Abstract
A teichoic acid (TA)-like polysaccharide in Enterococcus faecalis has previously been shown to induce opsonic antibodies that protect against bacteremia after active and passive immunization. Here we present new data providing a corrected structure of the antigen and the epitope against which the opsonic antibodies are directed. Capsular polysaccharide isolated from E. faecalis strain 12030 by enzymatic digestion of peptidoglycan and chromatography (enzyme-TA) was compared with lipoteichoic acid (LTA) extracted using butanol and purified by hydrophobic-interaction chromatography (BuOH-LTA). Structural determinations were carried out by chemical analysis and nuclear magnetic resonance spectroscopy. Antibody specificity was assessed by enzyme-linked immunosorbent assay and the opsonophagocytosis assay. After alanine ester hydrolysis, there was structural identity between enzyme-TA and BuOH-LTA of the TA-parts of the two molecules. The basic enterococcal LTA structure was confirmed: 1,3-poly(glycerol phosphate) nonstoichiometrically substituted at position C-2 of the glycerol residues with d-Ala and kojibiose. We also detected a novel substituent at position C-2, [d-Ala→6]-α-d-Glcp-(1→2-[d-Ala→6]-α-d-Glcp-1→). Antiserum raised against enzyme-TA bound equally well to BuOH-LTA and dealanylated BuOH-LTA as to the originally described enzyme-TA antigen. BuOH-LTA was a potent inhibitor of opsonophagocytic killing by the antiserum to enzyme-TA. Immunization with antibiotic-killed whole bacterial cells did not induce a significant proportion of antibodies directed against alanylated epitopes on the TA, and opsonic activity was inhibited completely by both alanylated and dealanylated BuOH-LTA. In summary, the E. faecalis strain 12030 enzyme-TA is structurally and immunologically identical to dealanylated LTA. Opsonic antibodies to E. faecalis 12030 are directed predominantly to nonalanylated epitopes on the LTA molecule.
Enterococci are gut commensals with generally low virulence for humans and animals. However, due to medical progress that has produced many patients surviving for prolonged periods under immunosuppressed conditions, along with high intrinsic and acquired resistance to a broad range of antimicrobial agents, these pathogens have attained increasing importance as serious causes of nosocomial infections. In the United States, the rate of infection with vancomycin-resistant enterococci has been rising steadily in recent years and is now approaching 29% of enterococcal infections in patients in intensive care units (24). In Europe, several countries, including Portugal, Greece, Italy, Ireland, and Cyprus, have reported rates of vancomycin resistance exceeding 20% (5). The limited choice of antimicrobials still available for treatment of serious enterococcal infections has spurred a renewed interest in immunotherapy and vaccine-based regimens to control this infection.
It has been postulated that protective immunity to encapsulated bacteria is dependent mainly on the presence of opsonic antibodies to surface or capsular polysaccharides (28). To date, five different capsular polysaccharides have been described for Enterococcus faecalis (32). A detailed structural analysis and characterization of the immune response and protection in vivo has been published for only one of them (14, 33). In 1999 Wang et al. described a novel, teichoic-acid (TA)-like capsular polysaccharide in E. faecalis strain 12030 (33). Antisera raised against purified polysaccharide killed the homologous strain, a variety of heterologous E. faecalis, as well as Enterococcus faecium strains, including some vancomycin-resistant strains, in an opsonophagocytic killing assay (14). Immunization with purified polysaccharide protected mice against E. faecalis bacteremia (13). The structure of this carbohydrate as described in the original publication has many similarities to that of lipoteichoic acid (LTA) of E. faecalis. In this report we demonstrate that the carbohydrate isolated by Wang et al. (33) is very similar to dealanylated LTA and that this LTA without alanine is in fact the main target of opsonic antibodies for this strain.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The strain used in this study was E. faecalis strain 12030, a clinical isolate also used in previous studies by Wang et al. (33) and Huebner et al. (13, 14). A mutant with a deletion in the first gene of dlt operon was created using the method described by Cieslewicz et al., with some modifications (2). The resulting mutant, 12030 ΔdltA was devoid of d-alanyl residues on its LTA (6). Starter cultures were grown for 18 h at 37°C in Colombia or tryptic soy broth supplemented with 1% glucose. The following day, the cultures were diluted 1:10 in fresh, prewarmed Colombia or tryptic soy broth plus glucose (total volume, 10 liters) and cultured for 2 h without shaking.
Purification of TA-like polysaccharide (enzyme-TA).
Bacterial cells were collected by centrifugation and washed in phosphate-buffered saline (PBS). Isolation of polysaccharide was performed as described by Huebner et al. (14). Briefly, bacterial cells were collected by centrifugation and resuspended in digestion buffer (PBS supplemented with 5 mM MgCl2, 1 mM CaCl2, and 0.05% NaN3), and cell wall material was digested by the addition of mutanolysin and lysozyme (each at 100 μg/ml) (Sigma Chemicals, St. Louis, MO) at 37°C for 18 h. Insoluble material was removed by centrifugation, and the supernatant was treated with nucleases (DNase I and RNase A, 100 μg/ml) at 37°C for 4 h, followed by treatment with proteinase K (100 μg/ml) (all from Sigma Chemicals) at 56°C for 18 h. The solution was then treated by the addition of 4 volumes of ethanol and the resulting precipitate collected by centrifugation. After resuspension and dialysis against deionized H2O, the soluble material was lyophilized.
For size exclusion chromatography, the material was dissolved in 0.01 M ammonium carbonate buffer (pH 8.6) and applied to a Sephacryl S-400 column (1.6 by 90 cm). Fractions were tested by immunoblot analysis with rabbit antiserum raised against the previously described purified TA-like capsular polysaccharide (14). Immunoreactive fractions were combined, dialyzed, and lyophilized. The material was redissolved in a buffer of 20 mM NaHCO3, pH 8.0, and applied on a 5-ml Q-Sepharose column (Q-Sepharose High Trap; Amersham Biotech, Uppsala, Sweden). Bound material was eluted with a gradient of 0 to 1,000 mM NaCl. Fractions were analyzed by hexose assay (4), phosphate assay (20), and immunoblotting. Fractions eluting at high ionic strength (600 mM NaCl) that tested positive by immunoblotting were pooled separately.
Purification of butanol-extracted LTA (BuOH-LTA).
