Abstract
Infection with Borrelia burgdorferi, the causative agent of Lyme disease, results in a Th1 response and proinflammatory cytokine production. Mice deficient for MKK3, an upstream activator of p38 mitogen-activated protein (MAP) kinase, develop a lower Th1 response and exhibit an impaired ability to produce proinflammatory cytokines upon infection with the spirochete. We investigated the contribution of p38 MAP kinase activity in gamma interferon (IFN-γ) production in CD4+ T cells in response to specific antigen through T-cell receptor (TCR)- and interleukin-12 (IL-12)-mediated signals. The specific inhibition of p38 MAP kinase in T cells and the administration of a pharmacological inhibitor of the kinase during the course of infection with the spirochete resulted in reduced levels of IFN-γ in the sera of infected mice. Our results also demonstrate that although p38 MAP kinase activity is not required for the differentiation of B. burgdorferi-specific CD4+ T cells, the production of IFN-γ by Th1 effector cells is regulated by the kinase. Both TCR engagement and IL-12 induced the production of the Th1 cytokine through the activation of the p38 MAP kinase pathway. Thus, the inhibition of this pathway in vitro resulted in decreased levels of IFN-γ during restimulation of B. burgdorferi-specific T cells in response to anti-CD3 and IL-12 stimulation. These results clarify the specific contribution of the p38 MAP kinase in the overall immune response to the spirochete and its role in the effector function of B. burgdorferi-specific T cells.
Lyme disease is the most common arthropod-borne disease in the United States. Recently, there has been a surge in the number of reported cases of Lyme disease, partially due to a shift in environmental conditions that favor the life cycle of Borrelia burgdorferi (20, 24). B. burgdorferi is a highly prevalent vector-transmitted spirochete. An early hallmark of infection with this spirochete is the development of a skin rash, erythema migrans, which is usually accompanied by flu-like symptoms (26). Moreover, infection with B. burgdorferi can result in pathology of the musculoskeletal, cardiovascular, and/or neurological systems (26). The development of pathology following infection with B. burgdorferi is a dynamic process affected by many variables, including, but not limited to, the number of spirochetes in the affected organs, spirochetal virulence, and the host immune response generated against B. burgdorferi (2). It has been speculated that one of the main factors influencing the pathology following infection is the development of a Th1 response. In fact, it has been shown that Th1 cells dominate the immune response in the synovial fluid of patients with Lyme disease and that the severity of arthritis directly correlates with the ratio of Th1 cells to Th2 cells in the synovium (14). Furthermore, experimental infections of mice have revealed a strong correlation between the production of proinflammatory cytokines, including interleukin-12 (IL-12) and gamma interferon (IFN-γ), and the development of acute murine Lyme arthritis and spirochetal adaptation (3, 4, 15, 19). However, infection of IFN-γ- or IFN-γRα-deficient mice with the spirochete in the footpad resulted in similar levels of arthritis (10, 13). These conflicting results may be largely attributed to the route of inoculation, which has been strongly associated with distinct pathological outcomes and spirochetal dissemination (6, 12, 22).
