Abstract
Plasmodium falciparum malaria kills roughly 2.5 million people, mainly children, annually. Much of this mortality is thought to arise from the actions of a malarial toxin. This toxin, identified as glycosylphosphatidylinositol (GPI), is a major pathogenicity determinant in malaria. A malarial molecule, Pfj, labeled by [3H]glucosamine like the GPIs, was identified as a non-GPI molecule. Here we show that Pfj is able to down-regulate tumor necrosis factor alpha (TNF-α) production induced by the GPI of P. falciparum. Mass spectrometry analysis showed that Pfj was not a single molecule but represented a number of molecules. Separation methods, such as cation-exchange chromatography and thin-layer chromatography, were used to isolate and identify the following four main fatty acids responsible for the inhibitory effect on TNF-α production: myristic, pentadecanoic, palmitic, and palmitoleic acids. This regulatory effect on cytokine production suggests that there is balanced bioactivity for the different categories of malarial lipids.
Infectious diseases caused by parasitic protozoans constitute one of the greatest public health problems of mankind, affecting 15% of the global population and having high morbidity and fatality rates. Plasmodium falciparum malaria alone infects about 500 million people worldwide and is responsible for approximately 2.5 million deaths per year. Several pathophysiological responses, such as cerebral malaria, metabolic acidosis, anemia, and hypoglycemia, are associated with malaria infection (32). Glycosylphosphatidylinositol (GPI) molecules that are free or linked to surface antigens of the parasites have been identified as dominant toxins involved in the pathogenic process. These molecules initiate the production of excess levels of the cytokines tumor necrosis factor alpha (TNF- α) and interleukin-1 (IL-1), leading to a systemic inflammatory cascade, renal failure, multiorgan inflammation, hypoglycemia, lactic acidosis, and death (33). Purified malarial GPIs also increase expression of cell adhesion molecules (ICAM-1, VCAM-1, and E-selectin) and nitric oxide production in human vascular endothelial cells through cytokine-independent pathways (35, 39). Thus, GPIs could participate in the increase in adherence of parasitized erythrocytes to brain vascular cells and in the increase in intracranial pressure observed in cerebral malaria. GPI toxicity is required for the development of severe disease syndromes in rodent models and may be the target of antidisease vaccination strategies (31, 34).
However, little is known about the role played by other parasitic lipids in the development of malaria pathogenesis. De novo biosynthesis of sphingolipids has been described in P. falciparum (13). Studies of intraerythrocytic development of P. falciparum have established that sphingomyelin is synthesized (1, 8, 14) by a parasite-specific enzyme and is important for parasite-mediated nutrient uptake (17, 18). In contrast to other eukaryotic cells, no discernible amounts of steryl esters are produced, and cholesterol is nearly absent in the parasite (42). During intraerythrocytic development of the parasite, the phospholipid composition of the erythrocyte membrane is strikingly altered. The plasma membrane of infected erythrocytes contains more phosphatidylcholine and phosphatidylinositol (PI) and less sphingomyelin than the plasma membrane of normal uninfected erythrocytes contains (15). Large increases in the levels of palmitic (C16:0) and oleic (C18:1) acids and major decreases in the levels of polyunsaturated fatty acids, such as arachidonic (C20:4) and docosahexaenoic (C22:6) acids, occur as a result of infection (15). This makes the phospholipid composition very similar to that of the parasite, indicating that there is intense dynamic phospholipid traffic between the erythrocyte membrane and the membrane of the intracellular parasite (15). Since mature erythrocytes have negligible lipid synthesis and metabolism, these modifications, according to Hsiao et al. (15), must be a result of parasite metabolism of erythrocyte lipids. Several studies have shown that the biosynthetic machinery of the parasite can provide all of the new phospholipid molecules. Two P. falciparum genes have been characterized; one of them encodes the choline-phosphate cytidylyltransferase, which catalyzes the rate-limiting step in the de novo synthesis of phosphatidylcholine, and the other encodes the CDP-diacylglycerol synthase, which synthesizes CDP-diacylglycerol, a central molecule for the metabolism of PI, phosphatidylglycerol, and phosphatidylserine (21, 44). A new pathway, which appears to be unique to Plasmodium, directly decarboxylates serine, thus providing the parasite with ethanolamine (7). The GPI biosynthetic pathway (5) and both the PI biosynthetic pathway and PI synthase in P. knowlesi and P. falciparum (41) have been described previously. Parasitized erythrocytes and hemozoin were found to contain large amounts of monohydroxy derivatives of polyenoic fatty acids in their ester lipids (36). A detailed structural analysis suggested that nonenzymatic, heme-catalyzed lipid peroxidation is the origin of these products. In hemozoin and parasitized erythrocytes a complex mixture of hydroxylinoleic acid and hydroxyarachidonic acid isomers was detected. The intraerythrocytic stages of P. falciparum accumulate triacylglycerol produced using oleate and diacylglycerol as substrates (26, 42). In contrast to other eukaryotic cells, neither steryl esters nor cholesterol esters, a second neutral lipid species reported to be important for a related apicomplexan, Toxoplasma gondii, were detected in P. falciparum (26, 42). PfDGAT, an enzyme similar to the acyl-coenzyme A:diacylglycerol acyltransferases (DGAT) involved in neutral lipid synthesis, is expressed in a stage-specific manner and is the most likely candidate for plasmodial triacylglycerol synthesis (26, 42). Synthesized neutral lipids are stored as lipid bodies in the parasite cytosol in a stage-specific manner. Their biogenesis is not modified upon incubation with excess fatty acids or lipoproteins or after depletion of lipoprotein in the culture medium (24).
After labeling of intraerythrocytic parasites with [3H]glucosamine, a radioactive peak having an unknown composition, designated Pfj, was observed (10). A similar peak was also detected after labeling of the cell-free system with UDP- [6-3H]GlcNAc but not after labeling with GDP-[2-3H]mannose (11). Furthermore, Pfj was not sensitive to GPI-specific phospholipase D and PI-phospholipase C, indicating that it is not a GPI (11). In the present study we aimed at understanding the biological effect of Pfj isolated from P. falciparum and characterizing it. Here we show that Pfj is able to inhibit TNF-α production induced by the GPI of P. falciparum. Also, we demonstrate that Pfj is not a single compound but a mixture of several molecules and that fatty acids are responsible for the inhibitory effect.
MATERIALS AND METHODS
Materials.
[3H]glucosamine hydrochloride (25 Ci/mmol) was purchased from Hartmann Analytic (Braunschweig, Germany). Myristic, pentadecanoic, palmitic, palmitoleic, stearic, and oleic acids were obtained from Sigma (Deisenhofen, Germany). All solvents used were analytical or high-performance liquid chromatography grade and were obtained from Riedel-de Haen (Seelze, Germany).
Parasites.
P. falciparum strain FCBR was obtained from B. Enders, Behring Co. (Marburg, Germany). It was maintained as previously described (29). Development and multiplication of plasmodial cultures were monitored by microscopic evaluation of Giemsa-stained smears. Parasite cultures were routinely checked for Mycoplasma contamination. Parasite multiplication was assessed as described previously (6). Trophozoites were isolated from rings and noninfected erythrocytes by magnetic separation using SuperMACS and D columns (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany).
