Abstract
Numerous bacterial pathogens use type III secretion systems (T3SSs) or T4SSs to inject or translocate virulence proteins into eukaryotic cells. Several different reporter systems have been developed to measure the translocation of these proteins. In this study, a peptide tag-based reporter system was developed and used to monitor the injection of T3S and T4S substrates. The glycogen synthase kinase (GSK) tag is a 13-residue phosphorylatable peptide tag derived from the human GSK-3β kinase. Translocation of a GSK-tagged protein into a eukaryotic cell results in host cell protein kinase-dependent phosphorylation of the tag, which can be detected with phosphospecific GSK-3β antibodies. A series of expression plasmids encoding Yop-GSK fusion proteins were constructed to evaluate the ability of the GSK tag to measure the injection of Yops by the Yersinia pestis T3SS. GSK-tagged YopE, YopH, LcrQ, YopK, YopN, and YopJ were efficiently phosphorylated when translocated into HeLa cells. Similarly, the injection of GSK-CagA by the Helicobacter pylori T4SS into different cell types was measured via phosphorylation of the GSK tag. The GSK tag provides a simple method to monitor the translocation of T3S and T4S substrates.
Many bacterial pathogens use type III secretion systems (T3SSs) or T4SSs to inject or translocate effector proteins into eukaryotic cells (12, 23, 34). Injected effector proteins function to disrupt host cell signaling pathways that normally function to limit bacterial growth. The T3S apparatus is a complex supramolecular structure that spans the bacterial inner and outer membranes and is topped by a needle-like structure. T4SSs also utilize a multicomponent membrane-bound secretion apparatus to translocate effector proteins or protein-DNA complexes into targeted eukaryotic cells. The mechanism by which T3SSs and T4SSs recognize substrates and transport these substrates across both bacterial and host membranes is not well understood.
The injection process can be divided into two distinct steps, (i) secretion of effector proteins across the bacterial membranes and (ii) translocation of effector proteins across a eukaryotic membrane. Recognition of cytosolic T3S substrates requires N-terminal signals, whereas recent analyses suggest that recognition of T4S substrates relies upon C-terminal signals (31, 55, 60, 61). A role for specific chaperone-like proteins has also been confirmed for some, but not all, T3S and T4S substrates (16, 17). Secretion chaperones may assist in the targeting of secretion substrates and/or maintain secretion substrates in a secretion-competent state.
The identity of the T3S or T4S apparatus components that specifically recognize T3S or T4S signals is not known. Recent studies suggest that a conserved T3S ATPase plays an important role early in the T3S process. The T3S ATPase has been shown to directly interact with both secretion substrates and T3S chaperones (1, 22). ATPase activity is required for unfolding of T3S substrates and for the release of chaperones from their substrates (1). Not surprisingly, multisubunit ATPases are also intimately involved in substrate recognition and energization of the T4S process (3).
Translocation of T3S effector proteins across the eukaryotic membrane requires the presence of specific secreted accessory proteins that assemble to form a pore-like structure, or “translocon,” in the eukaryotic membrane (6). Strains deficient in translocon assembly secrete T3S effector proteins into the extracellular medium but cannot translocate effector proteins across a eukaryotic membrane. A recent study by Mueller et al. (35) indicates that a needle tip complex, composed of LcrV in Yersinia spp., directs translocon assembly and mediates the needle-translocon connection. No structure analogous to the T3S translocon has been identified in T4SSs (12).
To investigate the effector protein signals and the secretion apparatus components required to inject effector proteins, an assay to measure the translocation of individual effector proteins is required. We previously developed a phosphorylatable tag, termed the ELK tag, which can be used to differentiate injected substrates from intrabacterial or extracellular substrates (13). The 35-residue ELK tag consists of the simian virus 40 (SV40) large tumor antigen nuclear localization sequence (NLS) fused to amino acids (aa) 375 to 392 of the eukaryotic transcription factor Elk-1. Translocation of an ELK-tagged effector protein into a eukaryotic cell results in host cell protein kinase-dependent phosphorylation of the ELK tag, which can subsequently be detected with phospho-specific Elk-1 antibodies. The ELK tag has been utilized to measure the injection of T3S effector proteins by Yersinia pestis (13), Y. pseudotuberculosis (48), and Salmonella enterica (14). Phosphorylation of the ELK tag requires NLS-mediated translocation of the tagged effector protein to the nucleus of the injected eukaryotic cell. Unfortunately, a number of ELK-tagged effector proteins are not phosphorylated upon injection into a eukaryotic cell, possibly because they cannot be transported to the cell's nucleus. The presence of the NLS in the ELK tag may also result in mislocalization of injected effector proteins and may disrupt or alter their function within the eukaryotic cell.
In this study, we attempted to identify a small “universal” translocation tag that can be efficiently phosphorylated in the cytosolic compartment of eukaryotic cells. The Y. pestis T3SS was used to evaluate the potential of four peptide tags that correspond to amino acid sequences that are recognized and phosphorylated by host cell protein kinases. The glycogen synthase kinase (GSK) tag is a 13-residue tag that (i) does not interfere with the secretion, translocation, or function of many effector proteins; (ii) is efficiently phosphorylated by cytosolic eukaryotic protein kinases; and (iii) can be used to measure the translocation of both T3S and T4S effector proteins.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The Escherichia coli and Y. pestis strains used in this study are listed in Table 1. All of the Y. pestis strains used are Pgm− and thus avirulent from peripheral routes of infection (59). Y. pestis strains were routinely grown in heart infusion broth (HIB) or on tryptose blood agar plates (Difco) at 28°C. For routine growth and secretion assays, Y. pestis strains were grown in TMH medium in the presence or absence of 2.5 mM CaCl2 as previously described (13). E. coli DH5α was used for routine cloning experiments. H. pylori strains were grown on GC agar plates as previously described (25). Antibiotics were routinely used at the following concentrations: ampicillin, 50 μg/ml; streptomycin, 50 μg/ml; kanamycin, 25 μg/ml; chloramphenicol, 20 μg/ml (for E. coli and Y. pestis) or 6 μg/ml (for H. pylori).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsb | Source or reference |
|---|---|---|
| Y. pestisa | ||
| KIM5-3001 | Smr pCD1 pPCP1 pMT1 | 30 |
| KIM5-3001.6 (ΔyopN) | Smr pCD1 yopN pPCP1 pMT1 | 46 |
| KIM5-3001.P39 (ΔyopE) | Smr pCD1 sycE yopE::Km pPCP1 pMT1 | 13 |
| KIM5-3001.P64 (ΔyopEB) | Smr pCD1 sycE yopE::Km yopB pPCP1 pMT1 | 58 |
| KIM5-3001.P55 (ΔyopN ΔtyeA) | Smr pCD1 yopN tyeA pPCP1 pMT1 | 18 |
| KIM5-3001.P62 (ΔyopN) | Smr pCD1 sycE yopE::Km yopN pPCP1 pMT1 | 19 |
| KIM5-3001.P68 (ΔyopN ΔsycN) | Smr pCD1 sycE yopE::Km yopN sycN pPCP1 pMT1 | 19 |
| KIM5-3001.P69 (ΔyopN ΔlcrG) | Smr pCD1 sycE yopE::Km yopN lcrG pPCP1 pMT1 | 19 |
| KIM5-3001.P70 (ΔyopN ΔyscB) | Smr pCD1 yopN yscB pPCP1 pMT1 | 19 |
| KIM5-3001.P48 (yop polymutant) | Smr pCD1 sycE yopE::Km yopJ yopT yopM yopH ypkA lcrQ pPCP1 pMT1 | 13 |
| KIM5-3001.P49 (yop polymutant yopB) | Smr pCD1 sycE yopE::Km yopJ yopT yopM yopH ypkA lcrQ yopB pPCP1 pMT1 | 13 |
| KIM5-3001.P72 (yop polymutant yopB yopN) | Smr pCD1 sycE yopE::Km yopJ yopT yopM yopH ypkA lcrQ yopB yopN pPCP1 pMT1 | 13 |
| KIM8-3002 (parent) | Smr pCD1 pMT1 | 64 |
| KIM8-3002.P39 (ΔyopE) | Smr pCD1 sycE yopE::Km pMT1 | 13 |
| E. coli DH5α | F− Φ80 dlacZ ΔM15 Δ(lacZYA argF)U169 endAl recAl hsdR17 deoR supE44 thi-1 gyrA96 relA1 | 7 |
| H. pylori P12ΔcagA | ΔcagA::Km | 40 |
| Plasmidsc | ||
| pBluescriptKS− | Cloning vector; Apr | Stratagene |
| pBAD30 | Expression vector; Apr | 24 |
| pYopE | 1.53-kb XhoI-BamHI fragment encoding YopE and SycE inserted into XhoI- and BamHI-digested pBluescriptKS− | 13 |
| pYopE129-ELK | 1.19-kb PstI-BamHI fragment encoding YopE1-129-ELK and SycE in pBluescriptKS− | 13 |
| pYopB2 | yopB complementation plasmid; Cmr | 13 |
| pIP9 | E. coli-H. pylori shuttle vector encoding GSK-CagA | 25 |
| pWS252 | E. coli-H. pylori shuttle vector encoding GSK-CagA1-1194 | 25 |
All Y. pestis strains are avirulent because of deletion of the pgm locus (59).
Native plasmids of Y. pestis include pCD1 (43); pPCP1 (54), encoding the outer membrane plasminogen activator (Pla) protease that has been shown to degrade exported Yops; and pMT1, encoding the capsular protein (47).
GSK tag and ELK tag expression plasmids are described in Materials and Methods.
Construction of the pYopE129-AKT, pYopE129-GSK, pYopE129-MEK, and pYopE129-STAT expression plasmids.
Synthetic oligonucleotides (Table 2) corresponding to the nucleotide sequence of each tag shown in Fig. 1 were heated to 94°C for 5 min and allowed to cool slowly to room temperature. The hybridized oligonucleotides were digested with PstI and XhoI and inserted into PstI- and XhoI-digested pYopE129-ELK (13), generating plasmids pYopE129-AKT, pYopE129-GSK, pYopE129-MEK, and pYopE129-STAT. DNA sequence analysis was used to confirm the sequence of each inserted tag.
