Abstract
Matrix metalloproteinases (MMP) are a family of host-derived enzymes involved in the turnover of extracellular matrix molecules. We have previously reported enhanced expression of matrix metalloproteinases in Chlamydia muridarum urogenital tract infection of female mice. Kinetics and patterns of MMP expression as well as enhanced expression in susceptible strains of mice in the prior study implied a role for MMP in pathogenesis. To explore this further, we infected a susceptible strain of mice (C3H/HeN) with C. muridarum and treated two groups of mice with either one of two chemical inhibitors of MMP (MMPi; captopril and a chemically modified tetracycline) and reserved infected sham-treated mice as controls. Neither of the treatments affected shedding of viable chlamydiae from the lower urogenital tract, but the administration of either MMPi protected mice from the formation of hydrosalpinx—a surrogate marker of oviduct occlusion and infertility. Interestingly, the mechanism of protection for mice treated with chemically modified tetracycline 3, appeared to be related to prevention of ascending upper genital tract infection. These results imply that MMP are involved in pathogenesis of chlamydial infection in this model by mediating ascension of the infection into the upper genital tract.
The mouse model of female urogenital tract infection with Chlamydia muridarum (previously, the mouse pneumonitis strain of Chlamydia trachomatis, MoPn) has been used for over 20 years to study immunological protection related to chlamydial infections (25). More recently, it has been used to study pathological immune responses during chlamydia infection. Hydrosalpinx formation, infertility, and fibrotic oviduct occlusion are routine consequences of infection following a single urogenital inoculation of Chlamydia muridarum in susceptible mouse strains. Each of these manifestations is positively correlated with the others and also occurs in resistant strains, but at a lower rate (8, 30).
We have found that nitrogen and oxygen radicals interact to have a major role in influencing pathogenesis in this model (23). In the absence of phagocyte oxidase, mice are spared from the chronic sequelae of hydrosalpinx and infertility following chlamydial infection, whereas the loss of inducible nitric oxide synthase significantly increases the rate of chronic sequelae. Interestingly, oxygen and nitrogen radicals have likewise been shown to have regulatory effects on host enzymes that influence the degradation of extracellular matrix (ECM) and fibrosis (13). The matrix metalloproteinases (MMP) are a class of zinc-dependent enzymes that are involved in the proteolysis and resynthesis of the ECM (18, 44), processing of cytokines to active forms (19), release of sequestered growth and signaling factors (14), and chemotaxis and migration of leukocytes through inflamed tissues (17, 40, 41). In addition to a pivotal role in fibrosis, MMP activity has been linked to atherosclerosis (11), angiogenesis associated with tumor growth and metastasis (5), and rheumatoid arthritis and other autoimmune disorders (10). With regard to chlamydial infections, a role for MMP has also been proposed in trachoma (1, 2) and enhanced MMP expression has been reported in an in vitro model of human fallopian tube infection (3). In the mouse urogenital infection model, MMP expression was elevated in susceptible strains when compared to resistant strains—especially during peak acute inflammatory responses (24). Particularly elevated in that study was MMP-9 (also known as gelatinase B), at least one significant source of which was infiltrating neutrophils. Recently, Pal et al. ruled out a role for the epithelial cell-derived MMP-7 (trivial name, matrilysin) in the murine urogenital tract but may have uncovered a role in intestinal infection (20).
In the present study, we hypothesized a role for MMPs in the pathogenesis of chlamydial infection in the mouse model. Mice were treated with either of two chemical inhibitors of MMP activity or sham treated, and outcomes of infection course, progression to upper genital tract involvement, and gross and microscopic pathology were assessed. The results indicate a role for MMP in the pathogenesis of acute disease, ascension of the infection into the upper genital tract, and chronic sequelae of disease in this model.
MATERIALS AND METHODS
Mice, infection, and infection assessment.
We selected a susceptible strain of mice for these experiments. Susceptibility is based primarily on hydrosalpinx formation, which is a surrogate marker of infertility (9, 34). Five- to 6-week-old female C3H/HeN mice were obtained from Harlan Sprague Dawley (Indianapolis, Indiana) and housed with rodent chow and water ad libitum with a 12:12 light-dark cycle. Following a 10-day acclimation period, mice were pretreated with 2.5 mg of medroxyprogesterone acetate (DepoProvera, P4; Upjohn, Kalamazoo, Michigan) and 7 days later inoculated intravaginally with 200 50% infective doses (104 inclusion-forming units [IFU]) of HeLa 229-grown C. muridarum MoPn, exactly as described elsewhere (22). In order to verify infection, cervicovaginal swabs were collected in all mice at day 4 postinfection and the infection course was followed in a subset of five mice at days 4, 7, 10, and 14 and every 7 days thereafter through day 49 postinfection in each experiment. C. muridarum was isolated and inclusions were visualized by indirect immunofluorescence and quantified as IFU in HeLa 229 cultures as previously described (22). In order to assess upper genital tract infection, mice were euthanized and urogenital tissue above the cervix but excluding the ovary was excised, homogenized in sucrose-phosphate-glutamate buffer, and sonicated, with the debris cleared by centrifugation and MoPn isolated as previously described (7).
MMPi.
To assess the validity of our hypothesis that MMP are involved in the sequelae of chlamydial infection in this model, we designed two types of experiments using two separate MMP inhibitors (MMPi). In each study, mice were divided into two groups in which only one group received the MMPi and the other remained untreated. In the first set of two experiments, captopril {1-[(2S)-3-mercapto-2-methylpropionyl]-l-proline} was purchased from Sigma-Aldirch (St. Louis, Missouri). Captopril is an angiotensin-converting enzyme (ACE) inhibitor used for the control of hypertension but has no known effect on normotensive animals or humans (16, 31). We selected this drug because it has also been shown to have efficacy as an MMPi and is water soluble and easily administered, with little toxicity in rodents even at relatively high doses (16, 31). Our initial experiments proved that captopril inhibited MMP activity by significantly reducing gelatin degradation on gelatin zymograms, as has also been shown elsewhere (33), but did not significantly inhibit chlamydial replication in vitro at a range of concentrations from 0.033 μM to 0.26 μM (or 7 μg/ml to 56 μg/ml; data not shown). The oral bioavailability of captopril in rodents is approximately 50%, and its half-life is approximately 71 min (31). In experiments involving captopril administration, drinking water was spiked with the drug at a concentration of 0.2 mg/ml according to the method of Singhvi et al. (31) and Volpert et al. (45). Because we have previously observed that the bulk of the increase in MMP (especially MMP-9) expression occurs during the first 21 days of infection and the accompanying inflammatory response, we commenced treatment on day 1 postinfection and continued it daily for 20 additional days. Controls were given regular drinking water for the same period.
