Abstract
Acyl homoserine lactones are synthesized by Pseudomonas aeruginosa as signaling molecules which control production of virulence factors and biofilm formation in a paracrine manner. We found that N-(3-oxododecanoyl)-l-homoserine lactone (3OC12-HSL), but not its 3-deoxo isomer or acyl-homoserine lactones with shorter fatty acids, induced the directed migration (chemotaxis) of human polymorphonuclear neutrophils (PMN) in vitro. By use of selective inhibitors a signaling pathway, comprising phosphotyrosine kinases, phospholipase C, protein kinase C, and mitogen-activated protein kinase C, could be delineated. In contrast to the well-studied chemokines complement C5a and interleukin 8, the chemotaxis did not depend on pertussis toxin-sensitive G proteins, indicating that 3OC12-HSL uses another signaling pathway. Strong evidence for the presence of a receptor for 3OC12-HSL on PMN was derived from uptake studies; by use of radiolabeled 3OC12-HSL, specific and saturable binding to PMN was seen. Taken together, our data provide evidence that PMN recognize and migrate toward a source of 3OC12-HSL (that is, to the site of a developing biofilm). We propose that this early attraction of PMN could contribute to prevention of biofilm formation.
Cell-to-cell communication among bacteria, such as Pseudomonas aeruginosa, relies on acyl-homoserine lactones (AHL) as the signaling molecules. AHL, also known as quorum-sensing molecules or autoinducers, are crucial for the production of virulence factors and for biofilm formation (6, 7, 8, 9, 11, 17, 21, 25, 33; for a review, see reference 10). Biofilm formation is common in P. aeruginosa infection and is the leading cause of morbidity and mortality in patients with cystic fibrosis (16, 18, 19). Moreover, opportunistic and nosocomial infections, wound infections, and implant-associated infections are caused by bacterial biofilms (for a review, see reference 5).
How the host defense reacts to biofilm infections is currently under investigation. It is assumed that in contrast to planktonic bacteria, the bacteria organized in biofilms are relatively resistant to the effector function of the immune system. A variety of escape mechanisms have been proposed, including the barrier function of the extracellular polymer substances (EPS), that could block access of immunocompetent cells to the embedded bacteria. The fact that bacteria, once released from the biofilm, become susceptible to killing, at least under experimental conditions, supports this proposal (12). Moreover, immunmodulatory activities of the EPS, particularly alginate, have been described (18, 23), as have effects of the quorum- sensing molecules. For N-(3-oxododecanoyl)-l-homoserine lactone (3OC12-HSL), a variety of effects on immunocompetent cells have been described, including inhibition of tumor necrosis factor alpha synthesis by activated macrophages, inhibition of proliferation of T-lymphocytes, and modulation of the B-cell response (3, 20, 27). Moreover, induction of apoptosis of mouse macrophages or neutrophils has been observed (26).
On the other hand, in response to biofilm infection the immune response is activated, and there is evidence that there is infiltration of immune effector cells into the infected site (29, 30, 31). Moreover, mechanisms to disrupt biofilm formation have been described; lactoferrin, which is also produced by phagocytic cells, prevents biofilm formation at doses below the doses required for bacterial killing (24). Moreover, epithelial cells inactivate AHL by a mechanism that has not been defined yet, which could prevent biofilm formation (4).
Here we report that 3OC12-HSL induces directed migration (chemotaxis) of neutrophils, which could represent a mechanism for attracting immune effector cells to the site of a developing biofilm.
MATERIALS AND METHODS
Acyl-homoserine lactones.
