Abstract
Placental Plasmodium falciparum infection affects birth outcomes and sensitizes fetal lymphocytes to parasite antigens. We assessed the influence of maternal P. falciparum infection on fetal myeloid dendritic cells (mDC) and plasmacytoid dendritic cells (pDC), analyzing the cord blood of offspring of Gabonese mothers with different infection histories. Cord blood from newborns of mothers with malarial infection at delivery had significantly more mDC than that from nonexposed newborns (P = 0.028) but mDC and pDC HLA-DR expression was unrelated to maternal infection history. Independently of these findings, cord blood mDC and pDC numbers declined significantly as a function of increasing maternal age (P = 0.029 and P = 0.033, respectively). The inducible antigen-specific interleukin-10-producing regulatory-type T-cell population that we have previously detected in cord blood of newborns with prolonged in utero exposure to P. falciparum may directly reflect the altered DC numbers in such neonates, while the maintenance of cord blood DC HLA-DR expression contrasts with that of DC from P. falciparum malaria patients.
Myeloid dendritic cells (mDC) and plasmacytoid dendritic cells (pDC) play a central role in orchestration of immune responses, in particular initiating primary CD4+ T-cell responses (15, 16), an essential step in establishing adaptive immune responses to pathogens such as the causal agents of malaria. Our current understanding of the complexity of DC function in malaria relies mostly on studies in rodent models, where specific DC subsets have been implicated as key players in the timely coordination of sequential Th1/Th2 regulation in Plasmodium chabaudi infection (23). While parasitized blood cells activate DC in various models, malarial parasites have evolved means to affect DC function, compromising the host's response to infection (20). This may extend to nonparasite antigens, as has recently been shown by demonstrating reduced cross-presentation in Plasmodium berghei infection (32) and impaired capacity to stimulate CD4+ T-helper cell proliferation and cytokine response after exposure to P. chabaudi (18). While some mechanisms of DC modulation, especially suppressive effects of the malarial pigment hemozoin, appear to be shared between mice and humans, additional pathways involving cell adhesion proteins confined to Plasmodium falciparum have been suggested for this most important malaria parasite of humans (30).
While the malaria-associated alterations of DC functions outlined above have been put forward as possible causes of reduced vaccine efficacy in regions where P. falciparum is endemic, and while small children are considered to constitute a high-risk group not least because of adverse immunological effects of prenatal exposure to P. falciparum, there are few published data on P. falciparum infection-related immunology in the neonatal compartment. DC function during fetal and neonatal development is generally not well defined, and although it is known that placental P. falciparum infection leads to sensitized fetal T cells (2-4, 8, 11, 17), the role of fetal DC in this context is unknown. Here, therefore, we investigated the effects of pregnancy-associated P. falciparum infection on fetal DC, using flow cytometry (fluorescence-activated cell sorting) to enumerate mDC and pDC in cord blood (CB) of offspring of women delivering in Lambaréné, Gabon. In parallel we assessed DC surface HLA-DR expression as a surrogate marker for DC capacity to present antigens to other immune cells. Data were analyzed as a function of maternal P. falciparum infection status.
MATERIALS AND METHODS
Study population.
The study was conducted at the Albert Schweitzer Hospital (HAS) in Lambaréné, Gabon, where malaria is holoendemic and transmission is perennial (31). Mothers were recruited at the maternity units of both HAS and Lambaréné General Hospital and were included after giving their written informed consent. Due to an unexpectedly low malaria incidence during the study period, women with a higher assumed risk of P. falciparum infection (e.g., primipara or reported malaria during pregnancy) were preferentially recruited towards the end of the work. The ethics committees of Tübingen University and the International Foundation of HAS approved the study.
Laboratory methods.