The mitogen-activated protein (MAP) kinase p38 is involved in the regulation of IFN-γ production and Th1 responses. Inhibition of p38 MAP kinase in the T-cell lineage causes impaired IFN-γ production by CD4+ Th1 cells (23). Impaired IFN-γ production by Th1 cells was also observed in mice deficient for MKK3, one of the upstream activators of p38 MAP kinase (18). In addition, GADD45β and GADD45γ are upregulated in Th1 cells and control the expression of IFN-γ through their association with an upstream activator of the p38 MAP kinase pathway, MEKK4 (11, 17). Moreover, Th1 cells deficient for MEKK4 are also defective in the production of IFN-γ (11). Thus, a number of findings support the role of this signaling pathway in the production of IFN-γ by Th1 cells. This pathway is therefore a potential target for therapies aimed at the modulation of proinflammatory Th1 responses against pathogens, such as B. burgdorferi. Mice that lack a specific upstream activator of p38 MAP kinase, MKK3, that are infected with B. burgdorferi develop a decreased Th1 response to the spirochete (1). However, it is not clear whether this effect is due to impairment of IFN-γ production by Th1 cells or whether the ubiquitous deficiency of the mkk3 gene during development can alter the development and/or function of other cell types, such as macrophages. Here we show that pharmacological inhibition of p38 MAP kinase during the course of infection with B. burgdorferi reduces the production of IFN-γ in vivo. Moreover, inhibition of p38 MAP kinase exclusively in T cells is sufficient to diminish the serum levels of the cytokine during infection with the spirochete. We also show that the activation of p38 MAP kinase appears to be more critical during the production of IFN-γ by effector Th1 cells in response to B. burgdorferi than during the initial phase of differentiation from naïve Th1 cells to effector Th1 cells. Overall, our results clarify the immune response to the spirochete mediated by CD4+ T cells and the role of the p38 MAP kinase pathway in the production of IFN-γ in response to a specific antigen.
MATERIALS AND METHODS
Mice, infection, and treatments.
Six-week-old female C3H/HeN mice were purchased from Charles River Laboratories (Wilmington, MA). Transgenic mice that express a dominant negative form of p38 MAP kinase have been described previously (23). Negative littermates (B10.BR) were used as controls. The mice were kept in microisolator cages and were provided food and water ad libitum. All the experiments involving animals were approved by the Institutional Animal Care and Use Committees of University of North Carolina at Charlotte and University of Massachusetts Amherst.
Low-passage N40, a clonal B. burgdorferi strain with proven infectivity and pathogenicity (1), was grown in BSK II media at 33°C, counted in a Hausser Meyerhoff counting chamber (Hausser Scientific, Horsham, PA), and used to inoculate the mice. All mice were infected with 105 spirochetes by subcutaneous injection in the midline of the back. C3H infected mice were inoculated in the peritoneal cavity daily with 1 mg of SB203580/kg (Calbiochem, San Diego, CA) during infection (5). Control C3H mice were inoculated in parallel with vehicle alone (1.3% dimethyl sulfoxide in phosphate-buffered saline). At sacrifice, the bladder and a fragment of skin from the inoculation site were cultured in BSK II media for 2 weeks to assess the infectious status of the mice.
Assessment of effector CD4+-T-cell function.
At sacrifice, splenic CD4+ T cells were purified by negative selection using biotinylated anti-mouse CD8a, CD11b, I-Ak/I-Ek, B220, Pan NK, and Ly-6G antibodies (BD Biosciences, San Diego, CA), followed by anti-biotin microbeads (Miltenyi Biotec, Auburn, CA), as previously described (1). Syngeneic antigen-presenting cells (APCs) were generated from total splenocytes by treatment with 50 μg/ml of mitomycin C (Sigma Chemical Co., St Louis, MO) for 40 min at 37°C.
B. burgdorferi-specific effector CD4+ T-cell responses were analyzed by incubating 106 purified CD4+ T cells plus 106 APCs per ml with 10 μg/ml of a B. burgdorferi cell sonicate. The effect of p38 MAP kinase inhibition on B. burgdorferi-specific effector CD4+ T-cell function was analyzed by addition of 5 μM SB203580 or the vehicle control. The specific contribution of IL-12 during recall responses was analyzed by antibody blockade (clone C17.8; BD Biosciences) during restimulation. CD4+ T cells from the infected mice were also stimulated in vitro with plate-bound anti-mouse CD3 (5 μg/ml; BD Biosciences). The restimulation supernatants were analyzed after 40 h of incubation for IFN-γ production.
Cytokine ELISA.
The levels of IFN-γ and IL-12p70 in the sera of the infected mice and restimulation supernatants were determined by capture enzyme-linked immunosorbent assay (ELISA), as previously described (1). The values were obtained by extrapolation using the values obtained with standard amounts of recombinant cytokines, as reported previously (1).
Statistical analysis.
