Abstract
Toxoplasma gondii induces a persistent central nervous system infection, which may be lethally reactivated in AIDS patients with low CD4 T-cell numbers. To analyze the role of CD4 T cells for the regulation of parasite-specific CD8 T cells, mice were infected with transgenic T. gondii expressing the CD8 T-cell antigen β-galactosidase (β-Gal). Depletion of CD4 T cells prior to infection did not affect frequencies of β-Gal876-884-specific (consisting of residues 876 to 884 of β-Gal) CD8 T cells but resulted in a pronounced reduction of intracerebral β-Gal-specific gamma interferon (IFN-γ)-producing and cytolytic CD8 T cells. After cessation of anti-CD4 treatment a normal T. gondii-specific CD4 T-cell response developed, but IFN-γ production of intracerebral β-Gal-specific CD8 T cells remained impaired. The important supportive role of CD4 T cells for the optimal functional activity of intracerebral CD8 T cells was also observed in mice that had been depleted of CD4 T cells during chronic toxoplasmosis. Reinfection of chronically infected mice that had been depleted of CD4 T cells during either the acute or chronic stage of infection resulted in an enhanced proliferation of β-Gal-specific IFN-γ-producing splenic CD8 T cells. However, reinfection of chronically infected mice that had been depleted of CD4 T cells in the acute stage of infection did not reverse the impaired IFN-γ production of intracerebral CD8 T cells. Collectively, these findings illustrate that CD4 T cells are not required for the induction and maintenance of parasite-specific CD8 T cells but, depending on the stage of infection, the infected organ and parasite challenge infection regulate the functional activity of intracerebral CD8 T cells.
In acute infections, primary CD8-T-cell responses are largely independent of CD4-T-cell help, whereas memory CD8 T cells and secondary CD8-T-cell responses to pathogen challenge in CD4-T-cell-deficient hosts decrease over time (3). In contrast to acute self-limited infections, the role of CD4 T cells for the induction, generation, and maintenance of functionally active pathogen-specific CD8 T cells is less well defined in persisting infections, although the reactivation of persistent intracellular infections in patients with disturbed CD4-T-cell responses, e.g., in AIDS patients, may partially be caused by impaired CD8-T-cell responses.
One of these reactivated infections is Toxoplasma encephalitis (TE) which may manifest in AIDS patients with low CD4-T-cell numbers (21, 24). In mice, oral ingestion of the obligate intracellular protozoal parasite induces an acute systemic infection followed by a chronic stage, during which the infection is confined to the brain (28). The course of TE is strongly dependent on the genetic background of mice, and T. gondii-resistant mice survive the infection, whereas susceptible mice succumb to a chronic progressive TE (4, 9). Control of intracerebral (i.c.) toxoplasms is partially mediated by parasite-specific antibodies (2, 12, 16, 17) and critically depends on gamma interferon (IFN-γ)-producing CD4 and CD8 T cells (12, 34). In contrast, perforin expression by CD8 T cells plays only a limited role for the control of i.c. toxoplasms (10). In T. gondii-susceptible mice a simultaneous depletion of CD4 and CD8 T cells results in a lethal exacerbation of chronic TE, whereas depletion of either CD4 or CD8 T cells does not increase mortality (12, 13). However, after cessation of anti-CD4 treatment, the reappearance of CD4 T cells is paralleled by an increased i.c. inflammation causing death of the mice (12, 13, 35).
In contrast to chronic toxoplasmosis, CD4-deficient mice die from an acute toxoplasmosis due to a defective parasite-specific antibody response (2, 16). In contrast, depletion of CD4 T cells in chronically infected mice does not affect parasite-specific antibody titers (35).
In acute toxoplasmosis of CD4−/− mice, activation of systemic CD8 T cells is normal although there is some evidence that systemic T. gondii-specific CD8 T cells produce less IFN-γ and are not effectively maintained (6, 16). However, a major technical limitation of some previous studies was the lack of a defined T. gondii-specific CD8-T-cell epitope which would allow a function-independent quantitation of parasite-specific CD8 T cells by tetramers. In addition, it is currently unknown whether an acquired CD4 deficiency impacts on established T. gondii-specific CD8-T-cell responses and whether CD4 T cells differentially regulate i.c. and systemic T. gondii-specific CD8-T-cell responses.
In order to address these open questions and to explore whether secondary CD8-T-cell responses are regulated by CD4 T cells at the very beginning of the infection as observed for nonpersisting infections, T. gondii-resistant mice were infected with a β-galactosidase (β-Gal) transgenic T. gondii strain, which induces a strong β-Gal876-884-specific (consisting of residues 876 to 884 of β-Gal) CD8 T cell response (19). Since toxoplasmosis is clinically particularly important in patients with dynamically changing CD4-T-cell frequencies, CD4-T-cell depletion experiments were performed. These experiments revealed that CD4 T cells regulate the functional activity of i.c. CD8 T cells in both the acute and chronic stages of disease, while the frequency of parasite-specific CD8 T cells was not affected. Upon reinfection, CD4 T cells restricted the proliferation of parasite-specific CD8 T cells irrespective of CD4-T-cell depletion either during the acute or chronic stage of infection. Clinically important, after cessation of CD4-T-cell depletion during acute infection, the repopulation of the CD4-T-cell compartment at later stages of infection was accompanied by the development of T. gondii-specific CD4 T cells.
MATERIALS AND METHODS
Animals.
Female CB6 (BALB/c × C57BL/6; H-2db) mice (6 to 8 weeks old) were purchased from Harlan-Winkelmann (Borchen, Germany). All animals were kept under conventional conditions in an isolation facility throughout the experiments. Experiments were approved and supervised by local governmental institutions.
Parasites and T. gondii infection.
Strain RH toxoplasms were grown in vitro in L929 fibroblasts in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum (FCS), penicillin (100 U/ml), and streptomycin (100 μg/ml) at 37°C and 5% CO2. Parasites were harvested from freshly lysed fibroblasts by centrifuging the tissue culture medium at 50 × g. The supernatant was passed through a 5-μm-pore-size syringe filter. Thereafter, RH toxoplasms were washed three times in 0.1 M phosphate-buffered saline (PBS) (20 min at 400 × g), counted microscopically, and heat killed at 65°C for 20 min. Heat-killed toxoplasms (HKT) were stored at −80°C until use. To infect mice with T. gondii, cysts of the low-virulent PruS1SPLACZ strain of T. gondii, which secretes an Ld-restricted CD8-T-cell epitope (TPHPARIGL) of β-Gal under control of the tachyzoite-specific SAG1 promoter (19), were harvested from the brains of chronically infected mice. Brain tissue of these animals containing approximately 50 cysts per total mouse brain was dispersed in 0.1 M PBS (pH 7.4). The final concentration of the infectious agents was adjusted to a dose of 5 cysts/0.5 ml, which was applied to the animals by gavage.
Isolation of splenocytes and cerebral leukocytes.
At the indicated days postinfection (p.i.), animals were anesthetized with Metofane (Janssen, Neuss, Germany) and intracardially perfused with 0.9% NaCl to remove contaminating intravascular leukocytes from the brain. Splenic leukocytes were isolated by passing spleens through a 70-μm-pore-size cell strainer (BD Biosciences, Heidelberg, Germany), and erythrocytes were lysed with ammonium chloride. Cerebral leukocytes were isolated from the brains as described previously (27). In brief, brain tissue was minced through a 100-μm-pore-size cell strainer, and leukocytes were separated by Percoll gradient centrifugation (Amersham-Pharmacia, Freiburg, Germany).
Generation of MHC-tetramer reagents.
The major histocompatibility complex (MHC)-peptide tetrameric complex Ld/β-Gal876-884 was generated as described before (19). Briefly, recombinant Ld heavy chain and β2-microglobulin were expressed as insoluble inclusion bodies in Escherichia coli and were further purified. The purified Ld heavy chain was refolded in vitro in the presence of a high concentration of the synthetic peptide TPHPARIGL (β-Gal876-884) to form stable and soluble MHC-peptide complexes and specifically biotinylated in vitro by adding the enzyme BirA (Avidity, Denver, Co), D-biotin, and ATP. Complexes were purified by gel filtration over a Superdex 200 HR column (Amersham-Pharmacia, Freiburg, Germany). Purified biotinylated MHC-peptide complexes were multimerized with streptavidin-phycoerythrin (PE; Molecular Probes). Tetrameric complexes were buffer exchanged with PBS containing 0.02% sodium azide, 1 μg/ml pepstatin, 1 μg/ml leupeptin, and 0.5 mM EDTA and adjusted to a concentration of 2 mg/ml by ultrafiltration. Peptides were supplied by Jerini (Berlin, Germany). A control tetramer with the Ld-restricted epitope (amino acids 118 to 126) of the nucleoprotein of the lymphocytic choriomeningitis virus was produced by the same procedure. Peptides were supplied by Jerini.