Metabolic labeling of P. falciparum GPIs.
Metabolic labeling of P. falciparum was performed in a glucose-free medium (Sigma) supplemented with 20 mM fructose (10). Cultures of 32- to 40-h-old parasites (trophozoites) were labeled with 0.5 mCi [3H]glucosamine for 4 h at 37°C. After labeling, parasites were released from erythrocytes by saponin lysis as described previously (10).
Extraction and purification of GPIs.
P. falciparum labeled and unlabeled GPIs were extracted three times with chloroform-methanol-water (10:10:3, by volume), dried under a nitrogen stream, subjected to “Folch” washing (9), and recovered in the n-butyl alcohol phase after partitioning between water and water-saturated n-butyl alcohol (1:1, by volume). Unlabeled GPIs were separated by thin-layer chromatography (TLC) on Merck Silica Gel 60 plates using a chloroform-methanol-water (4:4:1, by volume) solvent system. GPIs from metabolically labeled parasites were used as tracers. Chromatograms were scanned for radioactivity using a Berthold LB 2842 linear analyzer. The area corresponding to the GPI Pfα was scraped off, reextracted with chloroform-methanol-water (10:10:3, by volume) by sonication (Branson 3200; 47 MHz), and recovered in the n-butyl alcohol phase after water-saturated n-butyl alcohol-water partitioning. The area corresponding to the non-GPI molecule Pfj was scraped off the TLC plate, extracted from the silica with chloroform-methanol-water (10:10:3, by volume) by sonication, dried, and subjected to saponification using methanol-0.1 M KOH at 50°C for 4 h. After drying, partitioning between water and water-saturated n-butyl alcohol permitted recovery of the alkali-stable molecules in the n-butyl alcohol phase. Glycolipids were extracted from noninfected erythrocytes using the same procedure.
Purification of sphingolipid classes.
The lipids of P. falciparum trophozoites or of noninfected erythrocytes were extracted with chloroform-methanol-pyridine (90:60:1, by volume). After drying under a nitrogen stream, GPIs were eliminated by saponification using methanol-0.1 M KOH at 50°C for 4 h. Extracted lipids were partitioned between water and water-saturated n-butyl alcohol. Then the alkali-stable lipids recovered in the butanol phase were separated by using aminopropyl-bonded silica gel (LC-NH2) and weak cation-exchange (LC-WCX) cartridges and different solvents (3). The LC-NH2 column was preconditioned with 2 ml hexane. Then the parasite lipids diluted in 200 μl of chloroform were loaded onto the column. The first fraction (cholesterol [85%], cholesteryl esters, triglycerides, diglycerides, fatty alcohols, fatty acid methyl esters) was eluted with 1.4 ml of hexane-ethyl acetate (17:3, by volume). Then the LC-NH2 column was positioned on a LC-WCX column preconditioned with 1 ml of hexane, 2 ml of methanol-0.5 N acetic acid, 4 ml of methanol, and 4 ml of hexane. The second fraction (cholesterol [15%], monoglycerides, free ceramides, N-methyl derivatives of sphingoid bases) was eluted through both cartridges with 1.6 ml of chloroform-methanol (23:1, by volume), and the third fraction (normal and α-hydroxy free fatty acids) was eluted by passing 1.8 ml of di-isopropyl ether-acetic acid (98:5, by volume) through the two columns. The fourth fraction (ceramide mono-, di-, and trihexosides, globoside) was eluted with 2 ml of acetone-methanol (9:1.35, by volume) through the two columns. Next, the two cartridges were taken apart and processed further separately. The LC-WCX cartridge was eluted with 2 ml of chloroform-methanol (9:3, by volume) to recover the rest of the fourth fraction and with 2 ml of 1 N acetic acid in methanol to elute the fifth fraction (sphingosine, dihydrosphingosine, phytosphingosine). The LC-NH2 column was eluted with 2 ml of chloroform-methanol (2:1, by volume) to obtain the sixth fraction (sphingomyelin, phosphatidylcholine, lysolecithin, lysophosphatidylcholine, lecithin, phosphatidylethanolamine). All solvents were dried under a stream of nitrogen, and the molecules in the six fractions were tested using macrophages with or without GPI Pfα.
Thin-layer chromatography.
The third fraction containing fatty acids was separated by thin-layer chromatography using a chloroform-methanol (50:2, by volume) solvent system. Unsaturated fatty acids were visualized by staining with iodine vapor, and silica was scraped off in eight parts according to the positions of the yellow spots. Lipids obtained from each scraping were extracted with chloroform-methanol-water (10:10:3, by volume) by sonication, dried, and individually tested with macrophages in the presence of the Pfα. The fraction that had a biological effect was subjected to thin-layer chromatography again using a chloroform-methanol-2 M aqueous ammonia (65:25:4, by volume) solvent system. Unsaturated fatty acids were detected with iodine, and silica was scraped off in eight parts. Lipids were extracted from the silica with chloroform-methanol-water (10:10:3, by volume) by sonication, and each part was individually tested with macrophages.
Electrospray-mass spectrometry (ES-MS) analysis.
Glycosphingolipid fractions and standards were dissolved in 66% acetonitrile, 0.3% formic acid, loaded in Micromass type-F nanotips, and analyzed in positive-ion mode with a Micromass QTof2 mass spectrometer using a capillary voltage of 950V and a cone voltage of 40 V. Mass spectra were collected and processed with the MassLynx software. Fatty acid fractions and standards were dissolved in chloroform-methanol (1:1, by volume), loaded in Micromass type-F nanotips, and analyzed in negative-ion mode with an ABI QStarXL mass spectrometer using a capillary potential of 900 V and a declustering potential of 60 V. Mass spectra were collected and processed with the ABI Analyst software.
TNF-α production by macrophages.
RAW 264.7 macrophages were diluted to obtain a concentration of 106 cells/ml with the serum-free medium Panserin 401 (Pan Biotech GmbH, Aidenbach, Germany), and 0.2-ml aliquots were dispensed into the wells of a 96-well plate. The cells were allowed to adhere for 3 h, washed with medium, and incubated at 37°C in a 5% CO2 atmosphere for 24 h with medium containing the molecules tested. The GPI and its precursors were stored in n-butyl alcohol, and the different sphingolipid classes were stored in chloroform-methanol-water (40:30:4). The amount needed for one experiment was dried under a nitrogen stream to remove the solvent. The culture medium was added, and molecules were resuspended in this medium by sonication. For the negative control, macrophages were incubated with medium alone. The absence of endotoxin in all molecules was checked with a Limulus amebocyte lysate kit (QCL-100; Bio-Whittaker, Walkersville, MD). Measurement of TNF-α and IL-12 levels in the macrophage culture supernatants was performed using a specific sandwich enzyme-linked immunosorbent assay (ELISA) (BD Biosciences, San Jose, CA). Nitric oxide levels were measured using the Griess assay. Briefly, 1 volume of 0.1% naphthylethylenediamine (in water) and 1 volume of 1% sulfanilamide (in 5% phosphoric acid) were added to 2 volumes of culture supernatant. Absorbance was compared with the absorbance of NaNO2 standards.
