Abstract
Sequestration of Plasmodium falciparum-infected erythrocytes in the placenta is implicated in pathological outcomes of pregnancy-associated malaria (PAM). P. falciparum isolates that sequester in the placenta primarily bind chondroitin sulfate A (CSA). Following exposure to malaria during pregnancy, women in areas of endemicity develop immunity, and so multigravid women are less susceptible to PAM than primigravidae. Protective immunity to PAM is associated with the development of antibodies that recognize diverse CSA-binding, placental P. falciparum isolates. The epitopes recognized by such protective antibodies have not been identified but are likely to lie in conserved Duffy binding-like (DBL) domains, encoded by var genes, that bind CSA. Immunization of mice with the CSA-binding DBL3γ domain encoded by var1CSA elicits cross-reactive antibodies that recognize diverse CSA-binding P. falciparum isolates and block their binding to placental cryosections under flow. However, CSA-binding isolates primarily express var2CSA, which does not encode any DBLγ domains. Here, we demonstrate that antibodies raised against DBL3γ encoded by var1CSA cross-react with one of the CSA-binding domains, DBL3X, encoded by var2CSA. This explains the paradoxical observation made here and earlier that anti-rDBL3γ sera recognize CSA-binding isolates and provides evidence for the presence of conserved, cross-reactive epitopes in diverse CSA-binding DBL domains. Such cross-reactive epitopes within CSA-binding DBL domains can form the basis for a vaccine that provides protection against PAM.
Following repeated exposure to Plasmodium falciparum infections, adults in areas of endemicity develop immunity to clinical malaria (42, 46). However, women in areas of endemicity are uniquely susceptible to P. falciparum malaria during pregnancy (7, 36). Infection with P. falciparum is an important cause of maternal anemia and increases the risk of abortion, premature delivery, low birth weight, neonatal mortality, and infant anemia, especially in primigravidae (8, 28, 31, 35, 52). P. falciparum infections during pregnancy are frequently characterized by the sequestration of infected erythrocytes (IEs) in placental blood spaces (35), which can lead to inflammatory responses (54), deposition of fibrinoid material (57), and reduced blood flow to the fetus (18).
There has been considerable interest in understanding the molecular mechanisms that mediate placental sequestration of IEs and the reasons for the apparent lack of immunity to P. falciparum malaria in primigravidae residing in areas of endemicity. Multigravid women appear to be protected against the deleterious effects of P. falciparum infection during pregnancy (20, 36), suggesting that strain-transcending immunity develops rapidly following exposure to placental P. falciparum isolates. The mechanisms that mediate protective immunity against pregnancy-associated malaria (PAM) are not completely understood.
Adhesion studies have revealed that IEs derived from placentas predominantly bind chondroitin sulfate A (CSA) (1, 16, 24, 43). Binding to hyaluronic acid and normal immunoglobulins (Igs) may also play a minor role in placental sequestration (5, 6, 16, 23). In contrast, IEs derived from peripheral blood of P. falciparum-infected pregnant women or from that of nonpregnant donors, including children and adult men, commonly bind other endothelial receptors, such as CD36 (24). These findings suggest the possibility that the placenta may select for rare CSA-binding P. falciparum variants that are not commonly found in infected children or nonpregnant adults.
The cytoadherence of IEs to the host endothelium is mediated by variant surface proteins that belong to the P. falciparum erythrocyte membrane protein-1 (PfEMP-1) family (13). The P. falciparum genome contains ∼60 var genes that encode diverse PfEMP-1 variants (3, 48, 49, 53). Expression of PfEMP-1 undergoes antigenic variation due to the switching of var gene expression during blood-stage growth (48). Immune adults residing in areas of endemicity acquire antibodies that recognize diverse PfEMP-1 variants and agglutinate diverse P. falciparum isolates (33). Antibodies directed against PfEMP-1 are thought to be important components of naturally acquired immunity to P. falciparum malaria (10). While sera from immune adult men and primigravid women residing in areas of endemicity recognize a wide range of peripheral P. falciparum isolates, they exhibit poor recognition of placental P. falciparum isolates (4, 25) and CSA-binding laboratory strains (41, 51). Following P. falciparum infection during pregnancy, women develop antibodies that show improved recognition of a wide range of placental isolates and CSA-binding laboratory strains (4, 25, 41, 51). The levels of antibodies recognizing placental isolates or CSA-binding laboratory strains are significantly correlated with parity (4, 25, 41, 51). This indicates the development of antibodies that recognize conserved epitopes on the IE surfaces of diverse placental and CSA-binding isolates. The identity of such conserved epitopes has not yet been defined, but they are likely to lie within PfEMP-1 variants that mediate adhesion to CSA. The PfEMP-1 variants that were initially implicated in CSA binding include var1CSA from P. falciparum FCR3CSA (9) and CS2var from P. falciparum CS2 (39, 40). Adhesion to CSA is mediated by the DBL3γ domain of var1CSA (9) and CS2var (39, 40). Monoclonal antibodies raised against CHO cells expressing DBL3γ of var1CSA and antisera raised against recombinant DBL3γ expressed in insect cells recognize a wide range of placental P. falciparum isolates, suggesting that DBL3γ contains conserved, cross-reactive epitopes shared by diverse CSA-binding placental isolates (15, 32). However, although var1CSA was initially implicated as the var gene responsible for CSA binding in P. falciparum FCR3CSA, subsequent studies demonstrated that the expression of another var gene, var2CSA, and not that of var1CSA, is upregulated in diverse CSA-binding parasite lines and placental isolates (19, 21, 30, 44, 56). The var2CSA gene implicated in CSA binding does not encode any DBLγ domains. The reported reactivity of anti-rDBL3γ sera with placental CSA-binding P. falciparum isolates is thus paradoxical.
