Abstract
Mutation of tryptophan-101 in Clostridium difficile toxin A, a 308-kDa glucosyltransferase, resulted in a 50-fold-reduced cytopathic activity in cell culture experiments. The mutant toxin A was characterized and applied to distinguish between glucosyltransferase-dependent and -independent effects with respect to RhoB up-regulation as a cellular stress response.
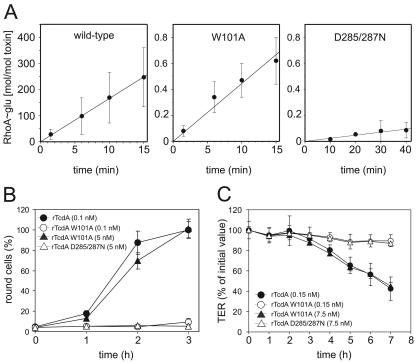
Clostridium difficile toxins A and B (TcdA and TcdB, respectively) are the major pathogenicity factors that are causative for antibiotic-associated pseudomembranous colitis (19). Several reports of the in vivo effects of TcdA in animal models reflect efforts to understand the cellular mechanism leading to clinical symptoms as well as to the release of mediators that are involved in the inflammatory process (2, 13, 15, 18, 20). The inherent glucosyltransferase (GT) activity of TcdA/B, which catalyzes monoglucosylation of the small GTPases Rho, Rac, and Cdc42, is well described (11, 12). However, Rho inactivation is not in accordance with the activation of Rho-dependent proinflammatory signal cascades (14). To address the issue of GT-independent effects, we used recombinant TcdA (rTcdA) (4, 10) and generated two mutant toxins by site-directed mutagenesis of the expression vector. Two highly conserved motifs were chosen for mutation, namely, tryptophan-101 and the DXD motif at positions 285 to 287 (3, 17). Analogous mutations of TcdB resulted in reductions of the in vitro GT activity, by factors of 1,000 and 5,000, respectively (5, 6, 16). The in vitro GT activities of rTcdA (wild type), rTcdA W101A (tryptophan mutant), and rTcdA D285/287N (DXD mutant) were determined from the linear phases of RhoA glucosylation kinetics (Fig. 1A), as described elsewhere (7). The GT activities of the W101A and D285/287N mutants were reduced 380-fold and 6,900-fold, respectively, compared to that of wild-type rTcdA. In contrast to the case in the cell-free system, the cytopathic activity of rTcdA W101A on Swiss 3T3 fibroblasts was estimated to be reduced only 50-fold (Fig. 1B). In addition, the colonic cell line Caco-2 was used to investigate the cytopathic property of mutant toxins with respect to transepithelial electrical resistance (TER). The TER of confluent Caco-2 cell monolayers grown on filter inserts (Falcon; BD, Germany) was determined, starting with an initial value of at least 150 Ω · cm2 (Fig. 1C). rTcdA W101A (7.5 nM) caused identical alteration of the TER (45% ± 4% of the initial value after 7 h) to that caused by 0.15 nM wild-type rTcdA (43% ± 11%). rTcdA W101A at an equimolar concentration (0.15 nM; 90% ± 6%) and rTcdA D285/287N (7.5 nM) did not significantly affect the TER (87% ± 3% of the initial value after 7 h).
FIG. 1.
(A) In vitro glucosyltransferase activities of wild-type and mutant rTcdA with the substrate RhoA. Enzyme activities were calculated for rTcdA (16.6 mol/mol · min), rTcdA W101A (0.044 mol/mol · min), and rTcdA D285/287N (2.4 × 10−3 mol/mol · min) (data are means ± standard deviations [SD]; n = 4 [for rTcdA D285/287N, n = 3]). (B) The kinetics of cell rounding by 0.1 nM wild-type rTcdA (•) and 5 nM rTcdA W101A (▴) were identical, whereas 0.1 nM rTcdA W101A (○) and rTcdA D285/287N (▵) did not cause rounding of cells within a period of 3 h (data are means ± SD; n = 5). (C) The integrity of Caco-2 cell monolayers was checked by measuring the TER after treatment with the indicated toxins applied to the apical site for 7 h. Only cells treated with 0.15 nM wild-type rTcdA (•) or 7.5 nM rTcdA W101A (▴) showed significant decreases in the TER, whereas cells treated with 0.15 nM rTcdA W101A (○) or 7.5 nM rTcdA D285/287N (▵) were not affected (data are means ± SD; n = 3).