Statistics.
The unpaired Student t test was used for statistical evaluation, and a P value of <0.05 was considered significant.
RESULTS
Pfj inhibits GPI-induced TNF-α production.
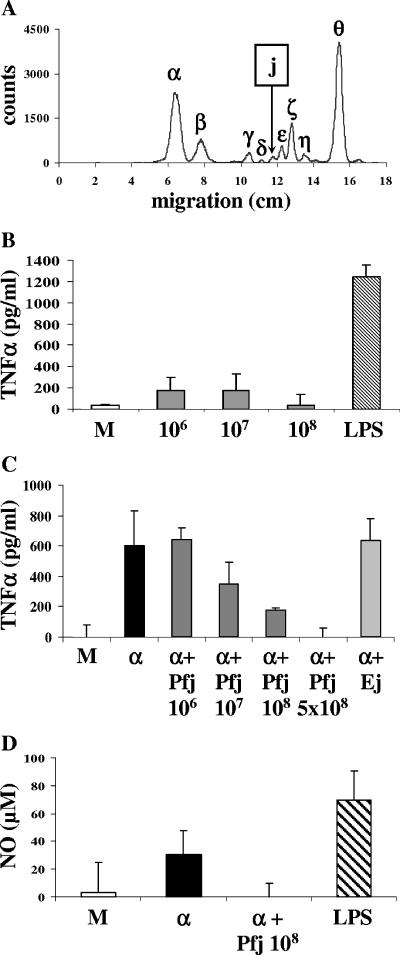
Pfj is a peak detected on TLC after labeling of parasites with [3H]glucosamine (Fig. 1A) and has been described as a non-GPI molecule (11). Since the malarial GPI Pfα induces TNF-α production in macrophages (33), we sought to determine whether Pfj could also stimulate the cells to produce TNF-α. To that end, macrophages were incubated with different amounts of Pfj, and TNF-α secretion in supernatants was measured using a sandwich ELISA. Pfj failed to induce TNF-α production by macrophages at any amount tested (Fig. 1B). We have previously shown that a crude extract of T. gondii glycolipids was unable to induce TNF-α production by macrophages (4). Purification of individual GPIs by TLC was necessary to obtain a response, suggesting that one or more inhibitory molecules were present in the crude extract. Such inhibitory molecules could also exist in Plasmodium. As Pfj did not have a stimulatory effect, it could have an inhibitory effect. To investigate this, Pfj was added to the malarial toxin GPI Pfα, which was isolated from the parasites and had the following structure (12): ethanolamine-phosphate-6(mannose [Man]α1-2)Manα1-2Manα1-6Manα1-4-glucosamine-phosphate-diacylglycerol (4GlcN-P-DAG). As described previously (35), TNF-α was found in supernatants of macrophages stimulated with Pfα (Fig. 1C). When Pfj was added 30 min prior to addition of Pfα, the production of TNF-α was inhibited in a dose-dependent manner (Fig. 1C). In the same way, Pfj reduced Pfα-induced NO production (Fig. 1D).
FIG. 1.
Pfj inhibits GPI-induced TNF-α production. (A) TLC scan of [3H]glucosamine-labeled glycolipids of P. falciparum using a Berthold LB 2842 linear analyzer. (B) Macrophages were incubated for 24 h in the presence of Pfj extracted from 106, 107, or 108 P. falciparum trophozoites. LPS (200 ng/ml) was used as a positive control. Medium alone was used as a negative control (lane M). (C) Macrophages were incubated for 24 h in the presence of the P. falciparum GPI Pfα (α) alone or with Pfj extracted from 106 to 5 × 108 trophozoites or with glycolipids extracted from noninfected erythrocytes and corresponding to the Pfj area after TLC (Ej). Pfj and Ej were added 30 min before Pfα was added. Supernatants were assayed for TNF-α production using a sandwich ELISA or for NO production using the Griess assay (D). The values are means ± standard deviations of triplicate samples in a representative experiment.
To be sure that this inhibitory effect was not due to a nonparasitic molecule, glycolipids from noninfected erythrocytes were subjected to the same isolation procedure. Erythrocyte glycolipids separated on a silica plate and located at the same position as Pfj were tested in the presence of Pfα. No diminution of TNF-α secretion was observed (Fig. 1C), confirming that the TNF-α inhibition obtained with Pfj had a parasitic origin. The inhibitory effect of Pfj was not due to cell death as the viability of macrophages was confirmed microscopically by trypan blue exclusion (data not shown).
ES-MS analysis of Pfj.
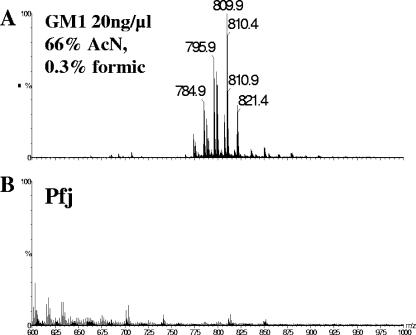
In contrast to P. falciparum GPIs, Pfj was not sensitive to alkaline treatment and deamination with nitrous acid (11), raising the possibility it may be a glycosphingolipid. ES-MS analysis of the ganglioside GM1 used as a glycosphingolipid control revealed doubly charged positive ions (Fig. 2A). The same analysis showed that Pfj contained a variety of different molecules, but none belonged to the sphingolipid family as only singly charged positive ions were present (Fig. 2B). Furthermore, the intensity of ions in the Pfj area was very low, although the sample was isolated from 5 × 109 parasites.
FIG. 2.
Pfj fraction does not contain glycosphingolipids: positive-ion electrospray mass spectra of ganglioside GM1 (A), used as an authentic glycosphingolipid standard to validate the analytical method, and of the Pfj fraction (B). The data were normalized to the intensity of the most abundant [M + 2H]2+ doubly charged GM1 ganglioside ion (m/z 809.9, 100%) in panel A. Only low-intensity, singly charged (nonglycosphingolipid) ions were observed in the Pfj sample.
TNF-α production in response to sphingolipid classes purified from P. falciparum.