TABLE 2.
Oligonucleotide primers used in this study
| Primer | Sequence (5′-3′) |
|---|---|
| AKT tag-1 | TTTCTGCAGCGCCCGCATTTCCCTCAGTTCTCGTACAGTGCAAGTGGAACTTGACTCGAGTTT |
| AKT tag-2 | AAACTCGAGTCAAGTTCCACTTGCACTGTACGAGAACTGAGGGAAATGCGGGCGCTGCAGAAA |
| GSK tag-1 | TTTCTGCAGATGAGTGGTCGCCCTCGCACTAC TAGTTTCGCTGAAAGTTGACTCGAGTTT |
| GSK tag-2 | AAACTCGAGTCAACTTTCAGCGAAACTAGTAG TGCGAGGGCGACCACTCATCTGCAGAAA |
| MEK tag-1 | TTTCTGCAGCTGATCGATAGTATGGCAAATTC GTTCGTGGGAACTCGCTGACTCGAGTTT |
| MEK tag-2 | AAACTCGAGTCAGCGAGTTCCCACGAACGAAT TTGCCATACTATCGATCAGCTGCAGAAA |
| STAT tag-1 | TTTCTGCAGGCAGATCCTGGTAGTGCAGCACCTTACCTGAAAACTAAATTCATCTGACTCGAGTTT |
| STAT tag-2 | AAACTCGAGTCAGATGAATTTAGTTTTCAGGTAAGGTGCTGCACTACCAGGATCTGCCTGCAGAAA |
| GSK-1 | CTGCAGATGAGTGGTCGCCCT |
| GSK-HindIII | TTTAAGCTTAATTAACCCTCACTAAAGGGA |
| GSK-XbaI | TTTTCTAGAAATTAACCCTCACTAAAGGGA |
| ELK-1 | CTGCATGCGGAATTAATTCCCGAG |
| ELK-HindIII | TTTAAGCTTAATTAACCCTCACTAAAGGGA |
| ELK-XbaI | TTTTCTAGAAATTAACCCTCACTAAAGGGA |
| YopH-1 | TTTGAATTCTAGGCGTGTATTTAATTAAGG |
| YopH-2 | GCTATTTAATAATGGTCGCCCTTG |
| YopD-1 | TTTCAATTGCATAATTTGGCCGGCAGACCC |
| YopD-2 | GACAACACCAAAAGCGGCTTTCAT |
| SycE-1 | TTTCAATTGTTGGTTAAGTTGATATTTTAT |
| SycE-2 | ACTAAATGACCGTGGTGGTGAGAT |
| LcrQ-1 | TTTGAATTCATAACTTAGAATATCGTAGAG |
| LcrQ-2 | GCCGTCAGCCGCCGTATCCTGGCG |
| YopT-1 | TTTCAATTGTCGGTATAGCAAAATAATGGC |
| YopT-2 | AACCTCCTTGGAGTCAAATGTTAA |
| YopK-1 | TTTGAATTCGGTTATTAAATAGTGTAGTTT |
| YopK-2 | TCCCATAATACATTCTTGATCGCA |
| YopN-1 | TTTGAATTCGGTAATTGTAATTATAAACTG |
| YopN-2 | GAAAGGTCGTACGCCATTAGTTTT |
| YopJ-1 | TTTGAATTCTTCATACCGCTGTTAATTCCC |
| YopJ-2 | TACTTTGAGAAGTGTTTTATATTC |
| LcrV-1 | TTTGAATTCAAGAAAACCAACGATGATGCG |
| LcrV-2 | TTTACCAGACGTGTCATCTAGCAG |
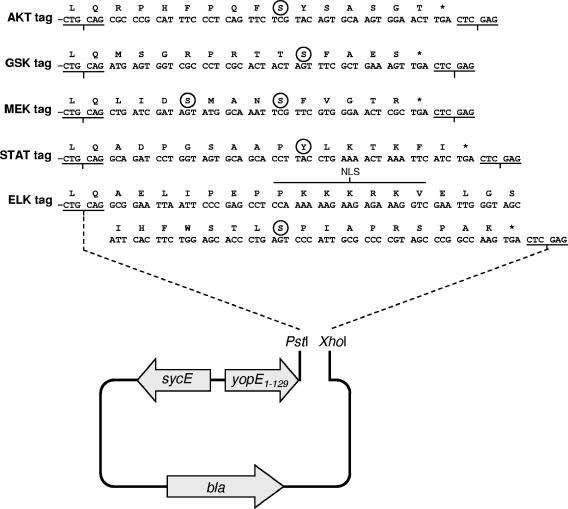
FIG. 1.
Nucleotide and amino acid sequences of the AKT, GSK, MEK, STAT, and ELK tags. DNA sequences encoding the AKT tag (aa 466 to 479 of AKT), the GSK tag (aa 1 to 13 of GSK-3β), the MEK tag (aa 214 to 226 of MEK1/2), the STAT tag (aa 697 to 711 of STAT3), and the ELK tag (SV40 large tumor antigen NLS fused to aa 375 to 392 of ELK-1), flanked by PstI and XhoI restriction sites, were inserted into PstI- and XhoI-digested plasmid pYopE, generating plasmids pYopE129-AKT, pYopE129-GSK, pYopE129-MEK, pYopE129-STAT, and pYopE129-ELK. Phosphorylation of circled residues, corresponding to AKT Ser473, GSK-3β Ser9, MEK1/2 Ser217/Ser221, STAT3 Tyr705, and ELK-1 Ser383, is required for detection with the corresponding phosphospecific antibody preparation (Cell Signaling Technology).
Construction of full-length YopH, YopD, YopT, YopK YopN, YopJ, LcrV, SycE, and LcrQ GSK tag and ELK tag expression plasmids.
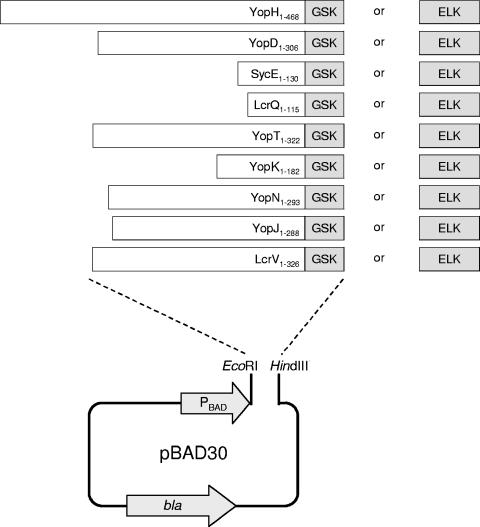
Expression plasmids encoding full-length YopD, YopT, YopK, YopN, YopJ, LcrV, SycE, or LcrQ carrying a C-terminal GSK tag or ELK tag were constructed by the PCR-ligation-PCR technique (2). Individual Y. pestis KIM genes and upstream sequences that include each gene's ribosomal binding site were amplified from plasmid pCD1 (43). Upstream primers that contained an EcoRI (or MfeI) restriction site were used in combination with downstream primers to amplify DNA fragments encoding each open reading frame lacking only its cognate stop codon (Table 2 contains the sequences of the primers used). The DNA fragment encoding the GSK tag plus the sequence encoding a two-amino-acid linker (LQ) was amplified from plasmid pYopE129-GSK with primers GSK-1 and GSK-HindIII (or GSK-XbaI), whereas the DNA fragment encoding the ELK tag was amplified from plasmid pYopE129-ELK with primers ELK-1 and ELK-HindIII (or ELK-XbaI). The yopD, yopT, yopK, yopN, yopJ, lcrV, sycE, and lcrQ fragments were ligated to the appropriate GSK or ELK fragment and reamplified with the upstream primer that contained an EcoRI (or MfeI) restriction site and primer GSK-HindIII, GSK-XbaI, ELK-HindIII, or ELK-XbaI. The resulting DNA fragments were digested with EcoRI (or MfeI) and HindIII (or XbaI) and inserted into EcoRI- and HindIII (or XbaI)-digested pBAD30 (24), generating plasmids pYopD-GSK, pYopD-ELK, pYopT-GSK, pYopT-ELK, pYopK-GSK pYopK-ELK, pYopN-GSK, pYopN-ELK, pYopJ-GSK, pYopJ-ELK, pLcrV-GSK, pLcrV-ELK, pSycE-GSK, pSycE-ELK, pLcrQ-GSK, and pLcrQ-ELK.
Tissue culture infections.
HeLa cells were grown at 37°C and 5% CO2 in RPMI medium (Invitrogen) supplemented with l-glutamine, 10% (vol/vol) fetal calf serum (FCS), and 100 μg/ml penicillin and streptomycin. HeLa cells were seeded into six-well tissue culture plates containing 2.5 ml of medium per well at a density of 5 × 105 cells per well. HeLa cells were allowed to adhere for 24 h. Before infection with Y. pestis, HeLa cells were washed twice with RPMI medium without FCS or antibiotics and incubated with 0.9 ml of the same medium for 30 min at 37°C in 5% CO2. Y. pestis strains were grown overnight at 30°C in HIB containing 2.5 mM CaCl2 and diluted the next day to an optical density at 620 nm of 0.40 in the same medium. After 2 h of growth at 30°C in a shaker bath and 1 h at 37°C, bacterial cells were washed once with RPMI medium and resuspended in the same medium. Y. pestis strains carrying derivatives of pBAD30 that express GSK- or ELK-tagged proteins were induced with l-arabinose (final concentration of 0.2%) 1 h prior to harvest and during the time of infection. HeLa cell monolayers were infected with Y. pestis strains at a multiplicity of infection (MOI) of 30 for 3 h at 37°C in 5% CO2. After 3 h, the culture supernatants were removed and the infected adherent HeLa cells were lysed by the addition of 100 μl of 1.5× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) lysis buffer containing mammalian cell protease (P-8340) and phosphatase (P-2850) inhibitor cocktails (Sigma). Samples were boiled for 3 min and analyzed by SDS-PAGE and immunoblotting with an Elk-1 (no. 9182), a phosphospecific Elk-1 (no. 9181), a GSK-3β (no. 9332), or a phosphospecific GSK-3β (no. 9336) antibody preparation (Cell Signaling Technology).
