Abstract
We have shown previously that gingipains from Porphyromonas gingivalis W83 can induce cell detachment, cell adhesion molecule (CAM) cleavage, and apoptosis in endothelial cells; however, the specific roles of the individual gingipains are unclear. Using purified gingipains, we determined that each of the gingipains can cleave CAMs to varying degrees with differing kinetics. Kgp and HRgpA work together to quickly detach endothelial cells. Interestingly, in the absence of active caspases, both gingipain-active W83 extracts and purified HRgpA and RgpB induce apoptotic morphology, suggesting that the gingipains can induce both caspase-dependent and caspase-independent apoptosis. Using z-VAD-FMK to inhibit Kgp activity and leupeptin to inhibit Rgp activity in gingipain-active W83 extracts, we investigated the relative significance of the synergistic role of the gingipains. z-VAD-FMK or leupeptin delayed, but did not inhibit, cell detachment induced by gingipain-active W83 extracts or purified gingipains. There was partial cleavage of N-cadherin and cleavage of VE-cadherin was not inhibited. Degradation of integrin β1 was inhibited only in the presence of z-VAD-FMK. These results further clarify the role P. gingivalis plays in tissue destruction occurring in the periodontal pocket.
Porphyromonas gingivalis has been implicated as a causative agent in chronic periodontitis (10, 25, 41; reviewed in reference 23), with strain W83 being very strongly associated with disease progression (22). Furthermore, there is growing evidence that P. gingivalis may be associated with other systemic illnesses, and there may be a direct relationship between P. gingivalis infection and cardiovascular disease (14; reviewed in references 20 and 38). The most notable and well-studied virulence factors of this black-pigmented anaerobe are its cysteine proteases, referred to as gingipains. The rgpA gene encodes the isoforms of the arginine-specific proteases HRgpA and RgpA(cat) and the membrane type mt-RgpA(cat), while rgpB encodes RgpB and the membrane type mt-RgpB. The lysine-specific protease Kgp is encoded by the kgp gene (11, 48). Not only do the gingipains provide a source of nutrition and heme for this asaccharolytic, gram-negative bacterium, but they also aid the bacterium in establishing and maintaining its ecological niche in the gingival pocket (35). They provide a means of attachment to other bacteria and host cells of the gingival crevice and aid in virulence by inducing degradation of extracellular matrix proteins, activation of matrix metalloproteinases, cleavage of cellular receptors, increasing vascular permeability, activation of coagulation factors, degradation of fibrinogen, and inactivation of members of the complement system (reviewed in references 27, 29, 36, 46, and 48). In the diseased periodontal pocket, there is a close proximity between the bacterial biofilm and endothelial cells, which can lead to endothelial cell damage (59). Furthermore, there is evidence for the presence of apoptotic cells in periodontitis (19, 34, 51, 57). Therefore, understanding the mechanisms of endothelial apoptosis induction in the periodontal pocket by P. gingivalis could provide new therapeutic targets of periodontitis and insights into the relationship between P. gingivalis and cardiovascular disease.
Apoptosis is a genetically programmed form of cell death that is typically driven by either an extrinsic pathway or an intrinsic pathway, ultimately activating the executioner caspases, such as caspase-3 (reviewed in reference 53). The cleavage of numerous intracellular substrates by executioner caspases produces the apoptotic morphology, characterized by cellular shrinkage, membrane blebbing, chromatin condensation and DNA fragmentation, mitochondrial membrane permeabilization, and plasma membrane changes signaling phagocytic uptake (37, 40). Apoptotic cell death can occur in the absence of active caspases (37, 39, 40). Further, inhibition of caspase activity may even reveal or enhance secondary caspase-independent cell death (37). It has been proposed that caspase-independent pathways of cell death exist to ensure that cell death will occur in cases where there may be nonfunctional members of the caspase-dependent pathway (37, 39). Caspase-independent apoptosis can be triggered by proteases, such as calpains, cathepsins, and granzymes, that cleave some of the same substrates cleaved by caspases (reviewed in references 39 and 43). While we have previously established a role for gingipains in endothelial cell caspase-dependent apoptosis (55), it is unclear whether these proteases also participate in caspase-independent processes.
Bioinformatic studies of the active site of legumain (family C13), a plant cysteine endopeptidase, revealed homology to three other families of cysteine proteases, clostripain (family C11) from Clostridium histolyticum, caspases (family C14), and gingipains (family C25), leading to the grouping of these proteases into the cysteine protease clan CD (6). They all have a conserved catalytic motif of His-Gly-spacer-Ala-Cys with a block of hydrophobic amino acids preceding each of the catalytic residues, which suggests that they may have similar enzymological properties and functions (6). These similarities have been further confirmed by the analysis of the crystal structure of RgpB. The three-dimensional structure of RgpB resembles a crooked one-root tooth, with the catalytic domain being the crown and topologically similar to caspase-1 and -3, while the root has an immunoglobulin-like fold (16). It appears that RgpB is also activated in a similar manner as the caspases (44). Moreover, natural caspase inhibitors have been found to modulate gingipain activity. For instance, p35, a baculovirus antiapoptotic protein that inhibits most known caspases, and a point mutant of cowpox viral cytokine-response modifier A (CrmA), which normally inhibits caspase-1 and -8, effectively abrogated the activity of Kgp (56). These results hint that other caspase-related proteases may also possess caspase-like activities (3).
Our previous work has shown that gingipains cause endothelial cell detachment, cell adhesion molecule cleavage, and apoptosis (55). Because the specific roles of the individual gingipains in these events are not known, the goal of this study was to determine the involvement of the different gingipains in bovine coronary artery endothelial cell (BCAEC) detachment, cell adhesion molecule (CAM) cleavage, and apoptosis. We report here that the gingipains synergistically cause cell detachment, CAM cleavage, and apoptosis. Both Kgp and HRgpA can quickly detach endothelial cells; HRgpA, Kgp, and RgpB can all cleave CAMs to varying degrees; and HRgpA and RgpB induce apoptosis in both a caspase-dependent and -independent manner. Our studies extend the relationship between caspases and gingipains by demonstrating that synthetic peptide caspase inhibitors inhibit Kgp but not Rgp activity. These results raise an important question about the functional similarities of caspases and gingipains: could gingipains have caspase-like activity in endothelial cells?
MATERIALS AND METHODS
Porphyromonas gingivalis culture conditions.
P. gingivalis strain W83 was grown as previously described (9) in brain heart infusion broth (Difco Laboratories, Detroit, MI) supplemented with 0.5% yeast extract (Difco Laboratories, Detroit, MI), hemin (5 μg/ml), vitamin K (0.5 μg/ml), and cysteine (0.1%) (all from Sigma-Aldrich, St. Louis, MO). W83 cultures were incubated at 37°C in an anaerobic chamber (Coy Manufacturing, Ann Arbor, MI) in 10% H2, 10% CO2, and 80% N2.
Gingipain extract preparation and protease assay.
Gingipain-active W83 extracts were prepared as previously described (55). Briefly, bacterial cultures were centrifuged and filtered to remove cells before acetone precipitation. This precipitate was centrifuged, and the pellet was resuspended in 150 mM NaCl (VWR Scientific, Brisbane, CA), 20 mM Bis-Tris, pH 7.4 (Sigma-Aldrich, St. Louis, MO), 5 mM CaCl2 (VWR Scientific, Brisbane, CA) and dialyzed against 4 liters of the same buffer with Aldrithiol-4 (Sigma-Aldrich, St. Louis, MO) to stabilize the gingipains. After dialysis, the sample was concentrated in a pressurized stirring concentrator (Millipore, Billerica, MA) with a 10,000-molecular-weight cutoff membrane at 4°C, clarified by centrifugation (192,000 × g, 1 h, 4°C), and stored in aliquots at −80°C.