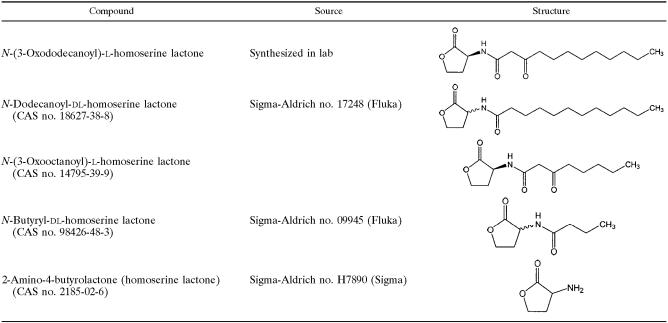
The chemical structures of the acyl-homoserine lactones are summarized in Table 1. N-Dodecanoyl-dl-homoserine lactone (C12-HSL) was purchased from Sigma Aldrich (München, Germany). N-Butyryl-dl-homoserine lactone (C4-HSL) was purchased from Sigma Aldrich, and 2-amino-4-butyrolactone was purchased from Fluka (Buchs, Switzerland). For all compounds, stock solutions (0.1 M) were prepared in dimethyl sulfoxide and diluted in Hanks balanced salt solution (HBSS) immediately before use (concentrations range, 0.1 μM to 100 μM).
TABLE 1.
Structures of the homoserine lactones used in this study
For synthesis of 3OC12-HSL and N-(3-oxooctanoyl)-l-homoserine lactone. (C8-HSL), the method described by Chhabra et al. (3) was used. In brief, equivalent amounts of Meldrums's acid (1 mmol; Aldrich) and the corresponding fatty acids (Fluka) were used along with equivalent amounts of 4-(dimethylamino)pyridine (Fluka) and N,N-dicyclohexylcarbodiimide (Aldrich). Each compound was purified to homogeneity by preparative liquid chromatography. 3OC12-HSL was radiolabeled during synthesis by using [14C]decanoic acid (50 mCi/mmol; Moravek Biochemicals, Brea, CA) as the precursor. The purity of the radiolabeled product was assessed by thin-layer chromatography. Three preparations were used, which differed to some extent with regard to the amount of radioactivity/mmol. The average radioactivity was 4.5 mCi/100 mmol.
Isolation of PMN from peripheral blood.
After informed consent was obtained, heparin blood was drawn from volunteers, mainly laboratory personal and students with no obvious symptoms of disease, observing the institutional guidelines. Polymorphonuclear neutrophils (PMN) were isolated by using PolymorphPrep (Nycomed, Oslo, Norway) and following the recommendations of the supplier. The PMN fraction was harvested, washed repeatedly in phosphate-buffered saline (pH 7.4), and suspended in HBSS containing 0.1% bovine serum albumin at a final concentration of 1 × 106 cells per ml.
Uptake of [14C]3OC12-HSL.
For the uptake studies 1 × 106 PMN suspended in 100 μl of HBSS supplemented with 5% bovine serum albumin were incubated with 5 μl of [14C]3OC12-HSL (0.01 μM to 100 μM) at 4°C for 20 min. After the cells were washed twice with HBSS, the radioactivity associated with the cells was measured using LSC Ultima.Gold MV cocktail (Packard, Groningen, The Netherlands) and an LS-1701 Coulter Counter (Beckman, München, Germany). To test for the specificity of uptake, increasing amounts of unlabeled 3OC12-HSL (5 to 100 μM) were added to the cells before [14C]3OC12-HSL (0.1 μM) was added.
Chemotaxis across a membrane filter.
A modified Boyden chamber assay was performed (2), using a chamber equipped with a nitrocellulose filter (pore size, 5 μm; thickness, 200 μm; Schleicher & Schuell GmbH, Dassel, Germany). As bona fide chemokines, recombinant human complement C5a (2 ng/ml; Sigma-Aldrich) and interleukin 8 (IL-8) (8 ng/ml; Immunotools, Friesoythe, Germany) were used. Random migration was assessed using HBSS. The cells (1 × 106 cells in 1 ml) were placed in the upper compartment, and the chemokines were placed in the lower compartment. After 90 min the cells that migrated into the filters were fixed with propanol, stained with hematoxylin, and evaluated using an Omnicon Alpha image analyzer (Bausch and Lomb, Heidelberg, Germany). Chemotaxis was measured by determining the position of the “leading front,” defined as the distance (in μm) from the top of the filter to a level where at least five cells could still be seen. Two parallel filters were prepared, and on each filter 10 different areas were evaluated. The mean ± standard deviation was calculated; differences between means were determined using a two-tailed t test. Because the chemotactic activities of PMN vary widely between individuals, all tests were carried out with cells from different donors. Cells were preincubated with C5a (2 ng/ml), IL-8 (8 ng/ml), or the corresponding acyl homoserine lactones at room temperature for 10 min.