Cord blood and maternal peripheral venous blood were collected into sterile heparin-containing tubes at or immediately after delivery, and specimens were obtained for placental thick smears. Cord blood mononuclear cells (CBMC) were isolated from diluted CB by a standard procedure with Ficoll (Apotheek AZL, The Netherlands), washed, and resuspended at 107 cells/ml in culture medium (Iscove’s modified Dulbecco’s medium; GIBCO) supplemented with 10% fetal calf serum (GIBCO), 1 mM pyruvate, 2 mM glutamate, 100 U/ml penicillin, and 100 μg/ml streptomycin. One-milliliter aliquots of this suspension were centrifuged and washed twice in 1 ml of phosphate-buffered saline containing 2 mM EDTA and 0.5% bovine serum albumin (SIGMA-Aldrich, Germany), and 10 μl of FcR blocking reagent (Miltenyi Biotec, Germany) was added to aliquots of 106 cells prior to incubation with specific antibodies.
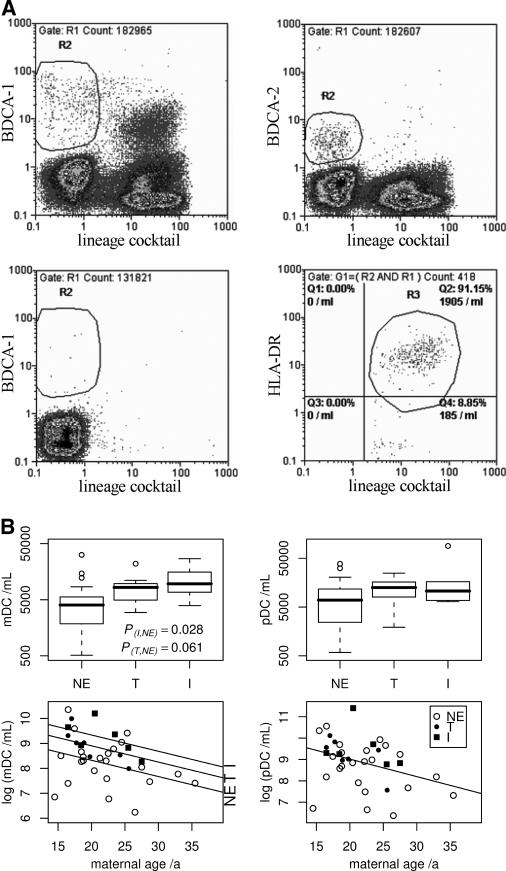
Fluorescence-labeled BDCA-1-/BDCA-2-specific antibodies (Miltenyi) were used to identify mDC and pDC. DC surface HLA-DR was revealed by HLA-DR-specific antibody (Becton Dickinson). Anti-CD34 and a lineage marker antibody cocktail (CD3, CD14, CD16, CD19, CD20, and CD56) were used to exclude non-DC populations, and controls comprised appropriately labeled mouse monoclonal antibody isotypes (all from Becton Dickinson). CBMC-antibody incubations were for 30 min at room temperature in the dark. Data from fluorescing DC were acquired with a flow cytometer (Partec, Germany), analyzing 300,000 events for DC and 200,000 events for isotype control enumerations. mDC/pDC were identified as BDCA-1+/BDCA-2+ lineage marker-negative (lin−) cells appearing in the forward-/side-scatter region of lymphocytes/monocytes (Fig. 1A). This identification of DC subsets follows their published characterization (7): in size and granularity, DC appear somewhat similar to lymphocytes and monocytes, and restricting the marker analysis to cells showing the corresponding forward-/side-scatter profile may reduce unspecific noise; in terms of surface antigens, mDC and pDC express with good specificity BDCA-1 (CD1c) and BDCA-2 (CD303), respectively, but lack common markers of various immune cell lineages. Using the lineage cocktail to identify and exclude cells expressing the respective DC marker but also any lineage-associated antigens further increases the specificity of our enumeration.
FIG. 1.
Flow cytometry for and distribution of CBDC subsets. (A) Flow cytometric identification of mDC (lin− BDCA-1+) and pDC (lin− BDCA-2+) in one subject's cord blood mononuclear cells from the lymphocyte/monocyte scatter region is shown in the upper left and right dot plots, respectively. Below, the isotype control for quantification of unspecific binding (mDC staining) and the gating of HLA-DR+ mDC are presented for the same sample. For details, see the text. (B) Concentrations of mDC and pDC were analyzed as a function of Plasmodium falciparum exposure in utero. The box-and-whisker plots illustrate medians and percentiles with outliers (open circles) and demonstrate higher mDC frequencies in CB of exposed offspring; dot plots show the negative associations between CB mDC/pDC numbers and maternal age, in the case of mDC including intercept shifts due to past or present exposure as revealed by two-way ANOVA. NE, nonexposed; T, treated; I, infected.
