Abstract
In monocytes, the fimbriae of the oral pathogen Porphyromonas gingivalis activate cross talk signaling from Toll-like receptor 2 (TLR2) to the β2 integrin CD11b/CD18, leading to the induction of the high-affinity state of the latter receptor. CD14 plays an important role in this “inside-out” proadhesive pathway by binding fimbriae and facilitating the activation of TLR2 and phosphatidylinositol 3-kinase signaling. In its high-affinity state, CD11b/CD18 mediates monocyte adhesion to endothelial cells and transmigration to sites of infection. We have now shown that P. gingivalis fimbriae function as both an activator and a ligand of CD11b/CD18; thus, fimbriae proactively promote their own binding to monocytes. Indeed, treatments that interfered with fimbria-induced activation of CD11b/CD18 (i.e., blockade of CD14, TLR2, or phosphatidylinositol 3-kinase signaling) also suppressed the cell binding activity of fimbriae, which was largely inducible and CD11b/CD18 dependent. Development of a recombinant inside-out signaling system in Chinese hamster ovary cells confirmed the ability of fimbriae to activate CD14/TLR2 signaling and induce their own CD11b/CD18-dependent binding. Induction of this proadhesive pathway by P. gingivalis fimbriae appeared to take place in lipid rafts. Indeed, methyl-β-cyclodextrin, a cholesterol-sequestering agent that disrupts lipid raft organization, was found to inhibit the fimbria-induced assembly of CD14/TLR2 signaling complexes and the activation of the high-affinity state of CD11b/CD18. Experiments using macrophages from mice deficient in various pattern recognition receptors indicated that the receptors involved in the inside-out proadhesive pathway (CD14, TLR2, and CD11b/CD18) are important for mediating P. gingivalis internalization within macrophages. It therefore appears that P. gingivalis proactively modulates β2 integrin adhesive activity for intracellular uptake.
CD11b/CD18 (CR3 or Mac-1) is a functionally versatile β2 integrin with significant roles in immunity and inflammation (5, 27). For instance, the ability of CD11b/CD18 to interact with its endothelial counterreceptor, intercellular adhesion molecule-1 (ICAM-1), is important for monocyte adhesion and transendothelial migration (17). The binding affinity of CD11b/CD18 for ICAM-1 is regulated by inside-out signaling, i.e., intracellular signals generated by other surface receptors such as chemotactic receptors (17, 27). The regulated shift from a low- to a high-affinity state of CD11b/CD18 is referred to as “CD11b/CD18 activation” (17, 27). We have recently described a novel Toll-like receptor 2 (TLR2)-mediated inside-out signaling pathway for CD11b/CD18 activation in response to Porphyromonas gingivalis fimbriae (12). P. gingivalis fimbria-activated CD11b/CD18 is capable of mediating monocyte adhesion to endothelial ICAM-1 and transendothelial migration (11). This TLR2 proadhesive pathway is conserved between humans and mice (11) and may constitute a defense mechanism for monocyte recruitment to sites of P. gingivalis infection, such as in periodontitis (37). However, the same process may contribute to monocyte transendothelial migration to atherosclerotic plaques, where P. gingivalis has been localized (16) and is suspected to contribute to the pathogenesis of atherosclerosis (7).
Strikingly, the P. gingivalis fimbria-induced signaling pathway for CD11b/CD18 activation may not be exclusively involved in innate defense or inflammation but may alternatively be exploited by the pathogen. We have found that the ability of mouse macrophages to elicit interleukin-12 (IL-12) p70 in response to P. gingivalis fimbriae is upregulated by CD11b deficiency but is eliminated by TLR2 deficiency (8). These findings suggest that fimbriae interact with CD11b/CD18 and inhibit TLR2-dependent induction of IL-12 p70, a major cytokine in mediating bacterial clearance (32). Through fluorescence resonance energy transfer (FRET) studies, we have additionally shown that fimbriae induce a coassociation of TLR2 with CD11b/CD18 as well as with CD14, an essential TLR2 coreceptor for fimbria-induced activation of nuclear factor κB and cytokine production (9). It therefore appears that fimbriae influence TLR2-dependent cellular activation through interactions with at least two functionally associated pattern recognition receptors (PRRs), CD14 and CD11b/CD18.
Our working hypothesis is that P. gingivalis has co-opted a proadhesive signaling pathway, normally involved in leukocyte-endothelial cell interactions, for enhancing the interaction of its cell surface fimbriae with CD11b/CD18. This proadhesive pathway is initiated when P. gingivalis fimbriae bind CD14 and activate TLR2- and phosphatidylinositol 3-kinase-mediated signaling, leading to the induction of an activation-specific neoepitope (CBRM1/5) on CD11b (12). The induction of the high-affinity conformation of CD11b/CD18, signified by the CBRM1/5 neoepitope, may be essential for the binding of fimbriae. However, direct evidence for a dual role of P. gingivalis fimbriae as both an activator and a ligand of CD11b/CD18 is missing. Moreover, the extent to which CD14 contributes to the binding of fimbriae after CD11b/CD18 becomes activated remains uncertain. To address these questions, we have now generated a recombinant inside-out signaling system in Chinese hamster ovary (CHO) cells and, moreover, developed a modified version of our standard binding assay for human monocytes. Specifically, the binding assay was dissected into an activation step (for stimulating the ligand-binding activity of CD11b/CD18) and a binding step. The latter step was carried out on ice and in the presence of metabolic inhibitors to inhibit further inside-out signaling. Using these methods, we have generated conclusive evidence that P. gingivalis fimbriae stimulate the adhesive activity of CD11b/CD18 and thereby promote their own CD11b/CD18-dependent cellular binding. Moreover, P. gingivalis fimbria-induced CD14/TLR2 cluster formation and the activation of the proadhesive pathway appeared to occur in membrane lipid rafts, signaling platforms used by several pathogens to invade host cells (1). Interestingly, the receptors involved in the inside-out signaling pathway for CD11b/CD18 activation (i.e., CD14, TLR2, and CD11b/CD18) were found to play important roles in mediating the internalization of fimbriated P. gingivalis by mouse macrophages.
MATERIALS AND METHODS
Reagents.
Monoclonal antibodies (MAbs) to TLR2 (clone TL2.1), TLR4 (HTA125), CD11b (CBRM1/5 and VIM12), and immunoglobulin (Ig) isotype controls (IgG1 and IgG2a) were purchased from eBioscience (San Diego, CA). MAb to CD11b (2LPM19c) was obtained from DakoCytomation (Carpinteria, CA). MAbs to major histocompatibility complex (MHC) class I (W6/32) and CD14 (Tük 4) were obtained from Abcam (Cambridge, United Kingdom). MAb to CD14 (MEM-18) was obtained from Caltag (Burlingame, CA). Anti-CD14 MAb (26ic) was purified from a hybridoma supernatant (HB246; ATCC, Manassas, VA). Murine-specific MAbs to CD14 (Sa14-2) and CD11b (M1/70) and their isotype controls were obtained from Cell Sciences (Canton, MA) and eBioscience, respectively. For FRET measurements (see below), MAbs were conjugated to Cy3 or Cy5 using labeling kits from Amersham Biosciences. Phorbol myristate acetate (PMA), N-formyl-Met-Leu-Phe (FMLP), wortmannin, LY294002, LY30351, methyl-β-cyclodextrin (MCD), and cholesterol were purchased from Sigma-Aldrich (St. Louis, MO). P. gingivalis 381 and an isogenic fimbria-deficient mutant (JH1004) (9) were grown anaerobically at 37°C in brain heart infusion broth supplemented with hemin (5 μg/ml) and menadione (1 μg/ml). Fimbriae were purified from P. gingivalis 381 as previously described (12). Purified fimbriae were free of contaminating substances on silver-stained sodium dodecyl sulfate-polyacrylamide gels and tested negative for endotoxin (<6 endotoxin units/mg protein) according to a quantitative Limulus amebocyte lysate assay (BioWhittaker, Walkersville, MD).