Inhibitors.
The extracellular signal-regulated kinase inhibitor PD98059 and the p38 mitogen-activated protein kinase inhibitor SB203580 were obtained from LC Laboratories, Woburn, MA. The phosphatidylinositol-3-kinase inhibitor Wortmannin, the phospholipase C inhibitor Staurosporine, and the phospholipase C or A2 inhibitor U73122 were purchased from Sigma-Aldrich. Pertussis toxin, an inhibitor of Gα1 GTP-binding protein, was obtained from Calbiochem (Marburg, Germany), Gefinitib, an inhibitor of phosphotyrosine kinases, was obtained from Biaffin (Kassel, Germany). PMN were pretreated with the inhibitors at various concentrations for 10 min at room temperature. Then the chemotaxis test was performed. The viability of PMN was assessed by trypan blue exclusion after 90 min (end of the chemotaxis test).
RESULTS
Induction of chemotaxis by AHL.
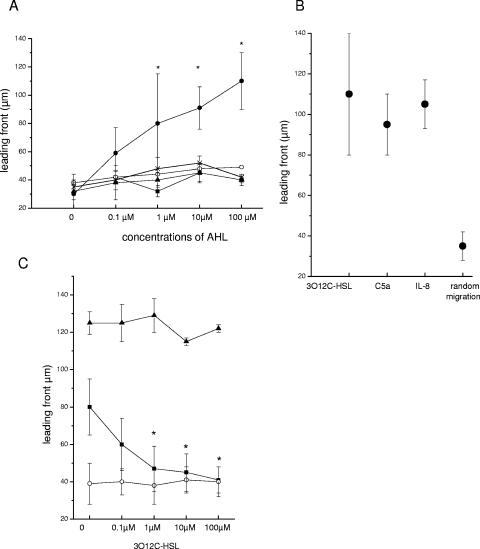
In the first series of experiments, the chemotaxis of PMN toward AHL was tested. Using a modified Boyden chamber assay, dose-dependent migration of PMN toward 3OC12-HSL was seen. At a concentration of 100 μM the migration (110 ± 25 μm [mean ± standard deviation for experiments with cells from seven different donors]) was similar to the migration seen in response to optimal concentrations of the well-studied chemokines complement C5a and IL-8 (Fig. 1A and B).
FIG. 1.
3OC12-HSL is chemotactic for PMN. (A) PMN were isolated from a healthy donor, and migration toward increasing concentrations of 3OC12-HSL (•), C12-HSL (×), C8-HSL (▴), C4-HSL (▪), or 2-amino-4-butyrolactone (○) was measured by determining the position of the leading front (μm). The data are means ± standard deviations for 20 different areas on two parallel filters. The asterisks indicate that the migration induced by 3OC12-HSL differs significantly from random migration and the migration induced by the other AHL (the largest P value was 0.01). (B) Chemotaxis toward 3OC12-HSL (100 μM), recombinant C5a (2 ng/ml), or IL-8 (8 ng/ml) compared to random migration. The data are means ± standard deviations for seven donors; differences between stimulated cells and random migration were significant with at a P value of <0.001. (C) Preincubation of PMN from one donor with increasing doses of 3OC12-HSL reduced chemotaxis toward IL-8 (8 ng/ml) (▪) but not toward C5a (2 ng/ml) (▴) (○, random migration). The data are means ± standard deviations for 20 different areas on two parallel filters; the asterisks indicate that the means are different from the means for the samples without 3OC12-HSL (P < 0.01).
The commercially available compound C12-HSL did not induce chemotaxis, nor did AHL with shorter fatty acids or 2-amino-4-butyrolactone, which lacks fatty acids (Fig. 1A).
Next, possible interactions between 3OC12-HSL and C5a or IL-8 were investigated. 3OC12-HSL did not act synergistically with either C5a or IL-8. Moreover, preincubation of PMN with IL-8 or C5a did not inhibit their migration toward 3OC12-HSL (data not shown). Preincubation of PMN with 3OC12-HSL, however, reduced the chemotaxis toward IL-8 but not the chemotaxis toward C5a (Fig. 1C).
