Abstract
The most aggressive form of anthrax results from inhalation of airborne spores of Bacillus anthracis and usually progresses unnoticed in the early stages because of unspecific symptoms. The only reliable marker of anthrax is development of bacteremia, which increases with disease progress. Rapid diagnosis of anthrax is imperative for efficient treatment and cure. Herein we demonstrate that the presence and level of a bacterial antigen, the protective antigen (PA), a component of B. anthracis toxins, in host sera can serve as a reliable marker of infection. This was tested in two animal models of inhalation anthrax, rabbits and guinea pigs infected by intranasal instillation of Vollum spores. In both models, we demonstrated qualitative and quantitative correlations between levels of bacteremia and PA concentrations in the sera of sick animals. The average time to death in infected animals was about 16 h after the appearance of bacteremia, leaving a small therapeutic window. As the time required for immunodetection of PA can be very short, the use of this marker will be beneficial for faster diagnosis and treatment of inhalation anthrax.
Anthrax is primarily a disease of herbivorous animals caused by Bacillus anthracis, a gram-positive, nonmotile, spore-forming rod (15). In humans, three types of anthrax have been recorded according to the route of infection: cutaneous, gastrointestinal, and inhalation (11). Inhalation anthrax is a rare disease associated with either industrial exposure to spores (7, 19) or bioterrorism (18). In humans, inhalation anthrax begins with a nonspecific influenza-like illness characterized by fever, myalgia, headache, a nonproductive cough, and mild chest discomfort. A second phase is marked by high fever, dyspnea, stridor, cyanosis, and shock. Blood smears in the later stages of illness may contain the characteristically gram-positive spore-forming bacilli (8, 28). Death is universal in untreated cases (7, 23). This form of disease is difficult to diagnose because of the lack of a specific marker and is virtually always fatal, even with vigorous antibacterial therapy (6, 18). Animal models used to study the progression of the disease showed a single-phase progression and no specific host physiological markers (1, 5, 20, 30). Appearance of B. anthracis in the sera of exposed animals is an unambiguous marker of infection (27).
The need for a reliable marker for the diagnosis of anthrax was exemplified during the bioterrorism-related anthrax outbreak in the United States (October and November 2001). Although 22 persons were reported to be infected by B. anthracis and of those, 10 patients were critically ill with confirmed inhalation anthrax (17, 18), thousands of people were treated just for the possibility of being exposed (9).
The virulence of B. anthracis is attributed to the anthrax tripartite toxin complex and the capsule (10, 25). The exotoxins are composed of three proteins, protective antigen (PA), lethal factor, and edema factor. Toxic activity is expressed only when PA is combined with lethal factor (forming the lethal toxin) or edema factor (forming the edema toxin). Lethal toxin, shown in vitro to cause lysis of macrophages and macrophage-like cell lines (14), plays a role in every step of disease progression, from the release of bacilli from the macrophages to host death (12, 21, 24, 29). A clear correlation between bacteremia and the PA concentration at the time of death was shown long ago (13). Consistent with the central role of PA in anthrax pathogenesis, we tested the possibility that the appearance and concentration of PA in the sera of exposed animals can serve as a reliable marker for the development and progression of anthrax.
MATERIALS AND METHODS
B. anthracis strain.
The strain used in this study was B. anthracis Vollum ATCC 14578 (Tox+ Cap+) from the Israel Institute for Biological Research collection (22).
Animals.
New Zealand White rabbits (2.5 to 3.5 kg) were obtained from Harlan (Israel). Hartley guinea pigs (300 to 400 g) were obtained from Charles River, Germany. The animals received food and water ad libitum. All animals were cared for according to the 1997 NIH guidelines for the care and use of laboratory animals; the Israel Institute for Biological Research animal use committee approved all experimental protocols. The animals were inoculated by intranasal instillation. The estimated 50% lethal doses (LD50) for rabbits and guinea pigs are 3 × 105 and 4 × 104 CFU, respectively (2, 31).
Determination of bacteremia.
Blood samples were taken from infected animals at 6 to 8 h intervals after exposure into sodium citrate tubes for bacteremia and into BD Vacutainer tubes for serum. Determination of bacteremia was done as described previously (2). Briefly, each blood sample was plated undiluted or after serial dilutions in saline. The lower limit of detection was 2 CFU/ml blood.