In our second study, chemically modified tetracycyline 3 (6-deoxy-6-demethyl-4-dedimethylaminotetracycline), or CMT-3 (also known as COL-3; a gift through a materials transfer agreement with Collagenex, Inc., Newtown, Pennsylvania), was administered. This experimental drug has been chemically modified in such a way that it has lost its antimicrobial effect but still retains activity as an MMPi that has been ascribed to all of the drugs in the tetracycline class of antimicrobials (12). While fairly selective for gelatinases, this drug has also been shown to inhibit other MMPs. According to Collagenex, Inc., the MICs (50%) for CMT-3 for the following human MMPs are as follows: MMP-2, 0.03 μM; MMP-9, 0.22 μM; MMP-13, 0.3 μM; MMP-8, 4 μM; and MMP-1, 34 μM. The oral bioavailability of CMT-3 is as follows: rat, 60 to 70% and primate, 30%. The half-life of this drug is as follows: mouse, 7 h; rat, 7 h; primate, 30 h; and, human, 40 h. This drug is hydrophobic in nature and thus was not suitable for administration in drinking water as was captopril. Instead, it was administered by oral gavage in a suitable vehicle. The experimental group received CMT-3 in the form of an oral gavage in concentrations at 200 mg/kg or 5 mg per mouse in 0.2 ml 2% carboxymethylcellulose (vehicle) daily, while the control received the same amount of 2% carboxymethylcellulose only. Treatment began 1 day after infection and continued until 21 days postinfection.
Although this drug has been proved to have greatly reduced antimicrobial effect, to the best of our knowledge, this drug had never been tested against Chlamydia (11). Because tetracyclines are well known for their antimicrobial activity against Chlamydia, it was important that the drug was proved to have reduced antimicrobial effect against chlamydiae and MoPn in particular. We tested this drug in a side-by-side MIC assay according to the method of Suchland et al. (37). Using their methods in L929 fibroblasts, we found a MIC for tetracycline of 0.032 μg/ml at the transition phase to morphologically aberrant inclusions (MIC-tp) and a MIC of 0.064 μg/ml where no inclusions were observed in triplicate cultures. These data were identical to those reported by Suchland et al. for human strains of chlamydiae (37). For CMT-3, we found a >30-fold reduction in antimicrobial effect with a MIC-tp of 1 μg/ml and a MIC of 2 μg/ml. Hence, this modified form of tetracycline had a significant reduction in antichlamydial effect in vitro, and as shown in the Results section below, had no discernible effect on shedding of viable MoPn in the lower urogenital tract of female mice. Su et al. have reported a subchlamydiacidal level of oxytetracycline at 1.2 mg per mouse injected subcutaneously every other day for 2 weeks that suppressed shedding of chlamydia (35). In a separate report, they used a chlamydiacidal level of doxycycline hyclate at 0.3 mg per mouse intraperitoneally per day for 2 weeks (36).
Collection of tissue samples and histological assessment.
In each experiment, we euthanized a subset of mice from each group on days 0, 7, 14, 21, and 56 postinfection. Based on previous observations in mice without interventions, these time points represent the following: immediately prior to infection (day 0), the onset of the acute the inflammatory response phase in the endometrium with minimal involvement of the oviduct (day 7), the peak of acute inflammation in the oviduct with some residual involvement of the endometrium (day 14), infection nadir and postacute inflammation (day 21), and postinfection with a chronic disease state (day 56), respectively (8, 30). At the time of necropsy and before excision, tissues were examined in situ for gross macroscopic evidence of pyosalpinx or hydrosalpinx formation and any other associated abnormalities in the viscera (30). Pyosalpinx is a gross measure of histologically evident salpingitis, and hydrosalpinx can be used as a correlate of fibrotic oviduct occlusion and infertility (9, 30, 35, 38, 39). A portion of the tissues was then processed for histological assessment, while another portion was homogenized and processed for other assays (see below).
Histological assessment.
Five to six excised genital tract samples at each of the time points days 7, 14, 21, and 28 postinfection were preserved in buffered 10% formalin at neutral pH and then embedded in paraffin and serially sectioned (5 μm) longitudinally. Attempts were made to include cervix, both uteri, and both oviducts and to include as much of the lumenal structures of each tissue to the greatest extent possible in each section (AML Labs, Rosedale, Maryland). The tissues were stained with hematoxylin and eosin (H&E) (30). Each H&E-stained tissue section was assessed by a board-certified pathologist (J.N.K.) who was blinded to the animal numbers, treatments, and groupings in the experiment, with sections from uninfected mouse urogenital tract tissues included for reference to normal tissue. Histopathological assessment was scored in the ectocervix, endocervix, uterus proximal to the cervix and prior to bifurcation (hereafter referred to as proximal uterus), uterine horns, and oviducts relative to normal, uninfected urogenital tract tissue. Parameters assessed were acute (neutrophils) and chronic (mononuclear cell) infiltrates, plasma cells, lumenal dilatation, and fibrosis. Each parameter was scored on a scale of 0 to 4 using a modification of the methods of Rank et al. (26) as follows: 0 = normal/no presence of parameter; 1 = rare or slight presence of parameter; 2 = scattered/mild or diffuse presence of parameter; 3 = consistent/numerous/frequent presence of parameter; and, 4 = confluent or severe presence of parameter. Assessments within the contralateral uterine horns and oviducts in the same tissue were averaged together. To control for variances in parameter foci according to cutting depth within the same tissue, we included three separate but roughly serial sections from each tissue. Scoring was done on each section, but the mean score for each serial section was used for average and data reporting.
Tissues from other mice at the same time points were embedded in optimum cutting temperature embedding medium (OCT; Sakura Finetek, Inc., Torrance, California). Once embedded, the sections were frozen immediately at −25°C and then transferred to −70°C. Serial 4-μm longitudinal sections were cut on a Thermo-Shandon cryotome, mounted on glass slides, fixed in methanol at −20°C for 10 min, rinsed in cold phosphate-buffered saline (PBS; pH 7.2) and blocked in 5% normal rabbit serum in PBS at room temperature for 20 min. Cryosections of tissues were then incubated with the primary antibody, goat anti-C. trachomatis (diluted 1:200 in PBS-2% rabbit serum; Fitzgerald Industries, Concord, Massachusetts). Following appropriate rinses in PBS, the secondary antibody, Vectastain ABC Elite for goat immunoglobulin G (Vector Labs, Burlingame, California) was diluted according to the manufacturer's protocol and incubated for 30 min at room temperature. The slides were then rinsed again and developed according to the manufacturer's protocol. Following a final rinse in PBS, the slides were then mounted with glass coverslips utilizing Aquapolymount (Polysciences, Inc, Warrington, PA) as the mounting medium. Mounted slides were viewed on a Ziess Axiovert 25 microscope with a mounted Axiocam MRc5 digital camera and Axiovision 4.2 image capturing software (Carl Zeiss USA, Inc., Thornwood, New York). Captured images were processed in Adobe Photoshop 6.0 and exported in an appropriate file format for publication purposes.
Zymography.