Data were expressed as averages ± standard errors. Differences between means were determined with the Student t test, using the software Prism (version 4.0). A difference was considered significant if the P value was <0.05.
RESULTS
Inhibition of p38 MAP kinase in T cells impairs the production of IFN-γ in response to B. burgdorferi.
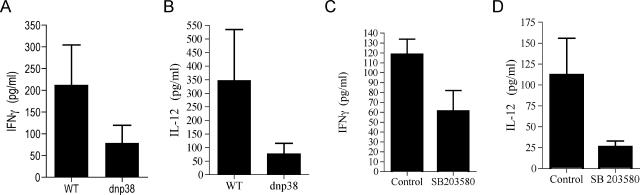
The production of IFN-γ during infection with B. burgdorferi is impaired in mice that are deficient for the p38 MAP kinase upstream activator, MKK3 (1). Since MKK3 is ubiquitously expressed, we first investigated whether p38 MAP kinase plays a role in the production of IFN-γ by CD4+ T cells in response to spirochetal antigens. We used transgenic mice that express a dominant negative mutant (dnp38) MAP kinase that inhibits the activity of endogenous p38 MAP kinase (23). The transgene is under the influence of the distal lck promoter, which drives its expression in T cells specifically (23). Groups of lck-dnp38 transgenic mice and B10.BR controls were infected with 105 spirochetes subcutaneously in the midline of the back. Two weeks postinfection the sera were analyzed to determine IFN-γ levels. Similar to MKK3 mice, the levels of IFN-γ in the sera of the dnp38 transgenic mice were reduced compared to the levels in the controls (Fig. 1A). The levels of IFN-γ in uninfected dnp38 transgenic and control mice were below the detection limit (data not shown). We also found reduced serum levels of IL-12 (Fig. 1B). These data confirm that p38 MAP kinase plays a role in the production of IFN-γ by cells that express the transgene, including CD4+ T cells, in vivo in response to B. burgdorferi.
FIG. 1.
T-cell p38 MAP kinase activity regulates the production of IFN-γ during infection with B. burgdorferi. lck-dnp38 transgenic and wild-type control (WT) mice were infected with 105 B. burgdorferi spirochetes subcutaneously in the midline of the back. Two weeks after infection, the levels of IFN-γ (A) and IL-12p70 (B) in the sera of the infected mice were determined by ELISA. C3H mice were infected as described above and treated with 1 mg of SB203580/kg or vehicle, and serum levels of IFN-γ (C) and IL-12 (D) were determined by ELISA. The data are the average values of two to five experiments, and each value was determined with pooled sera of three to five animals.
We also used a pharmacological approach by examining the effect of the pyridinyl imidazole compound SB203580, a specific inhibitor of p38 MAP kinase activity during the course of infection (5). Groups of C3H mice were infected as described above and divided into two groups. One group received daily intraperitoneal injections of SB203580 at a dose of 1 mg/kg in 100 μl. Two weeks after infection, the serum levels of IFN-γ were determined by ELISA. As observed with the dnp38 transgenic mice, the serum levels of IFN-γ in B. burgdorferi-infected, SB203580-treated mice were reduced compared to the levels in the control-treated mice (Fig. 1C). The IL-12 levels in the sera of the infected, SB203580-treated mice were also reduced compared to the levels in control-treated animals (Fig. 1D). These results demonstrate that the inhibition of p38 MAP kinase during infection with B. burgdorferi results in decreased production of IFN-γ and IL-12.
p38 MAP kinase mediates B. burgdorferi-induced IFN-γ production by effector CD4+ Th1 cells.