Flow cytometry analysis.
Brain-derived and splenic leukocytes were stained with rat anti-mouse CD4-fluorescein isothiocyanate (FITC) (clone RM4-5) and rat anti-mouse CD8-PE (clone 53-6.7). CD25 expression on CD4 T cells was detected by staining with rat anti-mouse CD25-FITC (clone PC61) and rat anti-mouse CD4-PE. The kinetics of β-Gal-specific CD8 T cells were determined by costaining isolated splenic and cerebral leukocytes with CD8-FITC and tetramer Ld/β-Gal876-884-PE, respectively. To analyze the activation state of β-Gal-specific CD8 T cells, splenic and cerebral T cells were costained with a rat anti-mouse CD8-cychrome (clone 53-6.7), Ld/β-Gal876-884-PE, and either rat anti-mouse CD62L-FITC (clone MEL-14) or rat anti-mouse CD44-FITC (clone IM7). Control staining was performed with a tetramer specific for an Ld-restricted epitope (amino acids 118 to 126) of the nucleoprotein of the lymphocytic choriomeningitis virus and isotype-matched control antibodies. All antibodies were obtained from Becton-Dickinson (Heidelberg, Germany). Flow cytometry was performed on a FACScan (Becton-Dickinson) instrument, and the data were analyzed with WinMDI or Cell Quest software.
Determination of IFN-γ and TNF production of β-Gal876-884-specific CD8 T cells.
Brain- and spleen-derived leukocytes were incubated with β-Gal876-884 peptide (10−7 M) and Golgi-Plug (1 μl/ml; Becton-Dickinson) containing brefeldin A in minimal essential medium alpha (MEM-α) for 3 h at 37°C. Thereafter, cells were stained with rat anti-mouse CD8-FITC. After fixation and permeabilization with cytofix/cytoperm (Becton-Dickinson), cells were stained with either rat-anti-mouse IFN-γ-PE or rat anti-mouse tumor necrosis factor (TNF)-PE (both from Becton-Dickinson). Controls included staining of unstimulated cells with anti-IFN-γ-PE or anti-TNF-PE in combination with CD8-FITC or staining with isotype-matched control antibodies.
ELISPOT assay.
The frequency of T. gondii-specific CD4 T cells in the spleen was analyzed by use of an IFN-γ enzyme-linked immunospot (ELISPOT) assay at the indicated time points p.i. CD4 T cells were isolated from spleens by magnetic cell sorting employing rat anti-mouse CD4-FITC and anti-FITC microbeads (Milteny, Bergisch Gladbach, Germany). Purity of isolated CD4 T cells was >90% as determined by flow cytometry. CD4 T cells at concentrations of 2 × 103 cells/well, 2 × 104 cells/well, and 2 × 105 cells/well, respectively, were placed in an ELISPOT plate coated with rat anti-mouse IFN-γ monoclonal antibody (Biosource, Camarilla, CA). Purified CD4 T cells were restimulated with syngeneic B6C spleen cells from noninfected mice (4 × 105 cells/well) preloaded with HKT (2 parasites/5 leukocytes). All ELISPOT plates were incubated overnight and developed with biotin-labeled rat anti-mouse IFN-γ, peroxidase-conjugated streptavidin, and amino-ethylcarbazole dye solution (Sigma, Deisenhofen, Germany). Controls included incubation of cells without HKT. The frequency of antigen-specific cells was calculated from triplicate wells as the number of spots per leukocyte seeded.
CD4-T-cell depletion and anti-CD25 treatment.
For depletion of CD4 T cells, mice were treated with rat anti-mouse CD4 (clone GK1.5), and for depletion of CD25+ cells mice were treated with rat anti-mouse CD25 (clone PC61) antibodies. Antibodies were purified from tissue culture supernatants by protein G chromatography, adjusted to a concentration of 2.5 mg/ml in 0.1 M PBS, sterile filtered, and stored at −80°C until used. Control mice were treated with rat immunoglobulin G (IgG) (Sigma). Anti-CD4 antibodies were injected intraperitoneally at a concentration of 0.5 mg/ml per mouse at the indicated time points p.i. For the first 3 days of treatment, antibodies were injected daily. Thereafter, anti-CD4 antibodies were injected every third day. Efficacy of CD4-T-cell depletion was >95%, and for CD25+ cell depletion, efficacy was >90%, as controlled by flow cytometry. For flow cytometry, the anti-CD4 RM4-4 clone, which is noncompetitive to the CD4-T-cell-depleting GK1.5 clone, was used.
BrdU experiments.
The drinking water of infected mice was supplemented with bromodeoxyuridine (BrdU; 2.0 mg/ml; Sigma) at the indicated days p.i. Water containing BrdU was prepared each day. One day after the termination of BrdU treatment, leukocytes were isolated from the spleen. Cells were stained with rat anti-mouse CD8-CyCrome and tetramer β-Gal876-884-PE, fixed permeabilized with 4% formaldehyde and 0.1% Triton X-100 in 0.1 M PBS, and stained with mouse anti-BrdU-FITC (Becton-Dickinson). Control staining was performed with isotype-matched control antibodies. Cells were analyzed by flow cytometry.
Measurement of cytotoxic T-lymphocyte activity.
Leukocytes were isolated from the spleens and brains of mice infected with PruS1SPLACZ at various time points after infection as indicated in Results. P815 (H-2d) cells were used as target cells and were coated with 10−7 M β-Gal876-884 peptide in MEM-α supplemented with 10% FCS at 37°C and 5% CO2. During the last hour of peptide incubation, P815 cells were labeled with 51Cr (100 μCi/1 × 106 cells) (Amersham-Pharmacia). Thereafter, target cells were washed three times with MEM-α supplemented with 10% FCS to remove unbound peptide and extracellular 51Cr. Isolated leukocytes and target cells were incubated at effector/target ratios of 200:1, 100:1, 30:1, 10:1, 3:1, 1:1, and 0.3:1 in triplicate. After incubation at 37°C with 5% CO2 for 5 h, 100 μl of the supernatant from each well was collected and the released 51Cr was counted in a gamma counter (Beckmann, Munich, Germany). The specific 51Cr release was calculated according to the following formula: 100 × [(amount in test release − amount in spontaneous release)/(amount in maximal release − amount in spontaneous release)], where test release was in the presence of effector cells, spontaneous release was in the presence of medium alone, and maximal release was in the presence of detergent.
Determination of anti-T. gondii IgM and IgG production.
Blood and cerebrospinal fluid (CSF) were obtained from uninfected and T. gondii-infected mice by puncture of the retro-orbital plexus and the cisterna magna as described previously. Anti-T. gondii-specific IgM and IgG antibodies were determined in 10-fold serially diluted sera and CSF by incubation in 96-well microtiter plates coated with T. gondii antigen (DADE Behring, Marburg, Germany). After incubation at 37°C for 2 h, microtiter plates were washed with PBS four times and incubated with biotinylated goat anti-mouse IgM and IgG, respectively (all from Sigma), at room temperature for 1.5 h. Plates were washed with PBS six times and incubated with alkaline phosphatase-conjugated ExtrAvidin (Sigma) for 60 min at 37°C. Thereafter, plates were washed with PBS six times, and AttoPhos substrate (Roche, Mannheim, Germany) was added and fluorescence was measured with a fluorimeter (Fluoroscan II; Labsystems, Helsinki, Finland). The highest positive dilution of sera and CSF from T. gondii-infected mice was determined, and values were considered as positive if they were twofold above the corresponding dilution of noninfected mice.
T. gondii immunohistochemistry.
For immunohistochemistry on frozen sections, spleens and brains of three animals per group were dissected, and blocks were mounted on thick filter paper with Tissue-Tek O.T.C. Compund (Miles Scientific, Naperville, IL), snap-frozen in isopentane (Fluka, Neu-Ulm, Germany), precooled on dry ice, and stored at −80°C. Immunohistochemistry was performed on 10-μm frozen sections as described previously (28). In brief, for the detection of T. gondii, an indirect immunoperoxidase protocol using a polyclonal rabbit anti-T. gondii antiserum (Biogenex, Duiven, The Netherlands) as primary antibody (Ab) and goat anti-rabbit IgG F(ab′)2 (Amersham, Freiburg, Germany) as secondary Ab was employed. Peroxidase reaction products were visualized using 3,3′-diaminobenzidine (Sigma) and H2O2 as cosubstrate. Sections were lightly counterstained with hemalum (Merck, Darmstadt, Germany). Numbers of T. gondii cells were determined microscopically on immunostained brain and spleen sections by counting 100 randomly selected high-power fields (40× objective) per mouse. The mean ± standard deviation (SD) of three mice per experimental group was determined.
Statistics.