LTA was obtained by butanol extraction as described previously (23). In summary, after growth as described above and centrifugation to collect bacterial cells, the organisms were resuspended in 0.1 M citrate buffer (pH 4.7) and cell walls disrupted by vibration with glass beads (Beadbeater; Glenn Mills, Clifton, NJ) followed by stirring with an equal volume of n-butanol for 30 min. After phase separation by centrifugation, the aqueous layer was removed, dialyzed against 0.1 M ammonium acetate (pH 4.7), and lyophilized. The material was redissolved in 15% n-propanol in 0.1 M ammonium acetate (pH 4.7) and applied to an octyl Sepharose column for hydrophobic interaction chromatography. Bound material was eluted with a gradient of 15 to 80% n-propanol. Fractions were assayed for total phosphorus and by immunoblot assay. Immunoreactive material eluting at around 50% n-propanol was combined and lyophilized repeatedly (23).
Dealanylation of BuOH-LTA.
BuOH-LTA was dissolved in 50 mM ammonium acetate buffer (pH 8.5) and incubated for 18 h at 20°C. After incubation, LTA was dialyzed against distilled water and lyophilized. The complete removal of alanyl residues was confirmed by 1H nuclear magnetic resonance (NMR) spectroscopy.
General and analytical chemical methods.
Composition analysis was carried out by first hydrolyzing samples with 2 M trifluoroacetic acid for 3 h at 120°C, followed by reduction with NaBH4 and peracetylation using acetic anhydride and pyridine. The absolute configurations of the sugars were determined by gas chromatography (GC) of their acetylated (S)-2-butyl glycosides (8, 11). GC conditions were as reported previously (16). For assignment of the absolute configuration of alanine after chemical dealanlyation, the sample was lyophilized and then treated with (S)-2-butanol (0.5 ml) and AcCl (0.1 ml). After acetylation, the product was dried and analyzed by GC using a temperature gradient starting at 100°C (held for 3 min) and progressing to 150°C at 3°C/min. Elution profiles were compared with standards prepared from d-alanine, l-alanine, and (S)-2-butanol.
NMR spectroscopy.
NMR experiments were carried out after exchange of the samples into 99.9% 2H2O. All one-dimensional (1H, 13C, and 31P), two-dimensional homonuclear (1H,1H correlation spectroscopy [COSY], total correlation spectroscopy [TOCSY], and nuclear Overhauser enhancement spectroscopy [NOESY]), as well as two-dimensional heteronuclear (1H,13C heteronuclear multiple quantum coherence [HMQC], HMQC-TOCSY, and 1H,31P HMQC) scans were recorded at 27°C with a Bruker DRX Avance 600 MHz spectrometer (operating frequencies of 600.31 MHz for 1H NMR, 150.96 MHz for 13C NMR, and 243.01 MHz for 31P NMR) using standard Bruker software. Chemical shifts were reported relative to an internal standard of acetone (δH 2.225; δC 31.45), and an external standard of 85% H3PO4 (δP 0.00). COSY, TOCSY, and NOESY experiments were recorded using data sets (t1 by t2) of 2,048 by 512 points, and 24 scans were acquired for each t1 value. The TOCSY and NOESY experiments were carried out in the phase-sensitive mode with mixing times of 100 ms and 250 ms, respectively. The 1H,13C correlations were measured in the 1H-detected mode via HMQC with proton decoupling in the 13C domain, and HMQC-TOCSY spectra were acquired using data sets of 2,048 by 512 points, and 128 scans were acquired for each t1 value.
ELISA studies.
Enzyme-linked immunosorbent assay (ELISA) experiments were performed by standard methods as described previously (31). In brief, microtiter plates were coated with various carbohydrate antigens derived from E. faecalis strain 12030 (10 μg/ml in 0.04 M phosphate buffer, pH 6.0) and kept for 18 h at 4°C. Previous studies have shown that coating of LTA and enzymatically extracted TA does not differ at pH 6 and pH 7, but stability of ester-bound alanine is enhanced at the lower pH. Between incubation steps, plates were washed three times with PBS containing 0.05% Tween 20. Blocking was performed with 3% skim milk in PBS-0.02% sodium azide at 37°C for 2 h. Rabbit sera were applied in twofold serial dilutions. A goat anti-rabbit immunoglobulin G-alkaline phosphatase conjugate (Sigma Chemicals) diluted 1:1,000 was used as secondary antibody, and p-nitrophenyl phosphate (Sigma) was used as a substrate (1 mg/ml in diethanolamine buffer, 0.5 mM MgCl2, pH 9.8). After 60 min of incubation at 37°C, the absorbance was measured at 405 nm. For inhibition ELISAs, fivefold dilutions of inhibiting polysaccharide starting at 100 μg/ml (final concentration) were mixed with a single dilution of rabbit antiserum (1:200), which was previously shown by ELISA to be near the middle of the linear portion of the binding curve.
Opsonophagocytic assay.
An opsonophagocytic assay was used as previously described (14, 21, 31). Baby rabbit serum (Cedarlane Laboratories, Hornby, Ontario, Canada) absorbed with the target bacterial strain served as a source of complement. The opsonic activity of immune sera was compared to that of controls containing preimmune serum. Sera from a rabbit immunized with enzyme-extracted TA-like polysaccharide (14) and from a rabbit immunized with antibiotic-killed whole bacterial cells (14) were tested. Negative controls included tubes from which polymorphonuclear leukocytes, complement, or serum was omitted. The opsonic activity of the serum was calculated as follows: [1 − (CFU immune serum at 90 min/CFU of preimmune serum at 90 min)] × 100.
For studies on inhibition of opsonic killing, a rabbit antiserum at a concentration of 1:200 was incubated for 90 min at 4°C with 0.08 to 100 μg/ml of inhibitor. After this incubation step (1 h at 4°C), the opsonic killing assay was continued as described above.
Statistical analysis.
Comparisons between inhibitors in the opsonophagocytic assay were made by analysis of variance and Turkey's post hoc test, pairwise analysis, or the two-tailed t test using the Prism4 software package (GraphPad, San Diego, CA). A P value of <0.05 was considered significant.
RESULTS
Purification of antigens.