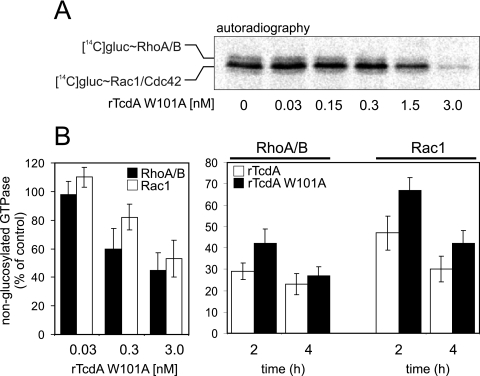
To check the extent of intracellular glucosylation of GTPases, unmodified GTPases were detected by either sequential 14C-glucosylation (RhoA/B and Rac1) (12), [32P]ADP ribosylation (RhoA/B) (1), or Western blot analysis (with anti-Rac1, which exclusively recognizes unmodified Rac1) (8). There was a concentration-dependent decrease in sequential 14C-glucosylation (Fig. 2A) that was obvious in cells incubated with 1.5 nM or higher concentrations of rTcdA W101A. Figure 2B shows the concentration- and time-dependent glucosylation of RhoA/B and Rac1 by rTcdA W101A. The amounts of nonglucosylated RhoA/B and Rac1 in 3T3 fibroblasts treated for 2 or 4 h with different concentrations of rTcdA W101A were almost identical in the respective cell lysates (Fig. 2B, left panel). The glucosylation kinetics of RhoA/B and Rac1 by rTcdA W101A (5 nM) did not differ significantly from those by rTcdA (0.1 nM) (Fig. 2B, right panel).
FIG. 2.
(A) Sequential 14C-glucosylation of lysates from toxin-treated Caco-2 cells. Reduced signals in the autoradiograph indicate the previous glucosylation of Rho GTPases by incubation with the indicated concentrations of rTcdA W101A for 16 h. (B) Specific rTcdA W101-catalyzed glucosylation of Rho and Rac from Swiss 3T3 fibroblasts was estimated by detection of unmodified GTPases. (Left) Concentration-dependent glucosylation of RhoA/B (black bars) and Rac1 (white bars) by rTcdA W101A. (Right) Time-dependent glucosylation of RhoA/B and Rac1 by rTcdA (white bars) and rTcdA W101A (black bars). Data are means ± SD (n = 3).
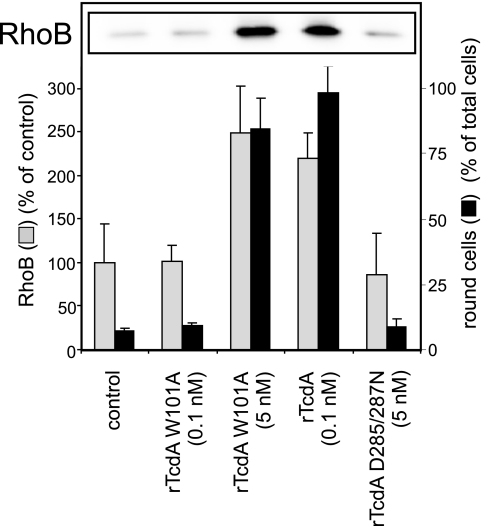
To check whether the previously reported up-regulation of the immediate-early gene product RhoB (9) is dependent on the GT activity of TcdA, enzyme activity-deficient rTcdA and rTcdA with reduced enzyme activity were applied. rTcdA (0.1 nM) induced strong synthesis of the RhoB protein in Swiss 3T3 fibroblasts after 4 h (Fig. 3, inset). Enzyme activity-deficient rTcdA D285/287N had no effect on RhoB up-regulation, even at a concentration of 100 nM, whereas the tryptophan mutant toxin (rTcdA W101A) showed a concentration-dependent effect. Thus, significant RhoB up-regulation was detected only with those concentrations of tryptophan mutant toxin that were sufficient to cause cytopathic effects. The concentration-dependent up-regulation of RhoB in correlation with cell rounding was determined in quadruplicate, and the results are displayed in Fig. 3.