Determination of PA by ELISA.
PA levels were determined by direct enzyme-linked immunosorbent assay (ELISA) in 96-well microtiter plates (Nunc, Roskilde, Denmark) with purified PA (26) as the reference standard. Plates were coated with 100 μg/ml diluted serum anti-PA (rabbit serum for determination of PA in rabbit blood or guinea pig serum for determination in guinea pig blood) in NaHCO3 buffer (50 mM, pH 9.6) and subsequently blocked with 5% skim milk (Becton Dickinson, Sparks, MD). The plates were washed with phosphate-buffered saline containing 0.05% Tween 20 (PBST; pH 7.4) and incubated with the test sera (diluted 1:2 in 0.5% skim milk) for 1 h at 37°C. For the standard curve, known concentrations of purified PA in 50% serum were used. The plates were washed with PBST and incubated with diluted anti-PA serum (rabbit serum for determination of PA in guinea pig blood or guinea pig serum for determination in rabbits blood). The plates were washed with PBST and developed with alkaline phosphatase-conjugated goat anti-rabbit or anti-guinea pig immunoglobulin G (Sigma, St. Louis, MO) as the detection reagent with p-nitrophenylphosphate (Sigma, St. Louis, MO) as the substrate. Absorbance at 405 nm was determined with a Spectramax 190 microplate reader (Molecular Devices, Sunnyvale, CA). The end point was defined as the highest dilution at which the absorbance was >3 standard deviations above that of the negative control. According to the standard curve, the lower limit of PA detection by this assay was determined to be 10 ng/ml.
Determination of PA by electrochemiluminescent immunoassay (ECLI).
The BioVeris detection technology (BioVeris Corporation, Gaithersburg, MD) was adapted for PA determination. Rabbit anti-PA antibodies were purified from hyperimmune serum with Aminolink columns according to the manufacturer's (Pierce, Rockford, IL) protocol. Rabbit anti-PA antibodies were then labeled with biotin and bound to paramagnetic beads. The same antibodies were also labeled with ruthenium and used as a detection tag in the assay. The homogeneous assay was started by addition of 75 μl of the test serum or standard PA (in naive rabbit serum) to the reaction mixture (containing paramagnetic beads and ruthenium-labeled antibodies). The plates were incubated for 30 min at room temperature. The results were measured with an M8/384 electrochemiluminescence reader (BioVeris Corporation, Gaithersburg, MD). According to the PA standard curve, the lower limit of PA detection by this assay was determined to be 1 ng/ml.
Statistical analysis.
The correlation between serum PA concentrations and bacteremia levels was tested by linear regression. The correlation between bacteremia appearance and infective dose was analyzed by analysis of variance. All tests were performed with GraphPad Prism version 4.03 for Windows (GraphPad Software, San Diego, CA).
RESULTS
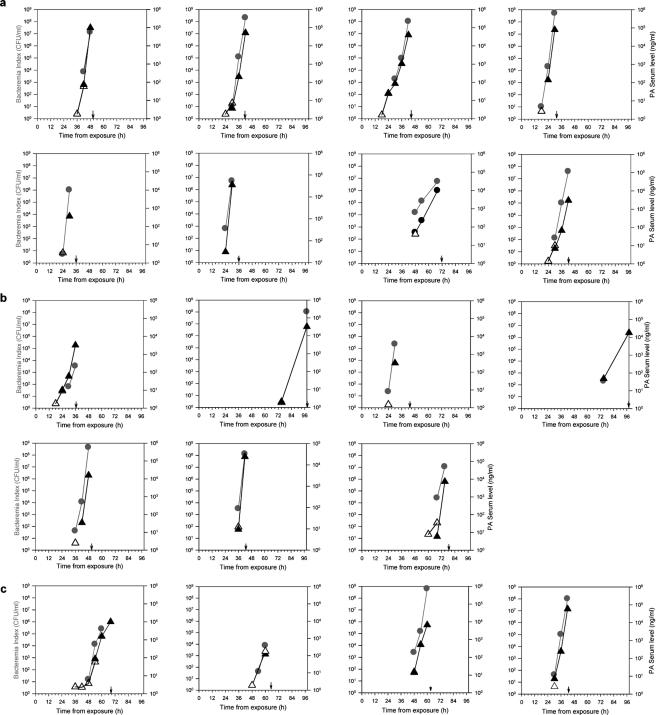
The rabbit model was chosen for this study as it allows repeated determinations of both bacteremia and PA content in sera of individual infected animals, thereby enabling close scrutiny of disease development (Fig. 1). Rabbits (eight per group) were intranasally exposed to three different doses of Vollum spores and monitored for various periods of time postexposure. On the basis of observations of individual animals, the time course for bacteremia development following infection with 1 to 30 LD50 shows no linear correlation with infection doses (P = 0.126). Detection of bacteremia starts at 18 to 90 h postinfection, with an average of 41.7 ± 20.1 h. All of the rabbits infected with 30 LD50 developed bacteremia and died, with a mean time to death (MTTD) of 43.8 ± 11.9 h (Fig. 1a); infection with 6 LD50 led to the deaths of seven of eight rabbits with an MTTD of 63.4 ± 25.6 h (Fig. 1b), and infection with 1 to 2 LD50 killed four of eight rabbits with an MTTD of 61.1 ± 11.1 h (Fig. 1c).