To optimize the purification of enzyme-TA, we revisited the protocol previously described by us (14). According to this protocol, cell walls of E. faecalis were digested with lysozyme and mutanolysin, and the material was fractioned by size exclusion chromatography after enzymatic removal of contaminating nucleic acid and proteins. Fractions from the size exclusion column were assayed by immunoblotting using an antiserum raised against enzyme-TA (14). Strong immunoreactivity of fractions eluting at the void volume was observed, but noticeable immunoreactivity was also seen in later fractions (data not shown). The void volume and later fractions were combined separately and then purified further by anion-exchange chromatography. Purified materials from the preparations were compared by NMR spectroscopy. Both had 1H NMR spectra typical of TA-like molecules, as described previously (33). The material eluting in the void volume of a Sephacryl S-400 column, however, showed additional signals at δ ∼1.3 and δ ∼0.9, suggestive of -CH2 and -CH3 residues, respectively (data not shown). To exclude contamination with glycolipids, the void-volume material was further extracted with chloroform-methanol as described by Bligh and Dyer (1), and the aqueous phase was applied to a hydrophobic interaction column as described by Morath et al. (23). The material retained on octyl-Sepharose was immunoreactive with antibodies against enzyme-TA and displayed the typical 1H NMR spectrum of the TA-like molecule from E. faecalis with additional signals suggestive of fatty acids. Since it has been reported previously that LTA is partially deacylated during enzymatic digestion of cell walls, these findings suggested that a proportion of the enzyme-TA was actually LTA (17, 18).
To elucidate the structural relationship of the enzyme-TA material to LTA in more detail, a second batch of antigen was purified using butanol extraction of cell walls followed by hydrophobic interaction chromatography under stringent acidic conditions, a method that specifically extracts LTA and conserves alanine substituents (23). From an octyl-Sepharose chromatography column, phosphorus-positive and immunoreactive material was retained then eluted around a concentration of 50% propanol. This material (BuOH-LTA) was combined and subjected to further analysis.
Structural analysis.
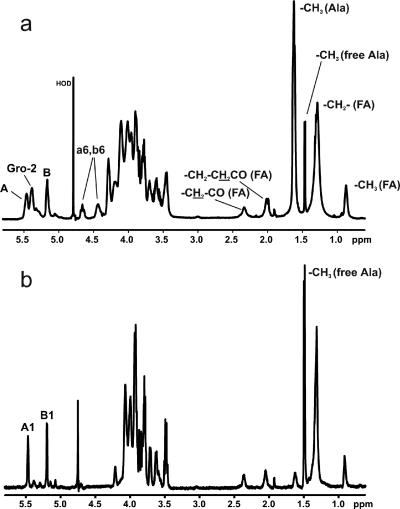
Results from the neutral sugar analysis and absolute configuration assignment of the monosaccharides by GC revealed the presence of d-glucose only. The 1H NMR spectrum of BuOH-LTA from strain 12030 of E. faecalis (Fig. 1a, Table 1) showed two broad anomeric signals at δ 5.461 (residue A), and at δ 5.165 (residue B), which were identified as substituted at position C-2, and a terminal α-d-Glcp residue, respectively. In addition, a signal at δ 5.388 was assigned to proton H-2 of the glycerol residue. The strong deshielding of this proton is caused by the substitution at position C-2 by an alanine residue (29). The doublet at δ 1.629 was recognized as a methyl group belonging to an alanine residue. Further, signals characteristic of fatty acids were identified: CH3 group (δ 0.861), CH2 groups (δ 1.283), -CH2-CH2-CO- (δ 2.018), and CH2-CH2-CO- (δ 2.341). All 1H and 13C chemical shifts of BuOH-LTA isolated from strain 12030 of E. faecalis were established from 1H,1H COSY, and TOCSY spectra, as well as 1H,13C HMQC and HMQC-TOCSY spectra (Table 1).
FIG. 1.
1H NMR spectra of lipoteichoic acids isolated from Enterococcus faecalis strain 12030. (a) BuOH-LTA; (b) BuOH-LTA after incubation at pH 8.5 for 4 h. Spectra were recorded at 27°C in 2H2O relative to an internal standard of acetone (δH 2.225; δC 31.45). The letters refer to the glycerol residue and the glucose residues in kojibiose (Fig. 4; Table 1), and the numerals refer to the protons in the respective residues. The protons belonging to the glucose residues substituted by alanine are in lowercase.
TABLE 1.
1H and 13C NMR chemical shifts of lipoteichoic acids isolated from Enterococcus faecalis LTA
| Residue |
1H and 13C chemical shifts (δ)a
|
||||||
|---|---|---|---|---|---|---|---|
| H1, C1b | H2, C2 | H3, C3b | H4, C4 | H5, C5 | H6, C6c | H6d | |
| →)-Gro-(3→P→ (unsubstituted) | 3.880 (3.945), 66.81 | 4.044, 70.03 | 3.945 (3.880), 66.81 | ||||
| →)-Gro-(3→P→ with Ala at C-2 | 4.091, 64.27 | 5.388, 74.55 | 4.091, 64.27 | ||||
| Ala | 170.91 | 4.282, 49.55 | 1.629, 16.08 | ||||
| →)-Gro-(3→P→ with kojibiose at C-2 | 3.989, 65.58 | 4.197, 75.55 | 3.989, 65.58 | ||||
| →2)-α-d-Glcp-(1→ (residue Α) | 5.461, 95.04 | 3.679, 74.85 | 3.862, 71.64 | 3.444, 70.03 | 3.952, 72.20 | 3.765, 60.99 | 3.885 |
| α-d-Glcp-(1→ (residue Β) | 5.165, 95.93 | 3.588, 71.66 | 3.790, 72.91 | 3.434, 70.03 | 3.902, 72.26 | 3.765, 60.99 | 3.842 |
| →)-Gro-(3→P→ with kojibiose at C-2 | 3.989, 65.58 | 4.197, 75.55 | 3.989, 65.58 | ||||
| →2)-α-d-Glcp-(1→ with Ala at C-6 (residue a) | 5.488, 95.04 | 3.695, 74.46 | 3.880, 71.64 | 3.470, 70.03 | 4.232, 70.10 | 4.428, 65.54 | 4.667 |
| α-d-Glcp-(1→ with Ala at C-6 (residue b) | 5.161, 95.93 | 3.611, 71.66 | 3.811, 72.91 | 3.486, 70.03 | 4.160, 70.19 | 4.397, 65.54 | 4.640 |
Spectra were recorded at 27°C in 2H2O relative to an internal standard of acetone (δH 2.225; δC 31.45).
Residues a and b, as defined in column 1.
Residue a, as defined in column 1.
Residue b, as defined in column 1.