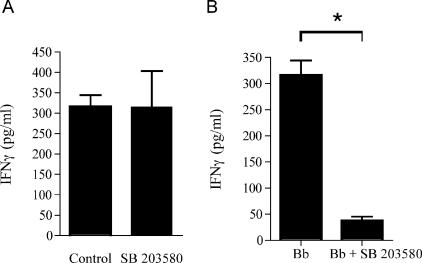
SB203580 effectively inhibits the production of IFN-γ by effector Th1 cells in vitro in a dose-dependent manner, but it only minimally affects the differentiation of naïve CD4+ T cells into effector Th1 cells (23). To determine whether the in vivo inhibition of p38 MAP kinase with SB203580 during the course of infection affected the frequency of B. burgdorferi-specific effector Th1 cells, we analyzed recall responses of purified CD4+ T cells from mice infected for 2 weeks. Purified splenic CD4+ T cells were restimulated with syngeneic mitomycin C-treated APCs in the presence of 10 μg/ml of a B. burgdorferi lysate. After 40 h, the supernatants were analyzed for the presence of IFN-γ by ELISA. We found that the amounts of IFN-γ produced by the effector CD4+ T cells were equivalent in the two groups of mice, regardless of the treatment during infection (P = 0.99, as determined by Student's t test) (Fig. 2A). However, the amount of IFN-γ in the restimulation supernatants of effector CD4+ T cells from B. burgdorferi-infected mice was reduced in the presence of SB203580 (P < 0.001) (Fig. 2B). No differences were observed in the production of IL-4 by B. burgdorferi-specific CD4+ T cells (data not shown). Together, these results suggested that in vivo, the inhibitor affected the effector function of B. burgdorferi-specific CD4+ T cells.
FIG. 2.
p38 MAP kinase regulates the production of IFN-γ by B. burgdorferi-specific effector Th1 cells. (A) CD4+ T cells were purified from mice infected with B. burgdorferi for 2 weeks and treated with either SB203580 or vehicle (1.3% dimethyl sulfoxide in phosphate-buffered saline) (Control) during infection. The cells were restimulated in the presence of a B. burgdorferi lysate (10 μg/ml) in the presence of mitomycin C-treated APCs. IFN-γ was quantified by capture ELISA 40 h after the initial stimulation. (B) Purified CD4+ T cells from infected C3H mice were stimulated with B. burgdorferi (Bb) in the absence or presence of 5 μM SB203580. IFN-γ was quantified 40 h later in the supernatants by ELISA. The data are representative of the results of three to five independent experiments. The asterisk indicates that the P value is <0.001, as determined by Student's t test.
IL-12 contributes to IFN-γ production by B. burgdorferi-specific effector Th1 cells.
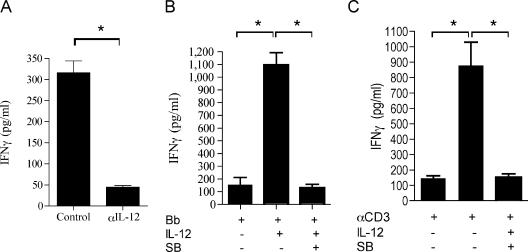
In addition to the activation of effector Th1 cells through antigen presentation and T-cell receptor (TCR) signals, B. burgdorferi can enhance IL-12 production by antigen-presenting cells (4). To investigate the relative contribution of APC-derived IL-12 to the overall IFN-γ production by B. burgdorferi-specific effector cells during restimulation in vitro, we restimulated purified CD4+ T cells from C3H mice infected for 2 weeks as described above in the absence or presence of 10 μg/ml of a blocking monoclonal antibody (MAb) specific for IL-12. The presence of the blocking MAb during restimulation resulted in reduced levels of IFN-γ in the supernatants (P < 0.001) (Fig. 3A), indicating that both TCR engagement and IL-12 contribute to the production of IFN-γ by B. burgdorferi antigen-specific effector cells.
FIG. 3.