Numbers of T. gondii cells and absolute numbers of tetramer-positive and IFN-γ-producing cells of spleen and brain were statistically analyzed by a Student's t test. A P value of <0.05 was accepted as significant.
RESULTS
The induction, frequency, and recruitment of T. gondii-specific CD8 T cells is independent of CD4 T cells in acute toxoplasmosis.
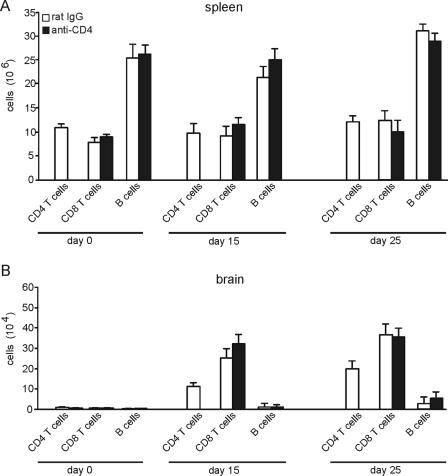
A depletion of CD4 T cells prior to the infection continued up to day 30 p.i. resulted in the absence of CD4 T cells in the spleen with an efficacy of more than 98% (Fig. 1A). In addition, depletion of peripheral CD4 T cells resulted in the complete absence of this cell population in the T. gondii-infected brain. CD4-T-cell-depleted mice recruited similar numbers of CD8 T cells and B cells into the parasite-infected brain (Fig. 1B), illustrating that recruitment of these cell populations is CD4 T cell independent.
FIG. 1.
Quantitation of splenic and i.c. T and B cells in acute toxoplasmosis. Spleen cells (A) and i.c. leukocytes (B) were isolated from noninfected mice and T. gondii-infected mice at days 15 and 25 p.i. Isolated leukocytes from rat IgG-treated (open bars) and anti-CD4-treated (filled bars) mice were stained for CD4 and CD8 T cells as well as for B cells. Data in both panels are the means ± SD from 6 mice per experimental group. CD4-T-cell depletion was initiated 3 days before infection and performed until day 30 p.i.
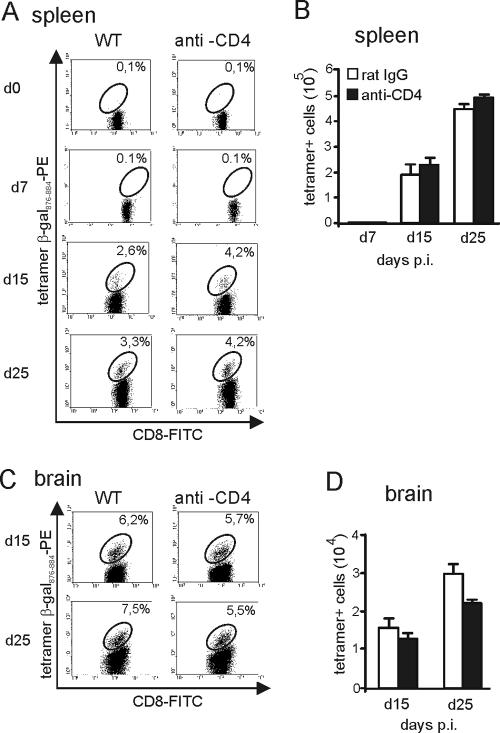
To determine the impact of CD4 T cells on the induction and frequency of splenic and i.c. T. gondii-specific CD8 T cells, the percentage of tetramer β-Gal876-884-positive cells within the CD8-T-cell population and the absolute number of β-Gal876-884-specific CD8 T cells were determined by tetramer staining after infection with β-Gal-transgenic T. gondii. In accordance with previously published data (19), β-Gal876-884-specific CD8 T cells were neither detected in noninfected mice (day 0) nor at day 7 p.i. (Fig. 2A and B). Although the relative number of T. gondii-specific CD8 T cells was slightly increased in the spleens of anti-CD4-treated mice compared to the rat IgG-treated control group at days 15 and 25 p.i. (Fig. 2A), absolute numbers of tetramer-positive CD8 T cells were identical at both days p.i. (P > 0.05 for day 15 and 25 p.i.) (Fig. 2B). In addition, in both groups, the absolute number of tetramer-positive cells significantly increased from day 15 to 25 p.i. (P < 0.005 for both groups) (Fig. 2B).
FIG. 2.
Frequency of β-Gal-specific CD8 T cells in acute toxoplasmosis. Mice were infected with a β-Gal-secreting T. gondii strain. The frequency of β-Gal876-884-specific CD8 T cells was determined by tetramer staining and flow cytometry. The percentage of β-Gal876-884 tetramer-positive specific CD8 T cells in spleen (A) and brain (C) is illustrated at the indicated time points p.i. In panel A, uninfected mice correspond to day 0. The absolute numbers of β-Gal tetramer-positive specific CD8 T cells in spleen (B) and brain (D) is shown at the indicated time points p.i. In panels A to D, 6 mice were analyzed per group. CD4-T-cell depletion was initiated 3 days before infection and performed until day 30 p.i.
In anti-CD4-treated and rat IgG-treated mice, absolute numbers of tetramer-positive cells increased in the brain from day 15 to 25 p.i. (P < 0.05 for both groups), and both experimental groups harbored similar relative and absolute numbers of β-Gal876-884-specific CD8 T cells in the brain at days 15 and 25 p.i. (for absolute numbers, P > 0.05 anti-CD4 versus rat IgG at days 15 and 25 p.i.) (Fig. 2C and D). These findings indicate that the induction, kinetics, and frequencies of T. gondii-specific CD8 T cells in brain and spleen were largely independent of CD4 T cells.
In accordance and extension of previously published data obtained in T. gondii-susceptible mice (1, 2, 16), CD4-T-cell-depleted T. gondii-resistant CB6 mice infected with β-Gal transgenic T. gondii had strongly reduced parasite-specific IgG and IgM titers in serum and CSF, and 80% of anti-CD4-treated mice succumbed to the infection up to day 30 p.i. due to an unrestricted parasite replication in the brain, whereas control Ab-treated mice developed a chronic TE with 100% survival (data not shown).
IFN-γ production and cytolytic activity of T. gondii-specific CD8 T cells are reduced in the brain but not in the spleen of CD4-T-cell-depleted mice.
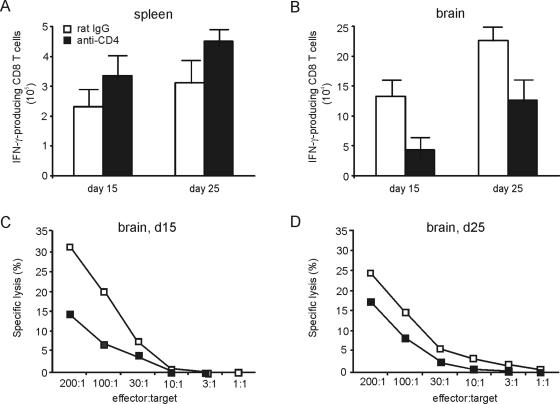
To determine whether CD4 T cells regulate the functional activity of T. gondii-specific CD8 T cells, the IFN-γ production and the cytotoxic activity of β-Gal-specific CD8 T cells were determined. Upon in vitro restimulation with β-Gal876-884 peptide, similar numbers of spleen-derived CD8 T cells from both rat IgG-treated and CD4-T-cell-depleted mice produced IFN-γ at days 15 and 25 p.i. (P > 0.05) (Fig. 3A). In contrast, significantly reduced numbers of i.c. CD8 T cells from anti-CD4-treated mice produced IFN-γ upon restimulation compared to control mice (P < 0.01 and P < 0.05 for day 15 and 25 p.i., respectively), although in both groups numbers of IFN-γ-producing CD8 T cells increased from day 15 to 25 p.i. (P < 0.01 for both groups) (Fig. 3B). At days 15 and 25 p.i., i.c. β-Gal876-884-specific CD8 T cells from mice with CD4-T-cell depletion also produced lower amounts of TNF (data not shown). Moreover, i.c. β-Gal-specific CD8 T cells of CD4-T-cell-depleted mice exerted a reduced ex vivo cytotoxic activity compared to rat IgG-treated mice at days 15 and 25 p.i. (56% and 30% reduction for anti-CD4 versus rat IgG at days 15 and 25, respectively) (Fig. 3C and D). In contrast to the brain, spleen-derived β-Gal-specific CD8 T cells of both experimental groups did not exhibit a direct ex vivo cytotoxic activity (data not shown). Collectively, these findings indicate that CD4 T cells are required to induce an optimal functional activity of T. gondii-specific CD8 T cells in the brain but not in the spleen.
FIG. 3.