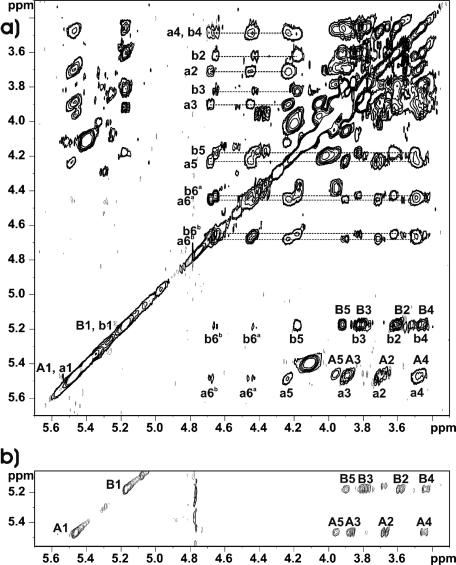
Four different glucose spin systems were identified by a TOCSY experiment (Fig. 2a). The spectrum showed that each anomeric signal (labeled A and B on the 1H NMR spectrum) includes two different anomeric protons. Two of them, δ 5.461 (A1) and δ 5.165 (B1), are typical for shift patterns of α-glucose residues. The remaining two spin systems, δ 5.488 (a1) and δ 5.161 (b1), consisted of several low-field-shifted protons. Two broad signals, δ ∼4.65 and δ ∼4.40 (a6, b6), in the 1H NMR proton spectrum (Fig. 1a) were attributed by analysis of COSY spectra (data not shown) to four H-6 protons of glucose residues (δ 4.667, δ 4.640, δ 4.428, and δ 4.397) deshielded due to substitution by alanine residues.
FIG. 2.
Sections of the TOCSY spectra of lipoteichoic acids isolated from Enterococcus faecalis strain 12030. (a) BuOH-LTA; (b) BuOH-LTA after incubation at pH 8.5 for 4 h. Spectra were recorded at 27°C in 2H2O relative to an internal standard of acetone (δH 2.225; δC 31.45). The letters refer to the glycerol residue and the glucose residues in kojibiose (Fig. 4; Table 1), and the numerals refer to the protons in the respective residues. The protons belonging to the glucose residues substituted by alanine at position C-6 are in lowercase.
Low-field-shifted signals of carbon atoms at δ 74.85 and δ 74.46 demonstrated substitutions at C-2 of residues A and a, respectively. The sequence of the residues in the repeating units of BuOH-LTA was established by NOESY experiments (data not shown). Strong interresidual NOE contacts were observed between H-1 B and H-2 A (H-1 b/H-2 a), as well as between H-1 A and H-2 Gro (H-1 a/H-2 Gro). These data were in agreement with previously proposed structures of LTA (i.e., a kojibiose residue attached to a glycerol residue at C-2).
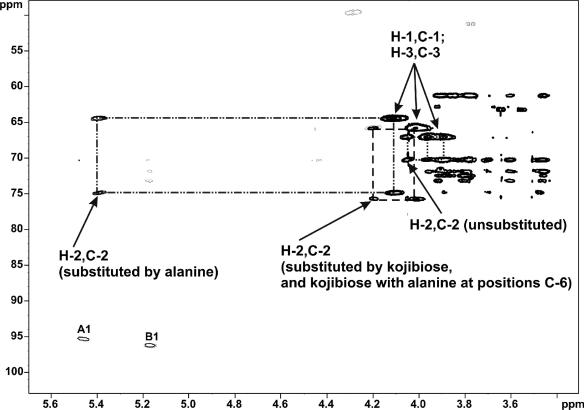
The 31P NMR spectrum showed two different broad phosphodiester resonances at δ −0.01 and δ −0.37. The first one was correlated to H-1 and H-3 of an unsubstituted glycerol residue, and the second one gave correlations to H-1 and H-3 of glycerol residues substituted at position C-2. Three different 1,3-poly(glycerol phosphate) residues were identified from the 1H,13C HMQC-TOCSY spectrum (Fig. 3), although there was no difference between glycerol residues substituted with kojibiose and kojibiose alanylated at position C-6 of both glucose residues.
FIG. 3.
Section of the HMQC-TOCSY spectrum of LTA isolated from E. faecalis strain 12030. Spectra were recorded at 27°C in 2H2O relative to an internal standard of acetone (δH 2.225; δC 31.45). The letters refer to the glycerol residues and the glucose residues in kojibiose (Fig. 4), and the numerals refer to the protons and 13C atoms in the respective residues. The rectangles show different types of 1,3-poly(glycerol phosphate) residues due to various substitutions at position C-2.
The 1H NMR spectrum of dealanylated BuOH-LTA showed only two narrow anomeric peaks (Fig. 1b). The above-described low-field shift (due to d-alanylation proton signals) had disappeared. Additionally, instead of the methyl signal (δ 1.629) attributed to chemically bound alanine residues, a large signal (δ 1.495) of a methyl group corresponding to a free alanine residue was present. These changes confirmed the d-alanylation of kojibiose and glycerol residues. The section of the TOCSY spectrum (Fig. 2b) of this sample showed only two glucose spin systems characteristic of a kojibiose residue.
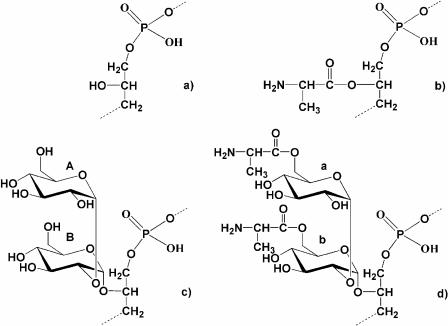
The chemical and the NMR results confirmed several aspects of the structures of LTA proposed by Wicken and Baddiley (34): 1,3-poly(glycerol phosphate) and 1,3-poly(glycerol phosphate) substituted with alanine or kojibiose at position C-2 of glycerol residues (Fig. 4a, b, and c, respectively). In addition, a novel alanine substitution was localized at positions C-6 of both glucoses in the kojibiose residue (Fig. 4d), identifying a new component of the LTA structure in enterococci.
FIG. 4.
Chemical structures of different repeating units of lipoteichoic acids isolated from Enterococcus faecalis strain 12030. (a) 1,3-Poly (glycerol phosphate); (b) 1,3-poly(glycerol phosphate) substituted at position C-2 of glycerol residues by d-Ala; (c) 1,3-poly(glycerol phosphate) substituted at position C-2 of glycerol residues by kojibiose; (d) 1,3-poly(glycerol phosphate) substituted at the position C-2 by [d-Ala→6]-α-d-Glcp-(1→2)-[d-Ala→6]-α-d-Glcp-(1→.
Immunological properties of enzyme-TA and BuOH-LTA.