Cell culture.
Monocytes were purified from human peripheral blood upon centrifugation using NycoPrep1.068 (Axis-Shield, Oslo, Norway) as previously described (12). Incidental nonmonocytes were removed by magnetic depletion using a cocktail of biotin-conjugated MAbs and magnetic microbeads coupled to anti-biotin MAb (Monocyte Isolation Kit II; Miltenyi Biotec, Auburn, CA). Purified monocytes were cultured at 37°C in a 5% CO2 atmosphere in RPMI 1640 (Invitrogen/Gibco, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum, 2 mM l-glutamine, 10 mM HEPES, 100 units/ml penicillin G, 100 μg/ml streptomycin, and 0.05 mM 2-mercaptoethanol (complete RPMI). Collection of human blood samples was conducted in compliance with established federal guidelines and institutional policies. CHO cells stably transfected with human complement receptor 1 (CR1) or CR3 (CD11b/CD18) were kindly provided by D. T. Golenbock (University of Massachusetts Medical School, Worcester, MA) (19). CHO cells were cultured in Ham's F-12 nutrient mixture (Invitrogen/Gibco) supplemented with 2 mM l-glutamine, 10% heat-inactivated fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin. Cell viability was monitored using the CellTiter-Blue assay kit (Promega, Madison, WI). None of the experimental treatments affected cell viability compared to medium-only control treatments.
CD11b/CD18 activation assay.
The CBRM1/5 epitope induction assay was used to monitor the activation state of CD11b/CD18 as previously described (12). The assay is based on the property of a reporter MAb (CBRM1/5) to detect a conformational change on CD11b that signifies the high-affinity binding state of CD11b/CD18 (4).
Binding assays.
Biotinylated fimbriae (1 μg/ml) were allowed to bind to human monocytes or human cell lines for 30 min at 37°C, as previously described (12). Subsequently, the cells were washed and incubated on ice with fluorescein isothiocyanate (FITC)-labeled streptavidin. After washing, binding was determined by measuring cell-associated fluorescence (in relative fluorescence units) on a microplate fluorescence reader (FL600; Bio-Tek Instruments, Winooski, VT) with excitation and emission wavelength settings of 485 and 530 nm, respectively. Background fluorescence was determined for cells treated with medium only and FITC-streptavidin.
To separate the CD11b/CD18 activation step from the binding stage in monocyte interactions with fimbriae, the basic protocol described above was modified as follows. The cells were pretreated for 10 min at 37°C with medium only or with agents that activate the ligand-binding capacity of CD11b/CD18 (10−7 M FMLP, 0.1 μg/ml PMA, or 1 μg/ml fimbriae) (“activation step”). The cells were then immediately washed with ice-cold Hanks' balanced salt solution containing 1% bovine serum albumin, 0.1% azide, and 50 mM 2-deoxy-d-glucose to inhibit metabolic activity. Subsequently, biotinylated fimbriae (1 μg/ml) were added to both nonactivated and activated cells for a 1-h incubation on ice (“binding step”), and binding was measured as described above. The reason for performing the binding step on ice and in the presence of metabolic inhibitors was because these conditions would permit binding to preactivated CD11b/CD18 but would not support further CD11b/CD18 activation, which requires higher temperatures (ambient or 37°C) and energy (20).
P. gingivalis internalization assay.
Mouse macrophages were incubated at 37°C with FITC-labeled P. gingivalis at a multiplicity of infection of 10:1 for various time points (5 to 60 min). Phagocytosis was stopped by cooling the incubation tubes on ice. After cell washing to remove nonadherent bacteria, extracellular fluorescence (representing attached but not internalized bacteria) was quenched with 0.2% trypan blue. The cells were washed again, fixed with 1% paraformaldehyde, and analyzed by flow cytometry using a fluorescence-activated cell sorter (FACSCalibur) and CellQuest software (Becton-Dickinson). The percentage of cells positive for FITC-P. gingivalis and the mean fluorescence intensity of the positive cells (giving a measure of the number of internalized bacteria) were recorded.
Recombinant inside-out signaling system in CHO cells.
To reconstitute the CD14/TLR2-dependent inside-out signaling system for CD11b/CD18 activation in CHO cells, CHO cell lines stably expressing CD11b/CD18 (CHO/CR3) or CD35 (CHO/CR1; control) (19) were transiently cotransfected with human CD14 and TLR2, with the latter in either the wild-type form or the signaling-deficient version, using plasmid constructs obtained from Invivogen (pUNO-hCD14, pUNO-hTLR2, pZERO-hTLR2tirless, or empty vectors as transfection controls). TLR plasmids were used at 100 ng per transfected well, and CD14 was used at 50 ng per transfected well. The total amount of plasmid DNA per well was kept constant (150 ng) by supplementation with empty control vector. Transfections were performed using the PolyFect transfection reagent (QIAGEN Inc., Valencia, CA) according to the manufacturer's instructions. Two days posttransfection, expression of transfected CD14 and TLR2 was confirmed by staining the cells with specific FITC-labeled anti-CD14 or anti-TLR2 MAb and measuring cell-associated fluorescence using a Bio-Tek microplate fluorescence reader. The binding of P. gingivalis fimbriae to transfected CHO cells was assessed using the basic protocol described above for monocytes.
MCD treatment and cholesterol reconstitution.
To deplete human monocytes of cholesterol using MCD and reconstituting cellular cholesterol in MCD-treated cells, we used a modification of a previously published methodology (3, 18). Briefly, human monocytes were incubated in the presence of 10 mM MCD for 30 min at 37°C to deplete the cells of cholesterol. The cells were washed and incubated for an additional 30 min with medium only or with 150 μM cholesterol. Subsequently, the cells (MCD-treated cells, MCD-treated and cholesterol-reconstituted cells, and cells treated with medium only) were used in functional assays.
FRET.
The procedures for measuring the efficiency of energy transfer between fluorescently labeled cell surface receptors have been previously described in detail (29, 30). Briefly, human monocytes were cultured on microchamber culture slides (Lab-tek; Invitrogen/Gibco). Following treatment for 10 min at 37°C with medium only or with P. gingivalis fimbriae (1 μg/ml), the cells were labeled with 100 μl of a mixture of Cy3-conjugated MAb to TLR2 (donor) and Cy5-conjugated MAb to CD14 or CD11b (acceptors). MAb to MHC class I conjugated to Cy5 was used for control purposes. The cells were rinsed twice with phosphate-buffered saline containing 0.02% bovine serum albumin and then fixed with 4% paraformaldehyde for 15 min. Cell fixation was necessary to prevent the potential reorganization of the proteins during the course of the experiment and energy transfer determinations. Energy transfer between different receptor pairs was calculated from the increase in donor fluorescence after acceptor photobleaching.