FIG. 3.
Up-regulation of RhoB is a sequel of Rho glucosylation. Western blot analysis showed the amounts of RhoB in toxin-treated cells (inset). The bar chart shows the correlation of toxin-induced cell rounding and RhoB expression (data are means ± SD; n = 4).
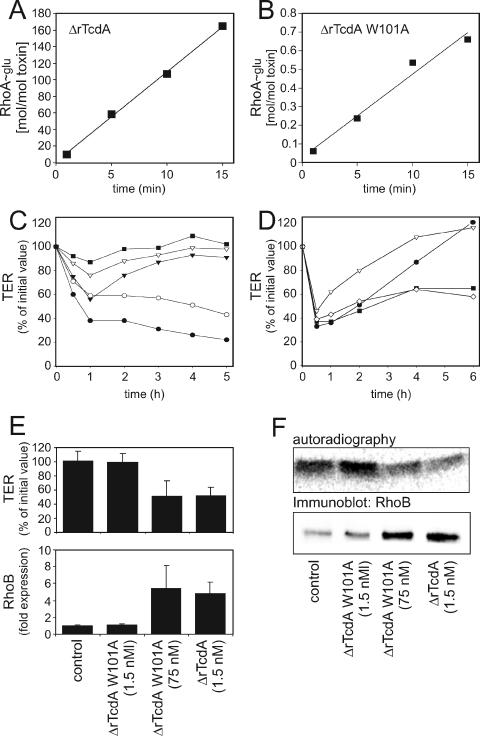
The N-terminal fragment of TcdA encompasses amino acids 1 to 1065 (ΔrTcdA) and consists of the minimal catalytic domain (amino acids 1 to 542) plus the portion up to the putative transmembrane region. However, ΔrTcdA lacks the transmembrane and receptor binding domains and is therefore incapable of entering target cells. The specific GT activities of ΔrTcdA and ΔrTcdA W101A were 10.3 mol/ mol · min and 0.04 mol/mol · min, respectively (Fig. 4A and B) and thus did not differ significantly from the GT activities of the corresponding holotoxins. To deliver the N-terminal fragments into intact cells by circumventing the active process of endocytosis, the electroporation technique (3 μF, 500 V) was applied (5). The minimal concentration of ΔrTcdA that induced an irreversible decrease in the TER over a period of 5 h was determined (Fig. 4C). A time course of TER measurements for Caco-2 cell monolayers was performed with ΔrTcdA, ΔrTcdA W101A, and 50 μl Bacillus megaterium protein fraction as a negative control for protein impurities (Fig. 4D). Mean values after 6 h of treatment for three separate experiments are shown in Fig. 4E. To complete the study of the intracellular effects of ΔrTcdA and ΔrTcdA W101A, sequential 14C-glucosylation of the cell lysates of electroporated monolayers was performed (Fig. 4F, upper panel). Compared to controls, there was no decrease in sequential 14C-glucosylation of lysates from cells treated with 1.5 nM ΔrTcdA W101A, but there was a reduction of about 40% in lysates from cells treated with either 75 nM ΔrTcdA W101A or 1.5 nM ΔrTcdA. In accordance with the effects on the TER and sequential 14C-glucosylation, up-regulation of RhoB was induced only by 1.5 nM ΔrTcdA and 75 nM ΔrTcdA W101A, as shown by Western blot analysis (Fig. 4F, lower panel). ΔrTcdA W101A (1.5 nM) did not induce the up-regulation of RhoB. A densitometric analysis of three separate experiments is shown in Fig. 4E, lower panel.
FIG. 4.