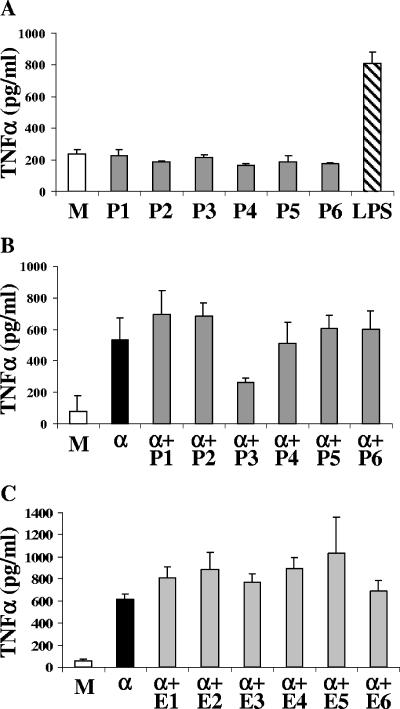
In order to identify the molecule responsible for the GPI inhibition, an alternative method was used to separate the molecules of P. falciparum. Lipids of the parasites were extracted with chloroform-methanol-pyridine and subjected to saponification to eliminate GPIs as described in Materials and Methods. Sphingolipid classes from 2 × 109 trophozoites were separated by using aminopropyl-bonded silica gel (LC-NH2) and weak cation-exchange (LC-WCX) cartridges and different organic solvents. Six fractions, one containing neutral lipids, one containing free ceramides, one containing fatty acids, one containing neutral glycosphingolipids, one containing free sphingoid bases, and one containing sphingomyelin, were obtained and individually tested. None of the six fractions induced TNF-α production by macrophages (Fig. 3A). When the six fractions were tested in the presence of the GPI Pfα, only fraction 3 containing fatty acids inhibited TNF-α secretion induced by the malarial toxin (Fig. 3B). The viability of macrophages was confirmed by trypan blue exclusion (data not shown). To check if this inhibitory effect was due to a nonparasitic molecule, glycolipids from noninfected erythrocytes were subjected to the same separation procedure. None of the six fractions obtained from erythrocytes reduced the GPI-induced TNF-α production (Fig. 3C). Since fatty acids cannot be labeled with [3H]glucosamine, we sought to determine how the inhibitory molecules present in Pfj could be fatty acids. To this end, we scraped off the Pfj radioactive peak from a TLC plate and separated it with both cartridges. While the inhibitory molecules were recovered in fraction 3 containing fatty acids, the radioactivity was recovered in the first fraction, in which neutral lipids eluted (data not shown). This fraction did not have an inhibitory effect on GPI-induced TNF-α production. Since neutral lipids cannot be labeled by [3H]glucosamine, the peak observed in the Pfj area might be another type of molecule coeluting with these lipids.
FIG. 3.
Fraction P3 containing fatty acids inhibits GPI-induced TNF-α production. (A) Macrophages were incubated for 24 h in the presence of the six different fractions (P1 to P6) isolated from 2 × 108 trophozoites. (B and C) Macrophages were incubated for 24 h in the presence of Pfα (α) alone or with fractions P1 to P6 (added 30 min before Pfα was added) (B) or with the six fractions extracted from noninfected erythrocytes (E1 to E6 added 30 min before Pfα was added) (C). Medium alone was used as a negative control (lanes M). The values are means ± standard deviations of triplicate samples in a representative experiment.
ES-MS analysis of fraction P3.
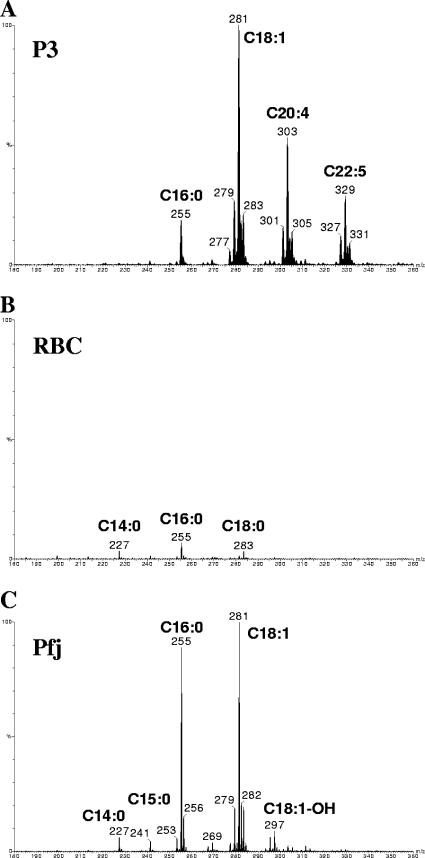
The method described above provided evidence indicating that fraction P3 should contain fatty acids. As shown by ES-MS analysis, fraction P3 contained several unsaturated fatty acids, mainly C18:0, C18:1, C20:4, and C22:5 (Fig. 4A). Fraction E3 from noninfected erythrocytes also contained singly charged ions, but the two spectra were not identical and E3 contained less fatty acids, mostly C14:0, C16:0, and C18:0 (Fig. 4B). With this in mind, the area corresponding to Pfj on the TLC plate was analyzed again, and it also contained fatty acids, mainly C14:0, C15:0, C16:0, C18:1, and C18:1-OH (Fig. 4C). The spectra of Pfj and P3 were not identical, but some fatty acids were common to both of these spectra.
FIG. 4.
Fatty acid analysis of Plasmodium and noninfected red cell fractions. Negative-ion electrospray-mass spectrometry was used to identify the carboxylate ([M-H]−) ions of free fatty acids present in the P3 fraction from P. falciparum (A), the E3 fraction from noninfected erythrocytes (RBC) (B), and the Pfj fraction from P. falciparum (C). The data in panel B were normalized to the intensity of the most intense fatty acid ion (m/z 281) in panel C to illustrate the paucity of fatty acids in the noninfected-erythrocyte-derived fraction.
Isolation of inhibitory fatty acids by thin-layer chromatography.
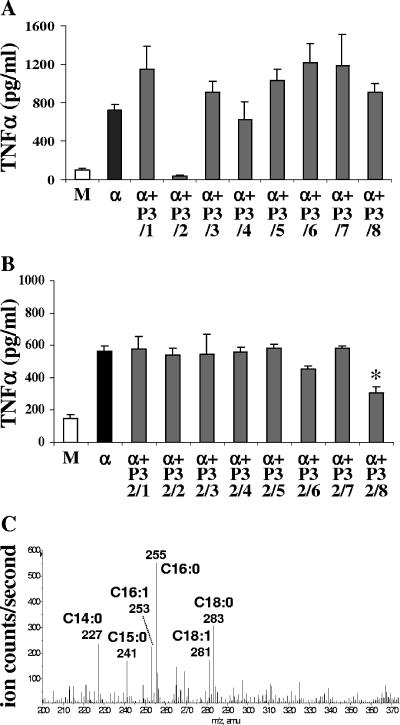
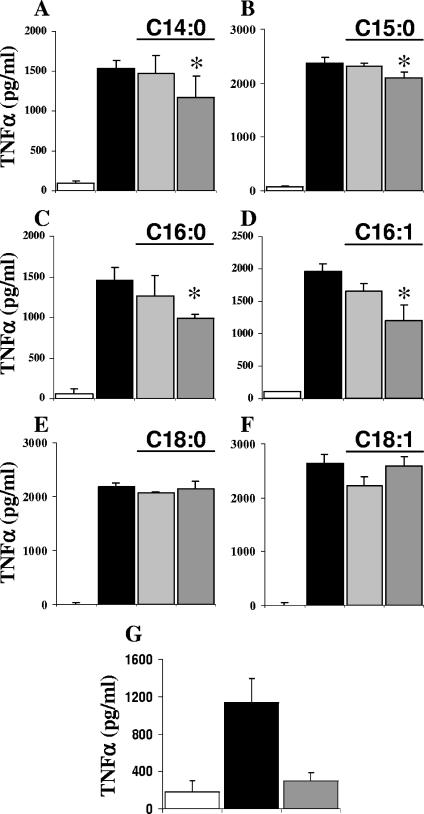
To determine which fatty acid in the panel was responsible for the inhibitory effect, TLC performed with the chloroform-methanol (50:2, by volume) solvent system was used to subfractionate fraction P3. Iodine vapors allowed staining of the unsaturated fatty acids, which were scraped off. The surfaces situated between the yellow spots, containing saturated fatty acids, were also scraped off in order to recover all molecules. Only the second area containing unsaturated fatty acids inhibited GPI-induced TNF-α production by macrophages (Fig. 5A). The yellow spot was large, suggesting that several molecules were present. A second TLC was done to further separate the fatty acids present in this inhibitory fraction. The chloroform-methanol-2 M aqueous ammonia (65:25:4, by volume) solvent system was used, and the unsaturated fatty acids were stained by using iodine vapors. Once again, areas were scraped off and tested with macrophages. The last fraction (migrating in the last 2 cm before the front [P3/2/8]) had an inhibitory effect (Fig. 5B). Analysis of this fraction by ES-MS revealed major ions at m/z 227, 241, 253, 255, 281, and 283, which were identified as myristic acid (C14:0), pentadecanoic acid (C15:0), palmitoleic acid (C16:1), palmitic acid (C16:0), oleic acid (C18:1), and stearic acid (C18:0), respectively (Fig. 5C). They were present at nanomolar concentrations in the sample. To discriminate between these six molecules, commercially available fatty acids were individually tested with macrophages stimulated by the P. falciparum GPI Pfα. Four of the six molecules, myristic, pentadecanoic, palmitic, and palmitoleic acids, were able to mimic the inhibitory effect of the natural compounds, but the inhibition was weaker (Fig. 6). This suggests that a specific proportional composition of fatty acids is required to obtain maximal down-regulation of TNF-α output. When the six fatty acids were added together in the same proportion as in the natural extract (1 part of C14:0, 0.7 part of C15:0, 1.5 parts of C16:0, 1 part of C16:1, 0.75 part of C18:0, and 0.7 part of C18:1), the TNF-α inhibition was stronger (Fig. 1G).