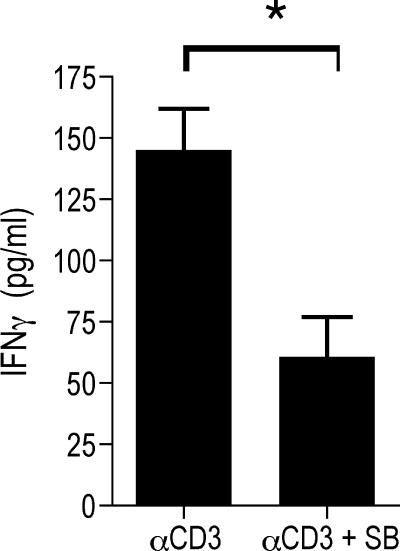
IL-12-mediated p38 MAP kinase activation contributes to IFN-γ production by effector Th1 cells. (A and B) CD4+ T cells were purified from C3H mice infected for 2 weeks, and they were restimulated with a B. burgdorferi lysate (Bb) in the absence or presence of a blocking MAb to IL-12 (αIL-12) (10 μg/ml) (A) or recombinant murine IL-12 (10 ng/ml) in the absence or presence of SB203580 (SB) (5 μM) (B) for 40 h. (C) Cells were also stimulated with anti-CD3 (αCD3) (5 μg/ml) in the absence of antigen-presenting cells and in the absence or presence of IL-12 and SB203580. The supernatants were analyzed to determine IFN-γ levels by ELISA. The results are representative of the results of three independent experiments. An asterisk indicates that the P value is <0.001, as determined by Student's t test.
p38 MAP kinase has been shown to be important for IL-12 production by APCs (18). To test whether the effect of SB203580 during the restimulation of effector Th1 cells in vitro with B. burgdorferi was due to impaired levels of IL-12, we restimulated B. burgdorferi-specific CD4+ T cells from mice infected for 2 weeks with syngeneic APCs and recombinant murine IL-12 in the presence or absence of SB203580. The presence of exogenous IL-12 during restimulation further enhanced the amount of IFN-γ produced by effector T cells, as expected (Fig. 3B), but it did not reverse the suppressive effect of SB203580 (Fig. 3B). A similar effect was observed when B. burgdorferi-specific CD4+ T cells were restimulated with anti-CD3 in the presence of exogenous IL-12 and in the absence of antigen-presenting cells (Fig. 3C), confirming that effector Th1 cells were targeted by the pharmacological inhibitor, resulting in reduced production of IFN-γ.
p38 MAP kinase controls B. burgdorferi-specific induction of IFN-γ by TCR engagement.
While the activation of p38 MAP kinase during stimulation of naïve CD4+ T cells shows slow kinetics and has minimal effects, TCR ligation rapidly activates p38 MAP kinase in effector Th1 (but not Th2) cells in vitro (23). More recently, it has been reported that p38 MAP kinase can also be activated by IL-12 in T cells (8, 21, 27). Since both TCR and IL-12R signals contribute to IFN-γ production by Th1 effector cells against B. bugdorferi (Fig. 3A), we examined which of these pathways could be the principal target for SB203580. We restimulated purified CD4+ T cells from infected mice with anti-CD3 in the absence of APCs and in the absence or presence of SB203580. The stimulation of the effector CD4+ T cells with anti-CD3 resulted in the production of IFN-γ (Fig. 4), which was dependent, at least partially, on p38 MAP kinase activity, since the presence of SB203580 reduced the amount of the cytokine in the restimulation supernatants (P < 0.001) (Fig. 4). These data demonstrate that TCR-mediated production of IFN-γ by effector T cells is regulated by p38 MAP kinase activity.
FIG. 4.
p38 MAP kinase mediates the production of IFN-γ in B. burgdorferi-specific effector Th1 cells in response to TCR signals. CD4+ T cells were purified from C3H mice infected for 2 weeks, and they were restimulated with plate-bound anti-CD3 (αCD3) in the absence or presence of 5 μM SB203580 (SB). IFN-γ levels in the restimulation supernatants were determined after 40 h by capture ELISA. The results are representative of the results of three independent experiments. The asterisk indicates that the P value is <0.01, as determined by Student's t test.