Electroporation of Caco-2 cell monolayers. (A and B) In vitro glucosyltransferase activities of ΔrTcdA (10.3 mol/mol · min) and ΔrTcdA W101A (0.04 mol/mol · min). (C) Determination of minimal effective concentrations of ΔrTcdA. ▪, 0.006 nM; ▿, 0.06 nM; ▾, 0.6 nM; ○, 1.5 nM; •, 3 nM. (D) Alteration of TER by 50 μl bone morphogenetic protein fraction (•) or 1.5 nM ΔrTcdA W101A (▿), ΔrTcdA W101A (75 nM, ▪), or wild-type ΔrTcdA (⋄). (E) Statistical evaluation of the TER measured after 6 h of treatment with the indicated toxins (top panel). (Bottom) Densitometric evaluation of RhoB immunoblots. The data shown are mean values for three separate experiments ± standard deviations. (F) (Top) Sequential 14C-glucosylation of cell lysates from electroporated Caco-2 cells. (Bottom) RhoB up-regulation in electroporated Caco-2 cells. The set of experiments shown is representative of three separate experiments.
In summary, this study evaluated tryptophan-101 mutant toxin A (rTcdA W101A) as a tool for studying glucosyltransferase-independent effects of C. difficile toxins because of its step-like concentration dependency on intact cells. rTcdA W101A has the same properties as wild-type rTcdA when applied at a 50-fold higher concentration than that of wild-type toxin to intact cells. At an equimolar concentration, the mutant toxin is inactive towards intact cells. In contrast to the enzyme activity-deficient DXD mutant, the remainder enzyme and cytopathic activity of the tryptophan mutant prove that it has correct toxic competence. The difference in GT activities in a cell-free system and in intact cells was shown to be due to intracellular conditions but not to uptake-mediated refolding of toxins.
Acknowledgments
This study was supported by Deutsche Forschungsgemeinschaft SFB 621 (project B5).
We thank Christiane Hotopp-Herrgesell for excellent technical assistance in cell culture and Markus Isermann for providing RhoA. We are also grateful to Karsten Heidrich, Institute of Physiological Chemistry, for sequencing the constructs.
Editor: J. T. Barbieri
REFERENCES
- 1.Ahnert-Hilger, G., M. Höltje, G. Grosse, G. Pickert, C. Mucke, B. Nixdorf-Bergweiler, P. Boquet, F. Hofmann, and I. Just. 2004. Differential effects of Rho GTPases on axonal and dendritic development in hippocampal neurons. J. Neurochem. 90:9-18. [DOI] [PubMed] [Google Scholar]
- 2.Anton, P. M., J. Gay, A. Mykoniatis, A. Pan, M. O'Brien, D. Brown, K. Karalis, and C. Pothoulakis. 2004. Corticotropin-releasing hormone (CRH) requirement in Clostridium difficile toxin A-mediated intestinal inflammation. Proc. Natl. Acad. Sci. USA 101:8503-8508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhattacharyya, S., A. Kerzmann, and A. L. Feig. 2002. Fluorescent analogs of UDP-glucose and their use in characterizing substrate binding by toxin A from Clostridium difficile. Eur. J. Biochem. 269:3425-3432. [DOI] [PubMed] [Google Scholar]
- 4.Burger, S., H. Tatge, F. Hofmann, I. Just, and R. Gerhard. 2003. Expression of recombinant Clostridium difficile toxin A using the Bacillus megaterium system. Biochem. Biophys. Res. Commun. 307:584-588. [DOI] [PubMed] [Google Scholar]
- 5.Busch, C., F. Hofmann, R. Gerhard, and K. Aktories. 2000. Involvement of a conserved tryptophan residue in the UDP-glucose binding of large clostridial cytotoxin glycosyltransferases. J. Biol. Chem. 275:13228-13234. [DOI] [PubMed] [Google Scholar]