FIG. 5.
Isolation by TLC of the inhibitory fatty acids. (A) Macrophages were incubated for 24 h in the presence of Pfα (α) alone or with the eight different fractions (P3/1 to P3/8) obtained after separation of fraction P3 (from 2 × 108 trophozoites) by TLC. (B) Macrophages were incubated for 24 h in the presence of Pfα alone or with the eight fractions (P3/2/1 to P3/2/8 added 30 min before Pfα was added) obtained after separation of fraction 3/2 by TLC. Medium alone was used as a negative control (lanes M). The values are means ± standard deviations of triplicate samples in a representative experiment. (C) Negative-ion mass spectrum of P. falciparum inhibitory fraction P3/2/8. The annotated ions correspond to [M-H]− fatty acid carboxylate ions with the numbers of carbon atoms and numbers of C=C double bonds indicated. The y axis shows the relative ion intensities. Nominal mass values are indicated on the spectrum, but the accurate mass values were all within 3 ppm of the theoretical masses for the corresponding fatty acid carboxylate ions.
FIG. 6.
Myristic, pentadecanoic, palmitic, and palmitoleic acids inhibit GPI-induced TNF-α production. Macrophages were incubated for 24 h with medium alone (open bars), with Pfα alone (black bars), or with myristic acid (A), pentadecanoic acid (B), palmitic acid (C), palmitoleic acid (D), stearic acid (E), or oleic acid (F) at a concentration of 1 μM (light gray bars) or 10 μM (dark gray bars) or the six fatty acids together (G) added 30 min before Pfα was added. The values are means ± standard deviations of triplicate samples in a representative experiment.
All precursors of the GPI Pfα activate TNF-α.
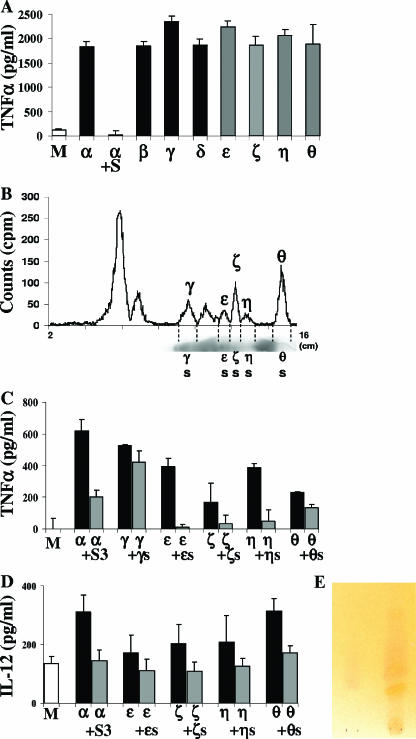
Pfj was located between the GPI precursors Pfδ [Man3-GlcN-inositol (Ino)(acyl)-P-DAG] and Pfɛ [Man2-GlcN-Ino(acyl)-P-DAG] (Fig. 1A). A previous study showed that Pfδ is able to induce production of TNF-α in macrophages, whereas Pfɛ and the precursors migrating farther on the plate, including Pfζ (GlcN-Ino-P-DAG), Pfη [Man1-GlcN-Ino(acyl)-P-DAG], and Pfθ [GlcN-Ino(acyl)-P-DAG], do not stimulate the cells (40). Our conclusion was that the minimal structure required to trigger a response in the cells should have three mannose residues (Manα1-2Manα1-6Manα1-4GlcN-inositol). The discovery of inhibitory fatty acids migrating after Pfδ raises the possibility that the TNF-α production could have been inhibited in previous experiments by other molecules comigrating with the smaller precursors (Pfɛ, Pfζ, Pfη, Pfθ). The GPI extraction procedure using chloroform-methanol-water followed by phase partitioning between water and water-saturated n-butanol simultaneously extracted phospholipids or other hydrophobic contaminants. To eliminate these unwanted molecules from the GPI preparation, the butanol phase was slowly dried under a nitrogen stream until the GPIs precipitated. This simple method efficiently separates the GPIs contained in the pellet from the contaminants present in the supernatant (2). The GPIs were dissolved in butanol and applied to a TLC plate. GPI Pfα and all its precursors were scraped off the TLC plate, extracted, and tested with macrophages. As shown in Fig. 7A, not only Pfα, Pfβ [EtN-P-Man3-GlcN-Ino(acyl)-P-DAG], Pfγ [Man4-GlcN-Ino(acyl)-P-DAG], and Pfδ but also the smaller precursors Pfɛ, Pfζ, Pfη, and Pfθ (containing less than three mannose residues) induced the production of TNF-α. This confirms that comigrating non-GPI molecules might down-regulate GPI activity. To further substantiate the presence of inhibitors in the supernatant obtained after GPI precipitation, the supernatant was added 30 min before Pfα was added. The TNF-α production induced by Pfα was then completely inhibited (Fig. 7A). The supernatant was separated by TLC with the same solvent system that was used for GPI separation (chloroform-methanol-water [4:4:1, by volume]), and a trace of radioactive GPIs was used to compare the migration results. The molecules present in the supernatant were visualized after the plate was sprayed with a 14% cupper sulfate-8% phosphoric acid solution and heated to 150°C for 20 min. Figure 7B shows that molecules in the supernatant migrated from Pfγ to the front of the plate. To determine whether these molecules had an inhibitory effect, they were scraped off and tested with macrophages. The silica areas ɛS, ζS, ηS, and θS comigrating with Pfɛ, Pfζ, Pfη, and Pfθ, respectively, inhibited the production of TNF-α (Fig. 7C) and IL-12 (Fig. 7D) induced by the GPI precursors. In contrast, molecules in area γS, which comigrated with Pfγ, did not have an inhibitory effect on the Pfγ-induced cytokine production (Fig. 7C). This confirms that inhibitory molecules migrating from Pfɛ to Pfθ were previously coextracted, masking the activity of these GPI precursors. In parallel, the contaminants in the supernatant were separated on both LC-NH2 and LC-WCX cartridges, and fraction 3 (S3) containing fatty acids was also able to inhibit Pfα-induced TNF-α and IL-12 production (Fig. 7C and D). To check the presence of free fatty acids in fraction S3, it was subjected to TLC (with hexane-chloroform-diethyl ether-acetic acid [80:10:10:1.5, by volume]), and free fatty acids were visualized after the plate was sprayed with 0.1% 2′,7′-dichlorofluorescein in ethanol, 1% aluminum chloride in ethanol, and 1% aqueous ferric chloride and warmed to 100°C after each spraying. Free fatty acids gave a rose-violet color (Fig. 7E).