St3051 human gastric epithelial cells and J774 mouse macrophage-like cells were grown in RPMI medium containing l-glutamine and 10% (vol/vol) FCS and infected with H. pylori at an MOI of 100 for 4 h at 37°C in 5% CO2 as described previously (39, 40). Infected St3051 or J774 cells were washed three times and suspended in phosphate-buffered saline containing 1 mM EDTA, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, 10 μg/ml pepstatin, and phosphatase inhibitor cocktail (P-2850; Sigma). Cells with adherent bacteria were collected by centrifugation and analyzed by SDS-PAGE and immunoblotting with a GSK-3β (no. 9332) or a phosphospecific GSK-3β (no. 9336) antibody preparation (Cell Signaling Technology).
SDS-PAGE and immunoblotting procedures.
Infected HeLa cell, St3051, or J774 lysates; bacterial cell pellet fractions; or culture supernatant fractions prepared as described previously (13) were loaded onto NuPAGE 12% Bis-Tris SDS-PAGE gels with a morpholineethanesulfonic acid (MES) or a morpholinepropanesulfonic acid (MOPS) buffer system (Invitrogen). Separated proteins were transferred to Immobilon-P membranes (Millipore) with a Tris-glycine buffer system, and membranes were blocked with 5% nonfat milk in TBS (20 mM Tris, 150 mM NaCl, pH 7.4) for 30 min at room temperature. Primary antibodies were diluted in TBS containing 5% bovine serum albumin and 0.05% Tween 20 and incubated with the blots overnight at 4°C. The Elk-1 (no. 9182), phosphospecific Elk-1 (no. 9181), GSK-3β (no. 9332), and phosphospecific GSK-3β (no. 9336) antibodies used were purchased from Cell Signaling Technology. Blots were washed three times with TBS containing 0.05% Tween 20 for 10 min each time. Secondary antibody (alkaline phosphatase-conjugated anti-rabbit immunoglobulin G) was diluted in TBS containing 5% nonfat milk and 0.05% Tween 20 and incubated with the blots for 2 h. Blots were washed as described above and developed with 1-Step 5-bromo-4-chloro-3-indolylphosphate (BCIP)-nitroblue tetrazolium (Pierce). Images of the blots were captured and quantitated with a ChemiImager 5500 digital imaging system and Alpha Innotech software.
RESULTS
Construction of YopE129-AKT, YopE129-GSK, YopE129-MEK, and YopE129-STAT expression vectors.
The 35-residue ELK tag has previously been used to measure the translocation of injected bacterial effector proteins that can be targeted to the cell nucleus via the ELK tag's NLS (13). Attempts to use the ELK tag with several other T3S effector proteins failed, possibly because of inefficient transport of the effector protein to the nucleus (data not shown; see Fig. 5). We sought to develop a more universal phosphorylatable translocation tag that possessed the following properties: (i) small size to minimize the effect of the tag on the secretion, translocation, and function of tagged proteins; (ii) efficient tag phosphorylation in the cytoplasmic compartment of the eukaryotic cell; (iii) no phosphorylation of the tag in the bacterial cell; and (iv) the availability of commercial antibodies that recognize the tag regardless of phosphorylation (anti-tag peptide antibodies) and that recognize only the phosphorylated form of the tag (phosphospecific anti-tag peptide antibodies).
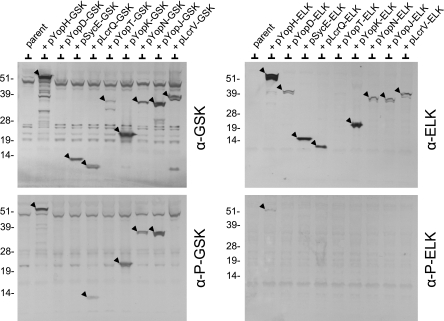
FIG. 5.
Injection and phosphorylation of YopH-GSK, LcrQ-GSK, YopK-GSK, YopN-GSK, and YopJ-GSK in HeLa cells. HeLa cell monolayers were infected at an MOI of 30 with Y. pestis KIM5-3001.P39 (ΔyopE) carrying derivatives of pBAD30 that express GSK-tagged (left panel) or ELK-tagged (right panel) proteins (arrowheads). Infected monolayers were analyzed by SDS-PAGE and immunoblotting with anti-GSK antibodies (α-GSK), anti-ELK antibodies (α-ELK), phosphospecific anti-GSK antibodies (α-P-GSK), or phosphospecific anti-ELK antibodies (α-P-ELK). The molecular masses (in kilodaltons) of protein standards are indicated on the left of each blot.
Four peptide sequences corresponding to portions of the AKT, GSK-3β, MEK1/2, and STAT3 proteins that carry residues (AKT Ser473, GSK-3β Ser9, MEK1/2 Ser217/221, and STAT3 Tyr705) that can be phosphorylated in the cytoplasmic compartment of a eukaryotic cell were evaluated in this study (Fig. 1). DNA sequences encoding these peptides were inserted in place of the sequence encoding the ELK tag in plasmid pYopE129-ELK, generating plasmids pYopE129-AKT, pYopE129-GSK, pYopE129-MEK, and pYopE129-STAT.
Expression and secretion of YopE129-AKT, YopE129-GSK, YopE129-MEK, YopE129-STAT, and YopE129-ELK by Y. pestis.
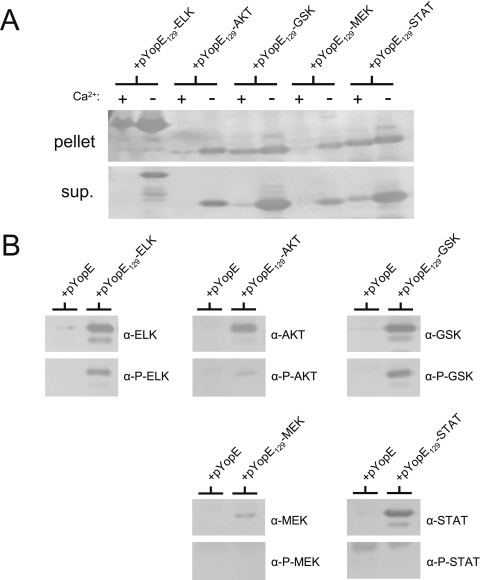
Plasmids pYopE129-AKT, pYopE129-GSK, pYopE129-MEK, pYopE129-STAT, and pYopE129-ELK were electroporated into a Y. pestis yopE deletion mutant (Y. pestis KIM8-3002.P39). The expression and secretion of the tagged YopE129 proteins were evaluated and compared after 5 h of growth in TMH medium at 37°C in the presence or absence of 2.5 mM CaCl2 (the presence of millimolar levels of extracellular calcium blocks T3S in Y. pestis). The amount of each tagged protein present in the cell pellet and in the culture supernatant was determined by SDS-PAGE and immunoblot analysis with antiserum specific for YopE (Fig. 2A). Each of the tagged proteins was expressed and secreted into the culture supernatant in the absence of CaCl2. The YopE129-GSK and YopE129-STAT proteins were secreted efficiently (>80% secreted) compared to the other tagged proteins (<50% secreted).
FIG. 2.
Secretion and translocation of YopE129-AKT, YopE129-GSK, YopE129-MEK, YopE129-STAT, and YopE129-ELK. (A) Expression and secretion of AKT-, GSK-, MEK-, STAT-, and ELK-tagged YopE129. Y. pestis KIM8-3002.P39 (ΔyopE) carrying plasmid pYopE129-AKT, pYopE129-GSK, pYopE129-MEK, pYopE129-STAT, or pYopE129-ELK was grown in the presence or absence of 2.5 mM CaCl2 in TMH medium for 5 h at 37°C. Expression and secretion of tagged YopE129 proteins were determined by SDS-PAGE and immunoblot analysis of cell pellet (pellet) and culture supernatant (sup.) fractions with antiserum specific for YopE. (B) Injection and phosphorylation of YopE129-GSK and YopE129-ELK in infected HeLa cells. Y. pestis KIM8-3002.P39 (ΔyopE) carrying plasmid pYopE, pYopE129-AKT, pYopE129-GSK, pYopE129-MEK, pYopE129-STAT, or pYopE129-ELK was grown in HIB for 2 h at 30°C and for 1 h at 37°C. HeLa cell monolayers (six-well dishes) were infected at an MOI of 30 for 3 h at 37°C. Infected monolayers were solubilized with SDS-PAGE sample buffer and analyzed by SDS-PAGE and immunoblotting with antibodies that recognize each tag sequence regardless of tag phosphorylation (α-ELK, α-AKT, α-GSK, α-MEK, or α-STAT) or that recognize the tag only if phosphorylated (α-P-ELK, α-P-AKT, α-P-GSK, α-P-MEK, or α-P-STAT).
Translocation and phosphorylation of YopE129-ELK and YopE129-GSK in HeLa cells.
To determine if an AKT, GSK, MEK, or STAT tag could be phosphorylated upon delivery into a eukaryotic cell, HeLa cell monolayers were infected with Y. pestis strains expressing YopE, YopE129-ELK, YopE129-AKT, YopE129-GSK, YopE129-MEK, or YopE129-STAT. At 3 h postinfection, the culture medium was discarded and the infected HeLa cell monolayers were analyzed by SDS-PAGE and immunoblotting with antibodies specific for the phosphorylated or nonphosphorylated form of each peptide tag (Cell Signaling Technology), i.e., anti-ELK (no. 9182), phosphospecific anti-ELK Ser383 (no. 9181), anti-AKT (no. 9272), phosphospecific anti-AKT Ser473 (no. 9271), anti-GSK (no. 9332), phosphospecific anti-GSK Ser9 (no. 9336), anti-MEK (no. 9122), phosphospecific anti-MEK Ser217/Ser221 (no. 9121), anti-STAT (no. 9132), and phosphospecific anti-STAT3 Tyr705 (no. 9131) (Fig. 2B). All five of the tagged YopE129 hybrid proteins were expressed during HeLa cell infection; however, only the ELK tag of YopE129-ELK and the GSK tag of YopE129-GSK were efficiently phosphorylated during infection and recognized by the relevant phosphospecific antibody preparations.