FIG. 1.
Development of bacteremia and appearance of PA in the sera of infected rabbits. Rabbits were exposed intranasally to 30 LD50 (a), 6 LD50 (b), or 1 to 2.5 LD50 (c) of B. anthracis spores. Every 6 to 8 h postinfection, bacteremia (•) and PA content (triangles) were determined. PA values were determined either by ELISA (▴) or by ECLI (▵). For each rabbit, the time of death is marked by a black arrow. Bacteremia was determined by plating of serial dilutions of whole blood.
Concurrently with bacteremia, detectable amounts of PA appeared in the sera of infected rabbits. In all of the animals tested, the blood PA concentration increased with time, similar to the rise in bacteremia (Fig. 1a to c). By ELISA, we were able to demonstrate the presence of PA in the sera of all sick animals (Fig. 1a to c, circles); however, there was some discrepancy between PA detection and bacteremia at lower bacteremia levels. PA was first detected concurrently with (10/18) or prior to (2/18) bacteremia detection, whereas in 6/18 rabbits bacteremia preceded PA detection in the serum by 6 h. We assumed that the discrepancy was due to the low sensitivity of the ELISA (10 ng/ml), and therefore we reexamined these samples with a more sensitive assay (ECLI; lower limit of detection, 1 ng/ml). This approach led to PA detection in all bacteremic samples. Furthermore, in 8/18 cases PA detection preceded bacteremia by 6 to 12 h (Fig. 1a to c, open triangles).
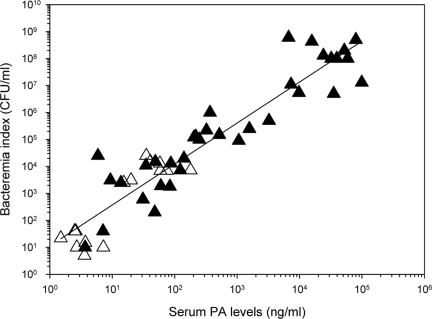
Qualitative detection of PA in the sera of bacteremic rabbits makes this antigen a reliable marker of the disease. As the bacteremia level is the only estimate of disease severity and progression, a quantitative correlation between the levels of bacteremia and PA would ensure the usefulness of this marker. A high positive correlation between the two parameters (log of bacteremia and PA concentration), exhibiting a coefficient of linearity (r2) of 0.864 (Fig. 2), permits a rough estimation of the bacteremia level from the PA concentration in serum.
FIG. 2.
Correlation between bacteremia and PA concentrations in the sera of the sick rabbits. PA values were determined by ELISA (▴) or ECLI (▵). Bacteremia was determined by plating of serial dilution of whole blood. The calculated correlation factor (r2) was 0.864.
To corroborate the findings in the rabbit model, we studied the correlation between bacteremia and PA concentration in the blood of infected guinea pigs. Intranasal inoculation of guinea pigs with 20 to 50 LD50 Vollum spores killed the animals, with an MTTD of 53.8 ± 3.75 h. To follow the development of bacteremia and PA accumulation in sera, groups of 20 infected guinea pigs were bled (single bleeding) every 6 h, starting at 24 h postinfection. Although a large variation was found in the time course of disease development, the almost simultaneous appearance of bacteremia and PA in the serum was observed (Fig. 3a). At 24 h postinfection, only PA was detected in 2/20 guinea pigs, and from 30 h postinfection, all bacteremic animals were toxemic. The results show a high positive correlation between these two parameters (r2 = 0.785; Fig. 3b), similar to the findings in the rabbit model.