DC counts were corrected according to isotype control staining. Absolute DC concentrations were calculated from detected DC, events in the lymphocyte gate, and the lymphocyte counts obtained with a CellDyn 3000 (Abbott).
CB samples were grouped according to maternal P. falciparum infection history (nonexposed, treated, or infected) determined by routine microscopic examination of Giemsa-stained thick smears at delivery, inspection of the mothers' health data, and thorough anamnesis.
Data analysis.
DC parameters were compared between groups by analysis of variance (ANOVA), applying log transformation where appropriate, with the significance of differences determined by Dunnett's test, using the statistical software “R” version 2.0.1 (R Foundation for Statistical Computing, Vienna, Austria) and its extension “multcomp” (F. Bretz et al., http://cran.r-project.org/bin/windows/contrib/r-release/multcomp_0.4-8.zip) for multiple-comparison procedures.
RESULTS AND DISCUSSION
A single CB sample contained P. falciparum-infected erythrocytes (iE). DC were detected in CBMC at various concentrations (range, 1,000 to 120,000/ml), with a geometric mean of ∼12,400/ml (95% confidence interval [CI], 8,800 to 17,600/ml). Consistent with the findings of others (26), our CB samples contained no CD34+ lin− DC marker-positive cells. Consequently, the stem cell marker was omitted after preliminary experiments.
Total and HLA-DR+ mDC numbers differed between groups (P = 0.021 and P = 0.019, respectively, by one-way ANOVA) and were found to be significantly higher in CB of the infected group compared to the nonexposed group (P = 0.028 and P = 0.027, respectively, by Dunnett's test), with similar but nonsignificant trends for more mDC/HLA-DR+ mDC in CB of the treated group (P = 0.061 and P = 0.053, respectively) (Fig. 1B; Table 1). When controlling for maternal age, which was the only confounding variable that was significantly associated with mDC numbers in one-way ANOVA, the association with malarial status was strengthened (P = 0.014 [total mDC] and P = 0.013 [HLA-DR+ mDC] by two-way ANOVA). Numbers of pDC showed similar trends, but these measurements were associated with higher dispersion than for mDC and remained statistically insignificant (Fig. 1B; Table 1). Our findings highlight clear differences between fetal DC exposed to P. falciparum in utero and DC in children with acute P. falciparum malaria, in whom DC (lin− CD83+) numbers are normal but numbers of lin− HLA-DR+ cells are decreased compared to those in healthy controls (29). In adults with acute P. falciparum malaria, pDC concentrations are decreased, while plasma alpha interferon levels are increased (21). Since pDC are major producers of alpha interferon, especially upon activation, these findings were interpreted as reflecting activation-induced migration of pDC into lymphoid sites. The observation of elevated mDC concentrations associated with in utero exposure to P. falciparum, together with identical trends for pDC, imply that placental P. falciparum infection indeed leads to activation of fetal DC, but clearly without any associated decrease in CBDC numbers. This may reflect the altered regulation of chemokine receptors in CBDC compared to adult DC (13). Such putatively impaired coordination of postactivation migratory processes in CBDC exposed to P. falciparum in utero merits further study. In any case, the offspring of mothers with placental P. falciparum infection at delivery have been shown to have earlier and more frequent parasitemia in the first year of life (5, 14, 19). Whether a causal link exists between this enhanced postnatal susceptibility to infection and the prenatal expansion of DC populations that we document here remains an open question.
TABLE 1.