Uterus and oviduct were excised and homogenized in cold PBS (pH 7.2) and processed exactly as previously described (7). The homogenate was then cleared of debris by centrifugation, and the supernatant was frozen in aliquots at −70°C. Total protein content was determined (Pierce BCA; Pierce-Endogen, Rockford, Illinois), and loading amounts were standardized to protein concentration. Precast gelatin zymogram gels (Bio-Rad, Inc., Hercules, California) were loaded and subjected to electrophoresis according to the manufacturer's specifications. Following electrophoresis, gels were renatured in Novex zymogram renaturing buffer (Invitrogen, Carlsbad, California) for 30 min at room temperature and then incubated overnight at 37°C in Novex zymogram developing buffer (Invitrogen). The gels were then washed and stained with 0.4% (wt/vol) Coomassie brilliant blue R-250 (Fisher Scientific, Inc., Fairlawn, New Jersey) for 45 min and then successively destained until distinct bands appeared against a blue background, indicating degradation of the substrate in the gel matrix. On each gelatin gel, human MMP-9 (trivial names, gelatinase B and type IV collagenase) and MMP-2 (trivial name, gelatinase A) were loaded as standards according to the manufacturer's specifications (Chemicon, Inc., Temecula, California). Analysis of zymogram activity was conducted essentially as previously described (24).
EIA for MMP-9.
The same homogenate samples prepared for zymography as described above were used for antigen-capture enzyme immunoassay (EIA) detection of total pro-MMP-9 according to the manufacturer's instructions (R&D Systems, Inc., Minneapolis, MN). Average values were obtained from duplicate samples. When values for a specific sample fell outside of the range of the standards, separate aliquots were thawed, rediluted to achieve a value within range of the standard curve of the assay, and assessed again. Values were then standardized to protein content and dilution factor (if used) and are reported as ng of total proMMP-9 per mg of protein for that sample.
Statistics.
Infection course as assessed by shedding of viable organisms from the lower genital tract and upper genital tract infection were compared by two-way analysis of variance (ANOVA) (treatment group, days) with repeated measures (days). Results for EIA for MMP-9 in tissue homogenates were compared among treated and control mice using a one-way unpaired t test. The incidence of acute (pyosalpinx) and chronic (hydrosalpinx) gross pathology was assessed using the chi-square statistic with Yates correction. The occurrence of histopathological parameters was analyzed using the Mann-Whitney U test.
RESULTS
MMPi protect mice from chronic chlamydial disease without affecting chlamydial replication in the lower urogenital tract.
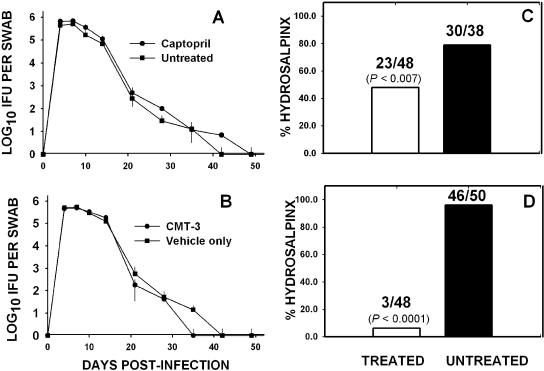
Figure 1 shows the results of administration of the two MMPi utilized in these experiments on the course of chlamydial infection and the resulting chronic disease as measured by hydrosalpinx formation. Figure 1A and C represent combined data from two separate experiments utilizing captopril to inhibit MMP. No significant difference was observed in the shedding of viable MoPn between the treated and untreated mice throughout the course of both experiments (Fig. 1A, P > 0.8 for both experiments, two-way repeated-measures ANOVA). However, when assessed for chronic disease, there was a significant reduction in the rate of hydrosalpinx formation at day 56 in the treated group of mice (two-sided P value <0.007, chi-square analysis with Yates correction).
FIG. 1.
Effect of MMPi on infection course and hydrosalpinx formation. Panel A depicts the course of infection in captopril-treated (filled circles) or untreated (filled squares) mice. Each point represents the mean IFU and standard deviation isolated from cervicovaginal swabs of at least 10 mice over the course of two experiments. Panel B shows the course of infection in mice treated with CMT-3 (filled circles) or carboxymethylcellulose vehicle only (filled squares), and each point represents the mean IFU and standard deviation of swabs of at least 15 mice over the course of three experiments. The infection course was unaffected by treatment with either MMPi when assessed by a two-factor (treatment group, days) analysis of variance with repeated measures (days). Panel C depicts the rate of hydrosalpinx formation at day 56 postinfection in mice treated with captopril for the first 21 days postinfection (white bars) and in untreated mice (black bars) over two iterations of this experiment. The numbers above the bars are the ratio of hydrosalpinges of total oviducts assessed. The number in parentheses is the two-sided P value achieved by chi-square analysis with Yates correction. Panel D is the same as panel C, except the values represent CMT-3-treated or vehicle-only-treated mice and are the total of three iterations of the experiment.
During the course of both captopril experiments, there were a number of unexpected deaths of mice (12 of 44 total mice) in the captopril-treated group. Each of the mice that expired had been monitored on a daily basis and did not appear otherwise overtly ill prior to death. On necropsy, we were unable to detect any discernible difference in the viscera, lungs, and other tissue in these animals when compared to untreated animals. Thus, we attributed these deaths to an undetermined and unintended consequence of captopril treatment specific to chlamydia-infected mice and we sought to acquire other MMPi that may not have the same adverse effect.
CMT-3 (also referred to as COL-3) is a chemically modified form of tetracycline that has been rendered essentially inert with regard to antimicrobial activity but retains the MMP inhibitory activity that is inherent to all tetracyclines. As can be seen in Fig. 1D, treatment with CMT-3 from day 1 to day 21 postinfection dramatically reduced the rates of hydrosalpinx formation assessed at day 56 postinfection when compared to mice treated with the vehicle only (two-sided P < 0.0001, chi-square analysis with Yates correction). The effect was observed without altering the infection course as assessed by shedding of viable organisms from the lower urogenital tract (Fig. 1B, P < 0.3 for three experiments, two-way ANOVA). The results were consistent for three iterations of the experiment, and a total of 98 oviducts were assessed.
It is important to note that both MMPi used in these studies were administered only for the first 21 days postinfection, whereas hydrosalpinx formation normally becomes apparent after resolution of infection (>35 days postinfection) (30). These results indicated that MMPi do not affect chlamydial replication in the lower urogenital tract but exert their effects on events occurring during acute disease that eventually lead to the chronic sequelae of chlamydial disease. Thus, we next refined our assessments to elucidate events during the first 21 days postinfection in treated and untreated mice. As a first step in this process, we assessed the incidence of gross pathological outcome of pyosalpinx as a measure of acute inflammation in treated and untreated mice.
Effects of MMPi on inflammation in the upper genital tract.