Statistical analysis.
Data were evaluated by analysis of variance and the Dunnett multiple-comparison test using the InStat program (GraphPad Software, San Diego, CA). Where appropriate (comparison of two groups only), two-tailed t tests were also performed. Statistical differences were considered significant at a P value of <0.05.
RESULTS
Induction of the CBRM1/5 neoepitope of CD11b correlates with increased cellular binding of P. gingivalis fimbriae.
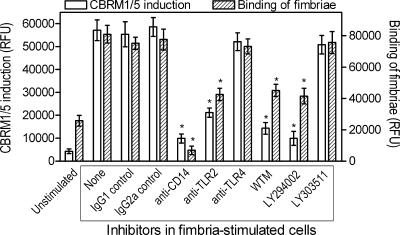
We have recently shown that P. gingivalis fimbriae activate the high-affinity binding state of CD11b/CD18 in human monocytes, as determined by the induction of the activation-specific CBRM1/5 neoepitope (12). Induction of the CBRM1/5 neoepitope is evident within 10 min following activation, peaks at 30 min, and slowly declines thereafter (12). That study, however, did not address whether the induction of the CBRM1/5 neoepitope promotes the cell binding activity of fimbriae. We have now found that upon a 30-min stimulation with fimbriae, monocytes display increased binding activity for both the CBRM1/5 reporter MAb and fimbriae (similarly treated but separate aliquots of cells were used in the two assays) (Fig. 1). Moreover, treatments that interfere with inside-out signaling and the induction of the CBRM1/5 neoepitope, namely, anti-CD14, anti-TLR2, or phosphatidylinositol 3-kinase inhibitors (12), also inhibited the cell binding of fimbriae (P < 0.05) (Fig. 1). Control treatments (anti-TLR4 or the inactive analog inhibitor LY303511) were without significant effects (Fig. 1). Therefore, induction of the CBRM1/5 neoepitope by fimbriae positively regulates the cell binding activity of fimbriae.
FIG. 1.
Induction of a CD11b activation-specific neoepitope (CBRM1/5) in monocytes correlates with the cell binding activity of P. gingivalis fimbriae. Human monocytes were pretreated for 30 min with medium only or with IgG1 or IgG2a isotype controls; MAbs to CD14, TLR2, or TLR4 (all at 10 μg/ml); or wortmannin (WTM; 50 nM), LY294002 (20 μM), or its inactive analog, LY30351 (20 μM). Subsequently, the cells were exposed for 30 min to medium only (unstimulated) or to 1 μg/ml of biotinylated P. gingivalis fimbriae. Similarly treated but separate aliquots of cells were assessed for induction of the CBRM1/5 neoepitope after staining with FITC-labeled CBRM1/5 MAb or for binding of fimbriae after staining with streptavidin-FITC. Cell-associated fluorescence was measured and expressed in relative fluorescence units (RFU). Data are presented as means ± standard deviations (SDs) of triplicate determinations from one of two independent experiments that yielded similar results. Asterisks indicate statistically significant (P < 0.05) inhibition of CBRM1/5 induction or of binding of fimbriae compared to corresponding uninhibited, stimulated controls.
P. gingivalis fimbriae display inducible binding to CD11b/CD18.
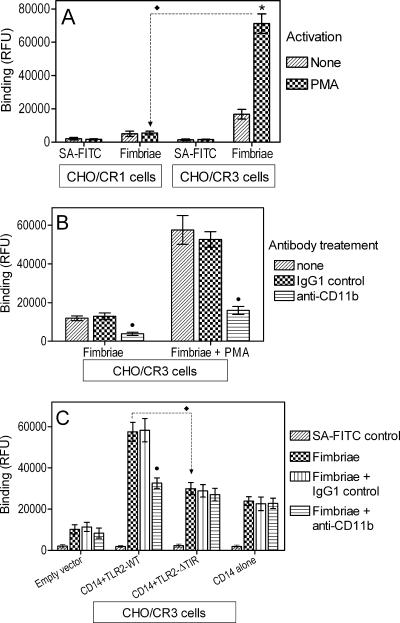
CHO/CR3 cells express human CD11b/CD18 (CR3), whereas CHO/CR1 cells express CD35 (CR1) (19). If CD11b/CD18 is an important cellular receptor of P. gingivalis fimbriae, then fimbriae would be expected to display enhanced binding to CHO/CR3 cells compared to CHO/CR1 cells, especially under conditions that activate the ligand-binding capacity of CD11b/CD18. Indeed, we found that fimbriae bound poorly to CHO/CR1 compared to CHO/CR3 cells, especially upon treatment with PMA, an activator of CD11b/CD18 that bypasses inside-out signaling (12). Specifically, PMA-treated CHO/CR3 cells were able to bind fimbriae at levels that were four- to fivefold higher than those untreated CHO/CR3 cells and at levels that were about 13-fold higher than those of PMA-treated CHO/CR1 cells (P < 0.05) (Fig. 2A). The treatment of CHO/CR1 cells with PMA did not upregulate the binding of fimbriae compared to the untreated control (Fig. 2A). The PMA-inducible binding activity of CHO/CR3 cells was abrogated by anti-CD11b MAb, confirming the involvement of CD11b/CD18 (Fig. 2B). We then developed a recombinant TLR2 inside-out signaling system in CHO/CR3 cells to determine if fimbriae themselves could activate CD11b/CD18 for promoting their cellular binding.
FIG. 2.
P. gingivalis fimbriae display inducible binding to CD11b/CD18. CHO cells stably transfected with CR1 (A) or CR3 (CD11b/CD18) (A to C) were exposed to biotinylated P. gingivalis fimbriae (1 μg/ml) for 30 min at 37°C. The binding of fimbriae was measured as cell-associated fluorescence after cell staining with streptavidin (SA)-FITC. Background binding was determined in cells treated with medium only and streptavidin-FITC. In panels A and B, the cells were incubated in the absence or presence of PMA (0.1 μg/ml), a CD11b/CD18 activator. In panel C, the cells were transiently cotransfected with human CD14 and TLR2 in either the wild type (TLR2-WT), the signaling-deficient mutant version (TLR2-ΔTIR), CD14 alone, or empty vector control. In panels B and C, the binding of fimbriae was assessed in the absence or presence of anti-CD11b or the IgG1 isotype control (both at 10 μg/ml). Data are presented as means ± SDs of triplicate determinations from one of two independent sets of experiments that yielded similar results. In panel A, asterisks denote statistically significant (P < 0.05) differences compared to the corresponding PMA-untreated control. Throughout, black diamonds (⧫) denote statistically significant (P < 0.05) differences between selected groups as indicated, whereas black circles (•) indicate statistically significant (P < 0.05) differences due to MAb treatment. RFU, relative fluorescence units.