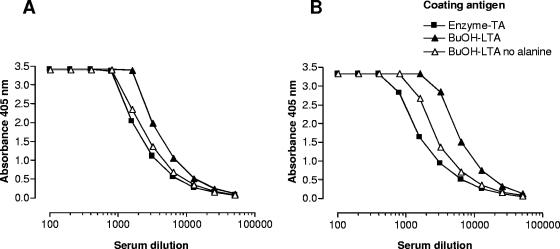
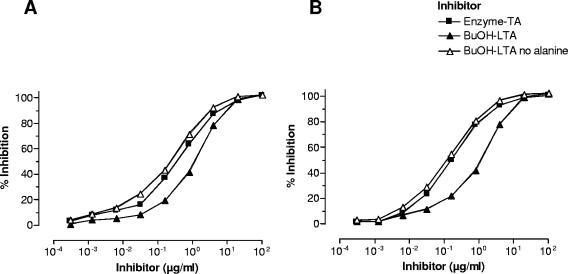
After confirming the structural identity of the TA part of enzyme-TA and dealanylated BuOH-LTA, we next compared the immunological characteristics of the two antigens. In a direct ELISA, sera raised against enzyme-TA and whole bacterial cells reacted equally well with either enzyme-TA, BuOH-LTA, or dealanylated BuOH-LTA (Fig. 5). Since the absence of fatty acids in enzyme-TA may reduce its ability to bind to polystyrene ELISA plates, we wanted to confirm the results by inhibition ELISA. With BuOH-LTA as a coating antigen, all three TA antigens could achieve full inhibition of the binding of anti-enzyme-TA and anti-whole-cell serum to BuOH-LTA, indicating a high degree of immunologic cross-reactivity among the different antigens (Fig. 6). Interestingly, BuOH-LTA was distinctly less potent at inhibiting the binding to itself of antibody raised to whole cells compared to the inhibition achieved with dealanylated LTA (Fig. 6). This finding implies that no d-alanine-specific antibodies are induced by immunization with whole bacterial cells. In addition, alanyl substituents present on native LTA used as a liquid phase inhibitor may interfere with the binding of antibodies recognizing nonalanylated epitopes on the molecule.
FIG. 5.
Binding of rabbit antiserum raised against antibiotic-killed whole bacterial cells (A) and of antiserum raised against enzyme-TA (B) against various coating antigens. Antigens used to coat ELISA plates are indicated. Data points represent averages of two determinations.
FIG. 6.
Inhibition ELISA of rabbit antiserum raised against antibiotic-killed whole bacterial cells (A) and of antiserum raised against enzyme-TA (B). The solid-phase antigen is the native BuOH-LTA, and the liquid phase antigen or inhibitor is as indicated. Data points represent averages of two determinations.
Epitope specificity of opsonic antibodies raised to enzyme-TA or whole bacterial cells.
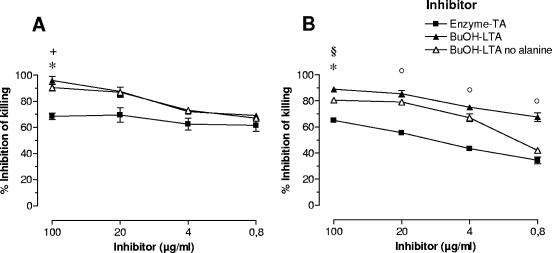
Opsonic activity of antiserum has been correlated with protective efficacy for many gram-positive and -negative bacteria (27, 28). We have shown previously that vaccination with enzyme-TA protects against bacteremia with E. faecalis and E. faecium when the infecting strains can be killed in an opsonic assay by antibodies raised to enzyme-TA (13). We therefore examined whether LTA could, in fact, be the target of opsonic antibodies induced by vaccination with whole bacterial cells (anti-whole cell) and enzyme-TA. The opsonophagocytic killing mediated by antibodies raised to whole bacterial cells was completely inhibited by BuOH-LTA, indicating that opsonic antibodies raised by this mode of immunization are directed only against LTA and that other surface antigens do not play a major role in the induction of opsonic antibodies (Fig. 7). Alanylated LTA (BuOH-LTA) and dealanylated LTA were equally effective at inhibiting opsonophagocytic killing, suggesting that the epitopes recognized by opsonic antibodies do not contain alanine residues (Fig. 7). Interestingly, both BuOH-LTA and dealanylated BuOH-LTA were significantly more potent inhibitors of opsonophagocytic killing than enzyme-TA, the original immunogen used for vaccination (Fig. 7).
FIG. 7.
Inhibition of opsonophagocytic killing of rabbit antiserum raised against antibiotic-killed whole bacterial cells (A) or enzyme-TA (B). Both antisera were used at a 1:200 dilution. Inhibitors used are indicated. Points represent averages of four values and error bars the standard errors of the means. *, P < 0.01 for enzyme-TA versus BuOH-LTA; °, P < 0.05 for enzyme-TA versus BuOH-LTA, §, P < 0.01 for enzyme-TA versus BuOH-LTA with no alanine; +, P < 0.05 for enzyme-TA versus BuOH-LTA with no alanine.
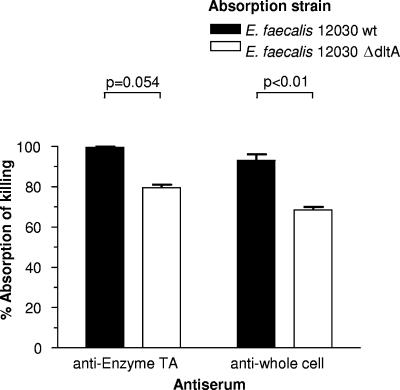
In order to clarify further the role of alanyl residues in the specificity of the opsonic antibodies, we used E. faecalis strain 12030 and an isogenic mutant lacking the dltA gene (ΔdltA strain), and thus devoid of the ability to alanylate LTA (6), to adsorb antiserum raised to either enzyme-TA or whole bacteria (Fig. 8). For the anti-whole-cell serum, the ΔdltA mutant strain absorbed significantly less opsonic activity than a comparable amount of cells of the wild-type strain (Fig. 8). Since the opsonic killing was still highly reduced when both antisera were adsorbed with either the parental or ΔdltA strain, it is unlikely that the ∼20% residual opsonic killing retained following adsorption with the ΔdltA strain was due to antibodies specific to alanylated epitopes in LTA. This is because antibodies to such an epitope do not exist in antisera raised to the enzyme-TA, as it lacks alanine. It is likely that the residual killing was due to a reduced efficiency of adsorption with the ΔdltA strain.