The AHL with short fatty acids (C4-HSL or C8-HSL) did not modulate the chemotaxis induced by 3OC12-HSL, nor did C12-HSL (data not shown).
Signaling pathway of 3OC12-HSL-induced chemotaxis.
Because all the known chemokines induce chemotaxis via surface receptors, we examined whether PMN expressed a receptor for AHL. In this context, we explored possible signaling pathways by preincubating PMN with inhibitors known to block second messengers or signal-transducing molecules. The inhibitors were used at concentrations that did not affect the viability. In all experiments, chemotaxis toward IL-8 was tested in parallel, because the signaling pathway for IL-8 and the IL-8 receptor has been well studied. The concentration that reduced the chemotaxis by 50% was calculated from the results of three independent experiments using cells from different donors. As shown in Table 2 and Fig. 2 and 3, inhibiting tyrosine kinases, phospholipase C, protein kinase C, and the mitogen-activated protein kinase reduced the chemotaxis toward 3OC12-HSL. Inhibition of extracellular protein kinase and of phosphoinositol-3-kinase, which are crucial for the IL-8-mediated chemotaxis, did not inhibit the 3OC12-HSL-induced chemotaxis. It is noteworthy that pertussis toxin, a specific inhibitor of G-protein-dependent signals, did not affect the chemotaxis in response to 3OC12-HSL but, in line with previously published data, affected the chemotaxis in response to IL-8 (Fig. 2). These data suggest the signaling pathway shown schematically in Fig. 3.
TABLE 2.
Effects of inhibitors of signaling pathways on 3O12C-HSL-induced chemotaxis
| Inhibitor | Target protein | IC50a |
|---|---|---|
| Gefinitib | Tyrosine kinase | 0.1 μM |
| Pertussis toxin | G protein | NI |
| U73122 | Phospholipase C | 1 μM |
| Staurosporin | Protein kinase C | 0.125 nM |
| SB203580 | Mitogen-activated protein kinase | 0.1 μM |
| PD98059 | Extracellular protein kinase | NI |
| Wortmannin | Phosphoinositol-3-kinase | NI |
IC50, concentration that inhibited chemotaxis by 50%. The values are means of three independent experiments performed using cells from different donors. NI, no inhibition.
FIG. 2.
Effects of inhibitors of various signaling pathways on the 3OC12-HSL-induced chemotaxis of PMN. PMN were preincubated with increasing doses (shown on the x axis) of Gefinitib (A) or pertussis toxin (B) for 10 min. After washing, chemotaxis toward 3OC12-HSL (100 μM) (•) was measured; the open circles show random migration, and the asterisks indicate that the means differed from the mean for the sample without Gefinitib (P < 0.002). For comparison chemotaxis toward IL-8 was measured (8 ng/ml) (right panels) (the results for only the highest concentration of the inhibitor are shown). The experiments were carried out with cells from different donors, which explains the differences in the chemotactic activities toward 3OC12-HSL and IL-8.
FIG. 3.
Schematic diagram of the most probable 3OC12-HSL signaling pathway. Because inhibition of tyrosine kinases (by Gefinitib), of phospholipase C (PLC) (by U73122), of protein kinase C (PKC) (by Staurosporin), and of mitogen-activated protein kinase (MEK) (by SB203580) inhibited the chemotaxis induced by 3OC12-HSL, we propose that the putative 3OC12-HSL receptor binds to a tyrosine kinase and induces the signaling pathway indicated on the left. This pathway differs from that for IL-8 because the IL-8 receptor is associated with G protein (the signal can be inhibited by pertussis toxin) (signaling pathway shown on the right). Triggering of the IL-8 receptor also leads to activation of phospholipase C and phosphatidylinositol-3-kinase (PI3K). The latter presumably activates mitogen-activated protein kinase and thus could represent an alternative or additional signaling pathway for IL-8, and this explains why preincubation of PMN with 3OC12-HSL reduced the chemotaxis toward IL-8 and not vice versa. On the right, the well-established signaling pathway for the IL-8-receptor (IL-8-R) is shown; activation of adenylate cyclase (AC) leads to upregulation of cyclic AMP (cAMP) and eventually upregulation of protein kinase A (PKA). DAG, diacylglycerol, Erk, extracellular signal-regulated kinase.