To confirm that the phosphorylation of the GSK tag observed during the HeLa cell infection experiment was representative of Yop translocation, we measured YopE129-GSK phosphorylation in HeLa cells infected with Y. pestis strains defective in Yop translocation (Fig. 3A). HeLa cell monolayers were infected with Y. pestis KIM5-3001.P39 (ΔyopE) and KIM5-3001.P64 (ΔyopE ΔyopB) carrying plasmid pYopE129-GSK. Analysis of the infected HeLa cell monolayers by SDS-PAGE and immunoblotting indicated that both strains expressed the YopE129-GSK protein; however, no phosphorylation of the tag was detected in the translocation-defective yopB deletion mutant (37, 38). Complementation of the yopB deletion mutant with plasmid pYopB2 restored YopE129-GSK phosphorylation to levels comparable to those of the parent strain. These results confirm that the GSK tag of the YopE129-GSK protein is phosphorylated only upon delivery into a eukaryotic cell, a process that requires the YopB protein. These results also indicate that the GSK tag, like the ELK tag, can be used to monitor both the expression and injection of YopE129 during a HeLa cell infection experiment. The anti-GSK antibodies can be used to detect the total amount of YopE129-GSK expressed during an infection, whereas the phosphospecific anti-GSK antibodies detect only the GSK-tagged YopE129 that is injected into the HeLa cells and subsequently phosphorylated.
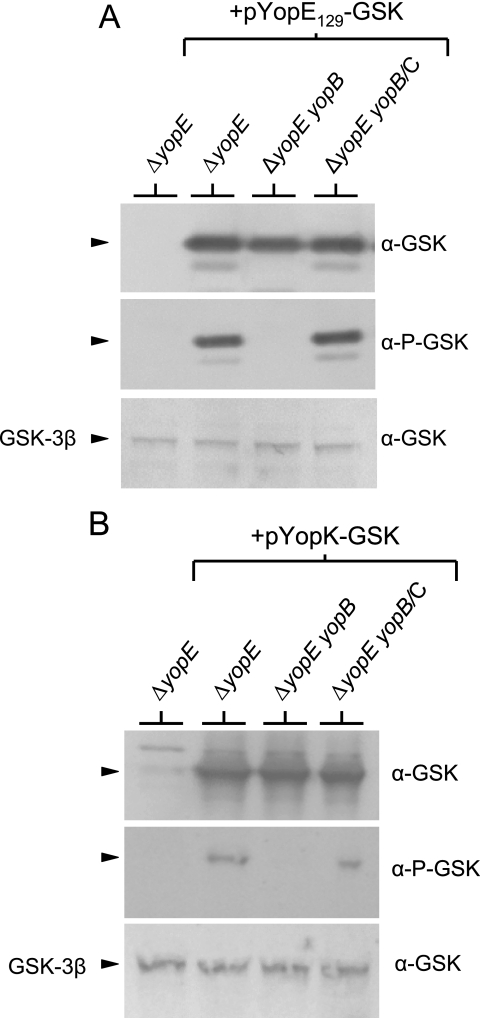
FIG. 3.
Injection and phosphorylation of YopE129-GSK and YopK-GSK require the expression of a functional YopB/YopD translocon. HeLa cell monolayers were infected at an MOI of 30 with Y. pestis KIM5-3001.P39 (ΔyopE) and KIM5-3001.P64 (ΔyopE ΔyopB) carrying plasmid pYopE129-GSK (A) or plasmid pYopK-GSK (B). Infected monolayers were subjected to SDS-PAGE and immunoblot analysis with anti-GSK antibodies (α-GSK) and phosphospecific anti-GSK antibodies (α-P-GSK). Levels of HeLa GSK-3β (used as a loading control) are shown. No translocation and phosphorylation of YopE129-GSK or YopK-GSK was detected in lysates from HeLa cell monolayers infected with the yopB deletion strain. Complementation (/C) of the yopB deletion strains with plasmid pYopB2 restored normal levels of YopE129-GSK or YopK-GSK translocation and phosphorylation.
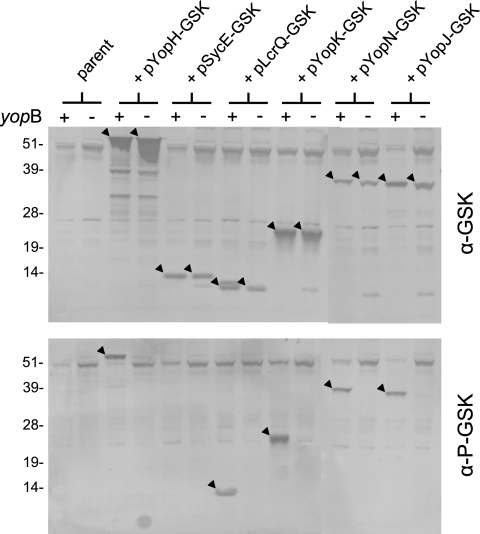
Translocation and phosphorylation of GSK-tagged YopH, LcrQ, YopK, YopN, and YopJ.
To evaluate and compare the abilities of the ELK tag and the GSK tag to measure the secretion and translocation of T3S substrates, we constructed low-copy-number pBAD30 expression plasmids (24) encoding full-length ELK- and GSK-tagged YopH, YopD, LcrQ, YopT, YopK, YopN, YopJ, LcrV, and SycE (Fig. 4). All of these proteins are known T3S substrates, with the exception of the SycE chaperone, which was included as a nonsecreted bacterial cytoplasmic control protein (29, 63). The pBAD30 expression plasmids encoding each of the ELK- and GSK-tagged proteins were electroporated into Y. pestis KIM5-3001.P39 (ΔyopE). The resulting Y. pestis strains were grown in the presence of 0.2% l-arabinose and used to infect HeLa cell monolayers. The injection and phosphorylation of the GSK-tagged proteins in the infected HeLa cells were examined by SDS-PAGE and immunoblot analysis with antibodies specific for the GSK (Cell Signaling Technology; no. 9332), the phosphorylated-GSK (no. 9336), the ELK (no. 9182), or the phosphorylated ELK (no. 9181) tag (Fig. 5).
FIG. 4.
Construction of plasmid vectors that express GSK-tagged or ELK-tagged YopH, YopD, SycE, LcrQ, YopT, YopK, YopN, YopJ, or LcrV. DNA fragments encoding full-length YopH, YopD, SycE, LcrQ, YopT, YopK, YopN, YopJ, or LcrV fused to sequences encoding the GSK tag or the ELK tag were generated by the PCR-ligation-PCR technique, digested with EcoRI (or MfeI) and HindIII (or XbaI), and inserted into EcoRI- and HindIII (or XbaI)-digested pBAD30 (see Materials and Methods).
All of the ELK- and GSK-tagged proteins, with the exception of YopD-GSK and YopT-ELK, were expressed during the infection experiment, although YopT-GSK, YopD-ELK, YopN-ELK, and YopJ-ELK were expressed only at low levels (Fig. 5, top panels). The low level of expression obtained with the tagged YopD and YopT proteins suggests that addition of an ELK or GSK tag can adversely affect the expression and/or stability of some proteins. The YopH-GSK, LcrQ-GSK, YopK-GSK, YopN-GSK, and YopJ-GSK proteins were efficiently expressed and translocated into the HeLa cells, and the GSK tag was phosphorylated (Fig. 5, lower left panel). No phosphorylation of the bacterial cytoplasmic GSK-tagged SycE chaperone was observed, confirming that the GSK tag is not phosphorylated in the bacterial cell. Finally, no translocation or subsequent phosphorylation of GSK-tagged LcrV was observed in infected HeLa cells. This observation is consistent with previous studies that indicate that the secreted LcrV protein is not injected into eukaryotic cells (15, 35, 45) but contrasts with another study that suggests that some LcrV enters HeLa cells via a T3S-independent pathway (20).
Translocation and subsequent phosphorylation of the YopH-ELK protein were demonstrated (Fig. 5, lower right panel); however, no phosphorylation of the remaining ELK-tagged proteins was observed. These results confirm that the ELK tag does not function as a translocation reporter with many T3S substrates. The YopN-ELK results are also in agreement with previous studies that showed that phosphorylation of the nonfunctional YopN-ELK protein was only detected when the protein was expressed in a yop polymutant strain of Y. pestis and from a high-copy-number pBluescript plasmid (13). The inability to observe phosphorylation of YopN-ELK expressed by Y. pestis KIM5-3001.P39 (ΔyopE) and from a pBAD30 vector (pYopN-ELK) was likely due to low expression from the low-copy-number pYopN-ELK plasmid or competition from other highly expressed secreted Yops. Other factors that could account for the poor performance of YopN-ELK and the other ELK-tagged proteins include the inability of some of the ELK-tagged proteins to be transported to the nucleus and poor secretion due to the addition of the ELK tag. Overall, these studies suggest that the GSK tag is superior to the previously characterized ELK tag for measuring the translocation of T3S substrates.
Previous studies have suggested that the secreted YopK protein (YopQ in Y. enterocolitica) is not translocated across the eukaryotic cell membrane (27, 29); however, YopK-GSK was phosphorylated during HeLa cell infection (Fig. 5, lower left panel), suggesting that YopK is translocated into targeted eukaryotic cells. To further confirm the translocation and phosphorylation of GSK-tagged YopK, we repeated the HeLa cell infection experiment with the ΔyopE parent strain and with an isogenic yopB deletion mutant, both of which express YopK-GSK (Fig. 3B). Phosphorylation of the GSK tag associated with YopK-GSK was observed in the parent strain but not in the yopB deletion mutant. Complementation of the yopB deletion mutant with plasmid pYopB2 restored the phosphorylation of YopK-GSK to levels comparable to those of the parent strain, confirming that phosphorylation of YopK-GSK requires the formation of a functional YopB/YopD translocon complex. These results suggest that at least a small amount of YopK is translocated into targeted eukaryotic cells or has some access to the cytoplasmic compartment of these cells.