Blood counts and dendritic cell measures in cord blood samples segregated according to maternal malaria historya
| Group (n) | White blood cells (103/μl) | Lymphocytes (%) | mDC/pDC ratio | HLA-DR+ mDC (% of all mDC) | HLA-DR+ pDC (% of all pDC) | HLA-DR+ mDC/ml | HLA-DR+ pDC/ml | MFIb of HLA-DR on:
|
|
|---|---|---|---|---|---|---|---|---|---|
| mDC | pDC | ||||||||
| Nonexposed (23) | 11.4 (9.6-12.4) | 45.8 (35.8-54.4) | 0.756 (0.441-0.878) | 81.4 (75.7-87.3) | 88.2 (84.6-93.3) | 2,888 (1,660-4,580) | 4,771 (1,926-10,830) | 18.74 (16.24-21.32) | 5.65 (4.45-6.90) |
| Treated (8) | 12.3 (10.9-14.7) | 41.9 (32.2-49.8) | 0.791 (0.542-0.931) | 85.6 (81.8-91.7) | 91.1 (86.4-97.7) | 6,382 (4,419-8,962)* | 9,259 (7,524-15,720) | 18.44 (13.88-20.52) | 5.36 (4.49-6.17) |
| Infected (6) | 14.0 (11.3-15.7) | 58.8 (49.9-71.4) | 0.880 (0.507-1.144) | 86.5 (81.9-90.8) | 92.2 (91.3-94.3) | 8,616 (6,349-13,220)† | 12,885 (7,066-13,490) | 18.04 (15.22-20.93) | 6.15 (5.33-6.87) |
Given are arithmetic means (or geometric means in the case of concentrations) and interquartile ranges. If ANOVA indicated significant group differences, the exposed groups were compared to the nonexposed group by Dunnett's test, and resulting P values are indicated (*, P = 0.053; †, P = 0.027). For technical reasons, two additional samples were analyzed only for mDC/pDC ratios in both the nonexposed and infected groups, while white blood cell counts and DC concentrations in the treated group were determined for seven samples only.
MFI, mean fluorescence intensity.
Maternal P. falciparum infection did not result in altered CBDC HLA-DR expression measured ex vivo either as the mean HLA-DR surface expression per cell or as the percentage of HLA-DR+ mDC or pDC (Table 1). This is somewhat surprising in light of the reportedly decreased frequencies of HLA-DR+ DC in individuals with P. falciparum malaria (21, 28, 29). HLA-DR expression and parasitemia are thought to be inversely correlated (25), and intact iE are required for at least some of the P. falciparum-associated DC modulation (27). In CB in our own and other settings, however, iE are very rarely observed (4, 12), but prenatal sensitization is comparatively frequent (2-4, 8, 11, 17). These observations thus clearly indicate that, rather than iE, transplacentally transferred parasite-derived components influence CBDC abundance in the majority of cases. In this context, it is notable that both malarial pigment (hemozoin), present at the materno-fetal interface (1), and plasmodial glycosylphosphatidylinositols interact with Toll-like receptors on DC (20, 25), and their influences on CBDC frequency and function therefore deserve further study.
CB contained pDC at a significantly higher concentration than mDC (P < 0.001 by paired t test after log transformation), resulting in a mean mDC/pDC ratio of 0.69 (95% CI, 0.58 to 0.82). There is no current consensus regarding the normal physiological CB mDC/pDC ratio. Intriguingly, both published studies that used the same CBDC markers as we did reported finding about fourfold more mDC (6, 22). These differences most likely reflect the different study populations: our study is the first to describe CBDC subset distributions in an African population.
Independently of exposure to P. falciparum in utero, the numbers of both mDC and pDC in CB declined with increasing maternal age, with coefficients of −0.065 (95% CI, −0.124 to −0.007; P = 0.029 [two-way ANOVA]) and −0.082 (95% CI, −0.157 to −0.007; P = 0.033 [one-way ANOVA]) for log-transformed mDC and pDC concentrations, respectively (Fig. 1B). We currently have no explanation for this intriguing observation. In the present study, our flow cytometric examinations were limited to neonatal samples due to resource constraints. Analyses of matched maternal samples would not only shed some light on the maternal age-CBDC relationship described but would also enable us to correlate the P. falciparum-associated neonatal changes with maternal equivalents.