CHO cells lack functional endogenous TLR2 (14) but transcribe TLR1 and TLR6, either of which is capable of cooperative signaling with TLR2 in TLR2-transfected CHO cells (13, 15, 23). Therefore, transfection of exogenous TLR2 in CHO/CR3 cells would permit the reconstitution of inside-out signaling upon activation with P. gingivalis fimbriae, which utilize either TLR1 or TLR6 as signaling partners of TLR2 (9). Specifically, CHO/CR3 cells were transiently transfected with human TLR2 in either the wild-type form (TLR2-WT) or the mutant version devoid of the intracellular Toll/interleukin-1 receptor (TIR) domain (TLR2-ΔTIR) (signaling-deficient control). The cells were additionally cotransfected with human CD14, which greatly facilitates fimbria-induced TLR2 inside-out signaling (12). We found that the binding of fimbriae to transfected CHO/CR3 cells was maximally increased (by almost sixfold compared to empty vector-transfected cells) when CD14 and TLR2-WT were cotransfected (Fig. 2C). Maximal binding was reduced by 48% when CD14 was cotransfected with the signaling-deficient TLR2-ΔTIR (P < 0.05) (Fig. 2B). This finding suggests that TLR2 signaling contributes to the cellular binding of fimbriae, presumably through the activation of CD11b/CD18. Indeed, that was the case, since a blocking anti-CD11b MAb reduced the binding of fimbriae in cells cotransfected with CD14 and TLR2-WT to levels similar to those seen in cells cotransfected with CD14 and TLR2-ΔTIR (P < 0.05) (Fig. 2C). Transfection with CD14 in the absence of TLR2 resulted in relatively modest CD11b/CD18-independent binding (Fig. 2C). In conclusion, P. gingivalis fimbriae readily bind to CD11b/CD18 upon its activation with an artificial agonist that bypasses inside-out signaling (PMA) or, more importantly, upon fimbria-induced TLR2-dependent inside-out signaling.
P. gingivalis fimbriae function as both an activator and a ligand of CD11b/CD18 in human monocytes.
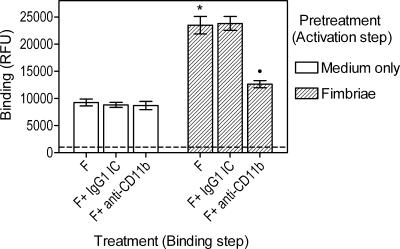
Using human monocytes and a modification of our fimbria binding assay (12), we tested fimbriae as an activator and a ligand of CD11b/CD18. Specifically, we dissected the binding assay into an activation step (for stimulating the ligand-binding activity of CD11b/CD18) and a binding step. The latter step was carried out on ice and in the presence of metabolic inhibitors to inhibit further inside-out signaling. Thus, human monocytes were pretreated at 37°C for 10 min with 1 μg/ml of unlabeled fimbriae (activation step), and subsequently, biotinylated fimbriae (1 μg/ml) were added to medium-only- or fimbria-pretreated monocytes for a 1-h incubation on ice (binding step). Under the experimental conditions of the binding step, added biotinylated fimbriae could not activate CD11b/CD18 in medium-only-pretreated monocytes because this is an energy-dependent process (20). Indeed, we found that fimbria-pretreated cells bound added labeled fimbriae at significantly (P < 0.05) higher levels than medium-only-pretreated cells (Fig. 3). Moreover, the observed binding in medium-only-pretreated cells could not be attributed to CD11b/CD18, as suggested by the lack of an inhibitory effect of anti-CD11b MAb (Fig. 3). In contrast, when fimbria-pretreated monocytes were incubated with labeled fimbriae in the presence of anti-CD11b MAb, the binding was significantly inhibited (P < 0.05) (Fig. 3) and was comparable to that seen in medium-only-pretreated monocytes (Fig. 3). The differences between medium-only- and fimbria-pretreated monocytes in the binding of labeled fimbriae are therefore attributable to activated CD11b/CD18 in the latter group. These results indicate that P. gingivalis fimbriae activate the ligand-binding capacity of CD11b/CD18, which can thereby efficiently recognize fimbriae.
FIG. 3.
P. gingivalis fimbriae activate the capacity of CD11b/CD18 to bind fimbriae. Human monocytes were pretreated for 10 min at 37°C with or without 1 μg/ml fimbriae (activation step). The cells were then immediately washed with ice-cold buffer containing metabolic inhibitors and incubated with 1 μg/ml biotinylated fimbriae (F), in the presence of anti-CD11b or IgG1 isotype control (IC) treatment, for 1 h on ice (binding step). Binding was measured as cell-associated fluorescence (relative fluorescence units [RFU]) after staining with streptavidin-FITC. Background fluorescence was determined using cells treated with medium only throughout the experiment, except for incubation with streptavidin-FITC, and is indicated by a discontinuous horizontal line. Data are shown as means ± SDs of triplicate determinations from one of two independent experiments that yielded similar results. Statistically significant (P < 0.05) enhancement of binding of fimbriae compared to the corresponding medium-only-pretreated group is indicated by an asterisk. A statistically significant (P < 0.05) inhibition of binding due to MAb treatment is indicated by a black circle (•).
Relative contribution of CD14 and CD11b/CD18 in the binding of P. gingivalis fimbriae.
The binding of fimbriae to CD14 is important for effective TLR2 inside-out signaling and activation of CD11b/CD18 (12). However, the extent to which CD14 contributes to the binding of fimbriae after CD11b/CD18 is activated remains uncertain. We have thus designed an experiment to assess the relative binding contribution of these receptors in human monocytes. Similarly to the previous experiment (Fig. 3), we dissected the binding assay into an activation step and a binding step. We found that under sham activation conditions (“medium only”), the binding of fimbriae to monocytes was almost entirely dependent upon CD14, without detectable participation of CD11b/CD18, as suggested by the strong inhibitory effect on binding by anti-CD14 MAb and the lack thereof by anti-CD11b MAb (Table 1). In stark contrast, when PMA or FMLP was used to activate CD11b/CD18, resulting in the enhanced cell binding of fimbriae (P < 0.05) (Table 1), both anti-CD14 and anti-CD11b displayed substantial inhibitory effects (Table 1). In fact, anti-CD11b appeared to have a somewhat stronger effect than anti-CD14 MAb (50 to 52% versus 36 to 39% inhibition, respectively) (Table 1). The concomitant use of both anti-CD14 and anti-CD11b MAbs resulted in an enhanced, additive inhibitory effect on binding (Table 1). Similar findings were obtained when the experiment was repeated using mouse macrophages and murine-specific MAbs to CD14 and CD11b (data not shown). In conclusion, both CD14 and CD11b/CD18 may serve as important binding receptors for P. gingivalis fimbriae in activated monocytes.
TABLE 1.