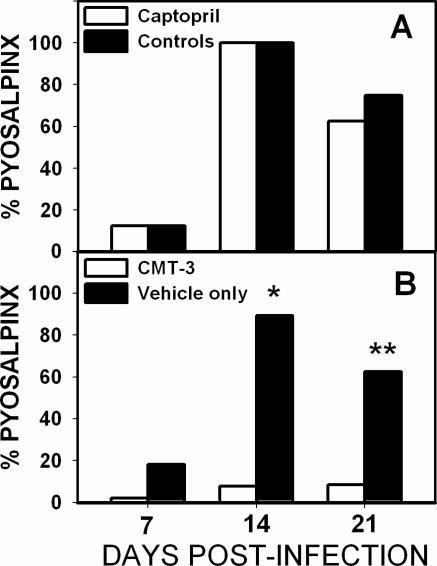
With a directed and selected inhibition of the activity of MMPs, one would anticipate that inflammatory cell accumulation would occur in infected animals but inflammation-associated tissue damage would be suppressed. Figure 2 depicts the incidence of the gross pathological observation of pyosalpinx in either captopril-treated mice or controls (panel A) or CMT-3-treated mice or vehicle-only controls (panel B) at days 7, 14, and 21. Despite its protective effect on the development of hydrosalpinx shown in Fig. 1, as can be seen in Fig. 2A, captopril treatment had no significant effect on the development of pyosalpinx (n = 8 per time point). Interestingly, CMT-3 all but completely prevented pyosalpinx formation (two-sided P values of <0.0001 at day 14 and <0.003 at day 21, chi-square analysis with Yates correction, n = 20 to 28 per time point). This protection from pyosalpinx was evident histologically, with CMT-3-treated mice showing rare to minimal evidence of salpingitis (see Fig. 5 below).
FIG. 2.
CMT-3 treatment inhibits pyosalpinx formation. Panel A represents the formation of pyosalpinx as evident on gross pathological examination in captopril-treated (white bars) and untreated (black bars) mice at days 7, 14, and 21 postinfection. No difference was seen between groups at any time using a chi-square test with Yates correction (one experiment, n = 8 per time point). Panel B represents the formation of pyosalpinx as evident on gross pathological examination in CMT-3-treated mice (white bars) or vehicle-only-treated mice (black bars) at days 7, 14, and 21 postinfection. CMT-3 treatment resulted in a statistically significant reduction in pyosalpinx at days 14 (*, P < 0.0001) and 21 (**, P < 0.003). The data represent a total of three experiments (n = 24 to 28 per time point).
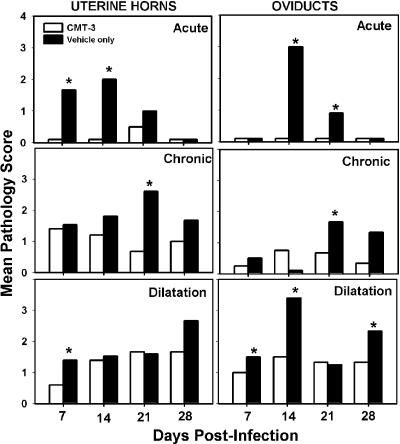
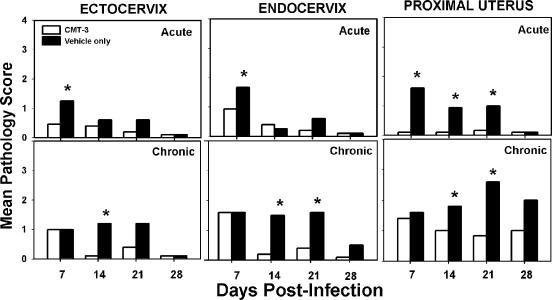
FIG. 5.
Histopathological assessment of the upper urogenital tract. The graphs depict the mean scores for CMT-3-treated mice (white bars) and vehicle-only-treated controls (black bars) relative to uninfected tissue. Each panel is labeled according to tissue and assessment type. The top row of panels represents scoring for acute inflammation, the middle row of panels represents scoring for chronic inflammation, and the bottom row of panels represents scoring for lumenal dilatation. Asterisks denote statistical significance by the Mann-Whitney U test. (P values varied but in each case were labeled if P was <0.05.) Scoring for fibrosis was conducted, but findings were inconsistent, not statistically different between groups, and are not shown.
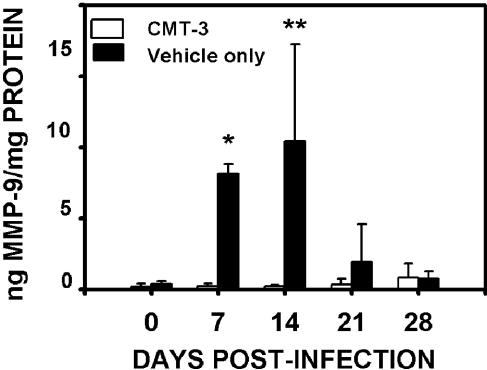
Figure 3 reflects the relative levels of MMP-9 in the tissues of CMT-3-treated mice and controls. Also known as gelatinase B, this MMP is a type IV collagenase that we have previously postulated to be involved in the tissue dissolution that accompanies upper genital tract infection in this model (24). A significant source for MMP-9 during infection is the acute inflammatory cell infiltrate. The level of MMP-9 in these tissues shown in Fig. 3 is likely reflective of the relative lack of acute inflammatory infiltrates. These results were confirmed by gelatin zymography as well. Gelatin zymograms showed a distinct absence of MMP-9 and reduced levels of MMP-2 (both type IV collagenases) in tissues of infected animals treated with CMT-3 (data not shown).
FIG. 3.
CMT-3 treatment decreases pro-MMP-9 levels in urogenital tract tissue. The results depicted in this graph represent the mean pro-MMP-9 ± the standard deviation as determined by antigen-capture EIA. Results are for CMT-3-treated mice (white bars) or vehicle-only-treated control mice (black bars) at the stated time points postinfection. Statistically greater pro-MMP-9 was detected in the tissues from control mice at days 7 (*, two-tailed P value of <0.0001) and 14 (**, P < 0.003) postinfection by an unpaired t test.
Figures 4 and 5 show the composite results of histopathological assessments of the lower and upper urogenital tracts, respectively. As depicted in Fig. 4, CMT-3 treatment significantly blunted acute inflammatory responses in the ectocervix and endocervix (day 7) and in the proximal uterus (days 7, 14, and 21). CMT-3 treatment also impeded chronic inflammatory responses in the ectocervix (day 14), endocervix (days 14 and 21), and proximal uterus (days 14 and 21). Fibrosis and lumenal dilatation of these tissues were inconsistently present during these time frames and did not vary significantly based on experimental groups. Hence, these data were not shown.
FIG. 4.
Histopathological assessment of the lower urogenital tract. The graphs depict the mean scores for CMT-3-treated mice (white bars) and vehicle-only-treated controls (black bars) relative to uninfected tissues. Each panel is labeled according to tissue and assessment type. The top row of panels represents scoring for acute inflammation. The bottom row of panels represents scoring for chronic inflammation. Asterisks denote statistical significance by the Mann-Whitney U test. (P values varied but in each case were labeled if P was <0.05.) While scoring for fibrosis and lumenal dilatation was occasionally observed, findings were inconsistent, not statistically different between groups, and are not shown.