In a majority of CB samples from those exposed to P. falciparum in utero, parasite antigen-specific T-cell responses show an interleukin-10 (IL-10) or Th2-like bias that contrasts with the Th1-like bias in P. falciparum-infected placentas (9, 11, 17). Despite their high functional plasticity, mDC are generally thought to induce Th1-type responses, but CB mDC have an impaired capacity for IL-12 production (10). Furthermore, partially activated DC lead to the expansion of regulatory T cells (24). Our own work has revealed inducible IL-10-producing Tr1-type regulatory T cells in CB of newborns with in utero P. falciparum exposure, associated with a Th2-type bias of parasite-specific T-cell responses (2-4). Taken together, our results are consistent with the hypothesis that sustained exposure to transplacentally transferred P. falciparum components stimulates immature fetal DC that induce an immunosuppressive/Th2-type immune milieu. The role of either mDC or pDC in this context is open to speculation, but we have shown that HLA expression by CB monocytes of the infected group is suppressed by parasite antigens in vitro (4), suggesting possible functional impairment of antigen-presenting cells in vivo. Neonates are the target population for malaria vaccines that are being developed, and it is of obvious concern that the efficacy of such vaccines in early life might potentially be compromised by inappropriately suppressed parasite-specific T-cell responses, the etiology of which may be related to the P. falciparum-related CBDC alterations that we describe here.
.
Acknowledgments
This work was supported by the German Government, via a Deutscher Akademischer Austausch Dienst (DAAD) studentship awarded to L.P.B., and by The Netherlands Foundation for the Advancement of Tropical Research (WOTRO, project no. W93-385).
We are grateful for the patient cooperation of all our study participants and acknowledge the kind support received from midwives, administrations, and staffs of the two hospitals and the Medical Research Unit. Fruitful discussions with Ilka Engelmann and Sanders Chai contributed to this work, and we thank Selidji Agnandji, Martin Kramer, and Daniela Schütte for their helping hands. The Leeds University Skin Research Centre assisted in the preparation of the fluorescence-activated cell sorter plots.
L.P.B. designed and performed research, analyzed data, and wrote the paper; R.F. and B.M. assisted with research methodology and analysis; A.A.A. performed research; P.G.K. designed and supervised research; and A.J.F.L. designed and supervised research and wrote the paper.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Brabin, B. J., C. Romagosa, S. Abdelgalil, C. Menendez, F. H. Verhoeff, R. McGready, K. A. Fletcher, S. Owens, U. D'Alessandro, F. Nosten, P. R. Fischer, and J. Ordi. 2004. The sick placenta—the role of malaria. Placenta 25:359-378. [DOI] [PubMed] [Google Scholar]
- 2.Brustoski, K., M. Kramer, U. Moller, P. G. Kremsner, and A. J. Luty. 2005. Neonatal and maternal immunological responses to conserved epitopes within the DBL-gamma3 chondroitin sulfate A-binding domain of Plasmodium falciparum erythrocyte membrane protein 1. Infect. Immun. 73:7988-7995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brustoski, K., U. Moller, M. Kramer, F. C. Hartgers, P. G. Kremsner, U. Krzych, and A. J. Luty. 2006. Reduced cord blood immune effector-cell responsiveness mediated by CD4+ cells induced in utero as a consequence of placental Plasmodium falciparum infection. J. Infect. Dis. 193:146-154. [DOI] [PubMed] [Google Scholar]