DISCUSSION
The molecular mechanisms that control the immune response to the spirochete B. burgdorferi are not completely understood. In spite of extensive work showing that the cell-mediated Th1 response induced by B. burgdorferi is responsible for the pathology associated with infection, little is known about the signal transduction pathways responsible for this response. p38 MAP kinase activity is involved in inflammation elicited during infection with B. burgdorferi (1). Thus, mice that are deficient in the specific upstream activator of p38 MAP kinase, MKK3, developed a decreased Th1 response and reduced arthritis. MKK3-deficient effector CD4+ T cells produced lower levels of IFN-γ, the hallmark cytokine produced by Th1 cells, in the presence of MKK3+/+ APCs (1). The role of p38 MAP kinase in the differentiation and effector function of CD4+ T cells remains unresolved. The previous studies were performed with in vitro differentiated CD4+ T cells from genetically modified mice or cell clones. Our results demonstrate for the first time that p38 MAP kinase activation in response to TCR- and IL-12-induced signals mediates the production of the Th1 cytokine in antigen-specific in vivo differentiated CD4+ T cells in a murine model of infection with B. burgdorferi.
We show that the inhibition of p38 MAP kinase during infection with B. burgdorferi did not affect the ability of ex vivo restimulated cells to produce IFN-γ in conditions in which the kinase is not inhibited, suggesting that this pathway is not involved in the differentiation of CD4+ T cells into B. burgdorferi-specific Th1 effector cells. In contrast, the levels of IFN-γ in the sera of the infected, SB203580-treated mice were consistently reduced. The specific inhibition of p38 MAP kinase in T cells also resulted in lower serum levels of IFN-γ, indicating that this pathway regulates the production of the cytokine in T cells. These results strongly suggest that the inhibition of p38 MAP kinase activity in vivo during infection with the spirochete affected the effector function of antigen-specific CD4+ T cells rather than their differentiation, which resulted in lower levels of serum IFN-γ. Indeed, the restimulation in the presence of the inhibitor in response to specific antigen or anti-CD3 resulted in reduced levels of IFN-γ; an effect that was also mediated by the blockade of IL-12 induced signals that enhanced TCR-mediated induction of the Th1 cytokine.
It is likely that the effect of the inhibitor in vivo during the course of infection also affected other cell types, including cells that may produce IL-12 in response to B. burgdorferi antigens. Since p38 MAP kinase is involved in IL-12 production by phagocytic cells (18), it is conceivable that the presence of SB203580 during infection with the spirochete affected the production of the cytokine by antigen-presenting cells, as well as the response of antigen-specific T cells to the cytokine. However, the presence of lower IFN-γ levels in the sera of the dnp38 transgenic mice infected with B. burgdorferi strongly suggests that Th1 p38 MAP kinase activation is responsible for the production of the cytokine. Moreover, in these mice the serum levels of IL-12 were also consistently reduced. The interaction of IFN-γ with phagocytic cells induces their activation and dramatically increases their phagocytic capacity and their ability to produce proinflammatory cytokines in response to specific ligands, such as B. burgdorferi antigens (Olson and Anguita, unpublished observations), underscoring the importance of p38 MAP kinase activity on Th1 cells not only for the production of IFN-γ but also for the effector function of this cytokine in other cell types, including phagocytic cells.
The modulation of proinflammatory cytokine production in response to B. burgdorferi has profound consequences for the ability of the spirochete to survive in the mammalian host and cause inflammation. The specific role of individual factors is still controversial. Conflicting findings regarding the development of murine inflammation are probably the result of several factors, including the route of infection and the identification of the pathological consequences of infection, such as arthritis. Indeed, three different reports on the pathology associated with the deficiency in the Toll-like receptor-mediated signaling molecule, MyD88, described contradictory results (7, 9, 16). Moreover, a previously unrecognized role of this adaptor protein in IFN-γ-mediated responses in innate immune cells (25) underscores the complexity of the signaling pathways that are involved in the response to the spirochete. These results therefore highlight the importance of a full understanding of the immune response associated with infection with B. burgdorferi. The data presented here demonstrate the regulation of IFN-γ production by B. burgdorferi-specific effector T cells mediated by p38 MAP kinase. Due to the effects of this cytokine on innate immune function, such as the activation of phagocytic cells, our findings shed light not only on the acquired immune response to the bacterium but also on the role of downstream targets of the T-cell cytokine, including phagocytic responses.