In Fig. 5, acute inflammation in the upper genital tract (uterine horns and oviduct) was also impeded in mice administered CMT-3, with statistically significant differences achieved at days 7 and 14 in the uterine horns and days 14 and 21 in the oviducts. Findings consistent with tubo-ovarian abscess were evident in several tissues from the control group but were never observed in the mice treated with the MMPi. These observations are consistent with the gross macroscopic observations relative to pyosalpinx formation described above for Fig. 2. Though chronic inflammatory responses in the upper genital tract of both groups were evident, a significantly dampened response was observed at day 21 in the group treated with the MMPi. Similarly, lumenal dilatation was observed in both groups but was more prominent in the untreated controls on day 7 in the uterine horns and days 7, 14, and 28 in the oviduct. The latter observation likely represents the early formation of hydrosalpinx in some of untreated mice, as was evidenced by blunted plicae indicating pressure atrophy in those tissues, whereas the earlier observations of dilatation appeared to be due to lumenal filling with inflammatory infiltrates. Fibrosis was not consistently observed during the time points assessed (data not shown), and this is consistent with our previous findings showing that fibrosis normally occurs ≥35 days postinfection (30).
We next used histological and microbiological methods to determine the incidence of ascending infection in CMT-3-treated mice when compared to vehicle-only-treated controls. Because of the unpredictable lethal effect of captopril on some chlamydia-infected mice, we did not further pursue the use of captopril as an MMPi modality in these experiments.
CMT-3 significantly impedes ascension of MoPn infection into the upper genital tract.
If CMT-3 protected mice from upper genital tract disease solely via inhibition of inflammatory cell infiltration of infected tissues (infection in the absence of inflammation), one would anticipate that mice treated with CMT-3 would exhibit similar evidence of upper genital tract infection as the control mice but be spared inflammation in the same regions. However, if the protection afforded by CMT-3 treatment was related to blocking an unknown mechanism by which the pathogen ascends the upper genital tract, one would anticipate a discernible delay or reduction in the rate of ascending infection in CMT-3-treated mice.
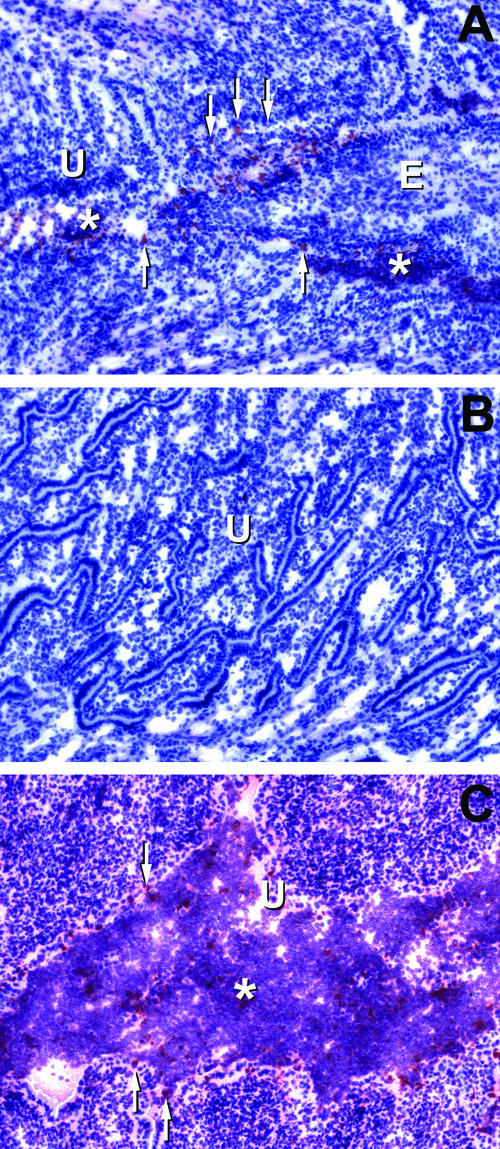
To address this issue, we first carefully examined several frozen sections (three to four per mouse, 40- to 50-μm intervals) of urogenital tract tissues from CMT-3-treated mice (n = 8 mice) and controls (n = 6 mice), for immunohistochemical (IHC) evidence of ascending chlamydial infection. Figure 6 shows typical results for chlamydial antigen staining in these sections. Panel A represents endocervix and proximal uterus in a CMT-3-treated mouse at day 7 postinfection. Panel B is the same section but is from the endometrium of the uterine horn, and panel C is the endometrium of a vehicle-only-treated mouse at day 7 postinfection.
FIG. 6.
Immunohistochemical localization of chlamydial infection in mice treated with CMT-3. All histological sections shown are from day 7 postinfection. Chlamydial antigen staining appears red, whereas the counterstain with hematoxylin leaves the tissue pale blue. Panel A shows the juncture between the endocervix and the proximal uterus in a CMT-3-treated mouse. Diffuse chlamydial antigen staining is present within the lumenal structures, and condensed staining consistent with chlamydial inclusions is present in epithelial layers and scattered throughout the lumen. Panel B is the uterine horn endometrium of the same mouse as panel A. There was a lack of chlamydial antigen staining or inflammation in this region, which is atypical for this time point in the infection in normal, untreated animals. Panel C is the endometrium of the uterine horn of a vehicle-only-treated mouse and shows typical findings relative to chlamydial antigen staining, lumenal dilatation, and vigorous inflammatory infiltrates. Control slides of uninfected tissue or infected tissue treated with the secondary antibody only showed no chlamydial antigen staining. U, uterine endometrium; E, endocervix. Asterisks show areas of inflammatory cell infiltration, and arrows point to focal staining consistent with the localization and morphology of chlamydial inclusions in columnar epithelium.
A consistent finding in each instance was that the upper genital tracts (uterine horns on day 7 and oviducts and uterus on day 14) of untreated mice were replete with staining for lumenal chlamydial antigen, and intact inclusions in the epithelium could often be observed. It was quite rare to detect any chlamydial antigen above the lower uterine body just prior to bifurcation of the uterine horns in CMT-3 mice. In fact, as shown in Fig. 6B, in the CMT-3-treated mice there was complete sparing of the uterine horns from infection by IHC assessment and the infection appeared to remain localized to the endocervix and proximal uterus.
While IHC analysis was useful for anatomically locating and tracking the progress of infection, detection of infection by this method is generally less sensitive than microbiological assessment. However, our IHC results were generally confirmed by microbiological assessment, and this is shown in Table 1. These data are the results of viable IFU counts from homogenates of the upper urogenital tract (includes all tissue above the cervix, including proximal uterus, uterine horns, and oviduct but excluding ovaries) from CMT-treated mice and controls as compared to IFU counts from cervicovaginal swabs at the same time points. These data show that while there was no difference in IFU counts in the lower urogenital tract (swab data; see also Fig. 1), there was a significantly lower infectious burden in the upper genital tract of CMT-3-treated mice (P < 0.006, two-factor ANOVA with repeated measures). The latter represents a time point at which the infection and accompanying inflammation normally have ascended into the oviducts. It should be noted that from a microbiological perspective, it appears there was indeed infection detected in upper genital tract tissues of CMT-3-treated mice. However, when taken together with our IHC results and the histopathological parameters observed, the histological source of viable chlamydia in the upper genital tract of CMT-3-treated mice in Table 1 was most likely proximal uterus. Also, some contamination from the endocervix was possible during even the most careful excision of this tissue since these tissues are lumenally contiguous.