Uptake of 3OC12-HSL by PMN.
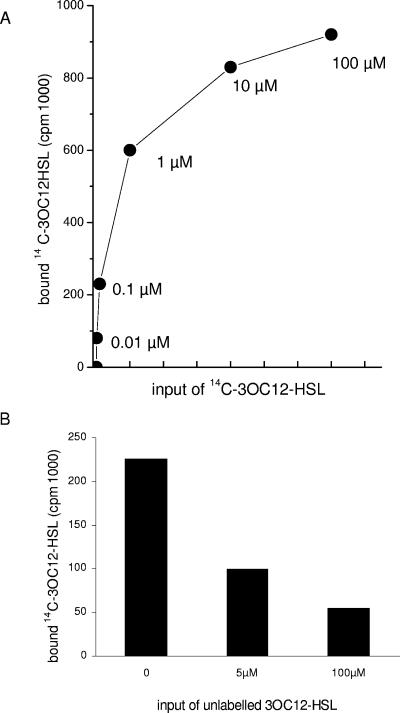
To test expression of a receptor for 3OC12-HSL more directly, binding studies with radiolabeled 3OC12-HSL were performed. 3OC12-HSL was labeled by using [14C]decanoic acid as the precursor for synthesis. Three different 14C-labeled homoserine lactone preparations were used and tested as follows: [14C]3OC12-HSL at various concentrations was incubated with PMN at 4°C for 20 min, and then the radioactivity associated with PMN was measured following repeated washing (the results of a representative experiment are shown in Fig. 4A). When a total of 100 μM [14C]3OC12-HSL was added, the uptake was about 1% (mean for the three preparations). Unlabeled 3OC12-HSL inhibited the uptake of the radiolabeled product (the results of a representative experiment are shown in Fig. 4B). From three experiments using 0.1 μM [14C]3OC12-HSL, a level of inhibition between 50 and 56% was calculated when the PMN were preincubated with 100 μM unlabeled 3OC12-HSL.
FIG. 4.
Uptake of 14C-labeled 3OC12-HSL by PMN. (A) PMN from a healthy donor were incubated with increasing concentrations of [14C]3OC12-HSL at 4°C for 20 min, and then the radioactivity associated with the cells was estimated (note that the x axis is linear for concentrations between 0 and 1 μM but logarithmic [log10] for concentrations from 1 to 100 μM). (B) Binding of [14C]3OC12-HSL (0.1 μM) to PMN could be inhibited by an excess of unlabeled 3OC12-HSL.
DISCUSSION
Bacterial biofilms are increasingly recognized as a major cause of persistent infections. Apparently, the biofilms protect the bacteria against host defense; there is, however, evidence that at least early in biofilm development, specific antibiofilm defense mechanisms are operative (4, 24). Lactoferrin, which is a major constituent of PMN, was shown to block biofilm development (24), leading to the question whether PMN can infiltrate a biofilm formation site. With this question in mind, we tested the effect of acyl homoserine lactones on PMN.
We found that 3OC12-HSL is chemotactic for PMN in a rather selective manner. Acyl homoserine lactones with shorter fatty acids (C4-HSL and C8-HSL) did not induce the migration of PMN, nor did C12-HSL or 2-amino-4-butyrolactone, the latter of which has an identical lactone ring but lacks a fatty acid chain (32). This indicates that the induction of chemotaxis is critically dependent on the three-dimensional structure of the molecule and possibly on its hydrophobicity.
Chemotaxis of PMN is crucial for host defense, particularly in bacterial infections. It is elicited by various cytokines, lipid mediators, or bacterial products. Receptors have been identified for all these chemoattractants, and these receptors link the extracellular signaling molecule to the nucleus and the cytoskeleton. Whether mammalian cells express a receptor(s) for 3OC12-HSL is not known yet, despite numerous studies showing effects of 3OC12-HSL on these cells and vice versa (4, 20, 27).