Functional activity of T. gondii-specific CD8 T cells. IFN-γ production of β-Gal876-884-specific splenic (A) and i.c. (B) CD8 T cells was determined by flow cytometry. Data represent the means ± SD of results from 6 mice. CD4-T-cell depletion was initiated 3 days before infection and performed until day 30 p.i. The cytotoxic activity of i.c. β-Gal876-884-specific CD8 T cells was determined at days 15 (C) and 25 (D) p.i. by a 51Cr release assay with β-Gal876-884 peptide-loaded P815 target cells. Data represent the means of two groups of 3 mice each.
Normal development of T. gondii-specific effector and memory CD8 T cells in CD4-T-cell-depleted mice.
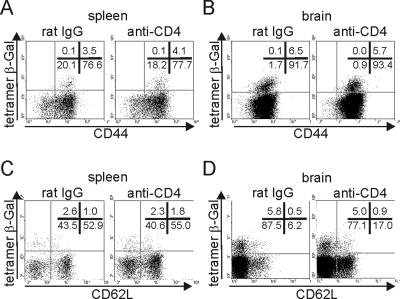
To determine whether CD4-T-cell depletion affects the development of T. gondii-specific effector and memory CD8 T cells, CD44 and CD62 expression of β-Gal-tetramer-positive and -negative CD8 T cells was determined. In both organs, all β-Gal876-884-specific CD8 T cells were CD44+ (Fig. 4A and B). In addition, the majority of β-Gal876-884-specific CD8 T cells was CD62L− in all groups (Fig. 4C and D). However, in the spleen some β-Gal876-884-specific CD8 T cells were CD62L+ (Fig. 4C). Thus, a depletion of CD4 T cells did not influence the development of T. gondii-specific CD44high CD62L− effector and CD44high CD62L+ memory CD8 T cells in brain or spleen.
FIG. 4.
CD44 and CD62L expression of T. gondii-specific CD8 T cells. At day 25 p.i., the frequency of CD44 (A and B) and CD62L (C and D) and β-Gal tetramer-positive and -negative CD8 T cells was determined in spleen (A and C) and brain (B and D) by flow cytometry. Data represent events gated on CD8+ T cells. In each dot plot, the percentage of positive cells for each quadrant is included. Six mice per group were analyzed.
Short-term depletion of CD4 T cells in acute toxoplasmosis results in normal frequencies of T. gondii-specific CD8 T cells and sustained functionally impaired intracerebal CD8 T cells in chronic toxoplasmosis.
To analyze whether the development of T. gondii-specific CD4 T cells and the persistence of β-Gal876-884-specific CD8 T cells require the presence of CD4 T cells from the very beginning of the infection or their presence only at later stages, CD4 T cells were depleted during the acute phase of disease until day 17 p.i. Thereafter, anti-CD4-treated and rat IgG-treated mice received sulfadiazine to prevent death of mice in the anti-CD4 group (day 17 to 30 p.i.). At day 30 p.i., mice of both groups had a mild TE without significant differences in their i.c. and splenic parasitic load (P > 0.05).
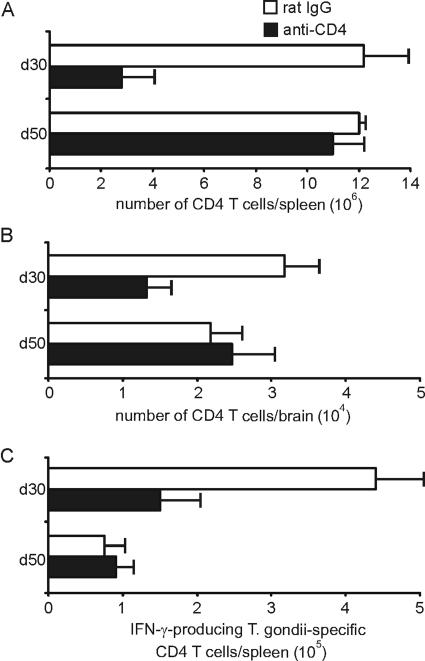
At day 30 p.i., the absolute numbers of CD4 T cells in spleen and brain were still reduced in the anti-CD4-treated group (P < 0.005 and P < 0.01 for spleen and brain, respectively) (Fig. 5A and B). However, at day 50 p.i., absolute numbers of splenic and i.c. CD4 T cells were completely restored in anti-CD4-treated mice and indistinguishable from control Ab-treated animals (P > 0.05 for both groups) (Fig. 5A and B). In addition, the frequency of splenic T. gondii-specific IFN-γ-producing CD4 T cells was reduced in anti-CD4-treated mice at day 30 p.i. (P < 0.01 p.i.) (Fig. 5C). However, at day 50 p.i. both groups of mice harbored equally low frequencies of T. gondii-specific IFN-γ-producing CD4 T cells (P > 0.05) (Fig. 5C).
FIG. 5.
Development of T. gondii-specific CD4 T cells after cessation of anti-CD4 treatment. After termination of anti-CD4 treatment at day 17 p.i. and sulfadiazine-treatment (day 17 to 30 p.i.), the numbers of CD4 T cells were determined in spleen (A) and brain (B) at days 30 and 50 p.i. Data represent the means ± SD from 6 mice per experimental group. (C) At days 30 and 50 p.i., the frequency of T. gondii-specific CD4 T cells was determined by an IFN-γ ELISPOT assay. In this assay, CD4 T cells were selectively isolated by magnetic cell sorting from the various groups of T. gondii-infected mice, and spleen cells of noninfected CB6 mice loaded with HKT served as APCs. Data represent the means ± SD of results from 3 mice per group and time point.
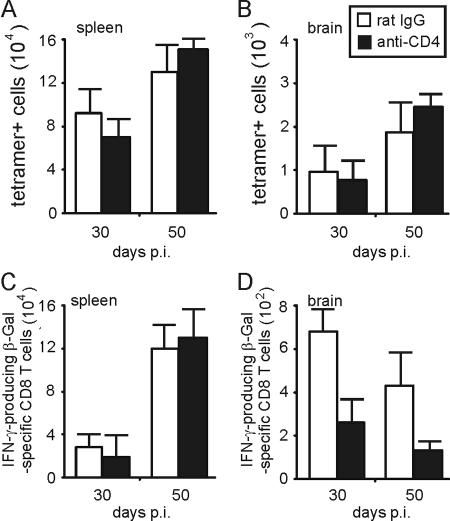
Numbers of splenic and i.c. tetramer β-Gal876-884-positive T. gondii-specific CD8 T cells were not significantly affected by anti-CD4 treatment, repopulation of the CD4-T-cell compartment, or expansion of T. gondii-specific CD4 T cells (P > 0.05 for anti-CD4 versus rat IgG application in spleen and brain at days 30 and 50 p.i.) (Fig. 6A and B). However, the frequency of i.c., but not splenic IFN-γ-producing β-Gal-specific CD8 T cells was consistently reduced in the anti-CD4-treated group at days 30 and 50 p.i. (P < 0.05 for spleen at days 30 and 50 p.i., P < 0.005 for brain at day 30, and P < 0.001 for brain at day 50 p.i.) (Fig. 6C and D). In contrast to the acute stage of disease, in the chronic phase of TE, i.c. β-Gal-specific CD8 T cells did not show a cytotoxic activity, irrespective of the experimental design (data not shown). At day 50 p.i., both groups still presented with a mild chronic TE without significant differences in the parasitic load as revealed by histopathology.
FIG. 6.
Frequency and IFN-γ production of T. gondii-specific CD8 T cells during repopulation of the CD4 T cell compartment. The frequency of splenic (A) and i.c. (B) β-Gal-specific CD8 T cells was determined by tetramer staining and flow cytometry in mice with anti-CD4 and rat IgG treatment, which was terminated at day 17 p.i. At days 30 and 50 p.i., the frequency of IFN-γ-producing β-Gal876-884-specific CD8 T cells was determined by flow cytometry in spleen (C) and brain (D). Data represent the means ± SD of results from 6 mice per group.
These findings illustrate that after cessation of CD4-T-cell depletion in the acute stage of infection, functionally active parasite-specific CD4 T cells develop at normal frequencies. In addition, in the chronic stage of infection, frequencies of parasite-specific CD8 T cells were not affected by CD4-T-cell depletion, while the functional activity of i.c. CD8 T cells did not recover in parallel to the reconstitution of the CD4-T-cell compartment.
Depletion of CD4 T cells in the chronic stage of infection results in a reduced frequency of IFN-γ-producing T. gondii-specific CD8 T cells.