Phosphorylation of YopH-GSK, LcrQ-GSK, YopK-GSK, YopN-GSK, and YopJ-GSK present in infected HeLa cell monolayers is dependent upon the expression of YopB.
To confirm that phosphorylation of the GSK tag associated with YopH-GSK, LcrQ-GSK, YopK-GSK, YopN-GSK, and YopJ-GSK is dependent upon the injection of the GSK-tagged proteins into a eukaryotic cell, we infected HeLa cells with Y. pestis strains that either express or fail to express a functional YopB/YopD translocon. The pYopH-GSK, pSycE-GSK, pLcrQ-GSK, pYopK-GSK, pYopN-GSK, and pYopJ-GSK plasmids were electroporated into a Y. pestis yop polymutant deletion strain (KIM5-3001.P48) and into an isogenic yopB deletion strain (KIM5-3001.P49). The yop polymutant strain was chosen to avoid competition for the T3S machinery between the pCD1-encoded YopJ, YopH, LcrQ, and SycE proteins and the pBAD30 vector-encoded GSK-tagged YopJ, YopH, LcrQ, and SycE proteins. Y. pestis mutants with the genes encoding YopK and YopN deleted were not utilized because of the roles of these gene products in the secretion and/or translocation process. The resultant strains were grown in the presence of 0.2% l-arabinose and used to infect HeLa cell monolayers. After a 3-h infection, infected HeLa cell monolayers were subjected to SDS-PAGE and immunoblot analysis with anti-GSK and phosphospecific anti-GSK antibodies. Each of the GSK-tagged proteins was expressed in the parent strain and in the yopB deletion strain (Fig. 6, top panel). Translocation and subsequent phosphorylation of GSK-tagged YopH, LcrQ, YopK, YopN, and YopJ were observed only in strains that expressed a fully functional T3SS. No phosphorylation of the bacterial cytoplasmic SycE-GSK protein was observed in the presence or absence of a functional YopB/YopD translocon. These results confirm that the GSK tag is only phosphorylated when a tagged protein is injected into a eukaryotic cell. Furthermore, these results indicate that the GSK tag can be used to measure the translocation of a variety of T3S substrates.
FIG. 6.
Injection and phosphorylation of YopH-GSK, LcrQ-GSK, YopK-GSK, YopN-GSK, and YopJ-GSK require the expression of a functional YopB/YopD translocon. HeLa cell monolayers were infected at an MOI of 30 with the Y. pestis yop polymutant deletion strain (KIM5-3001.P48) and an isogenic yopB deletion strain (KIM5-3001.P49) carrying plasmid pYopH-GSK, pSycE-GSK, pLcrQ-GSK, pYopK-GSK, pYopN-GSK, or pYopJ-GSK. Infected monolayers were subjected to SDS-PAGE and immunoblot analysis with anti-GSK antibodies (α-GSK) and phosphospecific anti-GSK antibodies (α-P-GSK). The YopH-GSK, LcrQ-GSK, YopK-GSK, YopN-GSK, and YopJ-GSK proteins expressed by Y. pestis KIM5-3001.P49 (arrowheads in top panel) were injected into the HeLa cells, and the GSK tag was phosphorylated (arrowheads in bottom panel). No translocation and phosphorylation of GSK-tagged proteins was observed in lysates from HeLa cell monolayers infected with the yopB deletion strain (− yopB). The molecular masses (in kilodaltons) of protein standards are indicated on the left of each blot.
Expression of YopN-GSK in a ΔyopN Y. pestis strain restores calcium-dependent regulation of Yop secretion.
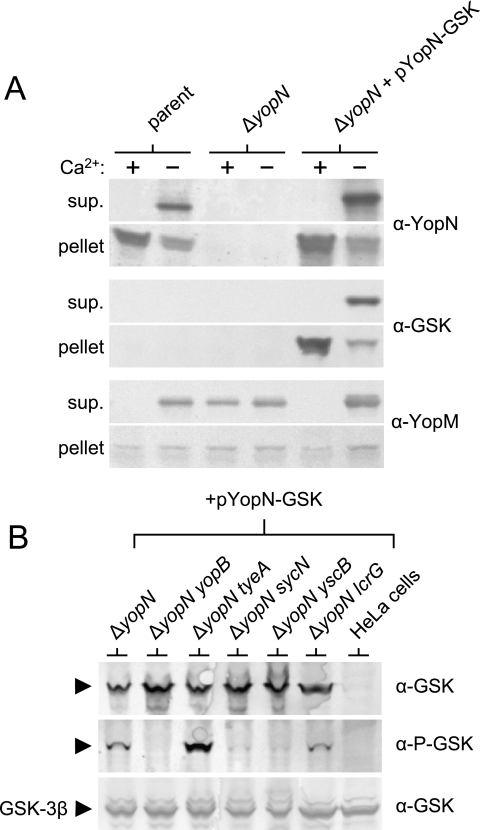
Previous studies have demonstrated that fusion of the 35-residue ELK tag to the C terminus of YopN disrupts the function of YopN in the regulation of Yop secretion (13). Therefore, translocation of YopN-ELK was monitored in the presence of an untagged copy of YopN that functioned to regulate the secretion process. To determine if the smaller 13-residue C-terminal GSK tag also disrupts the secretion-regulatory function of YopN, we moved the pYopN-GSK plasmid into a ΔyopN mutant Y. pestis strain. The parent strain, the ΔyopN strain, and the ΔyopN strain complemented with plasmid pYopN-GSK were grown in the presence or absence of 2.5 mM CaCl2 in TMH medium for 5 h at 37°C. Cell pellet and culture supernatant proteins were analyzed by SDS-PAGE and immunoblotting with antibodies specific for YopN, the GSK tag, and YopM (Fig. 7A). The parent secreted YopN and YopM in the absence of calcium but not in the presence of calcium (calcium-regulated secretion phenotype). As expected, the ΔyopN strain secreted YopM in both the presence and the absence of calcium (Fig. 7A; see anti-YopM supernatant; calcium-blind or constitutive-secretion phenotype). Providing plasmid pYopN-GSK to the ΔyopN strain completely restored the ability of this mutant to block Yop secretion in the presence of calcium, indicating that the addition of the small GSK tag to the C terminus of YopN does not disrupt the ability of YopN to regulate Yop secretion in response to extracellular calcium.
FIG. 7.
Analysis of YopN-GSK function and translocation. (A) Regulation of Yop secretion by YopN-GSK. Expression and secretion of YopN-GSK and YopM by Y. pestis KIM5-3001 (parent), KIM5-3001.6 (ΔyopN), and KIM5-3001.6 carrying plasmid pYopN-GSK grown in the presence (+) or absence (−) of 2.5 mM CaCl2 for 5 h in TMH medium. Volumes of culture supernatant (sup.) proteins and cell pellet fractions (pellet) corresponding to equal numbers of bacteria were subjected to SDS-PAGE and immunoblot analysis with antisera specific for YopN (α-YopN), the GSK tag (α-GSK), and YopM (α-YopM). (B) Roles of SycN, YscB, TyeA, and LcrG in the translocation of YopN-GSK. HeLa cell monolayers were infected at an MOI of 30 with Y. pestis KIM5-3001.6 (ΔyopN), KIM5-3001.P72 (ΔyopN ΔyopB), KIM5-3001.P55 (ΔyopN ΔtyeA), KIM5-3001.P68 (ΔyopN ΔsycN), KIM5-3001.P70 (ΔyopN ΔyscB), and KIM5-3001.P69 (ΔyopN ΔlcrG) carrying pYopN-GSK. Infected monolayers were subjected to SDS-PAGE and immunoblot analysis with anti-GSK antibodies (α-GSK) and phosphospecific anti-GSK antibodies (α-P-GSK). Levels of HeLa cell GSK-3β (used as a loading control) are shown.
Translocation of YopN-GSK by Y. pestis tyeA, sycN, yscB, and lcrG deletion mutants.
Several studies have determined that YopN is translocated into targeted eukaryotic cells (11, 13). These studies have shown that the SycN/YscB chaperone is required for efficient YopN translocation; in contrast, a lack of TyeA, which binds to a C-terminal domain of YopN, results in hypertranslocation of YopN. In addition, the translocation of YopN-ELK was specifically elevated in an lcrG deletion mutant (13). Since the YopN-ELK protein was unable to regulate Yop secretion, YopN-ELK translocation studies were carried out with a yop polymutant deletion strain that carried a wild-type copy of YopN.
The identification of a fully functional YopN-GSK protein prompted us to reinvestigate the roles of the SycN/YscB chaperone, TyeA, and LcrG in the translocation of YopN into HeLa cells. Plasmid pYopN-GSK was electroporated into the Y. pestis ΔyopN (KIM5-3001.62), ΔyopN ΔyopB (KIM5-3001.P72), ΔyopN ΔtyeA (KIM5-3001.P55), ΔyopN ΔsycN (KIM5-3001.P68), ΔyopN ΔyscB (KIM5-3001.P70), and ΔyopN ΔlcrG (KIM5-3001.P69) deletion mutants, and the resulting strains were used to infect cultured HeLa cells. Following a 3-h infection, infected HeLa cell lysates were subjected to SDS-PAGE and immunoblot analysis with anti-GSK and phosphospecific anti-GSK antibodies (Fig. 7B). All of the strains carrying plasmid pYopN-GSK expressed YopN-GSK at similar levels (Fig. 7B, top panel). The ΔyopN strain complemented with plasmid pYopN-GSK translocated YopN-GSK into the HeLa cells as previously shown (11, 13) (Fig. 7B, bottom panel). As expected, no translocation or subsequent phosphorylation of YopN-GSK was observed in the translocation-deficient ΔyopN ΔyopB mutant. As expected, the ΔyopN ΔyscB and ΔyopN ΔsycN mutants translocated only very low levels of the YopN-GSK protein; in contrast, the ΔyopN ΔtyeA strain hypertranslocated YopN into the infected HeLa cells. Surprisingly, translocation of YopN-GSK was reduced in the ΔyopN ΔlcrG strain compared to the ΔyopN strain. These results, which contrast with previous findings obtained with the YopN-ELK protein (13), suggest that LcrG does not play a direct role in regulating YopN translocation. Furthermore, these studies suggest that the elevated levels of YopN-ELK translocation observed previously in an lcrG yop polymutant strain may be an artifact of the strain used in those experiments or of the translocation tag used to monitor YopN injection.