Gingipain activity was determined using the substrate N-α-benzoyl-dl-arginine-p-nitroanilide (Sigma-Aldrich, St. Louis, MO) for Rgp activity or Ac-Lys-p-nitroanilide (Bachem, King of Prussia, PA) for Kgp activity, as previously described (55). One unit of gingipain activity is defined as the amount of enzyme releasing 1 pmol p-nitroanilide per minute as calculated based on maximum velocity and the extinction coefficient of p-nitroanilide of 9,200 at 405 nm. Gingipain activity was calculated based on the average of three measurements of the maximum velocity of the enzymatic reaction of specific substrate turnover. The concentration of gingipain activity used was within reported physiological levels of gingipain activity found in periodontal lesions (17).
Treatment of gingipain-active W83 extracts with a panel of peptide caspase inhibitors.
W83 extracts containing 2.1 or 3.3 U/μl of Rgp activity and 0.16 or 0.35 U/μl of Kgp activity were preincubated on ice for at least 30 min with a panel of peptide caspase inhibitors or Nα-p-tosyl-l-lysine chloromethyl ketone (TLCK; Sigma-Aldrich, St. Louis, MO). Rgp and Kgp activity were assayed in triplicate as described above. Percent inhibition was determined by comparison to untreated W83 extract. The standard error of the mean was calculated from three independent trials. The caspase inhibitors used are as follows (all from Enzyme Systems Products, Livermore, CA, unless otherwise indicated): z-VAD(OMe)-FMK (Biomol, Plymouth Meeting, PA) and Boc-D(OMe)-FMK, general caspase inhibitors; z-YVAD-FMK, a caspase-1 and -4 inhibitor; z-VDVAD(OMe)-FMK, a caspase-2 inhibitor; z-D(OMe)-E(OMe)-VD(OMe)-CH2F, a caspase-3 inhibitor; z-VEID-FMK, a caspase-6 inhibitor; z-IE(OMe)TD(OMe)-FMK, a caspase-8 inhibitor; and z-LE(OMe)HD(OMe)-FMK.TFA, a caspase-9 inhibitor.
Purification of gingipains from P. gingivalis strain HG66.
Comparisons of strain W83 and HG66 reveal that their Rgp is nearly identical in protein sequence (48) and is identical in substrate specificity. Kgp from these two strains is also nearly identical. Therefore, extracts were made from strain W83, and purified gingipains were isolated from strain HG66. HRgpA, Kgp, and RgpB were purified from the strain HG66 culture fluid as described previously (47, 49). Briefly, Kgp and HRgpA were purified using gel filtration and arginine-Sepharose chromatography, while RgpB was purified using a combination of gel filtration and anion-exchange chromatography on Mono Q (49). In this way, approximately 5 mg of each gingipain from 1 liter of bacterial culture was obtained in homogenous form, as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Eukaryotic cell culture and treatment with gingipains.
BCAEC were cultured according to the manufacturer's specifications, using their reagents (Cambrex, Walkersville, MD) as previously described (55). Briefly, cells from passages 6 through 10 were tested for viability prior to seeding in appropriate tissue culture dishes and allowed to reach near confluence. Culture media were removed from culture dishes and replaced with fresh media containing 5 mM l-cysteine to activate the gingipains. Gingipain-active W83 extracts or purified gingipains were added to the endothelial cells and allowed to incubate for the indicated times at 37°C in 5% CO2. To inhibit Rgp and Kgp activity, purified gingipains or gingipain-active W83 extracts were pretreated for at least 30 min on ice with 10 mM TLCK (Sigma, St. Louis, MO) and then added to the cells. To inhibit Kgp activity, the purified gingipains or gingipain-active W83 extracts were pretreated for at least 30 min on ice with 100 μM z-VAD-FMK. Leupeptin is known to inhibit Rgp activity (11); therefore, purified gingipains or gingipain-active W83 extracts were pretreated for at least 30 min on ice with 100 μM leupeptin (Axxora, LLC, San Diego, CA). Cells were also treated with 100 μM z-VAD-FMK for 1 h prior to the addition of gingipains, to inhibit caspase activity, and/or 100 μM leupeptin for at least 15 h prior to gingipain treatment. Untreated cells did not have P. gingivalis extracts or purified gingipains added to the cysteine-containing media. Numerous fields of BCAEC were monitored on an Olympus IX70 inverted microscope equipped with Hoffman modulation contrast for detachment and apoptosis, and representative fields were selected and photographed with a SPOT digital camera (Diagnostic Instruments, Inc., Sterling Heights, MI). For quantification of BCAEC detachment by gingipain-active W83 extracts in the presence and absence of inhibitors, cells were treated in triplicate as above. At the appropriate time points, the cells were gently rocked and the medium containing detached cells was transferred to a culture tube. An aliquot was then placed in a hemacytometer. Cells in the four corner grids were counted, and the average was recorded as the level of detachment. The standard error of the mean was calculated from two independent trials, and significance was determined using the two-tailed, nonpaired Student t test.
Caspase-3 (DEVDase) activity assay.
BCAEC were seeded in six-well plates and allowed to reach near confluence before the addition of 4 μM staurosporine (Str) (Alexis-Axxora, San Diego, CA) or gingipain-active W83 extracts in fresh cysteine-containing media in the absence and presence of 100 μM z-VAD-FMK. After 18 h, one-step caspase assay buffer (4) was added to the wells and allowed to incubate for 1 h at 37°C, 5% CO2. Three 150-μl aliquots from each well were placed in a black, clear-bottom 96-well plate and read at excitation and emission wavelengths of 380 and 460 nm, respectively, in a BIO-TEK Instruments FLX800 microplate fluorescence reader (Winooski, Vermont). To calculate the relative increase in DEVDase activity, the average relative fluorescence intensity of three treated wells was divided by the average relative fluorescence intensity of three untreated wells with and without z-VAD-FMK pretreatment. Standard deviation was calculated from five independent trials and significance was determined using the two-tailed, nonpaired Student t test.
Preparation of BCAEC lysates for SDS-PAGE and Western blot analysis.
Attached and detached BCAEC were washed once with Dulbecco's phosphate-buffered saline, lysed in cell lysis buffer containing complete protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN), and electrophoresed on NuPAGE 4 to 12% Bis-Tris gels (Invitrogen, Carlsbad, CA). Immunoblotting was performed as previously described (55). The primary antibodies used in these experiments were N-cadherin clone 32 and integrin β1 clone 18 (BD Biosciences, San Diego, CA), VE-cadherin clone C-19 (Santa Cruz Biotechnology, Santa Cruz, CA), poly(ADP-ribose) polymerase (PARP) clone C2-10 (R & D Systems, Minneapolis, MN), and a highly specific human autoantibody to topoisomerase I (Topo I) (generous gift from Eng M. Tan, The Scripps Research Institute, La Jolla, CA).
RESULTS
Kgp activity is inhibited by synthetic peptide caspase inhibitors.
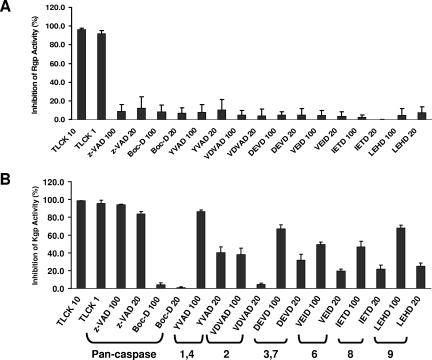
Because gingipains share similar features with caspases and Kgp is inhibited by a naturally occurring caspase-3 inhibitor (56), we investigated the effects of synthetic peptide caspase inhibitors on gingipain activity. As shown in Fig. 1A, the synthetic peptide caspase inhibitors tested had no inhibitory effect on Rgp activity. In contrast, Kgp activity was inhibited nearly 100% with z-VAD-FMK, a general caspase inhibitor, and Ac-YVAD-CMK, an inhibitor of caspase-1 and -4 (Fig. 1B). Moreover, Kgp activity was partially inhibited by all the caspase inhibitors tested except the general caspase inhibitor Boc-D-FMK.
FIG. 1.