Our data, although not conclusive, are compatible with the hypothesis that there is a receptor for 3OC12-HSL on PMN because (i) specific and saturable binding of [14C]3OC12-HSL to PMN could be demonstrated and (ii) dependence on an established, receptor-linked signaling cascade involving tyrosine kinase, phospholipase C, protein kinase C, and mitogen-activated protein kinase was found. In keeping with the hypothesis that there is a receptor for 3OC12-HSL is the requirement for a definite isomeric conformation. This finding corresponds to a finding of Chhabra et al. (3), who showed that the immunomodulatory activity of AHL, as determined in a splenocyte proliferation assay, depended critically on the l configuration. Another structural requirement is the length of the fatty acids. Again, this is in line with the finding of Chhabra et al. (3) that the lipophilicity is important for the functional activity.
It is noteworthy that in contrast to other chemoattractants, the putative 3OC12-HSL receptor is not linked to Gα1 protein, since the 3OC12-HSL-induced chemotaxis could not be inhibited by pertussis toxin. Receptors linked to pertussis toxin-insensitive Gq proteins, however, are not excluded. PMN express numerous Gq-linked receptors, some of which are orphan receptors and some of which mediate chemotaxis in response to fatty acids (1, 15). Alternatively, the putative 3OC12-HSL receptor could be a tyrosine kinase or could be linked directly to a tyrosine kinase.
Without a doubt 3OC12-HSL uses a signaling pathway different from that of IL-8 or C5a. However, there is a connection to the IL-8 signaling pathway; preincubation of PMN with 3OC12-HSL reduced the chemotaxis toward IL-8, most probably because activation of PMN by 3OC12-HSL resulted in activation and consequently in depletion of phospholipase C (Fig. 2).
While our data are compatible with the hypothesis that there is a receptor for 3OC12-HSL on PMN, definite proof is still not available, and other means of activating PMN (e.g., passive diffusion into the cells) cannot be ruled out. On the other hand, for all the chemotactic stimuli known so far, both polarization of PMN and sensing of a chemotactic gradient have been attributed to clustering, capping, and redistribution of chemokine receptors (13, 22). Moreover, in keeping with their function as major protagonists of innate, nonadaptive immunity, PMN are equipped with a variety of receptors recognizing bacterial products, either surface associated or released from the bacteria. These receptors include receptors for lipopolysaccharides, lipoteichoic acid, peptidoglycans, and bacterial DNA and also receptors recognizing a gradient of bacterium-derived peptides, exemplified by f-Met-Leu-Phe, which is widely used in vitro. Some of these receptors are more or less specific for their ligands, and others are promiscuous “pattern recognition” receptors. Thus, we think that a receptor for 3OC12-HSL is very likely, and experiments to identify such a receptor are now under way in our laboratories.
Regardless of the nature of binding, recognizing 3OC12-HSL is of utmost importance for host defense; assuming that 3OC12-HSL is released by the bacteria in the initial phase of biofilm formation, the sensing of a 3OC12-HSL gradient would attract PMN to the site of a developing biofilm. At an early stage, before bacteria are embedded in the EPS, phagocytosis and killing of the bacteria would still be possible, in contrast to the situation in a “mature” biofilm, where bacteria are obviously protected from phagocytic cells (12, 14, 28). Moreover, by releasing lactoferrin, PMN could contribute to the disruption of biofilm formation via chelation of iron (24). Thus, recognition of and attraction by 3OC12-HSL could be regarded as a way for PMN to control biofilm development.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Bockaert, J., and J. P. Pin. 1999. Molecular tinkering of G protein-coupled receptors: an evolutionary success. EMBO J. 18:1723-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brenneis, H., A. Schmidt, P. Blaas-Mautner, I. Wörner, R. Ludwig, and G. M. Hänsch. 1993. Chemotaxis of polymorphonuclear neutrophils (PMN) in patients suffering from recurrent infection. Eur. J. Investig. 23:693-698. [DOI] [PubMed] [Google Scholar]