Translocation of GSK-CagA by Helicobacter pylori.
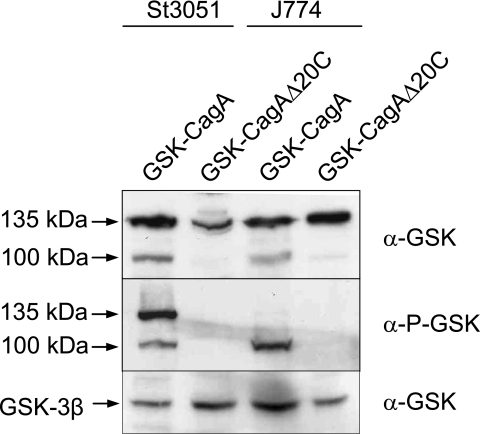
A number of T4SSs are also capable of directly injecting effector proteins into targeted eukaryotic cells. To investigate the ability of the GSK tag to monitor the translocation of T4SS substrates, we measured the injection of the H. pylori T4SS substrate CagA (40) with the GSK tag. The secretion signals associated with T4SSs are normally located at the C terminus of the protein; therefore, to avoid disrupting this portion of CagA, the GSK tag was inserted at the N terminus of CagA (25). An H. pylori cagA deletion mutant (P12ΔcagA) was transformed with plasmids pIP9 encoding GSK-CagA and pWS252 encoding GSK-CagA1-1194 (25). The GSK-CagA1-1194 protein is a truncated derivative of CagA that lacks the C-terminal 20 residues, a region that was previously shown to be required for CagA translocation. H. pylori P12ΔcagA expressing GSK-CagA or GSK-CagA1-1194 was used to infect St3051 gastric epithelial cells or J774 macrophages for 4 h at 37°C. SDS-PAGE and immunoblot analysis of infected St3051 and J774 cells demonstrated that both GSK-CagA and GSK-CagA1-1194 were expressed during the infection (Fig. 8, top panel). GSK-CagA, as shown previously with AGS cells (25), was injected into both St3051 and J774 cells, whereupon the GSK tag was phosphorylated (Fig. 8, bottom panel). As expected, no translocation and subsequent phosphorylation of the GSK-CagA1-1194 protein were observed, confirming (i) that the GSK tag can be used to monitor the translocation of T4S substrates, (ii) that the C-terminal 20 residues of CagA are required for injection of CagA, and (iii) that the GSK tag can function when expressed at either the N terminus or the C terminus of a secretion substrate.
FIG. 8.
H. pylori T4SS-mediated injection of GSK-CagA into St3051 gastric epithelial cells and J774 macrophage cells. St3051 and J774 cells were infected with H. pylori P12ΔcagA expressing GSK-CagA or GSK-CagA1-1194 (GSK-CagAΔ20C) at an MOI of 100 for 4 h at 37°C. Infected monolayers were examined by SDS-PAGE and immunoblot analysis with anti-GSK antibodies (α-GSK) and phosphospecific anti-GSK antibodies (α-P-GSK). Levels of HeLa cell GSK-3β (used as a loading control) are shown. Injected GSK-CagA was processed partially in St3051 cells, and completely in J774 cells, from ca. 135 kDa to an N-terminal ca.100-kDa fragment and a C-terminal ca. 35-kDa fragment (not shown).
Interestingly, GSK-CagA is proteolytically processed to an N-terminal ca. 100-kDa fragment and a C-terminal ca. 35-kDa fragment in both St3051 and J774 cells, although to different degrees. The proteolytic processing of CagA in J774 cells has been described previously (33, 39). With the GSK tag, we were able to show that only CagA inside adherent bacteria is present as an unprocessed 135-kDa protein (Fig. 8, top panel), whereas essentially no full-length CagA is present within the infected J774 cells (Fig. 8, bottom panel). This demonstrates that CagA is rapidly and completely processed following translocation into macrophage-like cells, whereas only partial processing occurs in St3051 cells.
DISCUSSION
Numerous bacteria use either T3SSs or T4SSs to directly inject effector proteins that function within host cells to modulate or disrupt specific cellular functions. Effector protein-dependent induction or suppression of key host cell signaling pathways is often an essential step in the infectious process. Several reporter systems have been developed to measure the injection of effector proteins into eukaryotic cells (5, 9, 13, 56, 60). These methods generally involve the construction of gene fusions that encode hybrid proteins that utilize the secretion and translocation signals present in the effector protein to transport a reporter domain into a eukaryotic cell. The reporter domain is either activated or modified or encounters its substrate only within the injected eukaryotic cell, providing an output signal that can be used to estimate the amount of hybrid protein present within the targeted cell.
The first translocation reporter used to measure the injection of T3S substrates was the ca. 400-residue catalytic domain of the Bordetella pertussis calmodulin-dependent adenylate cyclase toxin CyaA (56). Effector protein-CyaA hybrid proteins injected into eukaryotic cells are activated by binding calmodulin, resulting in an increase in intracellular cyclic AMP, which can be directly measured via an enzyme-linked immunosorbent assay. This technique has been widely used to measure the translocation of T3S (4, 28, 51) and T4S substrates (10, 36). The ELK tag is a small 35-residue tag that consists of the SV40 large tumor antigen NLS fused to aa 375 to 392 of the eukaryotic transcription factor Elk-1 (13). Translocation of an ELK-tagged T3S effector protein into a eukaryotic cell and subsequent transport to the cell nucleus result in host cell protein kinase-dependent phosphorylation of the ELK tag, which can be detected with phosphospecific anti-Elk-1 antibodies. Recently, TEM-1 β-lactamase hybrids have also been used to monitor the injection of effector proteins (9). Cleavage of the fluorescent β-lactamase substrate CCF2/AM by the TEM-1 β-lactamase domain of the injected hybrid protein disrupts CCF2/AM FRET, resulting in a change from a green fluorescence signal (520 nm) to a blue fluorescence signal (450 nm). Marketon et al. (32) used the TEM-1 β-lactamase-CCF2/AM system to identify, isolate, and analyze the eukaryotic cell populations injected by Y. pestis expressing Yop-TEM-1 β-lactamase hybrids in an infected mouse.
The Cre recombinase, which catalyzes the site-specific recombination and excision of DNA segments that are flanked by 34-bp recognition sequences (lox sequences), has been used as a translocation reporter for both T3S and T4S substrates (5, 52, 60). Injection of T3S effector protein-Cre hybrids or Cre-T4S effector protein hybrids into a eukaryotic cell that carries a Cre-dependent reporter construct catalyzes an essentially irreversible excision-based activation of the reporter construct. This technique has the advantage of creating a stable change in the targeted cells that can be measured long after the bacteria and injected effector proteins have been eliminated.
In contrast to most of the above-mentioned translocation reporters, the GSK tag is a small 13-residue tag that can be expressed at the N terminus or C terminus of a T3S or T4S effector protein. The small size of the tag appears to minimize its effect on the secretion and function of most tagged effector proteins. For example, attachment of the 35-residue ELK tag to the C terminus of YopN completely eliminated the ability of YopN to regulate Yop secretion (13), whereas a C-terminal GSK tag had no effect on YopN function. Similarly, attachment of CyaA toYopE129 significantly reduced YopE129-CyaA secretion (13); however, YopE129-GSK was efficiently secreted, even compared to YopE129-ELK. Preliminary studies also suggest that attachment of the GSK tag to YopJ and YopH did not block the antihost activities of these effectors. Expression of YopH-GSK in the yop polymutant strain restored the contact-dependent cytotoxicity of this strain (data not shown). Similarly, I. Lindner et al. (unpublished results) have shown that YopJ-GSK inhibits NFκB family members and blocks activation of the mitogen-activated protein kinase-extracellular signal-related kinase pathway. Likewise, an N-terminal GSK tag did not interfere with CagA function in gastric epithelial cells; for instance, injected GSK-CagA was tyrosine phosphorylated (25) and was able to induce characteristic actin rearrangements and cell elongation (the “hummingbird” phenotype), which represent major downstream effects of CagA translocation in AGS gastric epithelial cells (data not shown).
The GSK tag is phosphorylated in the cytosol of injected eukaryotic cells but not within bacterial cells. Commercial antibodies (Cell Signaling Technology) provide a means to detect the tagged protein regardless of tag phosphorylation (anti-GSK antibodies) or only if the tagged protein is translocated into a eukaryotic cell and the tag is subsequently phosphorylated (phosphospecific anti-GSK antibodies). This system provides a way to detect the total amount of the tagged protein expressed during an infection and, separately, a means to specifically detect only the tagged protein that was injected into a eukaryotic cell.
Another advantage of the GSK tag system is that no extensive postinfection processing, subcellular fractionation, or enzymatic assays are required to measure the translocation of tagged substrates. In addition, no special cell lines or postinfection treatments of infected cells are required. In contrast, analysis of infected cultures by the CyaA technique requires subcellular fractionation procedures and a subsequent assay for cyclic AMP (56). The use of Cre recombinase hybrids, on the other hand, requires that specific cell lines be used that carry a Cre-dependent reporter construct (5, 60). Overall, the GSK tag system is a simple, inexpensive system to specifically measure the translocation of tagged effector proteins into a variety of eukaryotic cells.