Relative contributions of CD14 and CD11b/CD18 to the binding of P. gingivalis fimbriaea
| Activation step treatment (37°C) | Binding step treatment (on ice) | Binding (RFU) (mean ± SD)d | % Inhibition by Ab treatment |
|---|---|---|---|
| Medium only | Medium only | 1,021 ± 177 | |
| Medium only | Fimbriae | 11,080 ± 1,123 | |
| Medium only | Fimbriae + IgG1 isotype control | 10,773 ± 895 | 2.8 |
| Medium only | Fimbriae + anti-CD14 | 2316 ± 348b | 79.1b |
| Medium only | Fimbriae + anti-CD11b | 10,013 ± 934 | 9.6 |
| Medium only | Fimbriae + anti-CD11b and anti-CD14 | 2,198 ± 456b | 80.2b |
| PMA | Fimbriae | 33,254 ± 3,453c | |
| PMA | Fimbriae + IgG1 isotype control | 31,991 ± 2,369 | 3.8 |
| PMA | Fimbriae + anti-CD11b | 16,072 ± 998b | 51.7b |
| PMA | Fimbriae + anti-CD14 | 21,149 ± 1,164b | 36.4b |
| PMA | Fimbriae + anti-CD11b and anti-CD14 | 9,660 ± 652b | 80.0b |
| FMLP | Fimbriae | 29,570 ± 3,086c | |
| FMLP | Fimbriae + IgG1 isotype control | 26,578 ± 2,023 | 10.1 |
| FMLP | Fimbriae + anti-CD11b | 14,953 ± 741b | 49.4b |
| FMLP | Fimbriae + anti-CD14 | 18,153 ± 1,021b | 38.6b |
| FMLP | Fimbriae + anti-CD11b and anti-CD14 | 8,457 ± 630b | 71.4b |
Human monocytes were pretreated for 10 min at 37°C with or without 0.1 μg/ml PMA or 10−7 M FMLP (activation step). The cells were immediately washed and incubated with biotinylated fimbriae, in the absence or presence of antibody treatment, for 1 h on ice (binding step). Binding was measured as cell-associated fluorescence (relative fluorescence units [RFU]) after staining with streptavidin-FITC. Background fluorescence was determined using cells treated with medium only throughout the experiment, except for incubation with streptavidin-FITC.
Statistically significant (P < 0.05) inhibition of binding due to MAb treatment.
Statistically significant (P < 0.05) enhancement of binding compared to the corresponding medium-only-pretreated group.
n = 3.
Lipid raft function is required for CD11b/CD18 activation by P. gingivalis fimbriae.
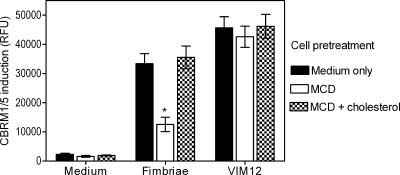
Lipid rafts are membrane microdomains that function as signaling platforms, the structural organization of which depends on cholesterol (2, 3, 30, 31). Because CD11b/CD18 activation (CBRM1/5 induction) by fimbriae requires TLR2 signaling (Fig. 1 and 2), we hypothesized that lipid raft-disrupting agents like MCD will inhibit the ability of fimbriae to induce the CD11b activation-specific CBRM1/5 neoepitope. To test this hypothesis, monocytes were pretreated for 30 min at 37°C with 10 mM MCD to deplete the cells of cholesterol. MCD-pretreated cells as well as medium-only-pretreated cells were then stimulated with fimbriae or with VIM12 MAb, for control purposes. Unlike most physiologic stimuli that activate CD11b/CD18 through inside-out signaling, VIM12 activates this integrin by binding to a CD11b site distal to the ligand-binding domain (28). As expected, both fimbriae and VIM12 induced the CBRM1/5 neoepitope in medium-only-pretreated cells (Fig. 4). However, MCD pretreatment significantly inhibited CBRM1/5 induction by fimbriae but not by VIM12 (P < 0.05) (Fig. 4). Cholesterol repletion of MCD-treated monocytes effectively reversed the inhibitory effect of MCD on fimbria-induced CBRM1/5 (Fig. 4), indicating that the MCD effect was specifically attributable to cholesterol sequestration.
FIG. 4.
MCD inhibits CBRM1/5 induction by P. gingivalis fimbriae. Monocytes were pretreated for 30 min with 10 mM MCD to deplete cholesterol or were pretreated for 30 min with 10 mM MCD followed by the addition of 150 μM cholesterol for an additional 30 min. The MCD- and MCD/cholesterol-pretreated monocytes, as well as cells pretreated with medium only, were subsequently stimulated for 30 min with fimbriae or the VIM12 MAb (control) or were left unstimulated with medium only. The cells were assessed for the induction of the CBRM1/5 epitope as outlined in the legend to Fig. 1. Data are presented as means ± SDs of triplicate determinations from one of three independent experiments that yielded similar results. Asterisks indicate statistically significant (P < 0.05) inhibition of CBRM1/5 induction compared to medium-only-pretreated cells.
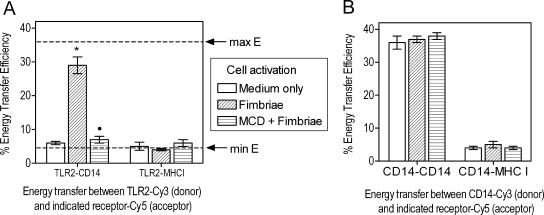
On the basis of our working model that fimbriae bind CD14 and initiate TLR2-mediated inside-out signaling (12), we next investigated the mechanism whereby MCD inhibits fimbria-induced CD11b/CD18 activation. When monocytes are exposed to fimbriae, TLR2 is expected to be recruited to lipid rafts, where it would associate with CD14 (a constitutive constituent of lipid rafts) (31), forming a binding/signaling receptor complex (9). However, lipid raft disruption by MCD could inhibit the association of TLR2 with CD14, thus impairing the formation of a CD14/TLR2 complex required for effective inside-out signaling. To test this hypothesis, we used FRET to determine the TLR2 coassociation with CD14 in the absence or presence of MCD. Specifically, energy transfer between Cy3-labeled TLR2 (donor) and Cy5-labeled CD14 (acceptor) was measured, whereas Cy5-labeled MHC class I (acceptor) was used as a negative control. As expected, there was minimal energy transfer between TLR2 and any of the acceptor receptors in unstimulated cells (Fig. 5A). In contrast, there was significant energy transfer from TLR2 to CD14, but not to MHC class I, in stimulated cells compared to unstimulated cells (P < 0.05) (Fig. 5A). Remarkably, when monocytes were pretreated with MCD prior to stimulation with fimbriae, the energy transfer between TLR2 and CD14 was significantly reduced to near-background levels (P < 0.05) (Fig. 5A). This suggests that MCD inhibits the assembly of CD14/TLR2 signaling complexes. To rule out the possibility that MCD inhibited energy transfer in a nonspecific way, we measured the energy transfer between two different epitopes of the same molecule (CD14) in unstimulated and stimulated cells with or without MCD pretreatment. Energy transfer between the two CD14 epitopes was consistently high and was not affected by the state of cell activation or the presence of MCD (Fig. 5B). Our findings from Fig. 4 and 5 collectively suggest that lipid raft function is essential for fimbria-induced CD14/TLR2 cluster formation and the ensuing inside-out signaling for CD11b/CD18 activation.
FIG. 5.