Acknowledgments
We have no conflicting financial interests.
This work was supported by grants from the American Heart Association and NIH (grant AR 048265) to J. Anguita and by a grant from the Arthritis Foundation to M. Rincón.
We thank Nataliya Kubalik for her technical assistance.
Editor: D. L. Burns
REFERENCES
- 1.Anguita, J., S. W. Barthold, R. Persinski, M. N. Hedrick, C. A. Huy, R. J. Davis, R. A. Flavell, and E. Fikrig. 2002. Murine Lyme arthritis development mediated by p38 mitogen-activated protein kinase activity. J. Immunol. 168:6352-6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anguita, J., M. N. Hedrick, and E. Fikrig. 2003. Adaptation of Borrelia burgdorferi in the tick and the mammalian host. FEMS Microbiol. Rev. 27:493-504. [DOI] [PubMed] [Google Scholar]
- 3.Anguita, J., D. H. Persing, M. Rincón, S. W. Barthold, and E. Fikrig. 1996. Effect of anti-interleukin 12 treatment on murine Lyme borreliosis. J. Clin. Investig. 97:1028-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anguita, J., V. Thomas, S. Samanta, R. Persinski, C. Hernanz, S. W. Barthold, and E. Fikrig. 2001. Borrelia burgdorferi-induced inflammation facilitates spirochete adaptation and variable major protein-like sequence locus recombination. J. Immunol. 167:3383-3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badger, A. M., J. N. Bradbeer, B. Votta, J. C. Lee, J. L. Adams, and D. E. Griswold. 1996. Pharmacological profile of SB 203580, a selective inhibitor of cytokine suppressive binding protein/p38 kinase, in animal models of arthritis, bone resorption, endotoxin shock and immune function. J. Pharmacol. Exp. Ther. 279:1453-1461. [PubMed] [Google Scholar]
- 6.Barthold, S. W. 1991. Infectivity of Borrelia burgdorferi relative to route of inoculation and genotype in laboratory mice. J. Infect. Dis. 163:419-420. [DOI] [PubMed] [Google Scholar]
- 7.Behera, A. K., E. Hildebrand, R. T. Bronson, G. Perides, S. Uematsu, S. Akira, and L. T. Hu. 2006. MyD88 deficiency results in tissue-specific changes in cytokine induction and inflammation in interleukin-18-independent mice infected with Borrelia burgdorferi. Infect. Immun. 74:1462-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berenson, L. S., J. Yang, B. P. Sleckman, T. L. Murphy, and K. M. Murphy. 2006. Selective requirement of p38{alpha} MAPK in cytokine-dependent, but not antigen receptor-dependent, Th1 responses. J. Immunol. 176:4616-4621. [DOI] [PubMed] [Google Scholar]
- 9.Bolz, D. D., R. S. Sundsbak, Y. Ma, S. Akira, C. J. Kirschning, J. F. Zachary, J. H. Weis, and J. J. Weis. 2004. MyD88 plays a unique role in host defense but not arthritis development in Lyme disease. J. Immunol. 173:2003-2010. [DOI] [PubMed] [Google Scholar]
- 10.Brown, C. R., and S. L. Reiner. 1999. Experimental Lyme arthritis in the absence of interleukin-4 or gamma interferon. Infect. Immun. 67:3329-3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chi, H., B. Lu, M. Takekawa, R. J. Davis, and R. A. Flavell. 2004. GADD45beta/GADD45gamma and MEKK4 comprise a genetic pathway mediating STAT4-independent IFNgamma production in T cells. EMBO J. 23:1576-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Souza, M. S., A. L. Smith, D. S. Beck, L. J. Kim, G. M. Hansen, Jr., and S. W. Barthold. 1993. Variant responses of mice to Borrelia burgdorferi depending on the site of intradermal inoculation. Infect. Immun. 61:4493-4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glickstein, L., M. Edelstein, and J. Z. Dong. 2001. Gamma interferon is not required for arthritis resistance in the murine Lyme disease model. Infect. Immun. 69:3737-3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gross, D. M., A. C. Steere, and B. T. Huber. 1998. T helper 1 response is dominant and localized to the synovial fluid in patients with Lyme arthritis. J. Immunol. 160:1022-1028. [PubMed] [Google Scholar]