- 4.Brustoski, K., U. Moller, M. Kramer, A. Petelski, S. Brenner, D. R. Palmer, M. Bongartz, P. G. Kremsner, A. J. Luty, and U. Krzych. 2005. IFN-gamma and IL-10 mediate parasite-specific immune responses of cord blood cells induced by pregnancy-associated Plasmodium falciparum malaria. J. Immunol. 174:1738-1745. [DOI] [PubMed] [Google Scholar]
- 5.Cot, M., J. Y. Le Hesran, T. Staalsoe, N. Fievet, L. Hviid, and P. Deloron. 2003. Maternally transmitted antibodies to pregnancy-associated variant antigens on the surface of erythrocytes infected with Plasmodium falciparum: relation to child susceptibility to malaria. Am. J. Epidemiol. 157:203-209. [DOI] [PubMed] [Google Scholar]
- 6.Darmochwal-Kolarz, D., J. Rolinski, J. Buczkowski, J. Tabarkiewicz, B. Leszczynska-Gorzelak, I. Zych, and J. Oleszczuk. 2004. CD1c(+) immature myeloid dendritic cells are predominant in cord blood of healthy neonates. Immunol. Lett. 91:71-74. [DOI] [PubMed] [Google Scholar]
- 7.Dzionek, A., A. Fuchs, P. Schmidt, S. Cremer, M. Zysk, S. Miltenyi, D. W. Buck, and J. Schmitz. 2000. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J. Immunol. 165:6037-6046. [DOI] [PubMed] [Google Scholar]
- 8.Engelmann, I., A. Santamaria, P. G. Kremsner, and A. J. Luty. 2005. Activation status of cord blood gamma delta T cells reflects in utero exposure to Plasmodium falciparum antigen. J. Infect. Dis. 191:1612-1622. [DOI] [PubMed] [Google Scholar]
- 9.Fried, M., R. O. Muga, A. O. Misore, and P. E. Duffy. 1998. Malaria elicits type 1 cytokines in the human placenta: IFN-gamma and TNF-alpha associated with pregnancy outcomes. J. Immunol. 160:2523-2530. [PubMed] [Google Scholar]
- 10.Goriely, S., B. Vincart, P. Stordeur, J. Vekemans, F. Willems, M. Goldman, and D. De Wit. 2001. Deficient IL-12(p35) gene expression by dendritic cells derived from neonatal monocytes. J. Immunol. 166:2141-2146. [DOI] [PubMed] [Google Scholar]
- 11.Ismaili, J., M. van der Sande, M. J. Holland, I. Sambou, S. Keita, C. Allsopp, M. O. Ota, K. P. McAdam, and M. Pinder. 2003. Plasmodium falciparum infection of the placenta affects newborn immune responses. Clin. Exp. Immunol. 133:414-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kassberger, F., A. Birkenmaier, A. Khattab, P. G. Kremsner, and M. Q. Klinkert. 2002. PCR typing of Plasmodium falciparum in matched peripheral, placental and umbilical cord blood. Parasitol. Res. 88:1073-1079. [DOI] [PubMed] [Google Scholar]
- 13.Langrish, C. L., J. C. Buddle, A. J. Thrasher, and D. Goldblatt. 2002. Neonatal dendritic cells are intrinsically biased against Th-1 immune responses. Clin. Exp. Immunol. 128:118-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Hesran, J. Y., M. Cot, P. Personne, N. Fievet, B. Dubois, M. Beyeme, C. Boudin, and P. Deloron. 1997. Maternal placental infection with Plasmodium falciparum and malaria morbidity during the first 2 years of life. Am. J. Epidemiol. 146:826-831. [DOI] [PubMed] [Google Scholar]
- 15.Lipscomb, M. F., and B. J. Masten. 2002. Dendritic cells: immune regulators in health and disease. Physiol. Rev. 82:97-130. [DOI] [PubMed] [Google Scholar]
- 16.MacDonald, K. P., D. J. Munster, G. J. Clark, A. Dzionek, J. Schmitz, and D. N. Hart. 2002. Characterization of human blood dendritic cell subsets. Blood 100:4512-4520. [DOI] [PubMed] [Google Scholar]