FIG. 3.
Bacteremia and PA concentrations in the blood of infected guinea pigs. (a) Animals were infected by intranasal spore instillation, and groups of 20 animals were bled every 6 h (starting at 24 h postinfection) for determination of bacteremia (•) or PA concentration (▴). (b) Correlation between bacteremia and PA concentrations in the sera of sick guinea pigs (r2 = 0.785). Bacteremia was determined by plating of serial dilutions of whole blood. PA was detected by ELISA (▴) or ECLI (▵).
DISCUSSION
In this study, we demonstrated that the accumulation of PA in the serum of sick animals may be used as a reliable surrogate marker for bacteremia, identifying and assessing the severity of the disease. Both markers show parallel appearance and a linear correlation between their cumulative levels in both of the models used—rabbits and guinea pigs.
The disease caused by B. anthracis in animal models differs from inhalation anthrax in humans, as it shows a single phase of development (1, 5, 20, 27, 30). Similar to the case in humans (3, 16-18), no significant changes were found in blood cells count, blood chemistry, or temperature of the host or in any other physiological marker (1, 5, 20, 30). The appearance of the characteristic gram-positive bacilli in the blood is a common phenomenon in both human and animal cases. Significant variation in the time of appearance of bacteremia, and thus in the development of the disease, was seen with our animal models. Furthermore, the appearance of bacteremia, and later the death of the animals, did not show a linear correlation with the infective dose used to expose the animals. Therefore, the variation in the time course of anthrax development following inhalational exposure to B. anthracis spores requires a simple, quick, and repeatable assay for identification of the disease. In this work, we demonstrated a strong positive correlation between first detection of bacteremia and the appearance of PA in the blood of rabbits and guinea pigs. Furthermore, the increase in bacteremia is accompanied by accumulation of PA, showing a linear correlation between the level of bacteremia and the concentration of PA in serum. This linear correlation allows determination of bacteremia as an estimation of the severity of the disease based on the PA concentration in blood.
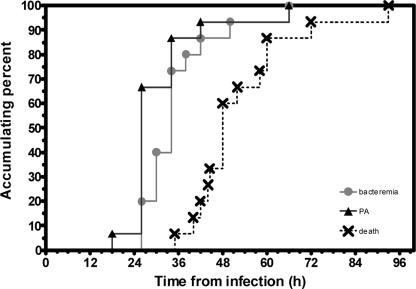
Figure 4 summarizes the pattern of the development of anthrax according to the appearance of bacteremia and PA, leading to the death of the exposed animals. The average time of death in the rabbits tested was 15.8 ± 5.9 h (range, 6 to 22 h) after the appearance of both parameters, leaving a small window for therapy. As quantitative immunodetection of PA can be done rapidly, the use of this approach can be very beneficial for the diagnosis of a bacteremic patient.
FIG. 4.
Progression of inhalation anthrax in a rabbit model. Disease development was monitored at various time points postinfection by bacteremia (•), serum PA concentrations (▴), and death (×).
During the bioterrorism-related anthrax outbreak in the United States (October and November 2001), there was a 2-day delay between preliminary identification of gram-positive rods in blood culture from a patient and the final identification of B. anthracis (4). This incident emphasizes the need for a quick and reliable marker that will identify and prove the development of anthrax. Herein, we suggest that determination of the PA concentration in the blood of patients suspected of having been exposed to B. anthracis spores may be used as a diagnostic tool, allowing a logical decision about the treatment required in positive cases.
Acknowledgments
We thank A. Shafferman and S. Reuveny for fruitful discussions. We thank N. Rothschild, Y. Shlomovich, O. Galan, and A. Tal for excellent technical assistance.
