] [PubMed] [Google Scholar]
- 18.Gaigg, B., B. Timischl, L. Corbino, and R. Schneiter. 2005. Synthesis of sphingolipids with very long chain fatty acids but not ergosterol is required for routing of newly synthesized plasma membrane ATPase to the cell surface of yeast. J. Biol. Chem. 280:22515-22522. [DOI] [PubMed] [Google Scholar]
- 19.Goldman, D. L., H. Khine, J. Abadi, D. J. Lindenberg, L. Pirofski, R. Niang, and A. Casadevall. 2001. Serologic evidence for Cryptococcus neoformans infection in early childhood. Pediatrics 107:E66. [DOI] [PubMed] [Google Scholar]
- 20.Heung, L. J., A. E. Kaiser, C. Luberto, and M. Del Poeta. 2005. The role and mechanism of diacylglycerol-protein kinase C1 signaling in melanogenesis by Cryptococcus neoformans. J. Biol. Chem. 280:28547-28555. [DOI] [PubMed] [Google Scholar]
- 21.Heung, L. J., C. Luberto, A. Plowden, Y. A. Hannun, and M. Del Poeta. 2004. The sphingolipid pathway regulates Pkc1 through the formation of diacylglycerol in Cryptococcus neoformans. J. Biol. Chem. 279:21144-21153. [DOI] [PubMed] [Google Scholar]
- 22.Holyoak, C. D., M. Stratford, Z. McMullin, M. B. Cole, K. Crimmins, A. J. P. Brown, and P. J. Coote. 1996. Activity of the plasma membrane H+-ATPase and optimal glycolytic flux are required for rapid adaptation and growth of Saccharomyces cerevisiae in the presence of the weak-acid preservative sorbic acid. Appl. Environ. Microbiol. 62:3158-3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ibrahim, A. S., S. G. Filler, M. S. Alcouloumre, T. R. Kozel, J. E. Edwards, Jr., and M. A. Ghannoum. 1995. Adherence to and damage of endothelial cells by Cryptococcus neoformans in vitro: role of the capsule. Infect. Immun. 63:4368-4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain, N., L. Li, D. C. McFadden, U. Banarjee, X. Wang, E. Cook, and B. C. Fries. 2006. Phenotypic switching in a Cryptococcus neoformans variety gattii strain is associated with changes in virulence and promotes dissemination to the central nervous system. Infect. Immun. 74:896-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawakami, K., Y. Koguchi, M. H. Qureshi, S. Yara, Y. Kinjo, K. Uezu, and A. Saito. 2000. NK cells eliminate Cryptococcus neoformans by potentiating the fungicidal activity of macrophages rather than by directly killing them upon stimulation with IL-12 and IL-18. Microbiol. Immunol. 44:1043-1050. [DOI] [PubMed] [Google Scholar]
- 26.Leemans, J. C., N. P. Juffermans, S. Florquin, N. van Rooijen, M. J. Vervoordeldonk, A. Verbon, S. J. van Deventer, and T. van der Poll. 2001. Depletion of alveolar macrophages exerts protective effects in pulmonary tuberculosis in mice. J. Immunol. 166:4604-4611. [DOI] [PubMed] [Google Scholar]
- 27.Lovchik, J. A., C. R. Lyons, and M. F. Lipscomb. 1995. A role for gamma interferon-induced nitric oxide in pulmonary clearance of Cryptococcus neoformans. Am. J. Respir. Cell Mol. Biol. 13:116-124. [DOI] [PubMed] [Google Scholar]
- 28.Luberto, C., B. Martinez-Marino, D. Taraskiewicz, B. Bolanos, P. Chitano, D. L. Toffaletti, G. M. Cox, J. R. Perfect, Y. A. Hannun, E. Balish, and M. Del Poeta. 2003. Identification of App1 as a regulator of phagocytosis and virulence of Cryptococcus neoformans. J. Clin. Investig. 112:1080-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luberto, C., D. L. Toffaletti, E. A. Wills, S. C. Tucker, A. Casadevall, J. R. Perfect, Y. A. Hannun, and M. Del Poeta. 2001. Roles for inositol-phosphoryl ceramide synthase 1 (IPC1) in pathogenesis of C. neoformans. Genes Dev. 15:201-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mare, L., R. Iatta, M. T. Montagna, C. Luberto, and M. Del Poeta. 2005. APP1 transcription is regulated by inositol-phosphorylceramide synthase 1-diacylglycerol pathway and is controlled by ATF2 transcription factor in Cryptococcus neoformans. J. Biol. Chem. 280:36055-36064. [DOI] [PubMed] [Google Scholar]
- 31.Missall, T. A., J. M. Moran, J. A. Corbett, and J. K. Lodge. 2005. Distinct stress responses of two functional laccases in Cryptococcus neoformans are revealed in the absence of the thiol-specific antioxidant Tsa1. Eukaryot. Cell 4:202-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mozaffarian, N., J. W. Berman, and A. Casadevall. 1995. Immune complexes increase nitric oxide production by interferon-gamma-stimulated murine macrophage-like J774.16 cells. J. Leukoc. Biol. 57:657-662. [DOI] [PubMed] [Google Scholar]