Induction of TLR2 association with CD14 by P. gingivalis fimbriae requires intact lipid raft function. Human monocytes were pretreated or not pretreated for 30 min with 10 mM MCD and then stimulated with P. gingivalis fimbriae (1 μg/ml) for 10 min. In panel A, energy transfer between TLR2 (Cy3) and CD14 (Cy5) or MHC class I (MHCI) (Cy5) was measured from the increase in donor (Cy3) fluorescence after acceptor (Cy5) photobleaching. The maximum (max) energy transfer efficiency (E) in the system is indicated by a horizontal discontinuous line and was 36 ± 2.0, determined as the energy transfer between Cy3-26ic MAb and Cy5-Tuk4 MAb, which recognize two different epitopes of the same molecule, CD14. The minimum (min) energy transfer efficiency is also shown and was 4 ± 0.5, determined as the energy transfer between molecules that do not engage in heterotypic associations (CD14 and MHC class I). Panel B shows a control experiment to investigate the effect of MCD on FRET measurements that should not be influenced by the status of lipid raft functionality, i.e., determining the energy transfer between two different epitopes of CD14 or between CD14 and MHC class I. Results are shown as means of percent energy transfer ± SDs calculated from three independent experiments. The asterisk indicates a statistically significant (P < 0.05) increase in energy transfer between TLR2 (donor) and the indicated receptor (acceptor) upon cell activation compared to energy transfer between the same donor-acceptor pair in unstimulated cells. The black circle indicates a statistically significant (P < 0.05) reversal of energy transfer increase due to MCD pretreatment.
Internalization of fimbriated P. gingivalis by macrophages is dependent upon CD14, TLR2, and CD11b/CD18.
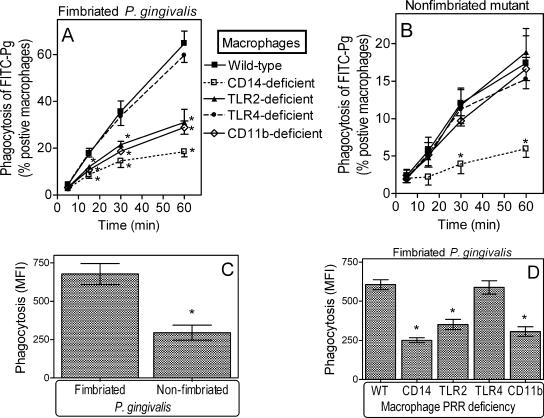
Since CD11b/CD18 can function as a phagocytic receptor (5), we next determined whether the binding of fimbriae to CD11b/CD18 mediates P. gingivalis internalization. For this purpose, peritoneal macrophages from wild-type or CD11b-deficient mice were incubated at 37°C for 5 to 60 min with FITC-labeled P. gingivalis 381 or an isogenic nonfimbriated mutant (JH1004). CD11b-deficient macrophages displayed significantly reduced efficiencies in the uptake of fimbriated P. gingivalis compared to wild-type macrophages (P < 0.05) (Fig. 6A), although no significant differences in the uptake of the nonfimbriated mutant were observed (Fig. 6B). Fimbriated P. gingivalis was internalized by >60% of wild-type macrophages after 60 min (Fig. 6A), whereas the nonfimbriated mutant was taken up by <20% of wild-type macrophages for the same time period (Fig. 6B). Moreover, wild-type macrophages positive for FITC-labeled P. gingivalis internalized significantly more fimbriated bacteria than nonfimbriated bacteria, as indicated by their respective mean fluorescence intensity values (P < 0.05) (Fig. 6C). Essentially no significant internalization was observed in assays performed at 4°C (data not shown). These data suggest that CD11b/CD18 plays an important role in the uptake of fimbriated P. gingivalis.
FIG. 6.
Effect of macrophage PRR deficiencies on P. gingivalis internalization. Peritoneal macrophages from wild-type mice or mice deficient in CD14, TLR2, TLR4, or CR3 were incubated with FITC-labeled P. gingivalis (Pg) 381 or P. gingivalis JH1004 (nonfimbriated mutant) at a multiplicity of infection of 10:1 for the indicated times at 37°C. Internalization was assessed by flow cytometry after washing the macrophages and quenching extracellular fluorescence and was expressed as percent FITC-positive macrophages (A and B). The mean fluorescence intensity (MFI) at the 60-min time point is also shown (C and D) as a relative measure of the number of internalized bacteria. Results are shown as means ± SDs (n = 3; for clarity, only the upper or lower SD is shown in A and B). Asterisks indicate statistically significant (P < 0.05) differences between PRR deficiencies and wild-type (WT) controls (A, B, and D) or between wild-type and mutant P. gingivalis (C).
We further examined CD14- and TLR2-deficient macrophages since CD14 and TLR2 are components of the inside-out signaling for CD11b/CD18 activation (12). TLR2 is not a phagocytic receptor; however, consistent with its role in inside-out signaling, TLR2 deficiency significantly inhibited the internalization of fimbriated P. gingivalis, as shown the by lower rates of FITC-labeled P. gingivalis-positive macrophages (P < 0.05) (Fig. 6A). This was in sharp contrast to TLR4 deficiency (control), which had no significant effect (Fig. 6A). The TLR2 effect was similar to that of CD11b/CD18, resulting not only in lower rates of FITC-labeled P. gingivalis-positive macrophages (P < 0.05) (Fig. 6A) but also in fewer internalized bacteria (P < 0.05) (Fig. 6D) compared to wild-type controls. CD14 deficiency significantly suppressed internalization (P < 0.05) (Fig. 6A and D), although it appeared to have a greater impact than CD11b/CD18, suggesting that CD14 may additionally mediate CD11b/CD18-independent phagocytosis. In fact, CD14 deficiency was the only condition examined that significantly affected the internalization of the nonfimbriated mutant (Fig. 6B). In summary, CD14, TLR2, and CD11b/CD18 play important roles in mediating the internalization of fimbriated P. gingivalis by mouse macrophages.
DISCUSSION
We have shown that P. gingivalis fimbriae proactively modulate the adhesive activity of CD11b/CD18 through TLR2 inside-out signaling, resulting in increased cellular binding and internalization of the organism by macrophages. The involvement of CD14 as an essential accessory receptor in cross talk signaling from TLR2 to CD11b/CD18 suggested the formation of CD14/TLR2 complexes when monocytes are exposed to P. gingivalis fimbriae. This was investigated using FRET, a biophysical technique that measures nonradiative transfer of energy from the excited state of a donor molecule to an appropriate acceptor. Because significant energy transfer can occur only when the molecules are located within close proximity (1 to 10 nm), FRET is useful for determining the sterical coassociation of molecules, such as the ligand-induced clustering of PRRs (25, 29, 30). Our FRET measurements and integrin activation experiments in conjunction with MCD interference (Fig. 4 and 5) suggest that TLR2 is recruited to lipid rafts of fimbria-stimulated monocytes and associates with CD14, forming a functional CD14/TLR2 receptor complex for inside-out signaling and CD11b/CD18 activation. Upon lipid raft disruption by MCD, TLR2-mediated inside-out signaling is abrogated, and CD11b/CD18 cannot be activated by signaling-dependent means. Interestingly, recent findings suggest that lipid raft function is also required for the ability of P. gingivalis to enter cells (33), although CD11b/CD18 has not yet been implicated as an internalization receptor for this pathogen. This possibility was addressed in this study and will be discussed below.