- 3.Chhabra, S. R., C. Harty, D. S. W. Hoii, M. Daykin, P. Williams, G. Telford, D. I. Pritchard, and B. W. Bycroft. 2003. Synthetic analogues of the bacterial signal (quorum sensing) molecule N-(3-oxododecanoyl)-l-homoserine lactone as immune modulators. J. Med. Chem. 46:97-104. [DOI] [PubMed] [Google Scholar]
- 4.Chun, C. K., E. A. Ozer, M. J. Welsh, J. Zabner, and E. P. Greenberg. 2004. Inactivation of a Pseudomonas aeruginosa quorum-sensing signal by human airway epithelia. Proc. Natl. Acad. Sci. USA 110:3587-3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costerton, W., R. Veeh, M. Shirtliff, M. Pasmore, C. Post, and G. Ehrlich. 2003. The application of biofilm science to the study and control of chronic bacterial infections. J. Clin. Investig. 112:1466-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donabedian, H. 2003. Quorum sensing and its relevance to infectious diseases. J. Infect. 46:207-214. [DOI] [PubMed] [Google Scholar]
- 7.Fagerlind, M. G., S. A. Rice, P. Nilsson, M. Harlen, S. James, T. Charlton, and S. Kjelleberg. 2004. The role of regulators in the expression of quorum-sensing signals in Pseudomonas aeruginosa. J. Mol. Microbiol. Biotechnol. 6:88-100. [DOI] [PubMed] [Google Scholar]
- 8.Fuqua, C., M. R. Parsek, and E. P. Greenberg. 2001. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu. Rev. Genet. 35:439-468. [DOI] [PubMed] [Google Scholar]
- 9.Fuqua, C., and E. P. Greenberg. 2002. Listening in on bacteria. Acyl-homoserine lactone signalling. Nat. Rev. Mol. Cell. Biol. 3:685-695. [DOI] [PubMed] [Google Scholar]
- 10.Greenberg, E. P. 2003. Bacterial communication and group behavior. J. Clin. Investig. 112:1288-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heurlier, K., V. Denervand, G. Pessi, C. Reimmann, and D. Haas. 2003. Negative control of quorum sensing by RpoN (σ54) in Pseudomonas aeruginosa PAO1. J. Bacteriol. 185:2227-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jesaitis, A. J., M. J. Franklin, D. Berglund, M. Sasaki, C. I. Lord, J. B. Bleazard, J. E. Duffy, H. Beyenal, and Z. Lewandowsky. 2003. Compromised host defense on Pseudomonas aeruginosa biofilms: characterization of neutrophil and biofilm interactions. J. Immunol. 171:4329-4339. [DOI] [PubMed] [Google Scholar]
- 13.Kannan, S. 2003. Neutrophil chemotaxis: potential role of chemokine receptors in extracellular nucleotide induced Mac-1 expression. Med. Hypotheses 61:577-579. [DOI] [PubMed] [Google Scholar]
- 14.Leid, J. G., M. E. Shirtliff, J. W. Costerton, and A. P. Stoodley. 2002. Human leukocytes adhere to, penetrate, and respond to Staphylococcus aureus biofilms. Infect. Immun. 70:6339-6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Poul, E., C. Loison, S. Strufy, J.-Y. Springael, V. Lannoy, M.-E. Decobecq, S. Brezillon, V. Dupriez, G. Vassart, J. Van Damme, M. Parmentier, and M. Detheux. 2003. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Microbiol. Chem. 278:25481-25489. [DOI] [PubMed] [Google Scholar]
- 16.Lyczak, J. B., C. L. Cannon, and G. B. Pier. 2002. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 15:194-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller, M. B., and B. L. Bassler. 2001. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55:165-199. [DOI] [PubMed] [Google Scholar]