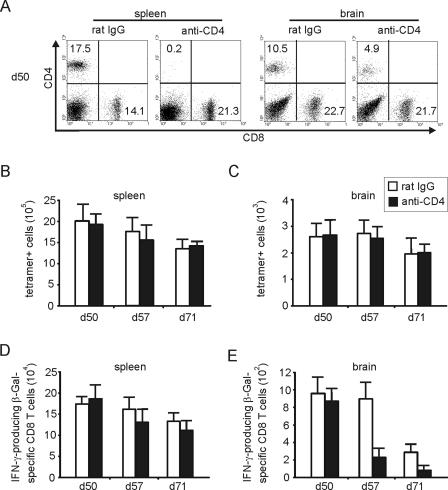
To further determine whether CD4 T cells regulate T. gondii-specific CD8 T cells that have been primed in the presence of CD4 T cells, CD4 T cells were depleted in chronically infected mice from day 50 to 71 p.i. At this stage of infection, administration of anti-CD4 Ab resulted in an efficient depletion of CD4 T cells in the spleen and caused a 50% reduction in the brain (Fig. 7A). The reduced capacity of the anti-CD4 Ab to deplete i.c. CD4 T cells is most probably explained by the limited capacity of the Ab to pass the blood-brain barrier and the low level of complement in the central nervous system. These findings are in accordance with previous observations illustrating that T cells are predominantly recruited to the central nervous system in acute TE and form a stable, nonproliferating cell pool in chronic TE (29).
FIG. 7.
Effect of CD4 depletion in chronically infected mice. CD4 T cells were depleted in chronically infected mice from day 50 to 71 p.i. (A) Flow cytometry of splenic and i.c. CD4 and CD8 T cells at day 50 p.i. The percentages of positive CD4 and CD8 T cells are shown in the respective quadrants. Cells of 3 mice per group and organ were analyzed. At days 50, 57, and 71 p.i., the frequency of β-Gal-specific CD8 T cells was determined by tetramer staining and flow cytometry in spleen (B) and brain (C). The absolute numbers of tetramer-positive CD8 T cells are shown as means ± SD from three spleens and brains per group. (D and E) At days 50, 57, and 71 p.i., the frequency of IFN-γ-producing β-Gal876-884-specific CD8 T cells was determined by flow cytometry in spleen (D) and brain (E). Data represent the means ± SD of results from 6 mice per group.
Depletion of CD4 T cells in the chronic stage of infection did not significantly alter frequencies of β-Gal-specific CD8 T cells in spleen and brain (P > 0.05 for brain and spleen at days 57 and 71 p.i., respectively) (Fig. 7B and C). In addition, the number of splenic IFN-γ producing β-Gal-specific CD8 T cells was not affected by CD4-T-cell depletion at days 57 and 71 p.i. (P > 0.05 at days 57 and 71 p.i.) (Fig. 7D). However, in the brain, the natural decline of parasite-specific IFN-γ producing β-Gal-specific CD8 T cells was more pronounced in CD4-T-cell-depleted animals, and anti-CD4-treated mice harbored only ∼25% of IFN-γ-producing β-Gal-specific CD8 T cells compared to nondepleted mice at days 57 and 71 p.i. (P < 0.05 for days 57 and 71 p.i.) (Fig. 7E). However, this reduction of i.c. IFN-γ-producing CD8 T cells was not sufficient to reactivate TE and to increase the i.c. parasite load (P > 0.05 for anti-CD4 versus rat IgG at day 71 p.i.) (data not shown).
Increased proliferation of T. gondii-specific CD8 T cells in reinfected mice depleted of CD4 T cells in the chronic or acute stage of infection.
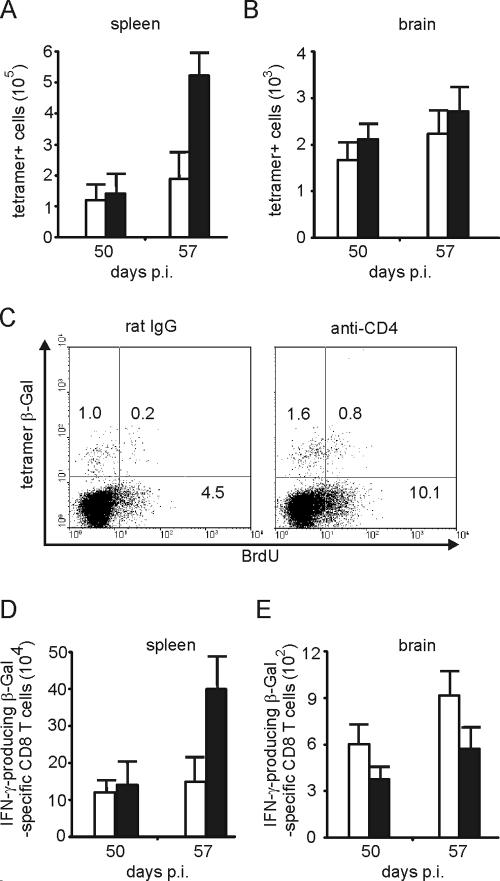
To delineate whether reinfection in the chronic stage of infection changes the impact of CD4 T cells on T. gondii-specific CD8 T cells primed in the presence or absence of CD4 T cells, chronically infected mice that had been depleted of CD4 T cells either in parallel to reinfection or in the acute stage of disease were orally reinfected with β-Gal transgenic T. gondii. For reinfection the same number of β-Gal transgenic parasites (5 cysts/mouse) was used.
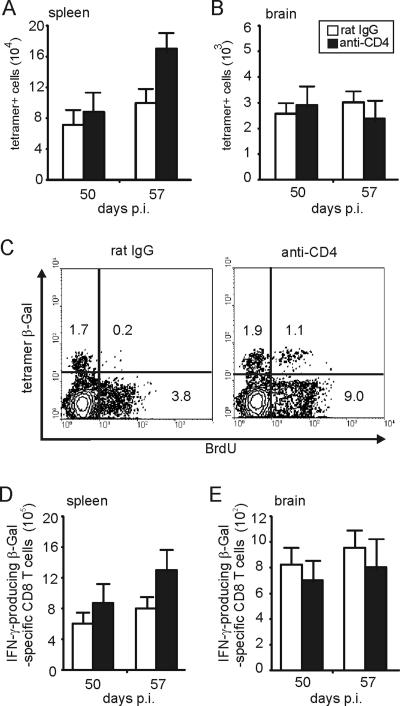
Combined CD4-T-cell depletion and low-dose reinfection, both initiated at day 50 p.i., resulted in a strong increase of β-Gal-specific CD8 T cells in the spleen at day 57 (P < 0.01 for anti-CD4 versus rat IgG at day 57 p.i.) (Fig. 8A). The rise of β-Gal-specific CD8 T cells in CD4-T-cell-depleted mice was accompanied by an increased BrdU incorporation of β-Gal tetramer-positive and -negative CD8 T cells compared to control Ab-treated animals (Fig. 8C). The increased number of splenic β-Gal-specific CD8 T cells in anti-CD4-treated animals was paralleled by a concomitant increase of splenic CD8 T cells producing IFN-γ after restimulation with β-Gal peptide, illustrating that functionally active CD8 T cells were induced upon reinfection in the absence of CD4 T cells (P < 0.05 for anti-CD4 versus rat IgG at day 57 p.i.) (Fig. 8D). In the brain, reinfection did not increase either the number of β-Gal887-884-specific CD8 T cells or the number of IFN-γ producing β-Gal-specific CD8 T cells in either experimental group from day 50 to 57 p.i. (P > 0.05 for tetramer-positive and IFN-γ-producing β-Gal-specific CD8 T cells in both groups of mice) (Fig. 8B and E). In addition, immunohistochemistry did not provide evidence for an increased splenic or i.c. parasitic load of CD4-T-cell-depleted animals at day 57 p.i. (P > 0.05 for brain and spleen) (data not shown).
FIG. 8.
Frequency and proliferation of splenic T. gondii-specific CD8 T cells in mice depleted of CD4 T cells in parallel to reinfection. At day 50 p.i., anti-CD4-treated and rat IgG-treated mice were orally reinfected with T. gondii (5 cysts/mouse). Both anti-CD4 and rat IgG treatment was initiated at day 47 p.i. At day 50 and 57 p.i., the frequency of β-Gal-specific CD8 T cells was determined by tetramer staining and flow cytometry in spleen (A) and brain (B). Data represent the means of results from 6 mice per group (A and B). Proliferation of CD8 T cells including β-Gal-specific CD8 T cells was determined by BrdU incorporation (C). Mice received BrdU from day 50 to 57 p.i. At day 57 p.i., the percentages of BrdU-positive CD8 T cells and β-Gal-specific CD8 T cells were determined by flow cytometry. Data are gated on CD8 T cells. Three mice per group were analyzed and representative data are shown. At days 50 and 71 p.i., the frequency of IFN-γ-producing β-Gal876-884-specific CD8 T cells was determined by flow cytometry in spleen (D) and brain (E). Data represent the means ± SD of results from 6 mice per group.