- 15.Keane-Myers, A., and S. P. Nickell. 1995. Role of IL-4 and IFN-gamma in modulation of immunity to Borrelia burgdorferi in mice. J. Immunol. 155:2020-2028. [PubMed] [Google Scholar]
- 16.Liu, N., R. R. Montgomery, S. W. Barthold, and L. K. Bockenstedt. 2004. Myeloid differentiation antigen 88 deficiency impairs pathogen clearance but does not alter inflammation in Borrelia burgdorferi-infected mice. Infect. Immun. 72:3195-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu, B., H. Yu, C. Chow, B. Li, W. Zheng, R. J. Davis, and R. A. Flavell. 2001. GADD45gamma mediates the activation of the p38 and JNK MAP kinase pathways and cytokine production in effector TH1 cells. Immunity 14:583-590. [DOI] [PubMed] [Google Scholar]
- 18.Lu, H. T., D. D. Yang, M. Wysk, E. Gatti, I. Mellman, R. J. Davis, and R. A. Flavell. 1999. Defective IL-12 production in mitogen-activated protein (MAP) kinase kinase 3 (Mkk3)-deficient mice. EMBO J. 18:1845-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matyniak, J. E., and S. L. Reiner. 1995. T helper phenotype and genetic susceptibility in experimental Lyme disease. J. Exp. Med. 181:1251-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCabe, G. J., and J. E. Bunnell. 2004. Precipitation and the occurrence of Lyme disease in the northeastern United States. Vector Borne Zoonotic Dis. 4:143-148. [DOI] [PubMed] [Google Scholar]
- 21.Morinobu, A., M. Gadina, W. Strober, R. Visconti, A. Fornace, C. Montagna, G. M. Feldman, R. Nishikomori, and J. J. O'Shea. 2002. STAT4 serine phosphorylation is critical for IL-12-induced IFN-gamma production but not for cell proliferation. Proc. Natl. Acad. Sci. USA 99:12281-12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Motameni, A. R., T. C. Bates, I. J. Juncadella, C. Petty, M. N. Hedrick, and J. Anguita. 2005. Distinct bacterial dissemination and disease outcome in mice subcutaneously infected with Borrelia burgdorferi in the midline of the back and the footpad. FEMS Immunol. Med. Microbiol. 45:279-284. [DOI] [PubMed] [Google Scholar]
- 23.Rincón, M., H. Enslen, J. Raingeaud, M. Recht, T. Zapton, M. S. Su, L. A. Penix, R. J. Davis, and R. A. Flavell. 1998. Interferon-gamma expression by Th1 effector T cells mediated by the p38 MAP kinase signaling pathway. EMBO J. 17:2817-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subak, S. 2003. Effects of climate on variability in Lyme disease incidence in the northeastern United States. Am. J. Epidemiol. 157:531-538. [DOI] [PubMed] [Google Scholar]
- 25.Sun, D., and A. Ding. 2006. MyD88-mediated stabilization of interferon-gamma-induced cytokine and chemokine mRNA. Nat. Immunol. 7:375-381. [DOI] [PubMed] [Google Scholar]
- 26.Van Solingen, R. M., and J. Evans. 2001. Lyme disease. Curr. Opin. Rheumatol. 13:293-299. [DOI] [PubMed] [Google Scholar]
- 27.Zhang, S., and M. H. Kaplan. 2000. The p38 mitogen-activated protein kinase is required for IL-12-induced IFN-gamma expression. J. Immunol. 165:1374-1380. [DOI] [PubMed] [Google Scholar]