Editor: D. L. Burns
REFERENCES
- 1.Albrink, W. S., and R. J. Goodlow. 1959. Experimental inhalation anthrax in the chimpanzee. Am. J. Pathol. 35:1055-1065. [PMC free article] [PubMed] [Google Scholar]
- 2.Altboum, Z., Y. Gozes, A. Barnea, A. Pass, M. White, and D. Kobiler. 2002. Postexposure prophylaxis against anthrax: evaluation of various treatment regimens in intranasally infected guinea pigs. Infect. Immun. 70:6231-6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartlett, J. G., T. V. Inglesby, Jr., and L. Borio. 2002. Management of anthrax. Clin. Infect. Dis. 35:851-858. [DOI] [PubMed] [Google Scholar]
- 4.Begier, E. M. 2005. Gram-positive rod surveillance for early anthrax detection. Emerg. Infect. Dis. 11:1483-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berdjis, C. C., C. A. Gleiser, H. A. Hartman, R. W. Kuehne, and W. S. Gochenour. 1962. Pathogenesis of respiratory anthrax in Macaca mulatta. Br. J. Exp. Pathol. 43:515-524. [PMC free article] [PubMed] [Google Scholar]
- 6.Brachman, P. S. 1980. Inhalation anthrax. Ann. N. Y. Acad. Sci. 353:83-93. [DOI] [PubMed] [Google Scholar]
- 7.Brachman, P. S., A. F. Kaufmann, and F. G. Dalldorf. 1966. Industrial inhalation anthrax. Bacteriol. Rev. 30:646-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brook, I. 2002. The prophylaxis and treatment of anthrax. Int. J. Antimicrob. Agents 20:320-325. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. 2001. Update: investigation of bioterrorism-related anthrax and adverse events from antimicrobial prophylaxis. Morb. Mortal. Wkly. Rep. 50:973-976. [PubMed] [Google Scholar]
- 10.Collier, R., and J. Young. 2003. Anthrax toxins. Annu. Rev. Cell Dev. Biol. 19:45-70. [DOI] [PubMed] [Google Scholar]
- 11.Dixon, T. C., M. Meseslson, J. Guilemin, and P. Hanna. 1999. Anthrax. N. Engl. J. Med. 341:815-826. [DOI] [PubMed] [Google Scholar]
- 12.Duesbery, N. S., C. P. Webb, S. H. Leppla, V. M. Gordon, K. R. Klimpel, T. D. Copeland, N. G. Ahn, M. K. Oskarsson, K. Fukasawa, K. D. Paull, and G. F. Vande Woude. 1998. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science 280:734-737. [DOI] [PubMed] [Google Scholar]
- 13.Fish, D. C., and R. E. Lincoln. 1968. In vivo-produced anthrax toxin. J. Bacteriol. 95:919-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guidi-Rontani, C., and M. Mock. 2002. Macrophage interactions. Curr. Top. Microbiol. Immunol. 271:115-141. [DOI] [PubMed] [Google Scholar]
- 15.Hanna, P. 1998. Anthrax pathogenesis and host response. Curr. Top. Microbiol. Immunol. 225:13-35. [DOI] [PubMed] [Google Scholar]
- 16.Inglesby, T. V., T. O'Toole, D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. M. Friedlander, J. Gerberding, J. Hauer, J. Hughes, J. McDade, M. T. Osterholm, G. Parker, T. M. Perl, P. K. Russell, and K. Tonat. 2002. Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA 287:2236-2252. [DOI] [PubMed] [Google Scholar]
- 17.Jernigan, D. B., P. L. Raghunathan, B. P. Bell, R. Brechner, E. A. Bresnitz, J. C. Butler, M. Cetron, M. Cohen, T. Doyle, M. Fischer, C. Greene, K. S. Griffith, J. Guarner, J. L. Hadler, J. A. Hayslett, R. Meyer, L. R. Petersen, M. Phillips, R. Pinner, T. Popovic, C. P. Quinn, J. Reefhuis, D. Reissman, N. Rosenstein, A. Schuchat, W. J. Shieh, L. Siegal, D. L. Swerdlow, F. C. Tenover, M. Traeger, J. W. Ward, I. Weisfuse, S. Wiersma, K. Yeskey, S. Zaki, D. A. Ashford, B. A. Perkins, S. Ostroff, J. Hughes, D. Fleming, J. P. Koplan, and J. L. Gerberding. 