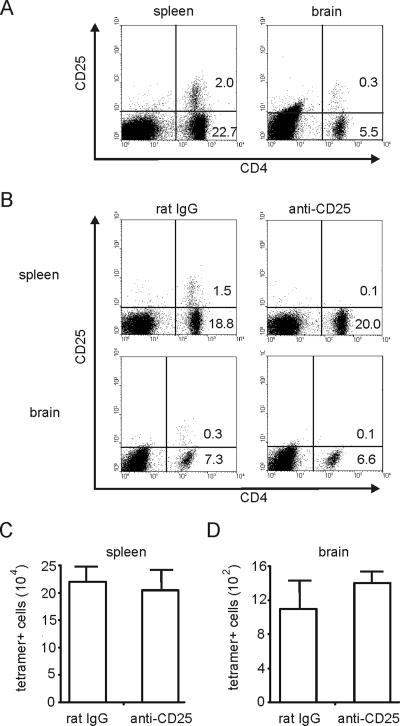
Since it has been shown previously that CD4 CD25+ regulatory T cells can limit the expansion of memory CD8 T cells (18), we determined whether the increased proliferation of CD8 T cells in mice that were reinfected and that had also been depleted of CD4 T cells was caused by a depletion of the CD4 CD25+-T-cell subset. At day 50 p.i., CD4 CD25+ T cells were present in spleen and brain of T. gondii-infected mice (Fig. 9A), and anti-CD25 treatment resulted in an efficient depletion of this T-cell subset in both organs (Fig. 9B). However, reinfection of CD25-depleted animals did not result in an increased frequency of β-Gal-specific CD8 T cells in the spleen or in the brain (P > 0.05 for brain and spleen) (Fig. 9C and D), indicating that the suppressive effect of CD4 T cells on the proliferation of CD8 T cells in reinfected mice is not mediated by CD4 CD25+ T cells.
FIG. 9.
Effect of anti-CD25 treatment on frequencies of T. gondii-specific CD8 T cells in reinfected mice. (A) At day 50 p.i., a subset of CD4 T cells is CD25+ in spleen and brain as determined by flow cytometry. The percentages of CD4+ C25− and CD4+ CD25+ cells are given in the respective quadrants. (B) Chronically infected mice were treated with either anti-CD25 or rat IgG from day 50 to 57 p.i. At day 57 p.i., the percentage of CD4+ and CD4+ CD25+ cells was determined by flow cytometry. (C and D) At day 57 p.i., the frequency of β-Gal-specific CD8 T cells was determined by tetramer staining and flow cytometry in spleen (C) and brain (D). In panels A and B, spleens and brains of 3 mice were analyzed. In panels C and D, data represent the means ± SD of results from 6 mice per group.
Reinfection of mice with CD8-T-cell priming in the absence of CD4 T cells also resulted in a strong increase of splenic tetramer-positive CD8 T cells, whereas the increase of tetramer-positive CD8 T cells was moderate in control Ab-treated animals (P < 0.005 for anti-CD4 versus rat IgG at day 57 p.i.) (Fig. 10A). In mice depleted of CD4 T cells before primary infection, the increase of β-Gal-specific CD8 T cells was paralleled by an increased incorporation of BrdU in both tetramer-positive (0.8%) as well as tetramer-negative CD8 T cells (10.1%) compared to non-CD4 T-cell-depleted mice (0.2% BrdU in tetramer-positive CD8 T cells; 4.5% BrdU in tetramer-negative CD8 T cells) (Fig. 10C). In the brains of both experimental groups, the number of β-Gal-specific CD8 T cells slightly increased within 7 days after reinfection without significant differences between the two groups (P > 0.05 at day 57 p.i.) (Fig. 10B). In anti-CD4-treated mice, reinfection induced the expansion of functionally active, IFN-γ-producing parasite-specific CD8 T cells in the spleen (P < 0.01 for anti-CD4 versus rat IgG at day 57 p.i.) (Fig. 10D). However, reinfection did not alter the impaired capacity of i.c. β-Gal-specific CD8 T cells to produce IFN-γ upon restimulation with β-Gal peptide (P < 0.05 for anti-CD4 versus rat IgG at days 50 p.i. and 57 p.i.) (Fig. 10E).
FIG. 10.
Frequency and proliferation of splenic T. gondii-specific CD8 T cells in reinfected mice that had been depleted of CD4 T cells during the acute stage of infection. Mice that had received either anti-CD4 or rat IgG treatment up to day 17 p.i. were orally reinfected with T. gondii (5 cysts/mouse) at day 50 p.i. At days 50 and 57 p.i., the frequency of β-Gal-specific CD8 T cells was determined by tetramer staining and flow cytometry in spleen (A) and brain (B) of 6 mice in each experimental group. (C) Proliferation of CD 8 T cells including β-Gal-specific CD8 T cells was determined by BrdU incorporation. Mice had received BrdU from day 50 to 57 p.i. At day 57 p.i., the percentages of BrdU-positive CD8 T cells and β-Gal-specific CD8 T cells were determined by flow cytometry. Data are gated on CD8 T cells. Three mice per group were analyzed, and representative data are shown. At days 50 and 57 p.i., the frequency of IFN-γ-producing β-Gal876-884-specific CD8 T cells was determined by flow cytometry in spleen (D) and brain (E). Data represent the means ± SD of results from 6 mice per group.
These findings indicate that (i) during reinfection CD4 T cells have a negative regulatory effect on the expansion of functionally active T. gondii-specific CD8 T cells irrespective of the time point of CD4 T cell depletion and that (ii) the i.c. T. gondii-specific CD8-T-cell response is largely unaffected by reinfection with the same strain of T. gondii.
DISCUSSION
These experiments were performed to determine the role of CD4 T cells in the regulation of CD8 T cells in the murine model of chronic toxoplasmosis providing four major findings. First, the frequency of T. gondii-specific CD8 T cells is independent of CD4-T-cell help in both acute and chronic toxoplasmosis. Second, upon reinfection CD4 T cells limit the proliferation of systemic parasite-specific CD8 T cells, irrespective of the time point of CD4-T-cell depletion. Third, even if CD4 T cells are completely absent at the onset of infection, a repopulation of the CD4-T-cell compartment is accompanied by the development of T. gondii-specific CD4 T cells. Fourth, CD4 T cells regulate the functional activity of i.c. CD8 T cells.
The frequencies of T. gondii-specific splenic and i.c. CD8 T cells in acute toxoplasmosis were equal in anti-CD4-treated and control Ab-treated mice, illustrating that the priming, expansion, and recruitment of these cells to the brain were CD4 T cell independent, which is in accordance with previous studies in acute toxoplasmosis of T. gondii-susceptible CD4−/− mice (6, 16). In addition, in most acute viral and bacterial infections, priming and expansion of pathogen-specific CD8 T cells is independent of CD4-T-cell help (5, 14, 22, 25, 31, 32), although a supportive role of CD4 T cells for primary pathogen-specific CD8-T-cell responses has also been described (22, 26). However, primary CD8-T-cell responses may not be completely cell autonomous, and a recent study in T. gondii-susceptible CD4-deficient mice provided some evidence that NK cells may be critical for the induction of T. gondii-specific CD8 T cells in the absence of CD4 T cells (7). The observation that frequencies of T. gondii-specific CD8 T cells in brain and spleen were not affected by depletion of CD4 T cells in the chronic stage of infection (Fig. 7) illustrates that the maintenance of T. gondii-specific CD8 T cells in lymphatic and parenchymatous target organs is also CD4 T cell independent. This is in contrast to findings in T. gondii-susceptible CD4−/− mice, which exhibited a reduced precursor CD8-T-cell frequency in the chronic stage of disease (6). However, assuming that the precursor CD8-T-cell frequency adequately measures the absolute numbers of parasite-specific CD8 T cells, one may envisage that in T. gondii-resistant and -susceptible mice, the importance of CD4-T-cell help for the maintenance of T. gondii-specific CD8 T cells is different. In addition, our observations are in marked contrast to acute nonpersisting infections, in which non-pathogen-specific CD4 T cells are required to maintain memory CD8 T cells after clearance of the infectious agent (33).
In contrast to nonpersisting bacterial and viral infections (31, 32), the frequencies of splenic and i.c. T. gondii-specific CD8 T cells in chronically infected mice were independent of the presence of CD4 T cells during the first 3 weeks of infection. Several mechanisms may compensate for CD4 deficiency in the acute stage, fostering T. gondii-specific CD8-T-cell responses. One factor is the persistence of Toxoplasma antigen, which may finally overcome defective CD4-T-cell help. Noteworthy, parasite load was equal in the brains of anti-CD4- and control Ab-treated mice at days 30 and 50 p.i. due to the antibiotic treatment performed from to day 17 to 30 p.i. in order to prevent death of CD4-depleted mice. Thus, stimulation by the persisting antigen per se instead of an increased exposure to antigen may compensate for the absence of CD4 help in acute toxoplasmosis.