Inhibition of Kgp activity, but not Rgp activity, after treatment with synthetic peptide caspase inhibitors. Gingipain-active W83 extracts were incubated with 10 mM or 1 mM TLCK or 100 μM or 20 μM concentrations of a panel of caspase inhibitors for at least 30 min on ice and then assayed for Rgp activity (A) or Kgp activity (B). TLCK inhibited nearly 100% of the Rgp and Kgp activity. Caspase inhibitors had no effect on Rgp activity. Caspase inhibitors z-VAD-FMK and Ac-YVAD-CMK at 100 μM inhibited Kgp activity by 90%. Other caspase inhibitors, except Boc-D, had partial inhibitory effects on Kgp activity. Error bars indicate standard errors of the means of results from three independent trials run in triplicate.
Selective inhibition of gingipain activity by z-VAD-FMK and leupeptin inhibits synergistic gingipain-induced BCAEC detachment and apoptosis.
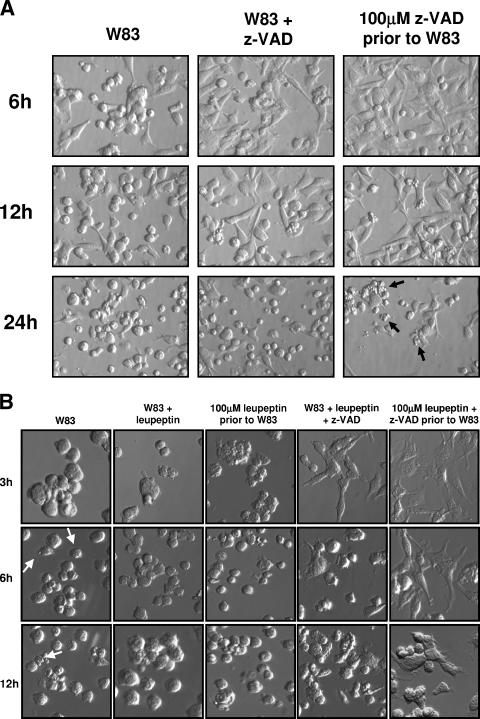
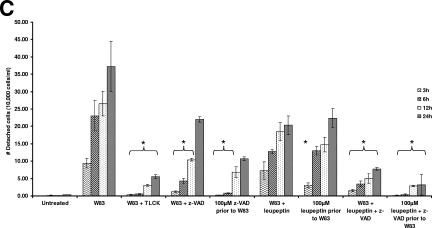
z-VAD-FMK was used to monitor the specific function of Kgp in the induction of the morphological changes seen in BCAEC after treatment with gingipain-active W83 extract. Gingipain-active W83 extracts preincubated with 100 μM z-VAD-FMK to inhibit Kgp were used to treat BCAEC (this treatment condition was designated W83 + z-VAD). Under these conditions, the final concentration of z-VAD-FMK subsequently exposed to the cells was too low (approximately 3 μM) to inhibit the caspases (1, 18, 42); thus, only Kgp should have been inhibited. A time course showed that, at early time points, cells treated with gingipain-active W83 extracts pretreated with 100 μM z-VAD-FMK displayed significantly (P ≤ 0.05 at 3 h, 6 h, and 12 h) less detachment than cells treated with gingipain-active W83 extracts (Fig. 2A and C). To assess the involvement of caspases in the morphological changes, BCAEC were pretreated with 100 μM z-VAD-FMK for 1 h and then the gingipain-active W83 extracts were added (this treatment condition was designated 100 μM z-VAD-FMK prior to W83). This treatment inhibited Kgp (Fig. 1B) and should inhibit all of the caspases (15). As shown in Fig. 2A (6 h panel) and 2C, this treatment significantly (P ≤ 0.03 at 3 h, 6 h, and 12 h) delayed cell detachment. However, by 24 h, there was more-pronounced apoptotic morphology in BCAEC pretreated with z-VAD-FMK prior to addition of the gingipain-active W83 extracts (Fig. 2A). To further ensure that all of the caspases were inhibited and Kgp was completely inhibited, a cocktail of caspase inhibitors that included z-VAD-FMK, z-YVAD-FMK, and z-DEVD-FMK was used to pretreat BCAEC. Apoptotic morphology was still observed in the presence of these inhibitors (data not shown), suggesting that Rgp activity present in the gingipain-active W83 extracts induced caspase-independent apoptosis.
FIG.2.
Gingipain-induced BCAEC detachment was inhibited in the presence of z-VAD-FMK and leupeptin. (A) BCAEC were treated, in the presence of 5 mM l-cysteine, with 200 μg/ml gingipain-active W83 extract (70 U Rgp activity/ml media and 5.3 U Kgp activity/ml media) and 100 μM z-VAD-FMK prior to exposure to gingipain-active W83 extract (100 μM z-VAD prior to W83) or gingipain-active W83 extract preincubated with 100 μM z-VAD-FMK (W83 + z-VAD). Arrows indicate cells with significant apoptotic morphology in BCAEC in the presence of 100 μM z-VAD-FMK. (B) BCAEC were treated, in the presence of 5 mM l-cysteine, with 200 μg/ml gingipain-active W83 extract (307.5 U Rgp activity/ml media and 19.4 U of Kgp activity/ml media), 100 μM z-VAD-FMK, and/or 100 μM leupeptin prior to exposure to gingipain-active W83 extracts or gingipain-active W83 extracts preincubated with 100 μM z-VAD-FMK and/or 100 μM leupeptin. Arrows indicate cells with apoptotic morphology. (C) BCAEC were treated in the presence of 5 mM l-cysteine with 119 μg/ml media gingipain-active W83 extract (113 U Rgp activity/ml media and 9 U Kgp activity/ml media) with and without 10 mM TLCK pretreatment, 100 μM z-VAD-FMK, and/or 100 μM leupeptin prior to exposure to gingipain-active W83 extracts or gingipain-active W83 extracts preincubated with 100 μM z-VAD-FMK and/or 100 μM leupeptin. Detached cells were counted in a hemacytometer at the indicated time points. Error bars indicate standard errors of the means of results from two independent trials run in triplicate in which gingipain-active W83 extracts in the presence of inhibitors were compared to gingipain-active W83 extracts alone at the same time point. *, P ≤ 0.05.
To further elucidate the individual roles of the gingipains in cell detachment and induction of apoptosis, endothelial cells and gingipain-active W83 extracts were treated with z-VAD-FMK to inhibit caspases and Kgp and leupeptin to inhibit Rgp. Similar to the experiments described above, we treated gingipain-active W83 extracts with 100 μM z-VAD-FMK and/or 100 μM leupeptin before their addition to BCAEC cells. We also preincubated BCAEC with these inhibitors before the addition of gingipain-active W83 extracts. Inhibition of Rgp activity either by treatment of gingipain-active W83 extracts or pretreatment of BCAEC with leupeptin produced only slight inhibition of detachment compared to the control (Fig. 2B). Only when BCAEC were treated with 100 μM leupeptin prior to the addition of gingipain-active W83 extracts at 3 h was there a significant decrease (P = 0.05) in detachment compared to BCAEC treated with gingipain-active W83 extracts (Fig. 2C). However, a significant inhibition (P ≤ 0.05 at all time points) in cell detachment was apparent when both Rgp and Kgp activities were inhibited with both z-VAD-FMK and leupeptin (Fig. 2B, far right panels, and Fig. 2C). Furthermore, treatment of BCAEC or gingipain-active W83 extracts with z-VAD-FMK and leupeptin produced levels of detachment that were not significantly different from those caused by treatment of gingipain-active W83 extracts with TLCK (P ≥ 0.06 at all time points for both W83 plus leupeptin plus z-VAD-FMK and 100 μM leupeptin plus z-VAD-FMK prior to W83) (Fig. 2C).
FIG. 2—
Continued.