Although the binding of fimbriae to CD14 would necessarily occur prior to the interaction with CD11b/CD18, the latter molecule functions as a major receptor upon the activation of its ligand-binding capacity. In fact, about 50% of the binding of fimbriae to stimulated monocytes is CD11b/CD18 dependent as determined by the use of blocking anti-CD11b MAb (Fig. 3 and Table 1). On the other hand, substantial binding of fimbriae to CD14 (35 to 40% of total cell binding) (Table 1) is detectable even after activation of the ligand-binding capacity of CD11b/CD18. Therefore, both CD14 and CD11b/CD18 could simultaneously function as important cellular receptors for fimbriae, although they may differentially influence TLR2-dependent cell activation. Whereas CD14 is essential for fimbria-induced and TLR2-mediated activation of NF-κB and induction of proinflammatory cytokines (9), CD11b/CD18 contributes partially to TLR2-dependent induction of tumor necrosis factor alpha (9) and is involved in the specific downregulation of IL-12 p70 (8). It is thus tempting to speculate that P. gingivalis fimbriae do not behave as a typical microbe-associated molecular pattern but rather behave as a virulence factor when interacting with PRRs. Microbe-associated molecular patterns are essential for performing microbial physiologic functions, and their relatively conserved structure renders them ideal targets for detection by the similarly conserved PRRs (22). On the other hand, virulence factors such as protein adhesins contribute to microbial adaptation within a particular host environment and are thus relatively variable structures. This suggests that virulence factors are not likely to have been selected as targets of pattern recognition during the course of evolution (22). However, the converse notion, that virulence protein adhesins may have evolved to interact with and exploit certain PRRs, constitutes a plausible hypothesis.
It is therefore possible that P. gingivalis, through its surface fimbriae, has evolved the ability to activate and bind CD11b/CD18 for enhancing its own survival. Our initial report that P. gingivalis fimbriae activate a CD14/TLR2-mediated inside-out signaling pathway for regulating the adhesive activity of CD11b/CD18 (12) was published concomitantly with a study by an independent group that demonstrated that mycobacterial lipoarabinomannan also stimulates this proadhesive pathway (26). Interestingly, this pathway is exploited by mycobacteria for promoting their uptake by monocytes via activated CR3 (CD11b/CD18) (26). It can thus be speculated that the TLR2 inside-out signaling pathway for CD11b/CD18 (CR3) activation may represent a universal pathway exploited by different pathogens. Although mycobacteria interact with CD11b/CD18 as a mechanism for intracellular parasitism (6), it has not been established whether P. gingivalis can similarly induce its uptake by monocytes/macrophages through fimbria-CD11b/CD18 interactions. We were thus prompted to investigate this possibility, and we found that all the receptors associated with the inside-out proadhesive pathway (i.e., CD14, TLR2, and CD11b/CD18) play important roles in mediating the internalization of fimbriated P. gingivalis by mouse macrophages (Fig. 6).
It remains to be established whether the TLR2 proadhesive pathway is hijacked by P. gingivalis to also promote intracellular survival following CD11b/CD18-mediated internalization. If this is true, fimbriae may play an instrumental role in this putative evasion strategy, as they both activate and subsequently bind CD11b/CD18. Moreover, the ability of P. gingivalis fimbriae to inhibit the induction of biologically active IL-12 in a CD11b/CD18-dependent way (8) may help reduce intracellular clearance of P. gingivalis. The fact that CD11b/CD18 does not readily activate the oxidative burst or other microbicidal mechanisms and is thus exploited by intracellular pathogens such as Legionella pneumophila and Mycobacterium tuberculosis for finding a replicative niche (21, 24, 34-36) suggests that CD11b/CD18 may similarly be exploited by other CD11b/CD18-interacting pathogens.
Acknowledgments
This work was supported by U.S. Public Health Service grant DE015254 from the NIDCR, National Institutes of Health (to G.H.), and by the Wellcome Trust and the Heart Research Fund, United Kingdom (to K.T.).
Editor: J. T. Barbieri
REFERENCES
- 1.Abraham, S. N., M. J. Duncan, G. Li, and D. Zaas. 2005. Bacterial penetration of the mucosal barrier by targeting lipid rafts. J. Investig. Med. 53:318-321. [DOI] [PubMed] [Google Scholar]
- 2.Cherukuri, A., M. Dykstra, and S. K. Pierce. 2001. Floating the raft hypothesis: lipid rafts play a role in immune cell activation. Immunity 14:657-660. [DOI] [PubMed] [Google Scholar]
- 3.Christian, A. E., M. P. Haynes, M. C. Phillips, and G. H. Rothblat. 1997. Use of cyclodextrins for manipulating cellular cholesterol content. J. Lipid Res. 38:2264-2272. [PubMed] [Google Scholar]
- 4.Diamond, M. S., and T. A. Springer. 1993. A subpopulation of Mac-1 (CD11b/CD18) molecules mediates neutrophil adhesion to ICAM-1 and fibrinogen. J. Cell Biol. 120:545-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehlers, M. R. W. 2000. CR3: a general purpose adhesion-recognition receptor essential for innate immunity. Microbes Infect. 2:289-294. [DOI] [PubMed] [Google Scholar]
- 6.Ernst, J. D. 1998. Macrophage receptors for Mycobacterium tuberculosis. Infect. Immun. 66:1277-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibson, F. C., III, C. Hong, H. H. Chou, H. Yumoto, J. Chen, E. Lien, J. Wong, and C. A. Genco. 2004. Innate immune recognition of invasive bacteria accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation 109:2801-2806. [DOI] [PubMed] [Google Scholar]
- 8.Hajishengallis, G., P. Ratti, and E. Harokopakis. 2005. Peptide mapping of bacterial fimbrial epitopes interacting with pattern recognition receptors. J. Biol. Chem. 280:38902-38913. [DOI] [PubMed] [Google Scholar]
- 9.Hajishengallis, G., R. I. Tapping, E. Harokopakis, S.-I. Nishiyama, P. Ratti, R. E. Schifferle, E. A. Lyle, M. Triantafilou, K. Triantafilou, and F. Yoshimura. 2. May 2006, posting date. Differential interactions of fimbriae and lipopolysaccharide from Porphyromonas gingivalis with the Toll-like receptor 2-centred pattern recognition apparatus. Cell. Microbiol. [Online.] doi: 2010.1111/j.1462-5822.2006.00730.x. [DOI] [PubMed]
- 10.Hajishengallis, G., R. I. Tapping, M. H. Martin, H. Nawar, E. A. Lyle, M. W. Russell, and T. D. Connell. 2005. Toll-like receptor 2 mediates cellular activation by the B subunits of type II heat-labile enterotoxins. Infect. Immun. 73:1343-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harokopakis, E., M. H. Albzreh, M. H. Martin, and G. Hajishengallis. 2006. TLR2 transmodulates monocyte adhesion and transmigration via Rac1- and PI3K-mediated inside-out signaling in response to Porphyromonas gingivalis fimbriae. J. Immunol. 176:7645-7656. [DOI] [PubMed] [Google Scholar]