Previous studies have demonstrated that YopE, YopH, LcrQ, YopN, and YopJ are translocated into targeted eukaryotic cells via the Yersinia sp. plasmid-encoded T3SS (8, 11, 41, 44, 49, 50). In contrast, the cytosolic SycE chaperone and the secreted LcrV protein are not injected into targeted eukaryotic cells (15, 63). Measurement of YopE-GSK, YopH-GSK, LcrQ-GSK, YopN-GSK, YopJ-GSK, SycE-GSK, and LcrV-GSK expression and localization in infected HeLa cells with the GSK tag system confirms these previous results. In contrast, earlier studies with YopK (YopQ) have suggested that YopK is not translocated into eukaryotic cells (27), whereas results presented in Fig. 3, 5, and 6, obtained with GSK-tagged YopK, indicate that YopK is injected into targeted eukaryotic cells. These results suggest that YopK, which has been hypothesized to be involved in regulating the size and function of the translocation pore (26), could have an additional, as yet unidentified, function as an antihost effector protein. Interestingly, the antihost effector YopE, which is a GTPase-activating protein that targets members of the Rho family of small GTP binding proteins, has also been shown to downregulate translocation pore formation (62).
In contrast to the ELK tag, addition of the GSK tag to YopN did not alter YopN function. This allowed us to reevaluate the roles of the SycN/YscB chaperone, TyeA, and LcrG in YopN-GSK translocation in the absence of untagged YopN. The results obtained (Fig. 7) confirm that the SycN/YscB chaperone is required for efficient YopN translocation and that TyeA normally functions to suppress YopN translocation (11, 13). Previous studies with YopN-ELK suggested that LcrG also plays a role in specifically limiting YopN translocation (13); however, studies with YopN-GSK failed to support this finding. In contrast, an lcrG deletion mutant showed reduced YopN translocation, a phenotype that is consistent with the constitutive-secretion phenotype associated with this mutant (53). These results suggest that, in contrast to our previous findings, LcrG plays no direct role in regulating YopN translocation.
The identity of the eukaryotic protein kinase or kinases responsible for the phosphorylation of the GSK tag is not known. Serine 9 of GSK-3β is known to be phosphorylated by the AKT kinase; however, addition of LY294002 or wortmannin, two inhibitors that block activation of AKT (21, 42), failed to significantly inhibit GSK tag phosphorylation (data not shown). This was not unexpected, given that protein kinase recognition sites removed from their normal context are often recognized and phosphorylated in a nonspecific manner by alternative kinases (57). The fact that the GSK tag is efficiently phosphorylated in the presence or absence of activated AKT suggests that multiple kinases can recognize and phosphorylate the tag. These results indicate that phosphorylation of the tag is not tightly regulated by specific environmental or cellular conditions. In fact, all of the GSK-tagged proteins, regardless of their abilities to inhibit specific signaling pathways, appear to be rapidly phosphorylated upon injection into a eukaryotic cell. Furthermore, results presented here and studies by Lindner et al. (unpublished results) indicate that the GSK tag is rapidly phosphorylated in a variety of cell types, suggesting that the tag can be used to study a number of different bacterial pathogens that use either T3SSs or T4SSs to manipulate different types of eukaryotic cells.
Acknowledgments
We thank Ken Fields for critical reading of the manuscript and for useful comments. We also acknowledge Yi Tan and Kathryn Abell at Cell Signaling Technology for helpful advice and for providing anti-GSK antibodies.
This work was supported by Public Health Service grant AI-39575 from the National Institutes of Health.
Editor: J. B. Bliska
REFERENCES
- 1.Akeda, Y., and J. E. Galan. 2005. Chaperone release and unfolding of substrates in type III secretion. Nature 437:911-915. [DOI] [PubMed] [Google Scholar]
- 2.Ali, S. A., and A. Steinkasserer. 1995. PCR-ligation-PCR mutagenesis: a protocol for creating gene fusions and mutations. BioTechniques 18:746-750. [PubMed] [Google Scholar]
- 3.Atmakuri, K., E. Cascales, and P. J. Christie. 2004. Energetic components VirD4, VirB11 and VirB4 mediate early DNA transfer reactions required for bacterial type IV secretion. Mol. Microbiol. 54:1199-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyd, A. P., N. Grosdent, S. Totemeyer, C. Geuijen, S. Bleves, M. Iriarte, I. Lambermont, J. N. Octave, and G. R. Cornelis. 2000. Yersinia enterocolitica can deliver Yop proteins into a wide range of cell types: development of a delivery system for heterologous proteins. Eur. J. Cell Biol. 79:659-671. [DOI] [PubMed] [Google Scholar]
- 5.Briones, G., D. Hofreuter, and J. E. Galan. 2006. Cre reporter system to monitor the translocation of type III secreted proteins into host cells. Infect. Immun. 74:1084-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buttner, D., and U. Bonas. 2002. Port of entry—the type III secretion translocon. Trends Microbiol. 10:186-192. [DOI] [PubMed] [Google Scholar]
- 7.Cambau, E., F. Bordon, E. Collatz, and L. Gutmann. 1993. Novel gyrA point mutation in a strain of Escherichia coli resistant to fluoroquinolones but not to nalidixic acid. Antimicrob. Agents Chemother. 37:1247-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cambronne, E. D., L. W. Cheng, and O. Schneewind. 2000. LcrQ/YscM1, regulators of the Yersinia yop virulon, are injected into host cells by a chaperone-dependent mechanism. Mol. Microbiol. 37:263-273. [DOI] [PubMed] [Google Scholar]
- 9.Charpentier, X., and E. Oswald. 2004. Identification of the secretion and translocation domain of the enteropathogenic and enterohemorrhagic Escherichia coli effector Cif, using TEM-1 β-lactamase as a new fluorescence-based reporter. J. Bacteriol. 186:5486-5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, J., K. S. de Felipe, M. Clarke, H. Lu, O. R. Anderson, G. Segal, and H. A. Shuman. 2004. Legionella effectors that promote nonlytic release from protozoa. Science 303:1358-1361. [DOI] [PubMed] [Google Scholar]
- 11.Cheng, L. W., O. Kay, and O. Schneewind. 2001. Regulated secretion of YopN by the type III machinery of Yersinia enterocolitica. J. Bacteriol. 183:5293-5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christie, P. J., K. Atmakuri, V. Krishnamoorthy, S. Jakubowski, and E. Cascales. 2005. Biogenesis, architecture, and function of bacterial type IV secretion systems. Annu. Rev. Microbiol. 59:451-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Day, J. B., F. Ferracci, and G. V. Plano. 2003. Translocation of YopE and YopN into eukaryotic cells by Yersinia pestis yopN, tyeA, sycN, yscB and lcrG deletion mutants measured using a phosphorylatable peptide tag and phosphospecific antibodies. Mol. Microbiol. 47:807-823. [DOI] [PubMed] [Google Scholar]
- 14.Day, J. B., and C. A. Lee. 2003. Secretion of the orgC gene product by Salmonella enterica serovar Typhimurium. Infect. Immun. 71:6680-6685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeBord, K. L., V. T. Lee, and O. Schneewind. 2001. Roles of LcrG and LcrV during type III targeting of effector Yops by Yersinia enterocolitica. J. Bacteriol. 183:4588-4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng, W., L. Chen, W. T. Peng, X. Liang, S. Sekiguchi, M. P. Gordon, L. Comai, and E. W. Nester. 1999. VirE1 is a specific molecular chaperone for the exported single-stranded-DNA-binding protein VirE2 in Agrobacterium. Mol. Microbiol. 31:1795-1807. [DOI] [PubMed] [Google Scholar]
- 17.Feldman, M. F., and G. R. Cornelis. 2003. The multitalented type III chaperones: all you can do with 15 kDa. FEMS Microbiol. Lett. 219:151-158. [DOI] [PubMed] [Google Scholar]
- 18.Ferracci, F., J. B. Day, H. J. Ezelle, and G. V. Plano. 2004. Expression of a functional secreted YopN-TyeA hybrid protein in Yersinia pestis is the result of a +1 translational frameshift event. J. Bacteriol. 186:5160-5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferracci, F., F. D. Schubot, D. S. Waugh, and G. V. Plano. 2005. Selection and characterization of Yersinia pestis YopN mutants that constitutively block Yop secretion. Mol. Microbiol. 57:970-987. [DOI] [PubMed] [Google Scholar]
- 20.Fields, K. A., and S. C. Straley. 1999. LcrV of Yersinia pestis enters infected eukaryotic cells by a virulence plasmid-independent mechanism. Infect. Immun. 67:4801-4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franke, T. F., S. I. Yang, T. O. Chan, K. Datta, A. Kazlauskas, D. K. Morrison, D. R. Kaplan, and P. N. Tsichlis. 1995. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell 81:727-736. [DOI] [PubMed] [Google Scholar]
- 22.Gauthier, A., and B. B. Finlay. 2003. Translocated intimin receptor and its chaperone interact with ATPase of the type III secretion apparatus of enteropathogenic Escherichia coli. J. Bacteriol. 185:6747-6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghosh, P. 2004. Process of protein transport by the type III secretion system. Microbiol. Mol. Biol. Rev. 68:771-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guzman, L. M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hohlfeld, S., I. Pattis, J. Puls, G. V. Plano, R. Haas, and W. Fischer. 2006. A C-terminal translocation signal is necessary, but not sufficient, for type IV secretion of the Helicobacter pylori CagA protein. Mol. Microbiol. 59:1624-1637. [DOI] [PubMed] [Google Scholar]