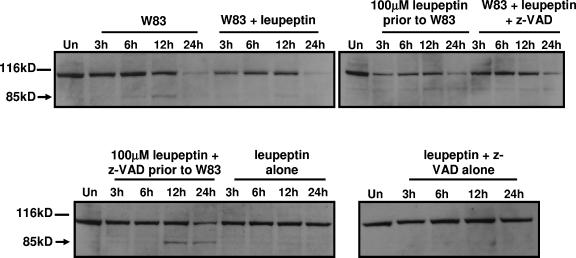
To determine the extent of caspase inhibition under these treatment conditions and to further differentiate caspase-independent apoptosis from necrosis, we monitored the cleavage of two caspase substrates. During apoptosis, caspases specifically cleave PARP and Topo I into 85-kDa and 70-kDa fragments, respectively. However, during necrosis, PARP is cleaved into a 50-kDa product and Topo I into 70-kDa and 45-kDa products (5). In cells pretreated with z-VAD-FMK to inhibit caspases (100 μM z-VAD-FMK prior to W83), the apoptotic cleavage product of PARP induced by treatment with gingipain-active W83 extracts was partially inhibited (Fig. 3) and there was no necrotic cleavage product (data not shown). Topo I cleavage into the apoptotic 70-kDa fragment was completely inhibited by pretreatment with 100 μM z-VAD-FMK prior to the addition of gingipain-active W83 extracts (Fig. 3). However, the presence of z-VAD-FMK did not inhibit the appearance of a faint 45-kDa cleavage product of Topo I. We have shown previously that cleavage of Topo I to a 45-kDa product occurs from the earliest time point of incubation with gingipain-active W83 extracts and is not indicative of necrosis based on the presence of apoptotic cell morphology, PARP cleavage into an 85-kDa fragment, and annexin V positivity (55). These results suggest that certain caspase substrates, such as PARP, may still be cleaved when Kgp and caspases are inhibited, most likely because of the presence of Rgp activity. Concomitant with a delay in cell detachment, there was also a partial inhibition of apoptotic PARP cleavage in cells pretreated with both 100 μM z-VAD-FMK and leupeptin, as suggested by the presence of both full-length PARP and its apoptotic 85-kDa fragment under these conditions at 24 h (Fig. 4). In the absence of z-VAD-FMK, leupeptin was unable to inhibit PARP cleavage (Fig. 4). Similar results were observed for apoptotic Topo I cleavage (data not shown). Collectively, it appears that the gingipains work together to detach endothelial cells and induce apoptosis.
FIG. 3.
In the presence of z-VAD-FMK, partial cleavage of caspase substrates still occurs in gingipain-induced apoptosis. Twenty micrograms of total protein from BCAEC treated for 24 h, in the presence of 5 mM l-cysteine, with gingipain-active W83 extract (70 U Rgp activity/ml media and 5.3 U Kgp activity/ml media), 100 μM z-VAD-FMK alone, 100 μM z-VAD-FMK prior to exposure to gingipain-active W83 extract (100 μM z-VAD prior to W83), or gingipain-active W83 extracts preincubated with 100 μM z-VAD-FMK (W83 + z-VAD) was separated by SDS-PAGE and immunoblotted with a monoclonal antibody to PARP and a highly specific human autoantibody to Topo I. Arrows indicate cleavage products, whereas lines indicate intact proteins. Un, untreated; +, present; −, absent.
FIG. 4.
Cleavage of PARP is partially inhibited in the presence of leupeptin and z-VAD-FMK. Twenty micrograms of total protein from BCAEC treated, in the presence of 5 mM l-cysteine, with gingipain-active W83 extract (307.5 U Rgp activity/ml media and 19.4 U Kgp activity/ml media), 100 μM z-VAD-FMK, and/or 100 μM leupeptin prior to exposure to gingipain-active W83 extracts (100 μM leupeptin prior to W83 or 100 μM leupeptin and z-VAD prior to W83) or gingipain-active W83 extracts preincubated with 100 μM z-VAD-FMK and/or 100 μM leupeptin (W83 + leupeptin or W83 + leupeptin + z-VAD) was separated by SDS-PAGE and immunoblotted with a monoclonal antibody to PARP. Arrows indicate cleavage products, whereas lines indicate intact proteins. Un, untreated.
Inhibition of Kgp activity with z-VAD-FMK and Rgp activity with leupeptin establishes a role for individual gingipains in the cleavage of specific CAMs.
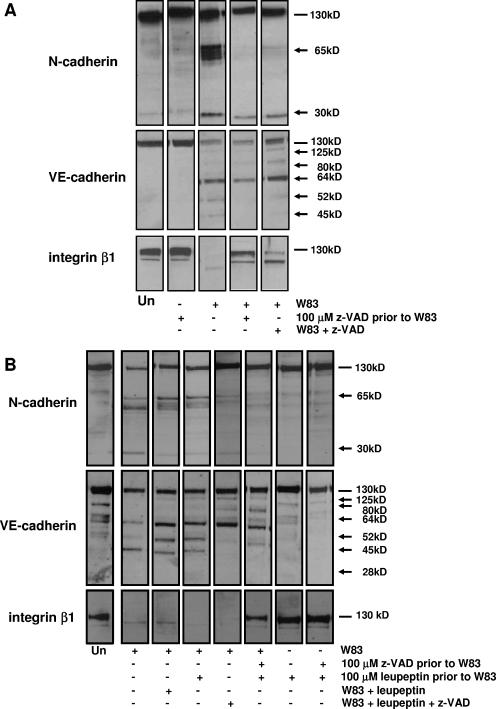
We have shown previously that treatment of BCAEC with gingipain-active W83 extracts induces cleavage of CAMs (55). We investigated the involvement of Kgp in CAM cleavage by inhibiting its activity with z-VAD-FMK. In the absence of z-VAD-FMK, N-cadherin was cleaved into products of approximately 65 kDa and 30 kDa in BCAEC treated with gingipain-active extracts. Interestingly, only a 30-kDa cleavage product was observed (Fig. 5A). VE-cadherin cleavage was not inhibited in the presence of z-VAD-FMK (Fig. 5A). BCAEC treated with gingipain-active W83 extracts also displayed complete degradation of integrin β1 (Fig. 5A). However, when Kgp was inactivated by treatment with z-VAD-FMK, degradation was inhibited (Fig. 5A). We also investigated CAM cleavage in the presence of leupeptin to further determine the specific roles of Rgp. As established by the inhibition of Kgp activity by z-VAD-FMK, treatment of BCAEC with leupeptin had little effect on the appearance of the 65-kDa cleavage products of N-cadherin but did reduce the extent of the 30-kDa cleavage product (Fig. 5B). Consistent with the studies with z-VAD-FMK (Fig. 5A), VE-cadherin cleavage was not inhibited in the presence of leupeptin (Fig. 5B). Leupeptin treatment had no effect on the degradation of integrin β1 (Fig. 5B). Only when Kgp was inhibited with 100 μM z-VAD-FMK was integrin β1 degradation inhibited (Fig. 5B).
FIG. 5.
Cleavage of CAMs is affected by z-VAD-FMK and leupeptin treatment. (A) Ten micrograms (N-cadherin and VE-cadherin) or 20 μg (integrin β1) of protein from BCAEC treated for 12 h, in the presence of 5 mM l-cysteine, with gingipain-active W83 extract (70 U Rgp activity/ml media and 5.3 U Kgp activity/ml media), 100 μM z-VAD-FMK alone, 100 μM z-VAD-FMK prior to exposure to gingipain-active W83 extract (100 μM z-VAD prior to W83), or gingipain-active W83 extracts preincubated with 100 μM z-VAD-FMK (W83 + z-VAD). (B) Twelve micrograms (N-cadherin and integrin β1) or 20 μg (VE-cadherin) of total protein from BCAEC treated for 6 h, in the presence of 5 mM l-cysteine, with gingipain-active W83 extract (307.5 U Rgp activity/ml media and 19.4 U Kgp activity/ml media), 100 μM z-VAD-FMK, and/or 100 μM leupeptin prior to exposure to gingipain-active W83 extract (100 μM leupeptin prior to W83) or gingipain-active W83 extracts preincubated with 100 μM z-VAD-FMK and/or 100 μM (W83 + leupeptin or W83 + leupeptin + z-VAD) was separated by SDS-PAGE and immunoblotted with a monoclonal antibody to N-cadherin, VE-cadherin, or integrin β1. Arrows indicate cleavage products, whereas lines indicate intact proteins. Un, untreated; +, present; −, absent.