FIG. 7.
All precursors of the GPI Pfα activate production of TNF-α. Macrophages were incubated for 24 h with GPI Pfα (α) or with its precursors Pfβ (β), Pfγ (γ), Pfδ (δ), Pfɛ (ɛ), Pfζ (ζ), Pfη (η), and Pfθ (θ). The supernatant obtained after GPI precipitation containing non-GPI species was added to macrophages 30 min before Pfα was added (α+S). The values are means ± standard deviations of triplicate samples in a representative experiment. (B) TLC scans of P. falciparum GPI precursors and of P. falciparum contaminating molecules (supernatant), showing their comigration with Pfγ to Pfθ. (C and D) The contaminant molecules migrating with Pfɛ, Pfζ, Pfη, and Pfθ, but not with Pfγ, inhibited production of TNF-α and IL-12 in macrophages (γS,ɛS, ζS, ηS, and θS were added 30 min before Pfγ, Pfɛ, Pfζ, Pfη, and Pfθ were added). Fraction 3, separated from the supernatant using LC-NH2 and LC-WCX cartridges, inhibited production of TNF-α and IL-12 in macrophages (S3 was added 30 min before Pfα was added). Medium alone was used as a negative control (lanes M). (E) TLC of S3. Free fatty acids are rose-violet. Myristic acid (My) was used as a positive control.
Altogether, our results show that malaria parasites produce different molecules (GPIs, fatty acids) with opposing activities affecting the cellular response of the host.
DISCUSSION
In this report, we show that fatty acids from P. falciparum are able to inhibit TNF-α production induced by the malarial toxin GPI Pfα in macrophages. An inhibitory activity was previously detected in lysates from erythrocytes infected with malarial parasites (38). When parasite extracts which did not induce the production of TNF-α were mixed with samples of malaria toxin before incubation with macrophages, no TNF-α was detectable in the culture supernatants, indicating that the nonstimulatory extracts contained an inhibitor. Furthermore, this inhibitor was able to abolish the tumoricidal activity of TNF-α with L929 cells (38). However, the nature of the inhibitor has not been identified, and it might be a fatty acid. Intraerythrocytic parasites have the capacity to synthesize triacylglycerol from the mature trophozoite stage to the schizont stage. Triacylglycerol is degraded into free fatty acids at the later stages (28). The resulting products, free fatty acids, are released into the medium during schizont rupture and/or merozoite release (28). This explains how the malarial fatty acids can act on host cells.
A remaining issue concerns the mechanisms by which the P. falciparum fatty acids trigger the intracellular signaling in the host cells. It is known that the malarial GPI induces the phosphorylation of several tyrosine kinases, leading to NF-κB activation (35, 40). A recent study showed that P. falciparum GPIs stimulate the production of TNF-α in macrophages through mainly Toll-like receptor 2 (TLR2) activation and to a lesser extent through TLR4 activation (16, 27). All unsaturated fatty acids tested by Lee et al., including docosahexaenoic acid (C22:6), eicosapentaenoic acid (EPA) (C20:5), arachidonic acid (C20:4), linoleic acid (C18:2), and oleic acid (C18:1), inhibited lipopolysaccharide (LPS)-induced NF-κB activation (19). Docosahexaenoic acid inhibited CD4-TLR4-induced NF-κB activation in 293T cells transfected with a constitutively active form of TLR4 (19). The fatty acid molecular target of GPI inhibition could be TLR2 or TLR4. An alternative hypothesis is that the fatty acids signal via the peroxisome proliferator-activated receptors (PPARs) that control a variety of genes in several pathways of lipid metabolism. Unsaturated and saturated fatty acids were identified as PPAR ligands participating in the PPAR-dependent transcriptional activity. Conjugated linoleic acid, a mixture of C18:2 derivatives, is a ligand and activator of PPARα and has an inhibitory effect on LPS-induced TNF-α production by RAW 264.7 macrophages compared with a linoleic acid control (25, 43). Oxidized EPA but not unoxidized EPA potently inhibited cytokine-induced activation of endothelial NF-κB (23). The inhibitory effect of oxidized EPA requires PPARα and acts possibly through direct interactions of PPARα with the p50/p65 subunits of NF-κB. Furthermore, PPARγ agonists reduce P. falciparum-induced TNF-α from human monocytes (37). All these data raise the possibility that myristic, palmitic, and palmitoleic acids from P. falciparum inhibit the GPI-induced TNF-α production via inhibition of NF-κB activation through PPAR. CD36 is the major receptor mediating the uptake of nonopsonized parasitized erythrocytes (22). Treatment of monocytes with PPARγ agonists (15d-PGJ2 or ciglitazone) resulted in an increase in surface CD36 and increased uptake of nonopsonized parasitized erythrocytes (37). Thus, it is conceivable that the malaria fatty acids participate in parasite uptake as PPAR agonists.
Our finding raises the possibility that there is a balance between the different lipid classes (GPIs and fatty acids) of P. falciparum in the inflammatory response of the host. If released when parasitized erythrocytes rupture in vivo, fatty acids could down-regulate the production of TNF-α and thus reduce its damaging effects on both parasite and host. Only about 1% of infected patients die of severe malaria complications. Lower production or release of fatty acids by the parasite might contribute to overproduction of TNF-α induced by the GPIs. However, GPI toxin activity is not restricted solely to the TNF-α pathway, and GPI toxicity in vivo may be partially cytokine independent (33, 35). GPI-induced TLR signaling is likely to induce complex gene expression profiles with many bioactivities, similar to other TLR agonists (30), of which TNF-α is simply one marker. Whether fatty acids down-regulate other relevant toxin-induced gene expression remains to be determined. Free fatty acids decreased parasitemia and improved the survival of mice infected by Plasmodium yoelii (20). It is possible that nonmalarial fatty acids also participate in the down-regulation of GPI bioactivity.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft, Fonds der Chemischen Industrie, Stiftung P.E. Kempkes, Hessisches Ministerium für Wissenschaft und Kunst, a British Council/DAAD ARC grant, the Wellcome Trust (grant 071463 to M.A.J.F.), the NH&MRC, NIH, WHO/TDR, HFSP, and Grand Challenge in Global Health (grant to L.S.). L.S. is an International Research Scholar of the Howard Hughes Medical Institute.