2002. Investigation of bioterrorism-related anthrax, United States, 2001: epidemiologic findings. Emerg. Infect. Dis. 8:1019-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jernigan, J. A., D. S. Stephens, D. A. Ashford, C. Omenaca, M. S. Topiel, M. Galbraith, M. Tapper, T. L. Fisk, S. Zaki, T. Popovic, R. F. Meyer, C. P. Quinn, S. A. Harper, S. K. Fridkin, J. J. Sejvar, C. W. Shepard, M. McConnell, J. Guarner, W. J. Shieh, J. M. Malecki, J. L. Gerberding, J. M. Hughes, and B. A. Perkins. 2001. Bioterrorism-related inhalational anthrax: the first 10 cases reported in the United States. Emerg. Infect. Dis. 7:933-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jernigan, J. A., D. S. Stephens, D. A. Ashford, and B. A. Perkins. 2003. Industry-related outbreak of human anthrax. Emerg. Infect. Dis. 9:1657-1659. [DOI] [PubMed] [Google Scholar]
- 20.Klein, F., J. S. Walker, D. F. Fitzpatrick, R. E. Lincoln, B. G. Mahlandt, W. I. Jones, Jr., J. P. Dobbs, and K. J. Hendrix. 1966. Pathophysiology of anthrax. J. Infect. Dis. 116:123-138. [DOI] [PubMed] [Google Scholar]
- 21.Klimpel, K. R., N. Arora, and S. H. Leppla. 1994. Anthrax toxin lethal factor contains a zinc metalloprotease consensus sequence which is required for lethal toxin activity. Mol. Microbiol. 13:1093-1100. [DOI] [PubMed] [Google Scholar]
- 22.Levy, H., M. Fisher, N. Ariel, Z. Altboum, and D. Kobiler. 2005. Identification of strain specific markers in Bacillus anthracis by random amplification of polymorphic DNA. FEMS Microbiol. Lett. 244:199-205. [DOI] [PubMed] [Google Scholar]
- 23.Meselson, M., J. Guillemin, M. Hugh-Jones, A. Langmuir, I. Popova, A. Shelokov, and O. Yampolskaya. 1994. The Sverdlovsk anthrax outbreak of 1979. Science 266:1202-1208. [DOI] [PubMed] [Google Scholar]
- 24.Moayeri, M., and S. H. Leppla. 2004. Anthrax toxins in pathogenesis. Curr. Opin. Microbiol. 7:19-24. [DOI] [PubMed] [Google Scholar]
- 25.Mourez, M. 2004. Anthrax toxins. Rev. Physiol. Biochem. Pharmacol. 152:135-164. [DOI] [PubMed] [Google Scholar]
- 26.Reuveny, S., M. White, Y. Adar, Y. Kafri, Z. Altboum, Y. Gozes, D. Kobiler, A. Shafferman, and B. Velan. 2001. Search for correlates of protective immunity conferred by anthrax vaccine. Infect. Immun. 69:2888-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross, J. M. 1957. The pathogenesis of anthrax following the administration of spores by respiratory route. J. Pathol. Bacteriol. 73:485-494. [Google Scholar]
- 28.Turnbull, P. C. B. 1998. Guidelines for the surveillance and control of anthrax in humans and animals (publication no. WHO/EMC/ZDI./98.6). Department of Communicable Diseases Surveillance and Response, World Health Organization, Geneva, Switzerland.
- 29.Vitale, G., R. Pellizzari, C. Recchi, G. Napolitani, M. Mock, and C. Montecucco. 1998. Anthrax lethal factor cleaves the N-terminus of MAPKKs and induces tyrosine/threonine phosphorylation of MAPKs in cultured macrophages. Biochem. Biophys. Res. Commun. 248:706-711. [DOI] [PubMed] [Google Scholar]
- 30.Walker, J. S., F. Klein, R. E. Lincoln, and A. L. Fernelius. 1967. Temperature response in animals infected with Bacillus anthracis. J. Bacteriol. 94:552-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiss, S., D. Kobiler, H. Levy, H. Marcus, A. Pass, N. Rothschild, and Z. Altboum. 2006. Immunological correlates for protection against intranasal challenge of Bacillus anthracis spores conferred by a protective antigen-based vaccine in rabbits. Infect. Immun. 74:394-398. [DOI] [PMC free article] [PubMed] [Google Scholar]