Here, we have produced recombinant DBL3γ (rDBL3γ) of var1CSA in its functional form and examined its immunogenicity. We demonstrate that immunization with rDBL3γ does elicit sera that cross-react with a wide range of placental isolates and block their binding to placental cryosections under static as well as physiologically relevant flow conditions. Importantly, we show that anti-rDBL3γ sera cross-react with one of two CSA-binding DBL domains, namely, the DBL3X domain of var2CSA. This observation suggests that the CSA-binding DBL domains DBL3γ and DBL3X share conserved B-cell epitopes and explains the paradoxical observations that anti-rDBL3γ sera recognize CSA-binding P. falciparum isolates even though these parasites express var2CSA that does not encode any DBLγ domains. Such conserved epitopes within diverse CSA-binding DBL domains may provide the basis for the development of vaccines that elicit cross-reactive antibodies against CSA-binding isolates and protect against PAM.
MATERIALS AND METHODS
Production and characterization of functional rDBL3γ.
DNA encoding the DBL3γ domain of var1CSA from P. falciparum FCR3 fused to a C-terminal six-histidine tag was amplified by PCR using primers 5′ ACT TGC CCA TGG GAA AAC GAT GGA AAG AAA C 3′ and 5′ ACG AGT GCG GCC GCT CAG TGA TGG TGA TGG TGA TGC CTG TTC AAG TAA TCT GTT G 3′ and a cloned fragment of the FCR3 var1CSA gene (kindly provided by Artur Scherf, Institut Pasteur, Paris, France) (9) as the template. The PCR product was cloned as a NcoI-NotI fragment downstream of the T7 promoter in expression vector pET28a+ (Novagen) and transformed into Escherichia coli BL21(DE3) (Novagen). Expression of rDBL3γ with a C-terminal six-histidine tag was induced by the addition of 1 mM isopropyl-1-thio-β-d-galactopyranoside (IPTG) to cultures of E. coli BL21(DE3)(pET28a+DBL3γ) grown to mid-logarithmic phase. Induced cultures were harvested after 4 h of growth at 37°C and lysed by sonication. Inclusion bodies were collected by centrifugation and solubilized in 6 M guanidine-hydrochloride. His-tagged rDBL3γ was purified under denaturing conditions by metal affinity chromatography using a Ni-nitrilotriacetic acid column (QIAGEN), refolded by the method of rapid dilution, and purified further to homogeneity by ion-exchange chromatography using SP-Sepharose (Pharmacia) and gel filtration chromatography using Superdex 75 as described earlier for other DBL domains (37, 47).
Refolded and purified DBL3γ was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) before and after reduction with 5 mM dithiothreitol and detected by silver staining. The homogeneity of rDBL3γ was analyzed by reverse-phase chromatography on a C8 column as previously described (37, 47). The presence of free thiols was detected by Ellman's method with 5′,5′-dithiobis(2-nitrobenzoic acid) (DTNB) (22, 37, 47).
Assessment of binding of rDBL3γ to biotinylated CSA immobilized on streptavidin-coated wells by ELISA.