We thank Miltenyi Biotec GmbH for lending the SuperMACS unit. We thank P. Gerold for his contribution in the initial phase of this work.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Ansorge, I., D. Jeckel, F. Wieland, and K. Lingelbach. 1995. Plasmodium falciparum-infected erythrocytes utilize a synthetic truncated ceramide precursor for synthesis and secretion of truncated sphingomyelin. Biochem. J. 308:335-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azzouz, N., H. Shams-Eldin, and R. T. Schwarz. 2005. Removal of phospholipid contaminants through precipitation of glycosylphosphatidylinositols. Anal. Biochem. 343:152-158. [DOI] [PubMed] [Google Scholar]
- 3.Bodennec, J., O. Koul, I. Aguado, G. Brichon, G. Zwingelstein, and J. Portoukalian. 2000. A procedure for fractionation of sphingolipid classes by solid-phase extraction on aminopropyl cartridges. J. Lipid Res. 41:1524-1531. [PubMed] [Google Scholar]
- 4.Debierre-Grockiego, F., N. Azzouz, J. Schmidt, J. F. Dubremetz, H. Geyer, R. Geyer, R. Weingart, R. R. Schmidt, and R. T. Schwarz. 2003. Roles of glycosylphosphatidylinositols of Toxoplasma gondii. Induction of tumor necrosis factor-alpha production in macrophages. J. Biol. Chem. 278:32987-32993. [DOI] [PubMed] [Google Scholar]
- 5.Delorenzi, M., A. Sexton, H. Shams-Eldin, R. T. Schwarz, T. Speed, and L. Schofield. 2002. Genes for glycosylphosphatidylinositol toxin biosynthesis in Plasmodium falciparum. Infect. Immun. 70:4510-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dieckmann-Schuppert, A., S. Bender, M. Odenthal-Schnittler, E. Bause, and R. T. Schwarz. 1992. Apparent lack of N-glycosylation in the asexual intraerythrocytic stage of Plasmodium falciparum. Eur. J. Biochem. 205:815-825. [DOI] [PubMed] [Google Scholar]
- 7.Elabbadi, N., M. L. Ancelin, and H. J. Vial. 1997. Phospholipid metabolism of serine in Plasmodium-infected erythrocytes involves phosphatidylserine and direct serine decarboxylation. Biochem. J. 324:435-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elmendorf, H. G., and K. Haldar. 1994. Plasmodium falciparum exports the Golgi marker sphingomyelin synthase into a tubovesicular network in the cytoplasm of mature erythrocytes. J. Cell Biol. 124:449-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Folch, J., M. Lees, and G. H. Sloane Stanley. 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226:497-509. [PubMed] [Google Scholar]
- 10.Gerold, P., A. Dieckmann-Schuppert, and R. T. Schwarz. 1994. Glycosylphosphatidylinositols synthesized by asexual erythrocytic stages of the malarial parasite, Plasmodium falciparum. Candidates for plasmodial glycosylphosphatidylinositol membrane anchor precursors and pathogenicity factors. J. Biol. Chem. 269:2597-2606. [PubMed] [Google Scholar]
- 11.Gerold, P., N. Jung, N. Azzouz, N. Freiberg, S. Kobe, and R. T. Schwarz. 1999. Biosynthesis of glycosylphosphatidylinositols of Plasmodium falciparum in a cell-free incubation system: inositol acylation is needed for mannosylation of glycosylphosphatidylinositols. Biochem. J. 344:731-738. [PMC free article] [PubMed] [Google Scholar]
- 12.Gerold, P., L. Schofield, M. J. Blackman, A. A. Holder, and R. T. Schwarz. 1996. Structural analysis of the glycosyl-phosphatidylinositol membrane anchor of the merozoite surface proteins-1 and -2 of Plasmodium falciparum. Mol. Biochem. Parasitol. 75:131-143. [DOI] [PubMed] [Google Scholar]
- 13.Gerold, P., and R. T. Schwarz. 2001. Biosynthesis of glycosphingolipids de-novo by the human malaria parasite Plasmodium falciparum. Mol. Biochem. Parasitol. 112:29-37. [DOI] [PubMed] [Google Scholar]
- 14.Haldar, K., L. Uyetake, N. Ghori, H. G. Elmendorf, and W. L. Li. 1991. The accumulation and metabolism of a fluorescent ceramide derivative in Plasmodium falciparum-infected erythrocytes. Mol. Biochem. Parasitol. 49:143-156. [DOI] [PubMed] [Google Scholar]
- 15.Hsiao, L. L., R. J. Howard, M. Aikawa, and T. F. Taraschi. 1991. Modification of host cell membrane lipid composition by the intra-erythrocytic human malaria parasite Plasmodium falciparum. Biochem. J. 274:121-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krishnegowda, G., A. M. Hajjar, J. Zhu, E. J. Douglass, S. Uematsu, S. Akira, A. S. Woods, and D. C. Gowda. 2005. Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum: cell signaling receptors, glycosylphosphatidylinositol (GPI) structural requirement, and regulation of GPI activity. J. Biol. Chem. 280:8606-8616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lauer, S. A., N. Ghori, and K. Haldar. 1995. Sphingolipid synthesis as a target for chemotherapy against malaria parasites. Proc. Natl. Acad. Sci. USA 92:9181-9185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lauer, S. A., P. K. Rathod, N. Ghori, and K. Haldar. 1997. A membrane network for nutrient import in red cells infected with the malaria parasite. Science 276:1122-1125. [DOI] [PubMed] [Google Scholar]
- 19.Lee, J. Y., A. Plakidas, W. H. Lee, A. Heikkinen, P. Chanmugam, G. Bray, and D. H. Hwang. 2003. Differential modulation of Toll-like receptors by fatty acids: preferential inhibition by n-3 polyunsaturated fatty acids. J. Lipid Res. 44:479-486. [DOI] [PubMed] [Google Scholar]
- 20.Levander, O. A., A. L. Ager, Jr., V. C. Morris, and R. G. May. 1990. Plasmodium yoelii: comparative antimalarial activities of dietary fish oils and fish oil concentrates in vitamin E-deficient mice. Exp. Parasitol. 70:323-329. [DOI] [PubMed] [Google Scholar]
- 21.Martin, D., L. Gannoun-Zaki, S. Bonnefoy, P. Eldin, K. Wengelnik, and H. Vial. 2000. Characterization of Plasmodium falciparum CDP-diacylglycerol synthase, a proteolytically cleaved enzyme. Mol. Biochem. Parasitol. 110:93-105. [DOI] [PubMed] [Google Scholar]
- 22.McGilvray, I. D., L. Serghides, A. Kapus, O. D. Rotstein, and K. C. Kain. 2000. Nonopsonic monocyte/macrophage phagocytosis of Plasmodium falciparum-parasitized erythrocytes: a role for CD36 in malarial clearance. Blood 96:3231-3240. [PubMed] [Google Scholar]