- 33.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution susceptibility testing of yeasts. Approved standard M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 34.Perfect, J. R. 2005. Cryptococcus neoformans: a sugar-coated killer with designer genes. FEMS Immunol. Med. Microbiol. 45:395-404. [DOI] [PubMed] [Google Scholar]
- 35.Rittershaus, P. C., T. B. Kechichian, J. C. Allegood, A. H. Merrill, Jr., M. Hennig, C. Luberto, and M. Del Poeta. 2006. Glucosylceramide synthase is an essential regulator of pathogenicity of Cryptococcus neoformans. J. Clin. Investig. 116:1651-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rivera, J., M. Feldmesser, M. Cammer, and A. Casadevall. 1998. Organ-dependent variation of capsule thickness in Cryptococcus neoformans during experimental murine infection. Infect. Immun. 66:5027-5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 38.Sawai, H., Y. Okamoto, C. Luberto, C. Mao, A. Bielawska, N. Domae, and Y. A. Hannun. 2000. Identification of ISC1 (YER019w) as inositol phosphosphingolipid phospholipase C in Saccharomyces cerevisiae. J. Biol. Chem. 275:39793-39798. [DOI] [PubMed] [Google Scholar]
- 39.Shao, X., A. Mednick, M. Alvarez, N. van Rooijen, A. Casadevall, and D. L. Goldman. 2005. An innate immune system cell is a major determinant of species-related susceptibility differences to fungal pneumonia. J. Immunol. 175:3244-3251. [DOI] [PubMed] [Google Scholar]
- 40.Sigler, K., and M. Hofer. 1991. Activation of the plasma membrane H(+)-ATPase of Saccharomyces cerevisiae by addition of hydrogen peroxide. Biochem. Int. 23:861-873. [PubMed] [Google Scholar]
- 41.Soteropoulos, P., T. Vaz, R. Santangelo, P. Paderu, D. Y. Huang, M. J. Tamas, and D. S. Perlin. 2000. Molecular characterization of the plasma membrane H+-ATPase, an antifungal target in Cryptococcus neoformans. Antimicrob. Agents Chemother. 44:2349-2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stadler, N., M. Hofer, and K. Sigler. 2001. Mechanisms of Saccharomyces cerevisiae PMA1 H+-ATPase inactivation by Fe2+, H2O2 and Fenton reagents. Free Radic. Res. 35:643-653. [DOI] [PubMed] [Google Scholar]
- 43.Toffaletti, D. L., T. H. Rude, S. A. Johnston, D. T. Durack, and J. R. Perfect. 1993. Gene transfer in Cryptococcus neoformans by use of biolistic delivery of DNA. J. Bacteriol. 175:1405-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tucker, S. C., and A. Casadevall. 2002. Replication of Cryptococcus neoformans in macrophages is accompanied by phagosomal permeabilization and accumulation of vesicles containing polysaccharide in the cytoplasm. Proc. Natl. Acad. Sci. USA 99:3165-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Rooijen, N., and A. Sanders. 1994. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J. Immunol. Methods 174:83-93. [DOI] [PubMed] [Google Scholar]
- 46.Walenkamp, A. M., P. Ellerbroek, J. Scharringa, E. Rijkers, A. I. Hoepelman, and F. E. Coenjaerts. 2003. Interference of Cryptococcus neoformans with human neutrophil migration. Adv. Exp. Med. Biol. 531:315-339. [DOI] [PubMed] [Google Scholar]
- 47.Wang, B., C. Biron, J. She, K. Higgins, M. J. Sunshine, E. Lacy, N. Lonberg, and C. Terhorst. 1994. A block in both early T lymphocyte and natural killer cell development in transgenic mice with high-copy numbers of the human CD3E gene. Proc. Natl. Acad. Sci. USA 91:9402-9406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang, B., S. J. Simpson, G. A. Hollander, and C. Terhorst. 1997. Development and function of T lymphocytes and natural killer cells after bone marrow transplantation of severely immunodeficient mice. Immunol. Rev. 157:53-60. [DOI] [PubMed] [Google Scholar]
- 49.Weinberg, P. B., S. Becker, D. L. Granger, and H. S. Koren. 1987. Growth inhibition of Cryptococcus neoformans by human alveolar macrophages. Am. Rev. Respir. Dis. 136:1242-1247. [DOI] [PubMed] [Google Scholar]
- 50.Zhao, L., F. Zhang, J. Guo, Y. Yang, B. Li, and L. Zhang. 2004. Nitric oxide functions as a signal in salt resistance in the calluses from two ecotypes of reed. Plant Physiol. 134:849-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.