CSA, CSB, and CSC (Calbiochem) were biotinylated using a biotinylation kit (Pierce) as described by the manufacturer. Biotinylated CSA (Bio-CSA), Bio-CSB, and Bio-CSC (100 μl of 5 μg ml−1) were immobilized in streptavidin-coated wells (Streptawell-96; Roche). Residual free sites were saturated with 3% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) with 0.05% Tween 20 (PBST) for 1 h at 37°C. rDBL3γ (5 μg ml−1) was added to wells and allowed to bind overnight at 4°C. Unbound rDBL3γ was removed by washing with PBST four times. A mouse monoclonal antibody against penta-histidine (penta-His) (QIAGEN) was added to each well at a 1:5,000 dilution and incubated at 37°C for 1 h to detect bound rDBL3γ. Bound mouse anti-penta-His monoclonal antibodies were detected with goat anti-mouse IgG peroxidase conjugate (Sigma) (1: 5,000 dilution) and O-phenylenediamine (OPD) (Sigma). The optical density at 490 nm was measured using an enzyme-linked immunosorbent assay (ELISA) plate reader (Molecular Devices) to estimate relative binding efficiencies. Biotinylated hyaluronic acid (Bio-HA) and the recombinant receptor for complement component C1q, also known as hyaluronic acid-binding protein 1 (gC1qR/HABP1) (17) (kindly provided by Anup Biswas and Kasturi Datta, Jawaharlal Nehru University, New Delhi, India) were used as controls.
Immunization of BALB/c mice.
BALB/c mice were immunized intraperitoneally with 25 μg of rDBL3γ formulated in 250 μl Freund's complete adjuvant and subsequently given three booster immunizations with 25 μg of rDBL3γ formulated with Freund's incomplete adjuvant on days 21, 42, and 73. Sera were collected on days 14, 35, 56, 70, and 87 and stored at −80°C until use.
Inhibition of binding of CSA to rDBL3γ by anti-rDBL3γ sera raised in mice.
rDBL3γ (100 μl of 5 μg ml−1 per well) was coated in 96-well polystyrene microtiter plates (Nunc) by incubation overnight at 4°C followed by blocking with 3% BSA. Microtiter plate wells were incubated for 1 h at 37°C with different dilutions of mouse sera raised against rDBL3γ or human sera from areas of endemicity. Pooled preimmune mice sera were used as controls. One hundred microliters of Bio-CSA (5 μg ml−1) was added to each well and allowed to bind for 1 h at 37°C. Following four washes with PBST, bound Bio-CSA was detected with Extravidin peroxidase and OPD (Sigma). The optical densities at 490 nm were used to estimate the relative binding efficiencies to CSA in the presence of test sera compared to those of controls.
Recognition of P. falciparum IEs by anti-rDBL3γ mouse sera using FACS.
Recognition of P. falciparum IEs by mouse anti-rDBL3γ sera was evaluated by fluorescence-assisted cell sorting (FACS) as described earlier (50). Briefly, 5 × 106 erythrocytes from asynchronous P. falciparum cultures were washed twice with RPMI medium, pH 6.8, and incubated for 30 min at 37°C with ethidium bromide (5 μg ml−1). After two washes, cells were incubated with anti-rDBL3γ mouse sera diluted 1:20 for 30 min at 4°C. Preimmune mouse serum was used as a negative control. Cells were washed two times with RPMI medium, pH 6.8, and incubated with anti-mouse IgG (heavy plus light chains) chicken at a dilution of 1:200 for 30 min on ice. Erythrocytes were washed twice with RPMI medium and incubated with a goat anti-chicken IgY conjugated to Alexa-488 (Molecular Probes) at a dilution of 1:200 for 30 min on ice. The recognition of intact IEs by antibodies was quantified with a Coulter EPICS flow cytometer and expressed as the mean Alexafluor intensity of ethidium bromide-gated IEs. Mouse sera raised against rDBL3γ were tested at a 1:20 dilution by both FACS and liquid immunofluorescence assay (L-IFA). Preimmune mouse sera were used as controls.
Inhibition of cytoadhesion of IE under static conditions by anti-rDBL3γ sera.
Static cytoadherence assays were performed as described previously (26). Briefly, Saimiri monkey brain endothelial C2 (SBEC C2) cells were grown to confluence on 12-well slides (Bio-Rad). Trophozoite- and schizont-stage IEs (20 μl of 107 IEs ml−1) were added to each well to allow binding. Heat-inactivated (56°C) anti-rDBL3γ mouse sera (dilution of 1:20) and soluble CSA (100 μg ml−1) (Calbiochem) were tested for inhibition of binding. Cytoadherence medium and mouse preimmune serum served as controls. In another experiment, SBEC C2 cells were preincubated with rDBL3γ (200 μg ml−1) prior to the addition of IEs. Adherent IEs were detected by Giemsa staining and scored by light microscopy. Results were expressed as percent inhibition of binding with respect to the appropriate controls.