- 17.Malhotra, I., P. Mungai, E. Muchiri, J. Ouma, S. Sharma, J. W. Kazura, and C. L. King. 2005. Distinct Th1- and Th2-Type prenatal cytokine responses to Plasmodium falciparum erythrocyte invasion ligands. Infect. Immun. 73:3462-3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Millington, O. R., C. Di Lorenzo, R. S. Phillips, P. Garside, and J. M. Brewer. 2006. Suppression of adaptive immunity to heterologous antigens during Plasmodium infection through hemozoin-induced failure of dendritic cell function. J. Biol. 5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mutabingwa, T. K., M. C. Bolla, J. L. Li, G. J. Domingo, X. Li, M. Fried, and P. E. Duffy. 2005. Maternal malaria and gravidity interact to modify infant susceptibility to malaria. PLoS Med. 2:e407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ndungu, F. M., B. C. Urban, K. Marsh, and J. Langhorne. 2005. Regulation of immune response by Plasmodium-infected red blood cells. Parasite Immunol. 27:373-384. [DOI] [PubMed] [Google Scholar]
- 21.Pichyangkul, S., K. Yongvanitchit, U. Kum-arb, H. Hemmi, S. Akira, A. M. Krieg, D. G. Heppner, V. A. Stewart, H. Hasegawa, S. Looareesuwan, G. D. Shanks, and R. S. Miller. 2004. Malaria blood stage parasites activate human plasmacytoid dendritic cells and murine dendritic cells through a Toll-like receptor 9-dependent pathway. J. Immunol. 172:4926-4933. [DOI] [PubMed] [Google Scholar]
- 22.Schibler, K. R., A. Georgelas, and A. Rigaa. 2002. Developmental biology of the dendritic cell system. Acta Paediatr. Suppl. 91:9-16. [DOI] [PubMed] [Google Scholar]
- 23.Sponaas, A. M., E. T. Cadman, C. Voisine, V. Harrison, A. Boonstra, A. O'Garra, and J. Langhorne. 2006. Malaria infection changes the ability of splenic dendritic cell populations to stimulate antigen-specific T cells. J. Exp. Med. 203:1427-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinman, R. M. 2003. The control of immunity and tolerance by dendritic cell. Pathol. Biol. (Paris). 51:59-60. [DOI] [PubMed] [Google Scholar]
- 25.Stevenson, M. M., and B. C. Urban. 2006. Antigen presentation and dendritic cell biology in malaria. Parasite Immunol. 28:5-14. [DOI] [PubMed] [Google Scholar]
- 26.Ueda, Y., M. Hagihara, A. Okamoto, A. Higuchi, A. Tanabe, K. Hirabayashi, S. Izumi, T. Makino, S. Kato, and T. Hotta. 2003. Frequencies of dendritic cells (myeloid DC and plasmacytoid DC) and their ratio reduced in pregnant women: comparison with umbilical cord blood and normal healthy adults. Hum. Immunol. 64:1144-1151. [DOI] [PubMed] [Google Scholar]
- 27.Urban, B. C., D. J. Ferguson, A. Pain, N. Willcox, M. Plebanski, J. M. Austyn, and D. J. Roberts. 1999. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature 400:73-77. [DOI] [PubMed] [Google Scholar]
- 28.Urban, B. C., T. T. Hien, N. P. Day, N. H. Phu, R. Roberts, E. Pongponratn, M. Jones, N. T. Mai, D. Bethell, G. D. Turner, D. Ferguson, N. J. White, and D. J. Roberts. 2005. Fatal Plasmodium falciparum malaria causes specific patterns of splenic architectural disorganization. Infect. Immun. 73:1986-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Urban, B. C., T. Mwangi, A. Ross, S. Kinyanjui, M. Mosobo, O. Kai, B. Lowe, K. Marsh, and D. J. Roberts. 2001. Peripheral blood dendritic cells in children with acute Plasmodium falciparum malaria. Blood 98:2859-2861. [DOI] [PubMed] [Google Scholar]
- 30.Urban, B. C., and S. Todryk. 2006. Malaria pigment paralyzes dendritic cells. J. Biol. 5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wildling, E., S. Winkler, P. G. Kremsner, C. Brandts, L. Jenne, and W. H. Wernsdorfer. 1995. Malaria epidemiology in the province of Moyen Ogoov, Gabon. Trop. Med. Parasitol. 46:77-82. [PubMed] [Google Scholar]
- 32.Wilson, N. S., G. M. Behrens, R. J. Lundie, C. M. Smith, J. Waithman, L. Young, S. P. Forehan, A. Mount, R. J. Steptoe, K. D. Shortman, T. F. de Koning-Ward, G. T. Belz, F. R. Carbone, B. S. Crabb, W. R. Heath, and J. A. Villadangos. 2006. Systemic activation of dendritic cells by Toll-like receptor ligands or malaria infection impairs cross-presentation and antiviral immunity. Nat. Immunol. 7:165-172. [DOI] [PubMed] [Google Scholar]