TABLE 1.
Viable C. muridarum IFU counts from homogenates of upper genital tract compared with cervicovaginal swabs
| Treatment group and samplea | Viable IFU (mean log10 IFU ± SD) at postinfection day:
|
|||||
|---|---|---|---|---|---|---|
| 4 | 7 | 10 | 14 | 21 | 28 | |
| CMT-3 | ||||||
| Swab | 5.66 ± 5.11 | 5.69 ± 5.06 | 5.52 ± 4.92 | 5.26 ± 4.77 | 2.26 ± 2.16 | 1.62 ± 1.7 |
| UGT tissue | 0.0 ± 0.0 | 4.25 ± 4.17 | 3.02 ± 3.28 | 2.48 ± 1.54 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Vehicle only | ||||||
| Swab | 5.77 ± 5.07 | 5.73 ± 5.09 | 5.46 ± 5.06 | 5.10 ± 4.84 | 2.75 ± 2.78 | 1.26 ± 0.95 |
| UGT tissue | 5.69 ± 5.06 | 5.32 ± 5.26 | 4.13 ± 4.10 | 4.00 ± 3.93 | 0.0 ± 0.0 | 0.0 ± 0.0 |
Statistically significant differences in the isolation of viable MoPn from the upper genital tract tissue (but not cervicovaginal swabs) in the two groups were observed by two-way (treatment group, days) ANOVA with repeated measures on the days shown (P < 0.006). n = 6 per time point for upper genital tract (UGT) tissue; n = 15 for swabs.
DISCUSSION
The central hypothesis of this study was that MMP are involved in the immunopathological responses leading to chronic chlamydial disease in the murine model. Previous observations had led us to postulate that an inflammatory insult early in the host response is likely to blame for the eventual formation of chronic chlamydial disease manifestations following infection resolution in this model (30). Hence, in the present study, we administered MMPi during the first 21 days postinfection to coincide with the time frame of acute infection and host response. We reasoned that if MMP were involved in the putative initial insult, inhibition of their activity would block the development of sequelae such as hydrosalpinx. In this respect, our hypothesis was proved correct but the result was not exactly as expected.
When assessed by shedding of MoPn from the lower genital tract, the infection course was normal in mice treated with both MMPi when compared to their respective controls. In the captopril-treated mice, acute manifestations of pyosalpinx and salpingitis were present at similar rates when compared to controls but captopril treatment during acute manifestations of the disease resulted in significantly reduced rates of hydrosalpinx when compared to untreated mice. From this observation, we postulate that while allowing a normal infection course and the accompanying acute inflammatory responses to occur, captopril impeded a critical pathological mechanism. A likely interventional point for captopril would be inhibition of proteolytic damage caused by host MMPs. An alternative explanation relates to the previous findings that angiotensin II binding of the AT-1 receptor has been shown to induce fibrotic effects. Hence, blocking ACE with captopril could lower angiotensin II and thereby impact fibrosis independent of MMP-induced mechanisms (15, 28). Unfortunately, certain untoward and still undefined effects of this drug in combination with chlamydial infection caused a significant number of deaths in this group and thus have impeded further exploration as to its mechanism of action.
In the application of CMT-3, our hypothesis that inhibiting MMP would protect mice again proved correct. However, we found an unexpected effect. Rather than impeding proteolytic activity at the point of inflammation, it appears to have blunted inflammation and spared the ascension of the infection into the upper genital tract by an unknown mechanism. To the best of our knowledge, this is the first report of a nonantimicrobial therapeutic intervention preventing ascending chlamydial infection.
One might argue that the drug had a minimal antimicrobial effect on the pathogen that slowed replication enough to prevent ascension until an effective adaptive immune response was initiated. In this regard, Balakrishnan et al. recently reported that certain MMPi could inhibit C. trachomatis serovar L2 replication in vitro through inhibiting a the metal-dependent enzyme peptide deformylase (4). However, it is unlikely that the drugs used in this study had a similar effect either in vitro or in vivo. The best supporting evidence of this assertion is that shedding of viable MoPn in the lower genital tract was completely unaltered by the administration of either MMPi. Other investigators using tetracyclines in the murine model have shown chlamydiacidal activity at far lower doses than those we used for treating mice with CMT-3 herein (6, 35). In addition, our in vitro assessments indicated that that CMT-3 is reduced at least 30-fold in its antimicrobial effects when compared to the parent molecule, tetracycline, and captopril similarly had no effect on replication at any concentration tested. Lastly, immunohistochemistry results indicated that the ascent of the pathogen stalled in the endocervix and lower endometrium of the proximal uterus, indicating that this was a likely source of the IFU isolated from cervicovaginal swabs. Taken together, these findings indicate that in vivo replication of MoPn was unaffected by the treatment and remained localized to the lower urogenital tract.
In studies by Su et al., tetracycline was used to induce “subclinical” infections that still stimulated significant protective adaptive immune responses (35). These studies were conducted in order to test the viability of developing a live, attenuated vaccine. Since there were some similarities between their results and our results with CMT-3 treatment, it is possible that the effect of tetracycline used in the studies by Su et al. were actually related to inhibition of MMP. Along these lines, we believe it would be interesting to assess the effects of MMPi on adaptive immunity in our model.
These novel observations raise still more interesting questions. How did CMT-3 impede ascension of the infection? Because there was very little acute inflammation present in tissues of CMT-3-treated mice, including the lower urogenital tract where the infection seemed to be confined, a plausible explanation would be that the inflammatory response assists in ascension of infection. It is well known that neutrophils appear to be involved in epithelial damage during chlamydial infection (21, 30, 38). Ultrastructural assessment of chlamydial infections in vivo often show infected epithelial cells apparently being pushed off their growing surface at the basal lamina (32). Hence, neutrophils and other inflammatory cells could assist in the dissemination and ascension of chlamydial infection into the upper genital tract by actively sloughing epithelial cells into the lumenal aspects of these tissues. Inflammatory cells could also assist in ascension by acting as a vehicle to carry viable chlamydia elementary bodies as passengers to a more susceptible cell type. While it has been shown that neutrophils do not support significant chlamydial growth, chlamydiae have been shown to have limited survival within these cells for several hours (27). In this respect, perhaps our findings are not so novel because in the same reference, Register et al. proposed a mechanism for neutrophils facilitating spread of the infection to cells that can support productive infection (27).
If these are proved to be potential mechanisms, another relevant question would involve the mechanism by which CMT-3 inhibited the normally vigorous inflammatory response in this model. While we selected this drug due to its potent ability to impede MMP-mediated turnover of the ECM, it and several other MMPi have been shown to inhibit inflammatory responses in general (29). In this regard, MMP are involved in the proteolytic modification of chemokines and cytokines to more active forms (19, 42, 43) and extracellular matrix proteolysis is involved in migration of inflammatory cells through tissues (17).