- 12.Harokopakis, E., and G. Hajishengallis. 2005. Integrin activation by bacterial fimbriae through a pathway involving CD14, Toll-like receptor 2, and phosphatidylinositol-3-kinase. Eur. J. Immunol. 35:1201-1210. [DOI] [PubMed] [Google Scholar]
- 13.Hasebe, A., A. Yoshimura, T. Into, H. Kataoka, S. Tanaka, S. Arakawa, H. Ishikura, D. T. Golenbock, T. Sugaya, N. Tsuchida, M. Kawanami, Y. Hara, and K. Shibata. 2004. Biological activities of Bacteroides forsythus lipoproteins and their possible pathological roles in periodontal disease. Infect. Immun. 72:1318-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heine, H., C. J. Kirschning, E. Lien, B. G. Monks, M. Rothe, and D. T. Golenbock. 1999. Cutting edge: cells that carry a null allele for Toll-like receptor 2 are capable of responding to endotoxin. J. Immunol. 162:6971-6975. [PubMed] [Google Scholar]
- 15.Henneke, P., O. Takeuchi, J. A. van Strijp, H. K. Guttormsen, J. A. Smith, A. B. Schromm, T. A. Espevik, S. Akira, V. Nizet, D. L. Kasper, and D. T. Golenbock. 2001. Novel engagement of CD14 and multiple Toll-like receptors by group B streptococci. J. Immunol. 167:7069-7076. [DOI] [PubMed] [Google Scholar]
- 16.Kozarov, E. V., B. R. Dorn, C. E. Shelburne, W. A. Dunn, Jr., and A. Progulske-Fox. 2005. Human atherosclerotic plaque contains viable invasive Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. Arterioscler. Thromb. Vasc. Biol. 25:e17-e18. [DOI] [PubMed] [Google Scholar]
- 17.Laudanna, C., J. Y. Kim, G. Constantin, and E. C. Butcher. 2002. Rapid leukocyte integrin activation by chemokines. Immunol. Rev. 186:37-46. [DOI] [PubMed] [Google Scholar]
- 18.Lawrence, J. C., D. E. Saslowsky, J. M. Edwardson, and R. M. Henderson. 2003. Real-time analysis of the effects of cholesterol on lipid raft behavior using atomic force microscopy. Biophys. J. 84:1827-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levitz, S. M., A. Tabuni, T. R. Kozel, R. S. MacGill, R. R. Ingalls, and D. T. Golenbock. 1997. Binding of Cryptococcus neoformans to heterologously expressed human complement receptors. Infect. Immun. 65:931-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li, R., J. Xie, C. Kantor, V. Koistinen, D. C. Altieri, P. Nortamo, and C. G. Gahmberg. 1995. A peptide derived from the intercellular adhesion molecule-2 regulates the avidity of the leukocyte integrins CD11b/CD18 and CD11c/CD18. J. Cell Biol. 129:1143-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowell, C. A. 2006. Rewiring phagocytic signal transduction. Immunity 24:243-245. [DOI] [PubMed] [Google Scholar]
- 22.Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1:135-145. [DOI] [PubMed] [Google Scholar]
- 23.Ozinsky, A., D. M. Underhill, J. D. Fontenot, A. M. Hajjar, K. D. Smith, C. B. Wilson, L. Schroeder, and A. Aderem. 2000. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between Toll-like receptors. Proc. Natl. Acad. Sci. USA 97:13766-13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Payne, N. R., and M. A. Horwitz. 1987. Phagocytosis of Legionella pneumophila is mediated by human monocyte complement receptors. J. Exp. Med. 166:1377-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfeiffer, A., A. Bottcher, E. Orso, M. Kapinsky, P. Nagy, A. Bodnar, I. Spreitzer, G. Liebisch, W. Drobnik, K. Gempel, M. Horn, S. Holmer, T. Hartung, G. Multhoff, G. Schutz, H. Schindler, A. J. Ulmer, H. Heine, F. Stelter, C. Schutt, G. Rothe, J. Szollosi, S. Damjanovich, and G. Schmitz. 2001. Lipopolysaccharide and ceramide docking to CD14 provokes ligand-specific receptor clustering in rafts. Eur. J. Immunol. 31:3153-3164. [DOI] [PubMed] [Google Scholar]
- 26.Sendide, K., N. E. Reiner, J. S. I. Lee, S. Bourgoin, A. Talal, and Z. Hmama. 2005. Cross-talk between CD14 and complement receptor 3 promotes phagocytosis of mycobacteria: regulation by phosphatidylinositol 3-kinase and cytohesin-1. J. Immunol. 174:4210-4219. [DOI] [PubMed] [Google Scholar]
- 27.Shimaoka, M., J. Takagi, and T. A. Springer. 2002. Conformational regulation of integrin structure and function. Annu. Rev. Biophys. Biomol. Struct. 31:485-516. [DOI] [PubMed] [Google Scholar]
- 28.Stöckl, J., O. Majdic, W. F. Pickl, A. Rosenkranz, E. Prager, E. Gschwantler, and W. Knapp. 1995. Granulocyte activation via a binding site near the C-terminal region of complement receptor type 3 alpha-chain (CD11b) potentially involved in intramembrane complex formation with glycosylphosphatidylinositol-anchored Fc gamma RIIIB (CD16) molecules. J. Immunol. 154:5452-5463. [PubMed] [Google Scholar]
- 29.Triantafilou, K., M. Triantafilou, and R. L. Dedrick. 2001. A CD14-independent LPS receptor cluster. Nat. Immunol. 2:338-345. [DOI] [PubMed] [Google Scholar]
- 30.Triantafilou, M., K. Brandenburg, S. Kusumoto, K. Fukase, A. Mackie, U. Seydel, and K. Triantafilou. 2004. Combinational clustering of receptors following stimulation by bacterial products determines lipopolysaccharide responses. Biochem. J. 381:527-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Triantafilou, M., K. Miyake, D. T. Golenbock, and K. Triantafilou. 2002. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J. Cell Sci. 115:2603-2611. [DOI] [PubMed] [Google Scholar]
- 32.Trinchieri, G. 2003. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3:133-146. [DOI] [PubMed] [Google Scholar]
- 33.Tsuda, K., A. Amano, K. Umebayashi, H. Inaba, I. Nakagawa, Y. Nakanishi, and T. Yoshimori. 2005. Molecular dissection of internalization of Porphyromonas gingivalis by cells using fluorescent beads coated with bacterial membrane vesicle. Cell Struct. Funct. 30:81-91. [DOI] [PubMed] [Google Scholar]
- 34.Vieira, O. V., R. J. Botelho, and S. Grinstein. 2002. Phagosome maturation: aging gracefully. Biochem. J. 366:689-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson, C. B., V. Tsai, and J. S. Remington. 1980. Failure to trigger the oxidative metabolic burst by normal macrophages: possible mechanism for survival of intracellular pathogens. J. Exp. Med. 151:328-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright, S. D., and S. C. Silverstein. 1983. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes. J. Exp. Med. 158:2016-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zambon, J. J., S. Grossi, R. Dunford, V. I. Harazsthy, H. Preus, and R. J. Genco. 1994. Epidemiology of subgingival bacterial pathogens in periodontal diseases, p. 3-12. In R. J. Genco, S. Hamada, J. R. Lehrer, J. R. McGhee, and S. Mergenhangen (ed.), Molecular pathogenesis of periodontal disease. American Society for Microbiology, Washington, D.C.