- 18.Pedersen, S. S., A. Kharazmi, F. Espersen, and N. Hoiby. 1990. Pseudomonas aeruginosa alginate in cystic fibrosis and the inflammatory response. Infect. Immun. 58:3363-3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pier, G. B., F. Coleman, M. Grout, M. Franklin, and D. E. Ohman. 2001. Role of alginate O acetylation in resistance of mucoid Pseudomonas aeruginosa in opsonic phagocytosis. Infect. Immun. 69:1895-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ritchie, A. J., A. O. Yam, K. M. Tanabe, S. A. Rice, and M. A. Cooley. 2003. Modification of in vivo and in vitro T- and B-cell-mediated immune responses by the Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl)-l-homoserine lactone. Infect. Immun. 71:4421-4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuster, M., C. P. Lostroh, T. Ogi, and E. P. Greenberg. 2003. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J. Bacteriol. 185:2066-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seely, A. J., J. F. Naud, G. Campisi, B. Giannias, S. Liu, A. DiCarlo, L. E. Ferri, J. L. Pascual, J. Tchervenko, and N. V. Christou. 2002. Alteration of chemoattractant receptor expression regulates human neutrophil chemotaxis in vivo. Ann. Surg. 235:550-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simpson, J. A., S. E. Smith, and R. T. Dean. 1989. Scavenging by alginate of free radicals released by macrophages. Free Radic. Biol. Med. 6:347-353. [DOI] [PubMed] [Google Scholar]
- 24.Singh, P. K., M. R. Parsek, E. P. Greenberg, and M. J. Welsh. 2002. A component of innate immunity prevents bacterial biofilm development. Nature 417:552-555. [DOI] [PubMed] [Google Scholar]
- 25.Smith, R. S., and B. H. Iglewski. 2003. Pseudomonas aeruginosa quorum sensing systems and virulence. Curr. Opin. Microbiol. 6:56-60. [DOI] [PubMed] [Google Scholar]
- 26.Tateda, K., Y. Ishii, M. Horikawa, T. Matsumoto, S. Miyairi, J. C. Pechere, T. J. Standiford, M. Ishiguro, and K. Yamaguchi. 2003. The Pseudomonas aeruginosa autoinducer N-3-oxododecanoyl homoserine lactone accelerates apoptosis in macrophages and neutrophils. Infect. Immun. 71:5785-5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Telford, G., D. Wheeler, P. Williams, P. T. Tomkins, P. Appleby, H. Sewell, G. S. Stewart, B. W. Bycroft, and D. I. Pritchard. 1998. The Pseudomonas aeruginosa quorum-sensing signal molecule N-(3-oxododecanoyl)-l-homoserine lactone has immunomodulatory activity. Infect. Immun. 66:36-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Underhill, D. M., and A. Oszinsky. 2002. Phagocytosis of microbes: complexity in action. Annu. Rev. Immunol. 20:825-852. [DOI] [PubMed] [Google Scholar]
- 29.Wagner, C., K. Kondella, T. Bernschneider, V. Heppert, A. Wentzensen, and G. M. Hänsch. 2003. Post-traumatic osteitis: analysis of inflammatory cells recruited into the site of infection. Shock 20:503-510. [DOI] [PubMed] [Google Scholar]
- 30.Wagner, C., A. Kaksa, W. Müller, B. Denefleh, V. Heppert, A. Wentzensen, and G. M. Hänsch. 2004. Polymorphonuclear neutrophils in posttraumatic osteomyelitis: cells recovered from the inflamed site lack chemotactic activity but generate superoxides. Shock 22:108-115. [DOI] [PubMed] [Google Scholar]
- 31.Wagner, C., D. Heck, K. Lautenschläger, C. Iking-Konert, V. Heppert, A. Wentzensen, and G. M. Hänsch. 2006. T-lymphocytes in implant-associated posttraumatic osteomyelitis: identification of cytotoxic T-effector cells at the site of infection. Shock 25:241-246. [DOI] [PubMed] [Google Scholar]
- 32.Welch, M., D. E. Todd, N. A. Whitehead, S. J. McGowan, B. W. Bycroft, and G. P. C. Salmond. 2000. N-acyl homoserine lactone binding to the CarR receptor determines quorum-sensing specificity in Erwinia. EMBO J. 19:631-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitehead, N. A., A. M. L. Barnard, H. Slater, N. J. L. Simpson, and G. P. C. Salmond. 2001. Quorum sensing in gram-negative bacteria. FEMS Microbiol. Rev. 25:365-404. [DOI] [PubMed] [Google Scholar]