With regard to involvement by specific MMP in pathogenesis, we have shown MMP-9 to be abundant in tissues in response to this infection, but its exact role remains uncertain (24). However, Pal et al. have recently ruled out a role for the epithelial cell-derived MMP-7 (trival name, matrilysin) in chlamydial urogenital infection in this model, but it may be involved in intestinal infection (20). Given our present findings, dissecting the roles of various MMP in the modulation of inflammatory responses, turnover of the ECM, and fibrogenesis and impeding ascending infection in this model will provide some useful insights into the pathogenesis of chlamydial infections in this and other animal models as well as in human infections.
Acknowledgments
This work was supported by Public Health Service grant AI49354 to K.H.R.
We wish to thank Collagenex, Inc., for supplying CMT-3 and Brad Zerler of Collagenex, Inc., for his kind review of the manuscript, information regarding pharmacology of the drug, and suggestions regarding experimental design and interpretation the results. We would also like to thank Raymond Johnson of Indiana University for his thoughtful suggestions with regard to immunohistochemistry and Laura Phelps for her kind assistance with digital image processing.
Editor: F. C. Fang
REFERENCES
- 1.Abu el-Asrar, A. M., K. Geboes, S. A. Al Kharashi, A. A. Al Mosallam, L. Missotten, L. Paemen, and G. Opdenakker. 2000. Expression of gelatinase B in trachomatous conjunctivitis. Br. J. Ophthalmol. 84:85-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abu el-Asrar, A. M., K. Geboes, and L. Missotten. 2001. Immunology of trachomatous conjunctivitis. Bull. Soc. Belge Ophtalmol. 280:73-96. [PubMed] [Google Scholar]
- 3.Ault, K. A., K. A. Kelly, P. E. Ruther, I. M. Sigar, and K. H. Ramsey. 2002. Chlamydia trachomatis enhances the expression of matrix metalloproteinases in an in vitro model of the human fallopian tube. Am. J. Obstet. Gynecol. 187:1377-1383. [DOI] [PubMed] [Google Scholar]
- 4.Balakrishnan, A., B. Patel, S. A. Sieber, D. Chen, N. Pachikara, G. Zhong, B. F. Cravatt, and H. Fan. 2006. Metalloprotease inhibitors GM6001 and TAPI-0 inhibit the obligate intracellular human pathogen Chlamydia trachomatis by targeting peptide deformylase of the bacterium. J. Biol. Chem. 281:16691-16699. (First published 24 March 2006; doi: 10.1074/jbc.M513648200.) [DOI] [PubMed] [Google Scholar]
- 5.Chantrain, C. F., P. Henriet, S. Jodele, H. Emonard, O. Feron, P. J. Courtoy, Y. A. DeClerck, and E. Marbaix. 2006. Mechanisms of pericyte recruitment in tumour angiogenesis: a new role for metalloproteinases. Eur. J. Cancer 42:310-318. [DOI] [PubMed] [Google Scholar]
- 6.Clark, R. A., and W. M. Nauseef. 1996. Isolation and functional analysis of neutrophils, p. 7.23.8.-7.23.10 In J. E. Coligan, A. M. Kruisbeek, D. H. Margulies, and W. Strober (ed.), Current protocols in immunology. John Wiley & Sons, Inc., New York, N.Y. [DOI] [PubMed]
- 7.Cotter, T. W., K. H. Ramsey, G. S. Miranpuri, C. E. Poulsen, and G. I. Byrne. 1997. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infect. Immun. 65:2145-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darville, T., C. W. Andrews, Jr., K. K. Laffoon, W. Shymasani, L. R. Kishen, and R. G. Rank. 1997. Mouse strain-dependent variation in the course and outcome of chlamydial genital tract infection is associated with differences in host response. Infect. Immun. 65:3065-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de la Maza, L. M., S. Pal, A. Khamesipour, and E. M. Peterson. 1994. Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infect. Immun. 62:2094-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Descamps, F. J., P. E. Van den Steen, I. Nelissen, J. Van Damme, and G. Opdenakker. 2003. Remnant epitopes generate autoimmunity: from rheumatoid arthritis and multiple sclerosis to diabetes. Adv. Exp. Med. Biol. 535:69-77. [DOI] [PubMed] [Google Scholar]
- 11.Dollery, C. M., and P. Libby. 2006. Atherosclerosis and proteinase activation. Cardiovasc. Res. 69:625-635. [DOI] [PubMed] [Google Scholar]
- 12.Greenwald, R. A., and L. M. Golub. 2006. Biologic properties of non-antibiotic, chemically modified tetracyclines (CMTs): a structured, annotated bibliography. Curr. Med. Chem. 8:237-242. [DOI] [PubMed] [Google Scholar]
- 13.Maeda, H., T. Okamoto, and T. Akaike. 1998. Human matrix metalloprotease activation by insults of bacterial infection involving proteases and free radicals. Biol. Chem. 379:193-200. [DOI] [PubMed] [Google Scholar]
- 14.Maitra, S. R., M. J. Shapiro, S. Bhaduri, and M. R. El Maghrabi. 2005. Effect of chemically modified tetracycline on transforming growth factor-beta1 and caspase-3 activation in liver of septic rats. Crit. Care Med. 33:1577-1581. [DOI] [PubMed] [Google Scholar]
- 15.Mezzano, S. A., M. Ruiz-Ortega, and J. Egido. 2001. Angiotensin II and renal fibrosis. Hypertension 38:635-638. [DOI] [PubMed] [Google Scholar]
- 16.Migdalof, B. H., M. J. Antonaccio, D. N. McKinstry, S. M. Singhvi, S. J. Lan, P. Egli, and K. J. Kripalani. 1984. Captopril: pharmacology, metabolism and disposition. Drug Metab. Rev. 15:841-869. [DOI] [PubMed] [Google Scholar]
- 17.Murphy, G., and J. Gavrilovic. 1999. Proteolysis and cell migration: creating a path? Curr. Opin. Cell Biol. 11:614-621. [DOI] [PubMed] [Google Scholar]
- 18.Nagase, H., and J. F. Woessner, Jr. 1999. Matrix metalloproteinases. J. Biol. Chem. 274:21491-21494. [DOI] [PubMed] [Google Scholar]
- 19.Opdenakker, G., P. E. Van den Steen, B. Dubois, I. Nelissen, E. Van Coillie, S. Masure, P. Proost, and J. Van Damme. 2001. Gelatinase B functions as regulator and effector in leukocyte biology. J. Leukoc. Biol. 69:851-859. [PubMed] [Google Scholar]
- 20.Pal, S., A. P. Schmidt, E. M. Peterson, C. L. Wilson, and L. M. De La Maza. 2006. Role of matrix metalloproteinase-7 in the modulation of a Chlamydia trachomatis infection. Immunology 117:213-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patton, D. L., D. V. Landers, and J. Schachter. 1989. Experimental Chlamydia trachomatis salpingitis in mice: initial studies on the characterization of the leukocyte response to chlamydial infection. J. Infect. Dis. 159:1105-1110. [DOI] [PubMed] [Google Scholar]
- 22.Ramsey, K. H., T. W. Cotter, R. D. Salyer, G. S. Miranpuri, M. A. Yanez, C. E. Poulsen, J. L. DeWolfe, and G. I. Byrne. 1999. Prior genital tract infection with a murine or human biovar of Chlamydia trachomatis protects mice against heterotypic challenge infection. Infect. Immun. 67:3019-3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramsey, K. H., I. M. Sigar, S. V. Rana, J. Gupta, S. M. Holland, and G. I. Byrne. 2001. Role for inducible nitric oxide synthase in protection from chronic Chlamydia trachomatis urogenital disease in mice and its regulation by oxygen free radicals. Infect. Immun. 69:7374-7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramsey, K. H., I. M. Sigar, J. H. Schripsema, N. Shaba, and K. P. Cohoon. 2005. Expression of matrix metalloproteinases subsequent to urogenital Chlamydia muridarum infection of mice. Infect. Immun. 73:6962-6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rank, R. G. 1999. Models of immunity, p. 239-295. In R. S. Stephens (ed.), Chlamydia: intracellular biology, pathogenesis, and immunity. American Society for Microbiology, Washington, D.C.