FIG. 8.
Inhibition of opsonophagocytic killing of E. faecalis strain 12030 after absorption of the indicated antiserum with either the homologous wild-type strain or an isogenic ΔdltA mutant lacking alanine incorporation into the LTA. Bars represent the means of four determinations and error bars the standard errors of the means.
DISCUSSION
Previously, we have described a novel TA-like capsular polysaccharide present in a number of E. faecalis and E. faecium strains that is a target of opsonic antibodies (14). Active and passive immunotherapy regimens using this antigen yielded protection in a mouse model of bacteremia and infection of kidneys, livers, and spleens (13). When antibodies were passively transferred up to 48 h after bacterial challenge, they were still protective (13). One- and two-dimensional NMR spectroscopy and chemical analysis initially revealed a repeating unit of →6)-[-α-d-Glcp→2]-α-d-Glcp-(1→ 2)-Gro-1-P-O→) in the isolated capsular polysaccharide (33). In this report we demonstrate the structural and immunological identity of the TA-like capsular polysaccharide molecule with the hydrophilic part of dealanylated LTA from the same enterococcal strain.
Several reports have described the enzymatic and chemical deacylation of LTA during culture and extraction (17, 18). In our initial work on purifying the enzyme-TA, we performed gel chromatography at a pH of 8.6. At this pH, however, the labile alanine-esters of LTA are rapidly cleaved, explaining why alanine residues were not previously detected (25). In the current study, LTA was extracted by butanol at pH 4.7, a purification method known to be LTA specific and to retain alanine residues (23) for comparison with the enzyme-TA material. The structure assigned by NMR spectroscopy and chemical analysis to the BuOH-LTA consisted of a poly(glycerophosphate) repeating unit with nonstoichiometric substitution of C-2 of glycerol with alanine or kojibiose. This results are in general agreement with previous reports on the structure of the enterococcal LTA (34). In addition, a novel substitution by alanine was found at position C-6 of both glucoses on some of the kojibiose residues. The alanylation of glycosyl substituents has been described previously for wall TA but not LTA (29). When d-alanine was removed by hydrolysis under mild alkaline conditions, NMR spectra of the hydrophilic part of the LTA molecule were identical to those of the TA-like enzyme-TA that we previously described (33).
Detailed analysis of our data from NMR spectroscopy and the previously published NMR assignments, however, revealed one misinterpretation. Wang et al. (33) attributed the H-1 and H-3 of the glycerol residue which was unsubstituted at position C-2 (Fig. 4a) to H-6 protons of →2)-α-d-Glcp-(1→. As a result of the misinterpretation of the 1H,31P TOCSY spectrum, an erroneous structure of TA was proposed (33). The 1H,13C HMQC-TOCSY spectrum (Fig. 3) showed a correlation between the H-1/H-3/C-3 cross-peak (δ 3.880/3.945/66.81) and the cross-peak at δ 4.044/70.03 which was assigned as the H-2/C-2 of the glycerol residue. Furthermore, the cross-peak H-5/C-5 of the →2)-α-d-Glcp-(1→ residue (δ 3.952/72.20) did not give any correlation to cross-peaks at δ 3.880/3.945/66.81 (H-1/H-3/C-3), which would have been necessary to confirm the originally proposed structure for the enzyme-TA.
With the confirmed structural identity of the TA-like enzyme-TA capsular polysaccharide and dealanylated LTA, we then set out to unravel the immunological relatedness of the two antigens. From our ELISA results we could conclude that the antiserum raised against the enzyme-TA capsule is fully cross-reactive with dealanylated LTA. No alanine-specific antibodies were present in this antiserum, but interestingly, antibodies requiring the presence of alanine to bind to LTA were also undetectable in serum raised against whole bacterial cells. We can only speculate on the reasons for this observation, which may relate to the alanine residues representing an overall poorly immunogenic epitope. Alternately, due to their lability, the alanine residues may be removed from LTA during antigen processing by antigen-presenting cells. A similar lack of immunogenicity for carbohydrate substituents such as O-acetate in other bacterial polysaccharides has been described (21, 26). Further studies are needed to clarify the role of alanine residues in the immune response to LTA in E. faecalis.
In agreement with our ELISA studies, opsonic antibodies were also cross-reactive with dealanylated LTA, demonstrating that the opsonic epitopes of the enzyme-TA molecule are also present on LTA. Again, we found no evidence that alanine is an essential component of opsonic epitopes on LTA. Our absorption studies using the homologous wild-type strain and a ΔdltA mutant showed that the mutant strain absorbed out less opsonic killing activity from serum raised against whole bacterial cells than did the wild-type strain. Since this effect was also present as a trend with the serum raised against purified enzyme-TA polysaccharide, it is unlikely that the lower adsorption by the ΔdltA strain is due to an inability to adsorb out specific antibodies to LTA with alanine present, because no alanine was present on the antigen used to raise antibody to enzyme-TA (33). It is more likely that the ΔdltA mutant either makes less LTA or retains less of it on the cell surface and, rather, sheds the LTA into the growth medium or perhaps synthesizes an altered LTA and thus is less efficient at adsorbing antibodies to this antigen from the opsonic antisera. Studies to evaluate these possibilities are under way.
Surprisingly, the enzyme-TA molecule was a less efficient inhibitor of opsonophagocytosis than LTA. This difference could reflect a shorter chain length of the enzymatically released material, a factor know to influence binding to length-stabilized epitopes (19).
Very few reports have dealt with LTA as a target of opsonic antibodies. A monoclonal antibody opsonic against pneumococcal TA has been described (22). Two other reports, however, have concluded that antibodies specific to LTA are not opsonic for coagulase-negative staphylococci (30) or for Staphylococcus hominis (7). The potential protective activity of antibody against enterococcal LTA may also involve inhibition of other functions of this molecule that could affect the host-pathogen interaction. Lipoteichoic acid has been linked to a variety of bacterial cellular functions, such as membrane elasticity and porosity, trafficking of molecules, anchoring of wall proteins, and determination of net charge and surface hydrophobicity (25). Lipoteichoic acids from several gram-positive bacteria have been shown to engage in interactions with Toll-like receptor 2 (12), and LTA is known to participate in attachment to epithelial and endothelial surfaces and to biomaterials (3, 9, 36) and is also involved in cell invasion (3). Thus, antibodies to LTA could inhibit a variety of bacterial functions or host responses.