Treatment of endothelial cells with HRgpA induces apoptotic cleavage of PARP.
Our previous work showed that gingipain-active W83 extracts and purified gingipains can cause endothelial cell detachment and apoptosis, as evidenced by the presence of apoptotic morphology and signature caspase cleavage products of PARP and Topo I (55). However, the individual roles of the gingipains in apoptosis induction in endothelial cells are not known. To confirm which purified gingipains induced apoptosis, we assayed lysates from BCAEC treated with individual gingipains for evidence of the 85-kDa PARP apoptotic cleavage product. Treatment with HRgpA induced apoptotic cleavage of PARP as early as 6 h, while treatment with RgpB induced slight PARP cleavage by 24 h (Fig. 6). Unexpectedly, treatment with Kgp induced a faint apoptotic cleavage product of PARP (Fig. 6). Similar kinetics of apoptotic cleavage were displayed in immunoblots of Topo I (data not shown).
FIG. 6.
Treatment with HRgpA induces apoptotic cleavage of PARP. Fifteen micrograms of total protein from BCAEC treated, in the presence of 5 mM l-cysteine, with 119 μg/ml media gingipain-active W83 extract (113 U Rgp activity/ml media and 9 U Kgp activity/ml media) with and without 10 mM TLCK pretreatment, purified HRgpA (8 μg/ml), Kgp (3 μg/ml), or RgpB (5.2 μg/ml) (all equivalent to 113 U of Rgp activity/ml media or 12.4 U of Kgp activity/ml media) and inhibited with 10 mM TLCK was separated by SDS-PAGE and immunoblotted with a monoclonal antibody to PARP. Arrows indicate cleavage products, whereas lines indicate intact proteins. Un, untreated.
Synergism of CAM cleavage is confirmed by treatment of BCAEC with purified gingipains.
To determine which gingipain(s) was responsible for CAM cleavage, BCAEC were treated with purified HRgpA, Kgp, and RgpB and then assayed for cleavage of CAMs. N-cadherin was cleaved to the 65-kDa cleavage products by Kgp (Fig. 7); however, a 30-kDa product was produced by both this enzyme and HRgpA. RgpB did not cleave N-cadherin. All of the gingipains appear to be involved in cleavage of VE-cadherin, with HRgpA having a predominant role in cleaving VE-cadherin and Kgp and RgpB exhibiting partial cleavage (Fig. 7). Kgp was the most efficient gingipain in degrading integrin β1 (Fig. 7).
FIG. 7.
Synergism of purified gingipains in induction of CAM cleavage. Fifteen micrograms of total protein from BCAEC treated for 6 h (N-cadherin), 24 h (VE-cadherin), or 12 h (integrin β1), in the presence of 5 mM l-cysteine, with purified HRgpA (8 μg/ml), Kgp (3 μg/ml), or RgpB (5.2 μg/ml) (all equivalent to 113 U of Rgp activity/ml media or 12.4 U of Kgp activity/ml media) were separated by SDS-PAGE and immunoblotted with antibodies to N-cadherin, VE-cadherin, and integrin β1. Arrows indicate cleavage products, whereas lines indicate intact proteins. Un, untreated; +, present.
HRgpA and RgpB induce cell detachment and apoptotic morphology in the absence of active caspases.
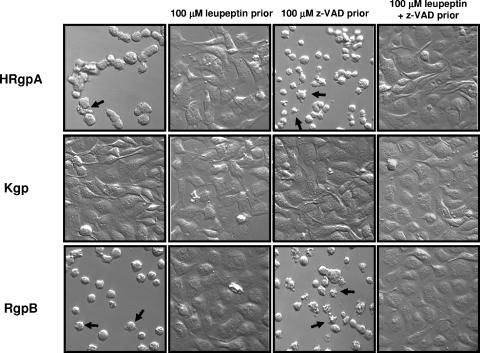
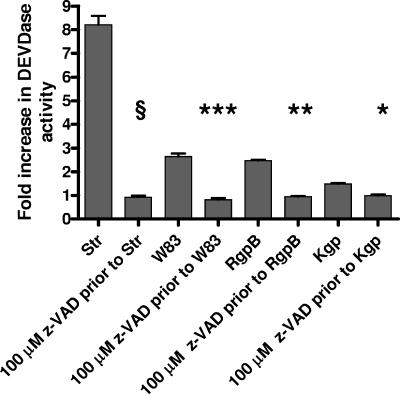
To determine if purified HRgpA and RgpB could induce apoptosis in the absence of active caspases, BCAEC were treated with 100 μM z-VAD-FMK prior to exposure to purified gingipains. HRgpA and RgpB were able to induce detachment and pronounced apoptotic morphology in the presence of z-VAD-FMK (Fig. 8). As expected, cell detachment and apoptosis induced by HRgpA and Rgp were inhibited in the presence of leupeptin (Fig. 8), and in the presence of z-VAD-FMK, Kgp did not induce initial detachment (data not shown). To confirm that z-VAD-FMK was in fact inhibiting caspase function under the different treatments, we monitored caspase-3 activity. Cells were pretreated with 100 μM z-VAD-FMK and then treated with 4 μM Str, which activates caspase-3. Pretreatment of BCAEC with z-VAD-FMK significantly inhibited Str-induced caspase-3 activation (P ≤ 0.0001) (Fig. 9). Furthermore, significant inhibition of caspase-3 activity was achieved upon BCAEC pretreatment with 100 μM z-VAD-FMK prior to the addition of W83 extract (P < 0.0003) and RgpB (P ≤ 0.002). Treatment with Kgp, which does not induce apoptosis, caused a slight increase in caspase-3 activity that could also be inhibited by z-VAD-FMK pretreatment (P < 0.03; data not shown), which may explain the slight induction of the 85-kDa apoptotic cleavage product of PARP (Fig. 6). These results strongly suggest that gingipains have the ability to trigger both caspase-dependent and caspase-independent apoptosis.
FIG. 8.
HRgpA and RgpB induce cell detachment and apoptotic morphology in the presence of 100 μM z-VAD-FMK. BCAEC were treated with purified HRgpA (40.5 μg/ml media), Kgp (9 μg/ml media), or RgpB (10 μg/ml media) (all equivalent to 113 U of Rgp activity/ml media or 6.2 U of Kgp activity/ml media) in the presence of 5 mM l-cysteine for 24 h. BCAEC were pretreated for at least 15 h with 100 μM leupeptin and/or 100 μM z-VAD-FMK for 1 h before the addition of individual gingipains. Arrows indicate cells with significant apoptotic morphology in BCAEC with and without active caspases.
FIG. 9.
Lack of DEVDase activity in BCAEC treated with gingipain-active W83 extract or individual gingipains in the presence of 100 μM z-VAD-FMK. BCAEC were treated for 18 h with 4 μM Str, 119 μg/ml media gingipain-active W83 extract (113 U Rgp activity/ml media and 9 U Kgp activity/ml media), and 10 μg/ml RgpB (113 U activity/ml media) with and without 1 h of pretreatment with 100 μM z-VAD-FMK to inhibit caspase activity and then assayed for cleavage of the fluorescent caspase-3 and -7 substrate Ac-DEVD-AMC. Error bars indicate standard deviations of the means of results from five independent trials. §, P ≤ 0.0001; ***, P < 0.0003; **, P < 0.002; *, P < 0.03.
DISCUSSION
This study has provided insights into the individual roles of the gingipains in endothelial cell detachment, CAM cleavage, and induction of apoptosis. We have shown through selective inhibition of gingipain activity and treatment of endothelial cells with purified gingipains that all of the gingipains cause cell detachment, with cells treated with Kgp reattaching after the initial detachment (55), and that HRgpA and RgpB can induce both caspase-dependent and -independent apoptosis. In addition, we demonstrate that all the gingipains participate in CAM cleavage. However, we cannot rule out the possibility that other factors in the gingipain-active W83 extracts may contribute to the BCAEC detachment and death. This alternative is currently being investigated in our laboratory. Our results further suggest that the gingipains can differentially interact and proteolyse specific CAMs. All three gingipains cleaved VE-cadherin and integrin β1. Both Kgp and HRgpA cleaved N-cadherin, but only Kgp cleaved N-cadherin to generate a group of fragments of approximately 65 kDa and a fragment of 30 kDa. In contrast, HRgpA cleaved N-cadherin to generate a 30-kDa fragment that was missing in the presence of RgpB.