- 26.Rank, R. G., M. M. Sanders, and D. L. Patton. 1995. Increased incidence of oviduct pathology in the guinea pig after repeat vaginal inoculation with the chlamydial agent of guinea pig inclusion conjunctivitis. Sex. Transm. Dis. 22:48-54. [DOI] [PubMed] [Google Scholar]
- 27.Register, K. B., P. A. Morgan, and P. B. Wyrick. 1986. Interaction between Chlamydia spp. and human polymorphonuclear leukocytes in vitro. Infect. Immun. 52:664-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruiz-Ortega, M., and J. Egido. 1997. Angiotensin II modulates cell growth-related events and synthesis of matrix proteins in renal interstitial fibroblasts. Kidney Int. 52:1497-1510. [DOI] [PubMed] [Google Scholar]
- 29.Sapadin, A. N., and R. Fleischmajer. 2006. Tetracyclines: nonantibiotic properties and their clinical implications. J. Am. Acad. Dermatol. 54:258-265. [DOI] [PubMed] [Google Scholar]
- 30.Shah, A. A., J. H. Schripsema, M. T. Imtiaz, I. M. Sigar, J. Kasimos, P. G. Matos, S. Inouye, and K. H. Ramsey. 2005. Histopathologic changes related to fibrotic oviduct occlusion after genital tract infection of mice with Chlamydia muridarum. Sex. Transm. Dis. 32:49-56. [DOI] [PubMed] [Google Scholar]
- 31.Singhvi, S. M., K. J. Kripalani, A. V. Dean, G. R. Keim, J. S. Kulesza, F. S. Meeker, J. J. Ross, J. M. Shaw, and B. H. Migdalof. 1981. Absorption and bioavailability of captopril in mice and rats after administration by gavage and in the diet. J. Pharm. Sci. 70:885-888. [DOI] [PubMed] [Google Scholar]
- 32.Soloff, B. L., R. G. Rank, and A. L. Barron. 1985. Electron microscopic observations concerning the in vivo uptake and release of the agent of guinea-pig inclusion conjunctivitis (Chlamydia psittaci) in guinea-pig exocervix. J. Comp. Pathol. 95:335-344. [DOI] [PubMed] [Google Scholar]
- 33.Sorbi, D., M. Fadly, R. Hicks, S. Alexander, and L. Arbeit. 1993. Captopril inhibits the 72 kDa and 92 kDa matrix metalloproteinases. Kidney Int. 44:1266-1272. [DOI] [PubMed] [Google Scholar]
- 34.Su, H., R. Messer, W. Whitmire, E. Fischer, J. C. Portis, and H. D. Caldwell. 1998. Vaccination against chlamydial genital tract infection after immunization with dendritic cells pulsed ex vivo with nonviable Chlamydiae. J. Exp. Med. 188:809-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su, H., R. Messer, W. Whitmire, S. Hughes, and H. D. Caldwell. 2000. Subclinical chlamydial infection of the female mouse genital tract generates a potent protective immune response: implications for development of live attenuated chlamydial vaccine strains. Infect. Immun. 68:192-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su, H., R. Morrison, R. Messer, W. Whitmire, S. Hughes, and H. D. Caldwell. 1999. The effect of doxycycline treatment on the development of protective immunity in a murine model of chlamydial genital infection. J. Infect. Dis. 180:1252-1258. [DOI] [PubMed] [Google Scholar]
- 37.Suchland, R. J., W. M. Geisler, and W. E. Stamm. 2003. Methodologies and cell lines used for antimicrobial susceptibility testing of Chlamydia spp. Antimicrob. Agents Chemother. 47:636-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swenson, C. E., E. Donegan, and J. Schachter. 1983. Chlamydia trachomatis-induced salpingitis in mice. J. Infect. Dis. 148:1101-1107. [DOI] [PubMed] [Google Scholar]
- 39.Swenson, C. E., M. L. Sung, and J. Schachter. 1986. The effect of tetracycline treatment on chlamydial salpingitis and subsequent fertility in the mouse. Sex. Transm. Dis. 13:40-44. [DOI] [PubMed] [Google Scholar]
- 40.Vaday, G. G., S. Franitza, H. Schor, I. Hecht, A. Brill, L. Cahalon, R. Hershkoviz, and O. Lider. 2001. Combinatorial signals by inflammatory cytokines and chemokines mediate leukocyte interactions with extracellular matrix. J. Leukoc. Biol. 69:885-892. [PubMed] [Google Scholar]
- 41.Vaday, G. G., R. Hershkoviz, M. A. Rahat, N. Lahat, L. Cahalon, and O. Lider. 2000. Fibronectin-bound TNF-alpha stimulates monocyte matrix metalloproteinase-9 expression and regulates chemotaxis. J. Leukoc. Biol. 68:737-747. [PubMed] [Google Scholar]
- 42.Van den Steen, P. E., S. J. Husson, P. Proost, J. Van Damme, and G. Opdenakker. 2003. Carboxyterminal cleavage of the chemokines MIG and IP-10 by gelatinase B and neutrophil collagenase. Biochem. Biophys. Res. Commun. 310:889-896. [DOI] [PubMed] [Google Scholar]
- 43.Van den Steen, P. E., G. Opdenakker, M. R. Wormald, R. A. Dwek, and P. M. Rudd. 2001. Matrix remodelling enzymes, the protease cascade and glycosylation. Biochim. Biophys. Acta 1528:61-73. [DOI] [PubMed] [Google Scholar]
- 44.Visse, R., and H. Nagase. 2003. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ. Res. 92:827-839. [DOI] [PubMed] [Google Scholar]
- 45.Volpert, O. V., W. F. Ward, M. W. Lingen, L. Chesler, D. B. Solt, M. D. Johnson, A. Molteni, P. J. Polverini, and N. P. Bouck. 1996. Captopril inhibits angiogenesis and slows the growth of experimental tumors in rats. J. Clin. Investig. 98:671-679. [DOI] [PMC free article] [PubMed] [Google Scholar]