- 26.Holmstrom, A., J. Petterson, R. Rosqvist, S. Hakansson, F. Tafazoli, M. Fallman, K. E. Magnusson, H. Wolf-Watz, and A. Forsberg. 1997. YopK of Yersinia pseudotuberculosis controls translocation of Yop effectors across the eukaryotic cell membrane. Mol. Microbiol. 24:73-91. [DOI] [PubMed] [Google Scholar]
- 27.Holmstrom, A., R. Rosqvist, H. Wolf-Watz, and A. Forsberg. 1995. YopK, a novel virulence determinant of Yersinia pseudotuberculosis. Contrib. Microbiol. Immunol. 13:239-243. [PubMed] [Google Scholar]
- 28.Kujat Choy, S. L., E. C. Boyle, O. Gal-Mor, D. L. Goode, Y. Valdez, B. A. Vallance, and B. B. Finlay. 2004. SseK1 and SseK2 are novel translocated proteins of Salmonella enterica serovar Typhimurium. Infect. Immun. 72:5115-5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee, V. T., and O. Schneewind. 1999. Type III machines of pathogenic yersiniae secrete virulence factors into the extracellular milieu. Mol. Microbiol. 31:1619-1629. [DOI] [PubMed] [Google Scholar]
- 30.Lindler, L. E., M. S. Klempner, and S. C. Straley. 1990. Yersinia pestis pH 6 antigen: genetic, biochemical, and virulence characterization of a protein involved in the pathogenesis of bubonic plague. Infect. Immun. 58:2569-2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lloyd, S. A., M. Norman, R. Rosqvist, and H. Wolf-Watz. 2001. Yersinia YopE is targeted for type III secretion by N-terminal, not mRNA, signals. Mol. Microbiol. 39:520-531. [DOI] [PubMed] [Google Scholar]
- 32.Marketon, M. M., R. W. DePaolo, K. L. DeBord, B. Jabri, and O. Schneewind. 2005. Plague bacteria target immune cells during infection. Science 309:1739-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moese, S., M. Selbach, U. Zimny-Arndt, P. R. Jungblut, T. F. Meyer, and S. Backert. 2001. Identification of a tyrosine-phosphorylated 35 kDa carboxy-terminal fragment (p35CagA) of the Helicobacter pylori CagA protein in phagocytic cells: processing or breakage? Proteomics 1:618-629. [DOI] [PubMed] [Google Scholar]
- 34.Mota, L. J., I. Sorg, and G. R. Cornelis. 2005. Type III secretion: the bacteria-eukaryotic cell express. FEMS Microbiol. Lett. 252:1-10. [DOI] [PubMed] [Google Scholar]
- 35.Mueller, C. A., P. Broz, S. A. Muller, P. Ringler, F. Erne-Brand, I. Sorg, M. Kuhn, A. Engel, and G. R. Cornelis. 2005. The V-antigen of Yersinia forms a distinct structure at the tip of injectisome needles. Science 310:674-676. [DOI] [PubMed] [Google Scholar]
- 36.Nagai, H., E. D. Cambronne, J. C. Kagan, J. C. Amor, R. A. Kahn, and C. R. Roy. 2005. A C-terminal translocation signal required for Dot/Icm-dependent delivery of the Legionella RalF protein to host cells. Proc. Natl. Acad. Sci. USA 102:826-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neyt, C., and G. R. Cornelis. 1999. Insertion of a Yop translocation pore into the macrophage plasma membrane by Yersinia enterocolitica: requirement for translocators YopB and YopD, but not LcrG. Mol. Microbiol. 33:971-981. [DOI] [PubMed] [Google Scholar]
- 38.Nordfelth, R., and H. Wolf-Watz. 2001. YopB of Yersinia enterocolitica is essential for YopE translocation. Infect. Immun. 69:3516-3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Odenbreit, S., B. Gebert, J. Puls, W. Fischer, and R. Haas. 2001. Interaction of Helicobacter pylori with professional phagocytes: role of the cag pathogenicity island and translocation, phosphorylation and processing of CagA. Cell Microbiol. 3:21-31. [DOI] [PubMed] [Google Scholar]
- 40.Odenbreit, S., J. Puls, B. Sedlmaier, E. Gerland, W. Fischer, and R. Haas. 2000. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science 287:1497-1500. [DOI] [PubMed] [Google Scholar]
- 41.Palmer, L. E., S. Hobbie, J. E. Galan, and J. B. Bliska. 1998. YopJ of Yersinia pseudotuberculosis is required for the inhibition of macrophage TNF-α production and downregulation of the MAP kinases p38 and JNK. Mol. Microbiol. 27:953-965. [DOI] [PubMed] [Google Scholar]
- 42.Pap, M., and G. M. Cooper. 1998. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-kinase/Akt cell survival pathway. J. Biol. Chem. 273:19929-19932. [DOI] [PubMed] [Google Scholar]
- 43.Perry, R. D., S. C. Straley, J. D. Fetherston, D. J. Rose, J. Gregor, and F. R. Blattner. 1998. DNA sequencing and analysis of the low-Ca2+-response plasmid pCD1 of Yersinia pestis KIM5. Infect. Immun. 66:4611-4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Persson, C., R. Nordfelth, A. Holmstrom, S. Hakansson, R. Rosqvist, and H. Wolf-Watz. 1995. Cell-surface-bound Yersinia translocate the protein tyrosine phosphatase YopH by a polarized mechanism into the target cell. Mol. Microbiol. 18:135-150. [DOI] [PubMed] [Google Scholar]
- 45.Pettersson, J., A. Holmstrom, J. Hill, S. Leary, E. Frithz-Lindsten, A. von Euler-Matell, E. Carlsson, R. Titball, A. Forsberg, and H. Wolf-Watz. 1999. The V-antigen of Yersinia is surface exposed before target cell contact and involved in virulence protein translocation. Mol. Microbiol. 32:961-976. [DOI] [PubMed] [Google Scholar]
- 46.Plano, G. V., and S. C. Straley. 1995. Mutations in yscC, yscD, and yscG prevent high-level expression and secretion of V antigen and Yops in Yersinia pestis. J. Bacteriol. 177:3843-3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Protsenko, O. A., P. I. Anisimov, O. T. Mozharov, N. P. Konnov, and A. Popov Iu. 1983. Detection and characterization of the plasmids of the plague microbe which determine the synthesis of pesticin I, fraction I antigen and “mouse” toxin exotoxin. Genetika 19:1081-1090. [PubMed] [Google Scholar]
- 48.Rosenzweig, J. A., G. Weltman, G. V. Plano, and K. Schesser. 2005. Modulation of Yersinia type three secretion system by the S1 domain of polynucleotide phosphorylase. J. Biol. Chem. 280:156-163. [DOI] [PubMed] [Google Scholar]
- 49.Rosqvist, R., A. Forsberg, and H. Wolf-Watz. 1991. Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect. Immun. 59:4562-4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosqvist, R., C. Persson, S. Hakansson, R. Nordfeldt, and H. Wolf-Watz. 1995. Translocation of the Yersinia YopE and YopH virulence proteins into target cells is mediated by YopB and YopD. Contrib. Microbiol. Immunol. 13:230-234. [PubMed] [Google Scholar]
- 51.Schechter, L. M., K. A. Roberts, Y. Jamir, J. R. Alfano, and A. Collmer. 2004. Pseudomonas syringae type III secretion system targeting signals and novel effectors studied with a Cya translocation reporter. J. Bacteriol. 186:543-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schulein, R., P. Guye, T. A. Rhomberg, M. C. Schmid, G. Schroder, A. C. Vergunst, I. Carena, and C. Dehio. 2005. A bipartite signal mediates the transfer of type IV secretion substrates of Bartonella henselae into human cells. Proc. Natl. Acad. Sci. USA 102:856-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Skryzpek, E., and S. C. Straley. 1993. LcrG, a secreted protein involved in negative regulation of the low-calcium response in Yersinia pestis. J. Bacteriol. 175:3520-3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sodeinde, O. A., A. K. Sample, R. R. Brubaker, and J. D. Goguen. 1988. Plasminogen activator/coagulase gene of Yersinia pestis is responsible for degradation of plasmid-encoded outer membrane proteins. Infect. Immun. 56:2749-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sorg, J. A., N. C. Miller, and O. Schneewind. 2005. Substrate recognition of type III secretion machines—testing the RNA signal hypothesis. Cell Microbiol. 7:1217-1225. [DOI] [PubMed] [Google Scholar]
- 56.Sory, M. P., and G. R. Cornelis. 1994. Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol. Microbiol. 14:583-594. [DOI] [PubMed] [Google Scholar]
- 57.Sparks, J. W., and D. L. Brautigan. 1986. Molecular basis for substrate specificity of protein kinases and phosphatases. Int. J. Biochem. 18:497-504. [DOI] [PubMed] [Google Scholar]
- 58.Torruellas, J., M. W. Jackson, J. W. Pennock, and G. V. Plano. 2005. The Yersinia pestis type III secretion needle plays a role in the regulation of Yop secretion. Mol. Microbiol. 57:1719-1733. [DOI] [PubMed] [Google Scholar]
- 59.Une, T., and R. R. Brubaker. 1984. In vivo comparison of avirulent Vwa− and Pgm− or Pstr phenotypes of yersiniae. Infect. Immun. 43:895-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vergunst, A. C., B. Schrammeijer, A. den Dulk-Ras, C. M. de Vlaam, T. J. Regensburg-Tuink, and P. J. Hooykaas. 2000. VirB/D4-dependent protein translocation from Agrobacterium into plant cells. Science 290:979-982. [DOI] [PubMed] [Google Scholar]
- 61.Vergunst, A. C., M. C. van Lier, A. den Dulk-Ras, T. A. Stuve, A. Ouwehand, and P. J. Hooykaas. 2005. Positive charge is an important feature of the C-terminal transport signal of the VirB/D4-translocated proteins of Agrobacterium. Proc. Natl. Acad. Sci. USA 102:832-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Viboud, G. I., and J. B. Bliska. 2001. A bacterial type III secretion system inhibits actin polymerization to prevent pore formation in host cell membranes. EMBO J. 20:5373-5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wattiau, P., and G. R. Cornelis. 1993. SycE, a chaperone-like protein of Yersinia enterocolitica involved in the secretion of YopE. Mol. Microbiol. 8:123-131. [DOI] [PubMed] [Google Scholar]
- 64.Williams, A. W., and S. C. Straley. 1998. YopD of Yersinia pestis plays a role in negative regulation of the low-calcium response in addition to its role in translocation of Yops. J. Bacteriol. 180:350-358. [DOI] [PMC free article] [PubMed] [Google Scholar]