- 23.Mishra, A., A. Chaudhary, and S. Sethi. 2004. Oxidized omega-3 fatty acids inhibit NF-kappaB activation via a PPARalpha-dependent pathway. Arterioscler. Thromb. Vasc. Biol. 24:1621-1627. [DOI] [PubMed] [Google Scholar]
- 24.Mitamura, T., K. Hanada, E. P. Ko-Mitamura, M. Nishijima, and T. Horii. 2000. Serum factors governing intraerythrocytic development and cell cycle progression of Plasmodium falciparum. Parasitol. Int. 49:219-229. [DOI] [PubMed] [Google Scholar]
- 25.Moya-Camarena, S. Y., J. P. Vanden Heuvel, S. G. Blanchard, L. A. Leesnitzer, and M. A. Belury. 1999. Conjugated linoleic acid is a potent naturally occurring ligand and activator of PPARalpha. J. Lipid Res. 40:1426-1433. [PubMed] [Google Scholar]
- 26.Nawabi, P., A. Lykidis, D. Ji, and K. Haldar. 2003. Neutral-lipid analysis reveals elevation of acylglycerols and lack of cholesterol esters in Plasmodium falciparum-infected erythrocytes. Eukaryot. Cell. 2:1128-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nebl, T., D. E. V. MJ, and L. Schofield. 2005. Stimulation of innate immune responses by malarial glycosylphosphatidylinositol via pattern recognition receptors. Parasitology. 130(Suppl. 1):S45-S62. [DOI] [PubMed] [Google Scholar]
- 28.Palacpac, N. M., Y. Hiramine, F. Mi-ichi, M. Torii, K. Kita, R. Hiramatsu, T. Horii, and T. Mitamura. 2004. Developmental-stage-specific triacylglycerol biosynthesis, degradation and trafficking as lipid bodies in Plasmodium falciparum-infected erythrocytes. J. Cell Sci. 117:1469-1480. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt, A., R. T. Schwarz, and P. Gerold. 1998. Plasmodium falciparum: asexual erythrocytic stages synthesize two structurally distinct free and protein-bound glycosylphosphatidylinositols in a maturation-dependent manner. Exp. Parasitol. 88:95-102. [DOI] [PubMed] [Google Scholar]
- 30.Schmitz, F., J. Mages, A. Heit, R. Lang, and H. Wagner. 2004. Transcriptional activation induced in macrophages by Toll-like receptor (TLR) ligands: from expression profiling to a model of TLR signaling. Eur. J. Immunol. 34:2863-2873. [DOI] [PubMed] [Google Scholar]
- 31.Schofield, L. 2002. Antidisease vaccines. Chem. Immunol. 80:322-342. [DOI] [PubMed] [Google Scholar]
- 32.Schofield, L., and G. E. Grau. 2005. Immunological processes in malaria pathogenesis. Nat. Rev. Immunol. 5:722-735. [DOI] [PubMed] [Google Scholar]
- 33.Schofield, L., and F. Hackett. 1993. Signal transduction in host cells by a glycosylphosphatidylinositol toxin of malaria parasites. J. Exp. Med. 177:145-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schofield, L., M. C. Hewitt, K. Evans, M. A. Siomos, and P. H. Seeberger. 2002. Synthetic GPI as a candidate anti-toxic vaccine in a model of malaria. Nature 418:785-789. [DOI] [PubMed] [Google Scholar]
- 35.Schofield, L., S. Novakovic, P. Gerold, R. T. Schwarz, M. J. McConville, and S. D. Tachado. 1996. Glycosylphosphatidylinositol toxin of Plasmodium up-regulates intercellular adhesion molecule-1, vascular cell adhesion molecule-1, and E-selectin expression in vascular endothelial cells and increases leukocyte and parasite cytoadherence via tyrosine kinase-dependent signal transduction. J. Immunol. 156:1886-1896. [PubMed] [Google Scholar]
- 36.Schwarzer, E., H. Kuhn, E. Valente, and P. Arese. 2003. Malaria-parasitized erythrocytes and hemozoin nonenzymatically generate large amounts of hydroxy fatty acids that inhibit monocyte functions. Blood 101:722-728. [DOI] [PubMed] [Google Scholar]
- 37.Serghides, L., and K. C. Kain. 2001. Peroxisome proliferator-activated receptor gamma-retinoid X receptor agonists increase CD36-dependent phagocytosis of Plasmodium falciparum-parasitized erythrocytes and decrease malaria-induced TNF-alpha secretion by monocytes/macrophages. J. Immunol. 166:6742-6748. [DOI] [PubMed] [Google Scholar]
- 38.Sheikh, N. A., H. N. Caro, J. Taverne, J. H. Playfair, and T. W. Rademacher. 1996. Malaria: a tumour necrosis factor inhibitor from parasitized erythrocytes. Immunology 87:461-466. [PMC free article] [PubMed] [Google Scholar]
- 39.Tachado, S. D., P. Gerold, M. J. McConville, T. Baldwin, D. Quilici, R. T. Schwarz, and L. Schofield. 1996. Glycosylphosphatidylinositol toxin of Plasmodium induces nitric oxide synthase expression in macrophages and vascular endothelial cells by a protein tyrosine kinase-dependent and protein kinase C-dependent signaling pathway. J. Immunol. 156:1897-1907. [PubMed] [Google Scholar]
- 40.Tachado, S. D., P. Gerold, R. Schwarz, S. Novakovic, M. McConville, and L. Schofield. 1997. Signal transduction in macrophages by glycosylphosphatidylinositols of Plasmodium, Trypanosoma, and Leishmania: activation of protein tyrosine kinases and protein kinase C by inositolglycan and diacylglycerol moieties. Proc. Natl. Acad. Sci. USA 94:4022-4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vial, H. J., M. L. Ancelin, J. R. Philippot, and M. J. Thuet. 1990. Biosynthesis and dynamics of lipids in Plasmodium-infected mature mammalian erythrocytes. Blood Cells 16:531-555. (Discussion, 16:556-561.) [PubMed] [Google Scholar]
- 42.Vielemeyer, O., M. T. McIntosh, K. A. Joiner, and I. Coppens. 2004. Neutral lipid synthesis and storage in the intraerythrocytic stages of Plasmodium falciparum. Mol. Biochem. Parasitol. 135:197-209. [DOI] [PubMed] [Google Scholar]
- 43.Yang, M., and M. E. Cook. 2003. Dietary conjugated linoleic acid decreased cachexia, macrophage tumor necrosis factor-alpha production, and modifies splenocyte cytokines production. Exp. Biol. Med. 228:51-58. [DOI] [PubMed] [Google Scholar]
- 44.Yeo, H. J., J. Sri Widada, O. Mercereau-Puijalon, and H. J. Vial. 1995. Molecular cloning of CTP:phosphocholine cytidylyltransferase from Plasmodium falciparum. Eur. J. Biochem. 233:62-72. [DOI] [PubMed] [Google Scholar]