The ability to bind and interact with N-cadherin could be a function of the adhesin domains of HRgpA and Kgp. These adhesin domains can modulate bacterial adherence (7, 8). Moreover, both HRgpA and Kgp can quickly cause cadherin cleavage. This would be consistent with the report that HRgpA was more effective than RgpA(cat) in inducing the loss of integrin β1 expression on human gingival fibroblasts (54). It is also possible that RgpB is inefficient in cleaving N-cadherin. Endothelial cells treated with gingipain-active W83 extracts displayed cleaved VE-cadherin prior to N-cadherin (55), indicating that VE-cadherin, in contrast to N-cadherin, is more readily cleaved by the gingipains. Since RgpB does cleave VE-cadherin, but significantly later than HRgpA (data not shown), it is possible that a longer incubation time may be required for N-cadherin to be cleaved by RgpB.
Our previous findings show that BCAEC treated with Kgp initially detach but, over time, reattach with little loss of cell viability (55). The inability of leupeptin to significantly affect gingipain-active W83 extract-induced detachment demonstrates that Kgp activity has a considerable role in detaching BCAEC in our experimental system. Based on our studies, Kgp appears to play a prominent role in BCAEC detachment and CAM cleavage, but under the conditions used for this treatment, it appears that BCAEC are able to counter the Kgp-induced effects in some way to maintain cell viability. This sizeable role for Kgp, however, is in contrast to previous reports that have suggested that Rgp had a greater effect than Kgp in inducing loss of cell adhesion and viability in human gingival fibroblasts or human umbilical vein endothelial cells (21, 33). The different responses previously observed most likely are related to differences in the eukaryotic cell lines used and, possibly, the strains of P. gingivalis used. In a previous report (55), we speculated that purified Kgp could have lost activity during the time course, allowing the cells to reattach. However, it appears that Kgp does remain active throughout the time course, since integrin β1 was degraded to completion throughout a 24-h time course (data not shown) and both N-cadherin and VE-cadherin showed Kgp-induced cleavages over 24 h (data not shown). Interestingly, during treatment of BCAEC with Kgp, it appears that full-length VE-cadherin may be up-regulated over the 24-h time course (data not shown), consistent with recent reports that cell adhesion expression can be modulated by P. gingivalis (26, 45, 58). VE-cadherin is essential for endothelial cell survival, acts as a seal at intercellular junctions, associating with β-catenin, plakoglobin, p120, and the actin cytoskeleton (13), and is a target of agents that increase vascular permeability. Inhibition of its function produced more damage to the endothelial monolayer in vivo than in vitro (2). It has been demonstrated that P. gingivalis can cause cleavage of p120, which can be inhibited by a caspase inhibitor (24) and, based on our findings, implicates Kgp involvement. Because p120 regulates VE-cadherin expression (30), it is tempting to speculate that the loss of function of p120 and VE-cadherin may contribute to the induction of apoptosis in endothelial cells. Therefore, it may be possible that BCAEC can up-regulate p120 and VE-cadherin expression to overcome the effects of Kgp treatment.
Based on our cumulative results, both Rgp and Kgp seem to be involved in producing cell detachment and CAM cleavage in BCAEC. There are several examples of the cooperative action or overlapping functions of the gingipains. Numerous reports that used selective Rgp and/or Kgp inhibitors uncovered the similar synergistic actions of Rgp and Kgp. Collagen degradation, cytokine degradation, enhancement of vascular permeability, and bacterial coaggregation were inhibited only in the presence of both Rgp and Kgp inhibitors (reviewed in reference 33). The synergistic actions of Rgp and Kgp are most clearly understood in the clotting system. Rgp is considered the major vascular permeability enhancement-inducing factor through activation of plasma prekallikrein, ultimately releasing bradykinin and enhancing vascular permeability. However, both Rgp and Kgp can release bradykinin from high-molecular-weight kininogen, also contributing to vascular permeability and production of gingival crevicular fluid in periodontitis (28; reviewed in reference 27). Therefore, in the periodontal pocket where all the gingipains are present, it is possible that there is cooperative action of the gingipains to sustain infection, create the pathology of periodontitis, cleave CAMs, and induce apoptosis.
The ability of peptide caspase inhibitors to inhibit Kgp activity is not surprising, but until this report, it had not been previously demonstrated. It has been shown that z-VAD-FMK and DEVD-FMK can inhibit many of the cathepsins, papain, and legumain, another clan CD protease (50, 52). Since RgpB is structurally similar to caspases (16), it could be expected that peptide caspase inhibitors would affect Rgp activity. Hintermann and colleagues implied this after determining that caspase inhibitor I, which inhibits caspase-3, prevented the P. gingivalis-induced cleavage of p120, paxillin, and FAK, while caspase inhibitor III, which is the general caspase inhibitor Boc-Asp(OMe)-CH2F, had no effect (24). However, our work with peptide caspase inhibitors would imply that Kgp activity, not Rgp activity, is most likely responsible for these cleavages. Our inhibition results are consistent with the report that Kgp activity could be inhibited by the natural caspase inhibitors p35 and a point mutant of CrmA (56). Because the gingipains are structurally similar to each other and can be assumed to all have similarity to the caspases (27), it will be interesting to determine the structural nature of selective Kgp inhibition by peptide caspase inhibitors.
Overlapping modes of cell death induced by the same stimulus appears to be a common phenomenon (31). It is becoming evident that there is a continuum between apoptosis and necrosis and that cells can die by alternative pathways with all or only some of the typical characteristics of apoptosis in caspase-independent apoptosis (32). Gingipains induce classic apoptosis with the characteristic morphology, caspase activation, phosphatidylserine exposure, and cleavage of the caspase substrates PARP and Topo I (55). However, to our knowledge, this is the first demonstration that gingipains can also induce caspase-3-independent apoptosis. The inability of z-VAD-FMK to completely inhibit PARP cleavage was also observed during etoposide-induced apoptosis, in the absence of DEVDase activity (12). Since we only assayed for caspase-3 and -7 activities, we cannot rule out the possibility of other caspases being active and mediating gingipain-induced apoptosis. However, this seems unlikely because z-VAD-FMK is a broad caspase inhibitor, especially when used at 100 μM, which was recently demonstrated to bind and inactivate all of the intracellular caspases (15). Interestingly, that study also reported more-pronounced apoptotic blebbing in the presence of z-VAD-FMK, with nuclear fragmentation as the only apoptotic feature that was inhibited by z-VAD-FMK (15). It should be noted that serine proteases were implicated in that caspase-independent apoptosis. Several classes of proteases, such as calpains, cathepsins, and the granzyme serine proteases, have also been found to induce caspase-independent apoptosis (reviewed in reference 43). It appears that the gingipains can be added to this list. Current efforts in the laboratory are aimed at elucidating the pathways involved in gingipain-induced caspase-dependent and -independent apoptosis in endothelial cells.
Acknowledgments
This work was supported by grant 3 PO4A 003 24 from the Committee of Scientific Research (KBN, Poland) (to J.P.), National Institutes of Health grant DE09761 (to J.T.), a Loma Linda University School of Dentistry, Loma Linda University School of Medicine Basic Research Support Grant, Public Health Service grants DE13664 and DE13664-S1 from the National Institute of Dental and Craniofacial Research (to H.M.F.), and National Institutes of Health grant A144088 (to C.A.C.).