A question that needs to be addressed is the cross-reactivity of LTA-specific antibodies against heterologous strains of E. faecalis, E. faecium, and other gram-positive bacteria expressing structurally related LTAs. When we initially examined the cross-reactivity of the antiserum raised against the enzyme-TA molecule, we could demonstrate opsonization of some heterologous enterococcal strains, but we did not obtain a universal cross-reactivity as one would expect from the highly conserved structure of LTA in enterococci (14). In another study, our group developed a serotyping system for E. faecalis (15). Using antisera raised against purified enzyme-TA polysaccharide and whole bacterial cells, 60% of examined strains could be grouped into four serotypes by cross-reactivity in an opsonophagocytosis assay, and 55% could be serogrouped by ELISA. This approach, though important for our understanding of the serodiversity of enterococcal strains, is confounded by using sera against multiple, surface-exposed antigens and arbitrary cutoff values for serologic classification of strains. In the face of our current data, the cross-reactivity of antibody to enterococcal LTA needs to be revisited using highly purified antigens and monospecific antisera. In this regard, it should be kept in mind that, despite an identical overall makeup of enterococcal LTA, the microheterogeneity, i.e., the amount and ratio of the substituents of C-2 of glycerol, leaves considerable potential for strain-to-strain variation and could partially explain the serodiversity of E. faecalis even when antibodies specific to a highly related surface antigen are used. Alternatively, opsonophagocytic killing of heterologous strains of E. faecalis may be mediated by antibodies against polysaccharide antigens not expressed by strain 12030. Hancock and Gilmore have described a novel polysaccharide containing glucose, galactose, and glycerol phosphate in E. faecalis strain FA2-2, which protects against antibody-independent phagocytic killing and increases persistence in host tissues (10). Using rabbit antiserum raised against whole bacteria of the same serogroup, we demonstrated that the opsonic antibodies were likely to be directed against the polysaccharide described by Hancock and Gilmore (15). In addition, Xu et al. have described a gene cluster (epa) encoding synthesis of a putative carbohydrate antigen that was implicated in resistance to killing mediated by polymorphonuclear phagocytes and complement (35). To date, no structural analysis is available on the material synthesized by proteins encoded in the epa gene cluster. In the face of these reports, it is conceivable that some strains of E. faecalis may express capsular polysaccharides more typical of gram-positive bacteria such as pneumococci that may leave LTA inaccessible to opsonic antibodies against this antigen.
In conclusion, we have shown that the target of opsonic antibodies in E. faecalis strain 12030 is LTA. The previously described TA-like molecule is structurally and immunologically identical to the immunodominant portion of LTA. Future work is needed to address the basis of serodiversity in enterococci and the binding epitopes of LTA-specific antibodies.
Acknowledgments
We thank Regina Engel and Heiko Kässner for their technical assistance and Ulrich Zähringer and Gerald Pier for helpful discussion.
J.H. was supported by an NIH/NIAID grant (R01 AI 50667) and Z.K. by grants from the Research Center Borstel and University of Gdansk (BW-8000-5-0290-6).
Editor: V. J. DiRita
REFERENCES
- 1.Bligh, E. G., and W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911-917. [DOI] [PubMed] [Google Scholar]
- 2.Cieslewicz, M. J., D. L. Kasper, Y. Wang, and M. R. Wessels. 2001. Functional analysis in type Ia group B Streptococcus of a cluster of genes involved in extracellular polysaccharide production by diverse species of streptococci. J. Biol. Chem. 276:139-146. [DOI] [PubMed] [Google Scholar]
- 3.Doran, K. S., E. J. Engelson, A. Khosravi, H. C. Maisey, I. Fedtke, O. Equils, K. S. Michelsen, M. Arditi, A. Peschel, and V. Nizet. 2005. Blood-brain barrier invasion by group B Streptococcus depends upon proper cell-surface anchoring of lipoteichoic acid. J. Clin. Investig. 115:2499-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubios, M., K. A. Gilles, J. K. Hamilton, P. A. Reabers, and F. Smith. 1956. Colorimetric method for the determination of sugars and related substances. Anal. Chem. 28:350-356. [Google Scholar]
- 5.European Antimicrobial Resistance Surveillance System. 2004. EARSS annual report 2004. [Online.] http://www.rivm.nl/earss/result/Monitoring_reports/.
- 6.Fabretti, F., C. Theilacker, L. Baldessarri, Z. Kaczynski, A. Kropec, O. Holst, and J. Huebner. 2006. Alaninie esters of enterococcal lipoteichoic acid play a role in biofilm formation and resistance to antimicrobial peptides. Infect. Immun. 74:4164-4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fattom, A., S. Shepherd, and W. Karakawa. 1992. Capsular polysaccharide serotyping scheme for Staphylococcus epidermidis. J. Clin. Microbiol. 30:3270-3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerwig, G. J., J. P. Kamerling, and J. F. Vliegenthart. 1979. Determination of the absolute configuration of mono-saccharides in complex carbohydrates by capillary G.L.C. Carbohydr. Res. 77:10-17. [DOI] [PubMed] [Google Scholar]
- 9.Gross, M., S. E. Cramton, F. Gotz, and A. Peschel. 2001. Key role of teichoic acid net charge in Staphylococcus aureus colonization of artificial surfaces. Infect. Immun. 69:3423-3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hancock, L. E., and M. S. Gilmore. 2002. The capsular polysaccharide of Enterococcus faecalis and its relationship to other polysaccharides in the cell wall. Proc. Natl. Acad. Sci. USA 99:1574-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haseley, S. R., O. Holst, and H. Brade. 1997. Structural and serological characterisation of the O-antigenic polysaccharide of the lipopolysaccharide from Acinetobacter haemolyticus strain ATCC 17906. Eur. J. Biochem. 244:761-766. [DOI] [PubMed] [Google Scholar]