In addition, the persistence of T. gondii is linked to the presence of T. gondii-specific CD44+ CD62L− effector CD8 T cells (reference 29 and this study). This may also contribute to the equal frequency of T. gondii-specific CD8 T cells independent of CD4 T cells during acute toxoplasmosis, since effector CD8 T cells are independent of CD4 help (3, 5, 25). In the brain, where T. gondii actively replicates continuously, more than 92% of β-Gal tetramer-positive and -negative CD8 T cells were CD44+ and CD62L− in all experimental groups (Fig. 4). In the spleen, all T. gondii-specific CD8 T cells were also CD44+, and 60% to 70% of these cells were CD62+, corresponding to an effector phenotype. However, irrespective of CD4-T-cell depletion during the acute stage, 30 to 40% of T. gondii-specific CD8 T cells were CD44+ CD62L+ memory cells (Fig. 4). Thus, in the spleen, an organ without parasite persistence, T. gondii-specific memory CD8 T cells develop without CD4-T-cell support.
In contrast, it appears unlikely that the resurrection of nonspecific or T. gondii-specific CD4 T cells after cessation of anti-CD4 Ab treatment contributes to equal frequencies of T. gondii-specific CD8 T cells in chronically infected mice, since depletion of CD4 T cells during the chronic phase of infection was not critical for the maintenance of T. gondii-specific CD8 T cells. Thus, in contrast to nonpersistent infections, CD4 T cells (33) do not provide essential survival signals for CD8 T cells in chronic toxoplasmosis.
Our novel observation of the development of T. gondii-specific CD4 T cells in parallel to repopulation of the CD4-T-cell compartment implies that the persisting antigen contributes to a rapid stimulation and expansion of these cells. At the time point of cessation of CD4 depletion (i.e., day 17 p.i.), acute toxoplasmosis was ongoing, and T. gondii-specific CD8 T cells still proliferated in the spleen (19). Thus, most probably, the expansion of T. gondii-specific CD4 T cells is additionally fostered by activated antigen-presenting cells (APCs) and proinflammatory cytokines. This assumption is supported by recent experiments in nonpersisting influenza virus infection (15), demonstrating that the amount of antigen available and the inflammatory milieu (infection ongoing versus cleared) strongly determine frequencies of virus-specific CD4 T cells. In contrast, virus-specific CD4 T cells do not develop in mice chronically infected with the lymphocytic choriomeningitis virus after CD4 T-cell depletion in the early stage of infection, implying that disease-specific factors determine the development of pathogen-specific CD4 T cells in hosts chronically infected.
In chronically infected mice, depletion of CD4 T cells in parallel to low-dose reinfection with T. gondii cysts resulted in an expansion of T. gondii-specific CD8 T cells, whereas control animals did not exhibit an increase of T. gondii-specific CD8 T cells. Thus, CD4 T cells limit the expansion of T. gondii-specific CD8 T cells after reinfection. Such an inhibitory function of CD4 T cells has also been observed in murine listeriosis, in which CD4 CD25+ T cells restrict the expansion of memory CD8 T cells after reinfection (18). Interestingly, the same protocol of anti-CD25 treatment did not result in an increased proliferation of T. gondii-specific CD8 T cells after reinfection although CD4 CD25+ T cells were present and effectively depleted by anti-CD25 treatment. This indicates that in toxoplasmosis the suppressive function of CD4 T cells is not contained in the CD25+ fraction of CD4 T cells but in other regulatory CD4-T-cell populations, e.g., foxp3+ CD4 T cells. The same observation of an increased proliferation of T. gondii-specific CD8 T cells was made in mice that had been depleted of CD4 T cells during the acute stage of infection and then reinfected after repopulation of the CD4-T-cell compartment in the chronic stage of disease. Although at present the underlying mechanism is yet unresolved, regulatory CD4 T cells may determine the capacity of T. gondii-specific CD8 T cells to respond to challenge infection as early as in the acute stage of infection.
In contrast, it appears unlikely that an increased antigenic stimulation due to an inefficient control of parasites applied secondarily in CD4-T-cell-depleted mice accounts for the increased proliferation of T. gondii-specific CD8 T cells, since a careful histological analysis of spleen and brain did not reveal differences in the amount of parasitic antigen between reinfected anti-CD4- and control Ab-treated mice. This is in line with previous data illustrating that even in the absence of CD4 T cells, chronic toxoplasmosis is efficiently controlled by CD8 T cells (12, 13). Moreover, the expansion of functionally active IFN-γ producing T. gondii-specific CD8 T cells in reinfected CD4-depleted mice may compensate for CD4 deficiency. Interestingly, the frequency of T. gondii-specific CD8 T cells in the brain remained constant after reinfection, which is most probably explained by the fact that reinfection of chronically infected mice with the same strain of T. gondii as used for primary infection does not result in an invasion of the brain by the parasite applied secondarily (8). In addition, the incomplete elimination of i.c. CD4 T cells in chronically infected anti-CD4-treated mice may also contribute to suppression of i.c. CD8-T-cell proliferation by the remaining CD4 T cells and may partially explain why T. gondii-specific T cells in general form a stable, nonproliferating cell pool in chronic TE (29).
In contrast to CD8-T-cell frequencies, the functional activity of i.c. CD8 T cells was partially dependent on CD4-T-cell help. In acute toxoplasmosis, depletion of CD4 T cells reduced the frequency of i.c. IFN-γ-producing and cytotoxic T. gondii-specific CD8 T cells. These findings extend previous observations illustrating that CD4−/−, but not CD4+/+, T. gondii-susceptible mice succumb to acute TE due to a massively impaired production of T. gondii-specific antibodies (8, 16). However, our data indicate that in addition to the defective Ab response, which was also observed in the present study (data not shown), and the absence of the protection of CD4 T cells, the functional impairment of i.c. CD8 T cells may contribute to death of CD4-T-cell-depleted animals from TE. Importantly, a functional impairment occurred only in i.c. but not in splenic CD8 T cells, illustrating that the functional importance of CD4 help is regulated in an organ-specific manner. Since the cellular compositions of spleen and brain are very different, even under inflammatory conditions such as toxoplasmosis, cells in the spleen, e.g., dendritic cell subpopulations which are absent from the brain, may compensate for the absence of CD4 T cells. Alternatively, the microenvironment of the brain may suppress CD8-T-cell responses, e.g., by the release of transforming growth factor β (11), and one may consider that CD4 T cells are required to overcome this immunosuppression and to guarantee an optimal activation of CD8 T cells.
Previous studies on the impact of CD4 T cells on CD8-T-cell responses in chronic TE have not been reported, and our results show that depletion of CD4 T cells in chronically infected mice also resulted in a fourfold reduction of i.c. IFN-γ-producing T. gondii-specific CD8 T cells, indicating that a continuous support by CD4 T cells is required to maintain an optimal functional activity of parasite-specific i.c. CD8 T cells. At present, the factors provided by CD4 T cells for the optimal functional activity of i.c. CD8 T cells are unknown. However, it appears unlikely that an insufficient activation of APCs accounts for the reduced frequency of functionally active i.c. T. gondii-specific CD8 T cells, since flow cytometry demonstrated that i.c. macrophages and microglia, the local i.c. APCs, expressed comparable levels of CD80, CD86, and MHC class I and II antigens in both anti-CD4- and control Ab-treated animals (data not shown). The observation that in chronic viral (20, 23, 36) and acute bacterial infections (30), frequencies of IFN-γ-producing or cytotoxic virus-specific CD8 T cells were also dependent on CD4-T-cell help illustrates that this supportive function of CD4 T cells is not restricted to TE.
Collectively, the present study shows that in toxoplasmosis, CD4 T cells support the protective function of i.c., but CD8 T cells limit the expansion of parasite-specific CD8 T cells after reinfection. These diverse functions of CD4 T cells may be performed by different subsets of CD4 T cells. In fact, ongoing experiments in our laboratory indicate that T. gondii-specific CD4 T cells are heterogeneous, including both regulatory and proinflammatory CD4 T cells. The relative importance of these different CD4-T-cell subsets may vary depending on the actual circumstances, which has implications for patients with disturbed CD4-T-cell responses.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (grant number Schl 392/6-1 to D.S.).
We thank Zoi Edinger, Nadja Schlüter, and Elena Fischer for excellent technical support.