Editor: V. J. DiRita
REFERENCES
- 1.Bannerman, D., M. Sathyamoorthy, and S. E. Goldblum. 1998. Bacterial lipopolysaccharide disrupts endothelial monolayer integrity and survival signaling events through caspase cleavage of adherens junction proteins. J. Biol. Chem. 273:35371-35380. [DOI] [PubMed] [Google Scholar]
- 2.Bazzoni, G., and E. Dejana. 2001. Pores in the sieve and channels in the wall: control of paracellular permeability by junctional proteins in endothelial cells. Microcirculation 8:143-152. [DOI] [PubMed] [Google Scholar]
- 3.Boyce, M., A. Degterev, and J. Yuan. 2004. Caspases: an ancient cellular sword of Damocles. Cell Death Differ. 11:29-37. [DOI] [PubMed] [Google Scholar]
- 4.Carrasco, R. A., N. B. Stamm, and B. K. Patel. 2003. One-step cellular caspase-3/7 assay. BioTechniques 34:1064-1067. [DOI] [PubMed] [Google Scholar]
- 5.Casiano, C. A., R. L. Ochs, and E. M. Tan. 1998. Distinct cleavage products of nuclear proteins in apoptosis and necrosis revealed by autoantibody probes. Cell Death Differ. 5:183-190. [DOI] [PubMed] [Google Scholar]
- 6.Chen, J. M., N. D. Rawlings, R. A. Stevens, and A. J. Barrett. 1998. Identification of the active site of legumain links it to caspases, clostripain and gingipains in a new clan of cysteine endopeptidases. FEBS Lett. 441:361-365. [DOI] [PubMed] [Google Scholar]
- 7.Chen, T., and M. J. Duncan. 2004. Gingipain adhesin domains mediate Porphyromonas gingivalis adherence to epithelial cells. Microb. Pathog. 36:205-209. [DOI] [PubMed] [Google Scholar]
- 8.Chen, T., K. Nakayama, L. Belliveau, and M. J. Duncan. 2001. Porphyromonas gingivalis gingipains and adhesion to epithelial cells. Infect. Immun. 69:3048-3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, Z., C. A. Casiano, and H. M. Fletcher. 2001. Protease-active extracellular protein preparations from Porphyromonas gingivalis W83 induce N-cadherin proteolysis, loss of cell adhesion, and apoptosis in human epithelial cells. J. Periodontol. 72:641-650. [DOI] [PubMed] [Google Scholar]
- 10.Christersson, L. A., J. J. Zambon, R. G. Dunford, S. G. Grossi, and R. J. Genco. 1989. Specific subgingival bacteria and diagnosis of gingivitis and periodontitis. J. Dent. Res. 68:1633-1639. [Google Scholar]
- 11.Curtis, M. A., J. Aduse-Opoku, and M. Rangarajan. 2001. Cysteine proteases of Porphyromonas gingivalis. Crit. Rev. Oral Biol. Med. 12:192-216. [DOI] [PubMed] [Google Scholar]
- 12.de Bruin, E. C., D. Meersma, J. de Wilde, I. den Otter, E. M. Schipper, J. P. Medema, and L. T. Peltenburg. 2003. A serine protease is involved in the initiation of DNA damage-induced apoptosis. Cell Death Differ. 10:1204-1212. [DOI] [PubMed] [Google Scholar]
- 13.Dejana, E., G. Bazzoni, and M. G. Lampugnani. 1999. Vascular endothelial (VE)-cadherin: only an intercellular glue? Exp. Cell Res. 252:13-19. [DOI] [PubMed] [Google Scholar]
- 14.Desvarieux, M., R. T. Demmer, T. Rundek, B. Boden-Albala, D. R. Jacobs, Jr., R. L. Sacco, and P. N. Papapanou. 2005. Periodontal microbiota and carotid intima-media thickness: the Oral Infections and Vascular Disease Epidemiology Study (INVEST). Circulation 111:576-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egger, L., J. Schneider, C. Rheme, M. Tapernoux, J. Hacki, and C. Borner. 2003. Serine proteases mediate apoptosis-like cell death and phagocytosis under caspase-inhibiting conditions. Cell Death Differ. 10:1188-1203. [DOI] [PubMed] [Google Scholar]
- 16.Eichinger, A., H. G. Beisel, U. Jacob, R. Huber, F. J. Medrano, A. Banbula, J. Potempa, J. Travis, and W. Bode. 1999. Crystal structure of gingipain R: an Arg-specific bacterial cysteine proteinase with a caspase-like fold. EMBO J. 18:5453-5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eley, B. M., and S. W. Cox. 1996. Correlation between gingivain/gingipain and bacterial dipeptidyl peptidase activity in gingival crevicular fluid and periodontal attachment loss in chronic periodontitis patients. A 2-year longitudinal study. J. Periodontol. 67:703-716. [DOI] [PubMed] [Google Scholar]
- 18.Gajdusek, C., K. Onoda, S. London, M. Johnson, R. Morrison, and M. Mayberg. 2001. Early molecular changes in irradiated aortic endothelium. J. Cell. Physiol. 188:8-23. [DOI] [PubMed] [Google Scholar]
- 19.Gamonal, J., A. Bascones, A. Acevedo, E. Blanco, and A. Silva. 2001. Apoptosis in chronic adult periodontitis analyzed by in situ DNA breaks, electron microscopy, and immunohistochemistry. J. Periodontol. 72:517-525. [DOI] [PubMed] [Google Scholar]
- 20.Gendron, R., D. Grenier, and L. Maheu-Robert. 2000. The oral cavity as a reservoir of bacterial pathogens for focal infections. Microbes Infect. 2:897-906. [DOI] [PubMed] [Google Scholar]
- 21.Grenier, D., S. Roy, F. Chandad, P. Plamondon, M. Yoshioka, K. Nakayama, and D. Mayrand. 2003. Effect of inactivation of the Arg- and/or Lys-gingipain gene on selected virulence and physiological properties of Porphyromonas gingivalis. Infect. Immun. 71:4742-4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffen, A. L., S. R. Lyons, M. R. Becker, M. L. Moeschberger, and E. J. Leys. 1999. Porphyromonas gingivalis strain variability and periodontitis. J. Clin. Microbiol. 37:4028-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haffajee, A. D., and S. S. Socransky. 1994. Microbial etiological agents of destructive periodontal diseases. Periodontol. 2000 5:78-111. [DOI] [PubMed] [Google Scholar]
- 24.Hintermann, E., S. K. Haake, U. Christen, A. Sharabi, and V. Quaranta. 2002. Discrete proteolysis of focal contact and adherens junction components in Porphyromonas gingivalis-infected oral keratinocytes: a strategy for cell adhesion and migration disabling. Infect. Immun. 70:5846-5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holt, S. C., J. Ebersole, J. Felton, M. Brunsvold, and K. S. Kornman. 1988. Implantation of Bacteroides gingivalis in nonhuman primates initiates progression of periodontitis. Science 239:55-57. [DOI] [PubMed] [Google Scholar]
- 26.Huang, G. T., D. Kim, J. K. Lee, H. K. Kuramitsu, and S. K. Haake. 2001. Interleukin-8 and intercellular adhesion molecule 1 regulation in oral epithelial cells by selected periodontal bacteria: multiple effects of Porphyromonas gingivalis via antagonistic mechanisms. Infect. Immun. 69:1364-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imamura, T. 2003. The role of gingipains in the pathogenesis of periodontal disease. J. Periodontol. 74:111-118. [DOI] [PubMed] [Google Scholar]
- 28.Imamura, T., J. Potempa, R. N. Pike, and J. Travis. 1995. Dependence of vascular permeability enhancement on cysteine proteinases in vesicles of Porphyromonas gingivalis. Infect. Immun. 63:1999-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imamura, T., J. Travis, and J. Potempa. 2003. The biphasic virulence activities of gingipains: activation and inactivation of host proteins. Curr. Protein Pept. Sci. 4:443-450. [DOI] [PubMed] [Google Scholar]