- 12.Henneke, P., S. Morath, S. Uematsu, S. Weichert, M. Pfitzenmaier, O. Takeuchi, A. Muller, C. Poyart, S. Akira, R. Berner, G. Teti, A. Geyer, T. Hartung, P. Trieu-Cuot, D. L. Kasper, and D. T. Golenbock. 2005. Role of lipoteichoic acid in the phagocyte response to group B Streptococcus. J. Immunol. 174:6449-6455. [DOI] [PubMed] [Google Scholar]
- 13.Huebner, J., A. Quaas, W. A. Krueger, D. A. Goldmann, and G. B. Pier. 2000. Prophylactic and therapeutic efficacy of antibodies to a capsular polysaccharide shared among vancomycin-sensitive and -resistant enterococci. Infect. Immun. 68:4631-4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huebner, J., Y. Wang, W. A. Krueger, L. C. Madoff, G. Martirosian, S. Boisot, D. A. Goldmann, D. L. Kasper, A. O. Tzianabos, and G. B. Pier. 1999. Isolation and chemical characterization of a capsular polysaccharide antigen shared by clinical isolates of Enterococcus faecalis and vancomycin-resistant Enterococcus faecium. Infect. Immun. 67:1213-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hufnagel, M., L. E. Hancock, S. Koch, C. Theilacker, M. S. Gilmore, and J. Huebner. 2004. Serological and genetic diversity of capsular polysaccharides in Enterococcus faecalis. J. Clin. Microbiol. 42:2548-2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaczynski, Z., G. Karapetyan, A. Evidente, N. S. Iacobellis, and O. Holst. 2006. The structure of a putative exopolysaccharide of Burkholderia gladioli pv. agaricicola. Carbohydr Res. 341:285-288. [DOI] [PubMed] [Google Scholar]
- 17.Kessler, R. E., and G. D. Shockman. 1979. Enzymatic deacylation of lipoteichoic acid by protoplasts of Streptococcus faecium (Streptococcus faecalis ATCC 9790). J. Bacteriol. 137:1176-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kessler, R. E., I. van de Rijn, and M. McCarty. 1979. Characterization and localization of the enzymatic deacylation of lipoteichoic acid in group A streptococci. J Exp. Med. 150:1498-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laferriere, C. A., R. K. Sood, J. M. de Muys, F. Michon, and H. J. Jennings. 1998. Streptococcus pneumoniae type 14 polysaccharide-conjugate vaccines: length stabilization of opsonophagocytic conformational polysaccharide epitopes. Infect. Immun. 66:2441-2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lowry, O. H., N. R. Roberts, K. Y. Leiner, M. L. Wu, and L. Farr. 1954. The quantative histochemistry of the brain. I. Chemical methods. J. Biol. Chem. 207:1-17. [PubMed] [Google Scholar]
- 21.Maira-Litran, T., A. Kropec, D. A. Goldmann, and G. B. Pier. 2005. Comparative opsonic and protective activities of Staphylococcus aureus conjugate vaccines containing native or deacetylated staphylococcal poly-N-acetyl-beta-(1-6)-glucosamine. Infect. Immun. 73:6752-6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDaniel, L. S., W. D. Waltman II, B. Gray, and D. E. Briles. 1987. A protective monoclonal antibody that reacts with a novel antigen of pneumococcal teichoic acid. Microb. Pathog. 3:249-260. [DOI] [PubMed] [Google Scholar]
- 23.Morath, S., A. Geyer, and T. Hartung. 2001. Structure-function relationship of cytokine induction by lipoteichoic acid from Staphylococcus aureus. J. Exp. Med. 193:393-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Nosocomial Infections Surveillance. 2004. National Nosocomial Infections Surveillance (NNIS) system report, data summary from January 1992 through June 2004. Am. J. Infect. Control 32:470-485. [DOI] [PubMed] [Google Scholar]
- 25.Neuhaus, F. C., and J. Baddiley. 2003. A continuum of anionic charge: structures and functions of d-alanyl-teichoic acids in gram-positive bacteria. Microbiol. Mol. Biol. Rev. 67:686-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pier, G. B., D. Boyer, M. Preston, F. T. Coleman, N. Llosa, S. Mueschenborn-Koglin, C. Theilacker, H. Goldenberg, J. Uchin, G. P. Priebe, M. Grout, M. Posner, and L. Cavacini. 2004. Human monoclonal antibodies to Pseudomonas aeruginosa alginate that protect against infection by both mucoid and nonmucoid strains. J. Immunol. 173:5671-5678. [DOI] [PubMed] [Google Scholar]
- 27.Pier, G. B., G. J. Small, and H. B. Warren. 1990. Protection against mucoid Pseudomonas aeruginosa in rodent models of endobronchial infections. Science 249:537-540. [DOI] [PubMed] [Google Scholar]
- 28.Robbins, J. B., R. Schneerson, and S. C. Szu. 1995. Hypothesis: serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J Infect. Dis. 171:1387-1398. [DOI] [PubMed] [Google Scholar]
- 29.Sadovskaya, I., E. Vinogradov, J. Li, and S. Jabbouri. 2004. Structural elucidation of the extracellular and cell-wall teichoic acids of Staphylococcus epidermidis RP62A, a reference biofilm-positive strain. Carbohydr. Res. 339:1467-1473. [DOI] [PubMed] [Google Scholar]
- 30.Takeda, S., G. B. Pier, Y. Kojima, M. Tojo, E. Muller, T. Tosteson, and D. A. Goldmann. 1991. Protection against endocarditis due to Staphylococcus epidermidis by immunization with capsular polysaccharide/adhesin. Circulation 84:2539-2546. [DOI] [PubMed] [Google Scholar]
- 31.Theilacker, C., F. T. Coleman, S. Mueschenborn, N. Llosa, M. Grout, and G. B. Pier. 2003. Construction and characterization of a Pseudomonas aeruginosa mucoid exopolysaccharide-alginate conjugate vaccine. Infect. Immun. 71:3875-3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Theilacker, C., W. A. Krueger, A. Kropec, and J. Huebner. 2004. Rationale for the development of immunotherapy regimens against enterococcal infections. Vaccine 22(Suppl. 1):S31-S38. [DOI] [PubMed] [Google Scholar]
- 33.Wang, Y., J. Huebner, A. O. Tzianabos, G. Martirosian, D. L. Kasper, and G. B. Pier. 1999. Structure of an antigenic teichoic acid shared by clinical isolates of Enterococcus faecalis and vancomycin-resistant Enterococcus faecium. Carbohydr. Res. 316:155-160. [DOI] [PubMed] [Google Scholar]
- 34.Wicken, A. J., and J. Baddiley. 1963. Structure of intracellular teichoic acids from group D streptococci. Biochem. J. 87:54-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu, Y., L. Jiang, B. E. Murray, and G. M. Weinstock. 1997. Enterococcus faecalis antigens in human infections. Infect. Immun. 65:4207-4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yokoyama, Y., and Y. Harabuchi. 2002. Decreased serum and pharyngeal antibody levels specific to streptococcal lipoteichoic acid in children with recurrent tonsillitis. Int. J. Pediatr. Otorhinolaryngol. 63:199-207. [DOI] [PubMed] [Google Scholar]