Editor: J. L. Flynn
REFERENCES
- 1.Araujo, F. G. 1992. Depletion of CD4+ T cells but not inhibition of the protective activity of IFN-gamma prevents cure of toxoplasmosis mediated by drug therapy in mice. J. Immunol. 149:3003-3007. [PubMed] [Google Scholar]
- 2.Araujo, F. G. 1991. Depletion of L3T4+ (CD4+) T lymphocytes prevents development of resistance to Toxoplasma gondii in mice. Infect. Immun. 59:1614-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bevan, M. J. 2004. Helping the CD8(+) T-cell response. Nat Rev. Immunol. 4:595-602. [DOI] [PubMed] [Google Scholar]
- 4.Brown, C. R., C. A. Hunter, R. G. Estes, E. Beckmann, J. Forman, C. David, J. S. Remington, and R. McLeod. 1995. Definitive identification of a gene that confers resistance against Toxoplasma cyst burden and encephalitis. Immunology 85:419-428. [PMC free article] [PubMed] [Google Scholar]
- 5.Buller, R. M., K. L. Holmes, A. Hugin, T. N. Frederickson, and H. C. Morse III. 1987. Induction of cytotoxic T-cell responses in vivo in the absence of CD4 helper cells. Nature 328:77-79. [DOI] [PubMed] [Google Scholar]
- 6.Casciotti, L., K. H. Ely, M. E. Williams, and I. A. Khan. 2002. CD8+-T-cell immunity against Toxoplasma gondii can be induced but not maintained in mice lacking conventional CD4+ T cells. Infect. Immun. 70:434-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Combe, C. L., T. J. Curiel, M. M. Moretto, and I. A. Khan. 2005. NK cells help to induce CD8+-T-cell immunity against Toxoplasma gondii in the absence of CD4+ T cells. Infect. Immun. 73:4913-4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dao, A., B. Fortier, M. Soete, F. Plenat, and J. F. Dubremetz. 2001. Successful reinfection of chronically infected mice by a different Toxoplasma gondii genotype. Int. J. Parasitol. 31:63-65. [DOI] [PubMed] [Google Scholar]
- 9.Deckert-Schlüter, M., D. Schlüter, D. Schmidt, G. Schwendemann, O. D. Wiestler, and H. Hof. 1994. Toxoplasma encephalitis in congenic B10 and BALB mice: impact of genetic factors on the immune response. Infect. Immun. 62:221-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denkers, E. Y., G. Yap, T. Scharton-Kersten, H. Charest, B. A. Butcher, P. Caspar, S. Heiny, and A. Sher. 1997. Perforin-mediated cytolysis plays a limited role in host resistance to Toxoplasma gondii. J. Immunol. 159:1903-1908. [PubMed] [Google Scholar]
- 11.Fontana, A., D. B. Constam, K. Frei, U. Malipiero, and H. W. Pfister. 1992. Modulation of the immune response by transforming growth factor beta. Int. Arch. Allergy Immunol. 99:1-7. [DOI] [PubMed] [Google Scholar]
- 12.Gazzinelli, R., Y. Xu, S. Hieny, A. Cheever, and A. Sher. 1992. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J. Immunol. 149:175-180. [PubMed] [Google Scholar]
- 13.Israelski, D. M., F. G. Araujo, F. K. Conley, Y. Suzuki, S. Sharma, and J. S. Remington. 1989. Treatment with anti-L3T4 (CD4) monoclonal antibody reduces the inflammatory response in toxoplasmic encephalitis. J. Immunol. 142:954-958. [PubMed] [Google Scholar]
- 14.Janssen, E. M., E. E. Lemmens, T. Wolfe, U. Christen, M. G. von Herrath, and S. P. Schoenberger. 2003. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421:852-856. [DOI] [PubMed] [Google Scholar]
- 15.Jelley-Gibbs, D. M., D. M. Brown, J. P. Dibble, L. Haynes, S. M. Eaton, and S. L. Swain. 2005. Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation. J. Exp. Med. 202:697-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson, L. L., and P. C. Sayles. 2002. Deficient humoral responses underlie susceptibility to Toxoplasma gondii in CD4-deficient mice. Infect. Immun. 70:185-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang, H., J. S. Remington, and Y. Suzuki. 2000. Decreased resistance of B cell-deficient mice to infection with Toxoplasma gondii despite unimpaired expression of IFN-gamma, TNF-alpha, and inducible nitric oxide synthase. J. Immunol. 164:2629-2634. [DOI] [PubMed] [Google Scholar]
- 18.Kursar, M., K. Bonhagen, J. Fensterle, A. Kohler, R. Hurwitz, T. Kamradt, S. H. Kaufmann, and H. W. Mittrucker. 2002. Regulatory CD4+ CD25+ T cells restrict memory CD8+ T cell responses. J. Exp. Med. 196:1585-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwok, L. Y., S. Lutjen, S. Soltek, D. Soldati, D. Busch, M. Deckert, and D. Schlüter. 2003. The induction and kinetics of antigen-specific CD8 T cells are defined by the stage specificity and compartmentalization of the antigen in murine toxoplasmosis. J. Immunol. 170:1949-1957. [DOI] [PubMed] [Google Scholar]
- 20.Liu, H., S. Andreansky, G. Diaz, T. Hogg, and P. C. Doherty. 2002. Reduced functional capacity of CD8+ T cells expanded by post-exposure vaccination of gamma-herpesvirus-infected CD4-deficient mice. J. Immunol. 168:3477-3483. [DOI] [PubMed] [Google Scholar]
- 21.Luft, B. J., and J. S. Remington. 1988. AIDS commentary. Toxoplasmic encephalitis. J. Infect. Dis. 157:1-6. [DOI] [PubMed] [Google Scholar]
- 22.Marzo, A. L., V. Vezys, K. D. Klonowski, S. J. Lee, G. Muralimohan, M. Moore, D. F. Tough, and L. Lefrancois. 2004. Fully functional memory CD8 T cells in the absence of CD4 T cells. J. Immunol. 173:969-975. [DOI] [PubMed] [Google Scholar]
- 23.Matloubian, M., R. J. Concepcion, and R. Ahmed. 1994. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 68:8056-8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montoya, J. G., and O. Liesenfeld. 2004. Toxoplasmosis. Lancet 363:1965-1976. [DOI] [PubMed] [Google Scholar]
- 25.Rahemtulla, A., W. P. Fung-Leung, M. W. Schilham, T. M. Kundig, S. R. Sambhara, A. Narendran, A. Arabian, A. Wakeham, C. J. Paige, R. M. Zinkernagel, et al. 1991. Normal development and function of CD8+ cells but markedly decreased helper cell activity in mice lacking CD4. Nature 353:180-184. [DOI] [PubMed] [Google Scholar]
- 26.Riberdy, J. M., J. P. Christensen, K. Branum, and P. C. Doherty. 2000. Diminished primary and secondary influenza virus-specific CD8+ T-cell responses in CD4-depleted Ig−/− mice. J. Virol. 74:9762-9765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlüter, D., N. Kaefer, H. Hof, O. D. Wiestler, and M. Deckert-Schlüter. 1997. Expression pattern and cellular origin of cytokines in the normal and Toxoplasma gondii-infected murine brain. Am. J. Pathol. 150:1021-1035. [PMC free article] [PubMed] [Google Scholar]
- 28.Schlüter, D., J. Lohler, M. Deckert, H. Hof, and G. Schwendemann. 1991. Toxoplasma encephalitis of immunocompetent and nude mice: immunohistochemical characterisation of Toxoplasma antigen, infiltrates and major histocompatibility complex gene products. J. Neuroimmunol. 31:185-198. [DOI] [PubMed] [Google Scholar]
- 29.Schlüter, D., T. Meyer, L. Y. Kwok, M. Montesinos-Rongen, S. Lutjen, A. Strack, M. L. Schmitz, and M. Deckert. 2002. Phenotype and regulation of persistent intracerebral T cells in murine Toxoplasma encephalitis. J. Immunol. 169:315-322. [DOI] [PubMed] [Google Scholar]
- 30.Serbina, N. V., V. Lazarevic, and J. L. Flynn. 2001. CD4(+) T cells are required for the development of cytotoxic CD8(+) T cells during Mycobacterium tuberculosis infection. J. Immunol. 167:6991-7000. [DOI] [PubMed] [Google Scholar]
- 31.Shedlock, D. J., and H. Shen. 2003. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 300:337-339. [DOI] [PubMed] [Google Scholar]
- 32.Sun, J. C., and M. J. Bevan. 2003. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 300:339-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun, J. C., M. A. Williams, and M. J. Bevan. 2004. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat. Immunol. 5:927-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki, Y., F. K. Conley, and J. S. Remington. 1989. Importance of endogenous IFN-gamma for prevention of toxoplasmic encephalitis in mice. J. Immunol. 143:2045-2050. [PubMed] [Google Scholar]
- 35.Vollmer, T. L., M. K. Waldor, L. Steinman, and F. K. Conley. 1987. Depletion of T-4+ lymphocytes with monoclonal antibody reactivates toxoplasmosis in the central nervous system: a model of superinfection in AIDS. J. Immunol. 138:3737-3741. [PubMed] [Google Scholar]
- 36.Zajac, A. J., J. N. Blattman, K. Murali-Krishna, D. J. Sourdive, M. Suresh, J. D. Altman, and R. Ahmed. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]