- 30.Iyer, S., D. M. Ferreri, N. C. DeCocco, F. L. Minnear, and P. A. Vincent. 2004. VE-cadherin-p120 interaction is required for maintenance of endothelial barrier function. Am. J. Physiol. Lung Cell. Mol. Physiol. 286:L1143-L1153. [DOI] [PubMed] [Google Scholar]
- 31.Jaattela, M. 2002. Programmed cell death: many ways for cells to die decently. Ann. Med. 34:480-488. [DOI] [PubMed] [Google Scholar]
- 32.Jaattela, M. 2004. Multiple cell death pathways as regulators of tumour initiation and progression. Oncogene 23:2746-2756. [DOI] [PubMed] [Google Scholar]
- 33.Kadowaki, T., and K. Yamamoto. 2003. Suppression of virulence of Porphyromonas gingivalis by potent inhibitors specific for gingipains. Curr. Protein Pept. Sci. 4:451-458. [DOI] [PubMed] [Google Scholar]
- 34.Koulouri, O., D. F. Lappin, M. Radvar, and D. F. Kinane. 1999. Cell division, synthetic capacity and apoptosis in periodontal lesions analysed by in situ hybridisation and immunohistochemistry. J. Clin. Periodontol. 26:552-559. [DOI] [PubMed] [Google Scholar]
- 35.Lamont, R. J., and H. F. Jenkinson. 2000. Subgingival colonization by Porphyromonas gingivalis. Oral Microbiol. Immunol. 15:341-349. [DOI] [PubMed] [Google Scholar]
- 36.Lamont, R. J., and H. F. Jenkinson. 1998. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol. Mol. Biol. Rev. 62:1244-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leist, M., and M. Jaattela. 2001. Four deaths and a funeral: from caspases to alternative mechanisms. Nat. Rev. Mol. Cell Biol. 2:589-598. [DOI] [PubMed] [Google Scholar]
- 38.Li, X., K. M. Kolltveit, L. Tronstad, and I. Olsen. 2000. Systemic diseases caused by oral infection. Clin. Microbiol. Rev. 13:547-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lockshin, R. A., and Z. Zakeri. 2004. Caspase-independent cell death? Oncogene 23:2766-2773. [DOI] [PubMed] [Google Scholar]
- 40.Lorenzo, H. K., and S. A. Susin. 2004. Mitochondrial effectors in caspase-independent cell death. FEBS Lett. 557:14-20. [DOI] [PubMed] [Google Scholar]
- 41.Machtei, E. E., R. Dunford, E. Hausmann, S. G. Grossi, J. Powell, D. Cummins, J. J. Zambon, and R. J. Genco. 1997. Longitudinal study of prognostic factors in established periodontitis patients. J. Clin. Periodontol. 24:102-109. [DOI] [PubMed] [Google Scholar]
- 42.Madge, L. A., J. H. Li, J. Choi, and J. S. Pober. 2003. Inhibition of phosphatidylinositol 3-kinase sensitizes vascular endothelial cells to cytokine-initiated cathepsin-dependent apoptosis. J. Biol. Chem. 278:21295-21306. [DOI] [PubMed] [Google Scholar]
- 43.Mathiasen, I. S., and M. Jaattela. 2002. Triggering caspase-independent cell death to combat cancer. Trends Mol. Med. 8:212-220. [DOI] [PubMed] [Google Scholar]
- 44.Mikolajczyk, J., K. M. Boatright, H. R. Stennicke, T. Nazif, J. Potempa, M. Bogyo, and G. S. Salvesen. 2003. Sequential autolytic processing activates the zymogen of Arg-gingipain. J. Biol. Chem. 278:10458-10464. [DOI] [PubMed] [Google Scholar]
- 45.Nassar, H., H. H. Chou, M. Khlgatian, F. C. Gibson III, T. E. Van Dyke, and C. A. Genco. 2002. Role for fimbriae and lysine-specific cysteine proteinase gingipain K in expression of interleukin-8 and monocyte chemoattractant protein in Porphyromonas gingivalis-infected endothelial cells. Infect. Immun. 70:268-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O'Brien-Simpson, N. M., P. D. Veith, S. G. Dashper, and E. C. Reynolds. 2003. Porphyromonas gingivalis gingipains: the molecular teeth of a microbial vampire. Curr. Protein Pept. Sci. 4:409-426. [DOI] [PubMed] [Google Scholar]
- 47.Pike, R., W. McGraw, J. Potempa, and J. Travis. 1994. Lysine- and arginine-specific proteinases from Porphyromonas gingivalis. Isolation, characterization, and evidence for the existence of complexes with hemagglutinins. J. Biol. Chem. 269:406-411. [PubMed] [Google Scholar]
- 48.Potempa, J., A. Banbula, and J. Travis. 2000. Role of bacterial proteinases in matrix destruction and modulation of host responses. Periodontol. 2000 24:153-192. [DOI] [PubMed] [Google Scholar]
- 49.Potempa, J., J. Mikolajczyk-Pawlinska, D. Brassell, D. Nelson, I. B. Thogersen, J. J. Enghild, and J. Travis. 1998. Comparative properties of two cysteine proteinases (gingipains R), the products of two related but individual genes of Porphyromonas gingivalis. J. Biol. Chem. 273:21648-21657. [DOI] [PubMed] [Google Scholar]
- 50.Rozman-Pungercar, J., N. Kopitar-Jerala, M. Bogyo, D. Turk, O. Vasiljeva, I. Stefe, P. Vandenabeele, D. Bromme, V. Puizdar, M. Fonovic, M. Trstenjak-Prebanda, I. Dolenc, V. Turk, and B. Turk. 2003. Inhibition of papain-like cysteine proteases and legumain by caspase-specific inhibitors: when reaction mechanism is more important than specificity. Cell Death Differ. 10:881-888. [DOI] [PubMed] [Google Scholar]
- 51.Sawa, T., F. Nishimura, H. Ohyama, K. Takahashi, S. Takashiba, and Y. Murayama. 1999. In vitro induction of activation-induced cell death in lymphocytes from chronic periodontal lesions by exogenous Fas ligand. Infect. Immun. 67:1450-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schotte, P., W. Declercq, S. Van Huffel, P. Vandenabeele, and R. Beyaert. 1999. Non-specific effects of methyl ketone peptide inhibitors of caspases. FEBS Lett. 442:117-121. [DOI] [PubMed] [Google Scholar]
- 53.Schultz, D. R., and W. J. Harrington, Jr. 2003. Apoptosis: programmed cell death at a molecular level. Semin. Arthritis Rheum. 32:345-369. [DOI] [PubMed] [Google Scholar]
- 54.Scragg, M. A., S. J. Cannon, M. Rangarajan, D. M. Williams, and M. A. Curtis. 1999. Targeted disruption of fibronectin-integrin interactions in human gingival fibroblasts by the RI protease of Porphyromonas gingivalis W50. Infect. Immun. 67:1837-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sheets, S. M., J. Potempa, J. Travis, C. A. Casiano, and H. M. Fletcher. 2005. Gingipains from Porphyromonas gingivalis W83 induce cell adhesion molecule cleavage and apoptosis in endothelial cells. Infect. Immun. 73:1543-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Snipas, S. J., H. R. Stennicke, S. Riedl, J. Potempa, J. Travis, A. J. Barrett, and G. S. Salvesen. 2001. Inhibition of distant caspase homologues by natural caspase inhibitors. Biochem. J. 357:575-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tonetti, M. S., D. Cortellini, and N. P. Lang. 1998. In situ detection of apoptosis at sites of chronic bacterially induced inflammation in human gingiva. Infect. Immun. 66:5190-5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walter, C., J. Zahlten, B. Schmeck, C. Schaudinn, S. Hippenstiel, E. Frisch, A. C. Hocke, N. Pischon, H. K. Kuramitsu, J. P. Bernimoulin, N. Suttorp, and M. Krull. 2004. Porphyromonas gingivalis strain-dependent activation of human endothelial cells. Infect. Immun. 72:5910-5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zoellner, H., C. C. Chapple, and N. Hunter. 2002. Microvasculature in gingivitis and chronic periodontitis: disruption of vascular networks with protracted inflammation. Microsc. Res. Tech. 56:15-31. [DOI] [PubMed] [Google Scholar]