- 6.Busch, C., F. Hofmann, J. Selzer, J. Munro, D. Jeckel, and K. Aktories. 1998. A common motif of eukaryotic glycosyltransferases is essential for the enzyme activity of large clostridial cytotoxins. J. Biol. Chem. 273:19566-19572. [DOI] [PubMed] [Google Scholar]
- 7.Genth, H., K. Aktories, and I. Just. 1999. Monoglucosylation of RhoA at threonine-37 blocks cytosol-membrane cycling. J. Biol. Chem. 274:29050-29056. [DOI] [PubMed] [Google Scholar]
- 8.Genth, H., J. Huelsenbeck, B. Hartmann, F. Hofmann, I. Just, and R. Gerhard. 2006. Cellular stability of Rho-GTPases glucosylated by Clostridium difficile toxin B. FEBS Lett. 580:3565-3569. [DOI] [PubMed] [Google Scholar]
- 9.Gerhard, R., H. Tatge, H. Genth, T. Thum, J. Borlak, G. Fritz, and I. Just. 2005. Clostridium difficile toxin A induces expression of the stress-induced early gene product RhoB. J. Biol. Chem. 280:1499-1505. [DOI] [PubMed] [Google Scholar]
- 10.Gerhard, R., S. Burger, H. Tatge, H. Genth, I. Just, and F. Hofmann. 2005. Comparison of wild type with recombinant Clostridium difficile toxin A. Microb. Pathog. 38:77-83. [DOI] [PubMed] [Google Scholar]
- 11.Just, I., J. Selzer, M. Wilm, C. Von Eichel-Streiber, M. Mann, and K. Aktories. 1995. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature 375:500-503. [DOI] [PubMed] [Google Scholar]
- 12.Just, I., M. Wilm, J. Selzer, G. Rex, C. Von Eichel-Streiber, M. Mann, and K. Aktories. 1995. The enterotoxin from Clostridium difficile (ToxA) monoglucosylates the Rho proteins. J. Biol. Chem. 270:13932-13936. [DOI] [PubMed] [Google Scholar]
- 13.Kim, H., S. H. Rhee, E. Kokkotou, X. Na, T. Savidge, M. P. Moyer, C. Pothoulakis, and J. T. LaMont. 2005. Clostridium difficile toxin A regulates inducible COX-2 and PGE2 synthesis in colonocytes via reactive oxygen species and activation of p38 MAP kinase. J. Biol. Chem. 280:21237-21245. [DOI] [PubMed] [Google Scholar]
- 14.Kyriakis, J. M., and J. Avruch. 2003. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 81:807-869. [DOI] [PubMed] [Google Scholar]
- 15.Mykoniatis, A., P. M. Anton, M. Wlk, C. C. Wang, L. Ungsunan, S. Blüher, M. Venihaki, S. Simeonidis, J. Zacks, D. Zhao, S. Souglioultzis, K. Karalis, C. Mantzoros, and C. Pothoulakis. 2003. Leptin mediates Clostridium difficile toxin A-induced enteritis in mice. Gastroenterology 124:683-691. [DOI] [PubMed] [Google Scholar]
- 16.Qa'Dan, M., M. Ramsey, J. Daniel, L. M. Spyres, B. Safiejko-Mroczka, W. Ortiz-Leduc, and J. D. Ballard. 2002. Clostridium difficile toxin B activates dual caspase-dependent and caspase-independent apoptosis in intoxicated cells. Cell. Microbiol. 4:425-434. [DOI] [PubMed] [Google Scholar]
- 17.Reinert, D. J., T. Jank, K. Aktories, and G. E. Schulz. 2005. Structural basis for the function of Clostridium difficile toxin B. J. Mol. Biol. 351:973-981. [DOI] [PubMed] [Google Scholar]
- 18.Savidge, T. C., W.-H. Pan, P. Newman, M. O'Brien, P. M. Anton, and C. Pothoulakis. 2003. Clostridium difficile toxin B is an inflammatory enterotoxin in human intestine. Gastroenterology 125:413-420. [DOI] [PubMed] [Google Scholar]
- 19.Voth, D. E., and J. Ballard. 2005. Clostridium difficile toxins: mechanism of action and role in disease. Clin. Microbiol. Rev. 18:247-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao, D., S. Kuhnt-Moore, H. Zeng, A. Pan, J. S. Wu, S. Simeonidis, M. P. Moyer, and C. Pothoulakis. 2002. Substance P-stimulated interleukin-8 expression in human colonic epithelial cells involves Rho family small GTPases. Biochem. J. 368:665-672. [DOI] [PMC free article] [PubMed] [Google Scholar]