Inhibition of cytoadherence of IEs to placental cryosections with anti-rDBL3γ mouse sera under flow conditions.
The cytoadherence of IEs on placental cryosections was tested under flow conditions as described earlier (2, 26). Briefly, slides of four cryosections from each of two different placentas were mounted in a cell adhesion flow chamber (CAF-10; Immunetics) held in place by vacuum. The system was connected to an infusion/withdrawal pump (model 210P; KD Scientific) to control the flow of IE suspension or cell-free medium through the perfusion chamber. Reservoirs containing the IE suspension or cytoadhesion medium (RPMI medium, pH 6.8) were connected to the outlet of the perfusion chamber through a three-way valve. The cryosections were perfused with IEs at 5% parasitemia (mature stages) and 25% hematocrit in RPMI medium for 10 min at a shear stress of 0.05 Pa. The chamber was then flushed with RPMI medium to remove nonadherent IEs. Adherent IEs were observed with an inverted microscope (Eclipse TE 200; Nikon) with a PlanFluor 40/0.60 objective (Nikon). Bound IEs were counted on 10 randomly distributed fields with an area of 0.081 mm2. Assays were performed in the presence of anti-rDBL3γ mouse serum (1:20 dilution) to test the ability of the IEs to block adhesion. Results were expressed as percent inhibition of binding with respect to binding in presence of preimmune sera.
Parasites and cells.
P. falciparum laboratory strains as well as field isolates were cultured in O+ erythrocytes in RPMI 1640 (Sigma, France) in candle jars as described previously (55). P. falciparum laboratory strains FCR3CSA (9) and BC-1-CSA (kindly provided by Artur Scherf, Institut Pasteur, Paris, France) and P. falciparum placental isolates 24-CSA, 42-CSA, 42DJ-CSA, 193-CSA, 938-CSA, and 939-CSA (29), all of which bind CSA, were used for the study. In addition, P. falciparum strains FCR3CD36 (9), D10, and T996 (kindly provided by David Walliker, University of Edinburgh) and peripheral isolates RAJ-68, RAJ-104, and JDP8 (collected from regions of India where malaria is endemic and kindly provided by C. R. Pillai, Malaria Research Centre, Delhi, India), which do not bind CSA, were used as controls. The CSA-binding ability of placental isolates and CSA-binding laboratory strains was maintained by periodic panning on Sc17 cells (29).
Placental cryosections.
Four cryosections from each of two different normal placentas collected in France were mounted sideways in the centers of 76- by 25-mm superfrost glass slides (Menzel-Glaser, Germany) for cytoadherence analysis under flow conditions. Placental cryosections (7 μm) were cut with a cryotome (CM 3050; Leica, Germany), air dried, fixed in 2.5% paraformaldehyde in PBS, pH 7.2, for 30 min, washed with PBS, dried, and preserved in an airtight box at −80°C until use. Before being used in adhesion assays, the slides were air dried quickly to prevent the condensation of water. Informed consent was obtained from all the participants. Biological material was sampled in strict accordance with the Mattei Law 666-8.
Identification of CSA-binding DBL domains of var2CSA by expression on the surface of mammalian cells.
Plasmids were constructed to express the DBL domains (DBL1X to DBL6ɛ) of var2CSA of P. falciparum 3D7 (PFL0030c) on the surface of mammalian cells as described previously for other DBL domains (12, 34, 38). Amino acid boundaries of each DBL domain construct were as follows: for DBL1X, amino acids (aa) 46 to 343; for DBL2X, aa 535 to 934; for DBL3X, aa 1214 to 1562; for DBL4ɛ, aa 1581 to 1931; for DBL5ɛ, aa 1983 to 2291; and for DBL6ɛ, aa 2333 to 2617. Briefly, the DBL domains were fused to the signal sequence and transmembrane domain of herpes simplex virus glycoprotein D (HSV gD) to allow targeting to the mammalian cell surface as described previously (12, 34, 38). The plasmid pRE4 (kindly provided by Roselyn Eisenberg and Gary Cohen), which contains the gene for HSV gD (14), was digested with PvuII and ApaI to excise the central region encoding amino acids 33 to 248 of HSV gD. DNA fragments encoding DBL domains of 3D7 var2CSA were amplified by PCR using Pyrococcus furiosus DNA polymerase (Stratagene) by use of var2CSA-specific primers and cloned into PvuII and ApaI sites in the vector pRE4 as previously described (12) to yield plasmids pRE4-DBL1X, pRE4-DBL2X, pRE4-DBL3X, pRE4-DBL4ɛ, pRE4-DBL5ɛ, and pRE4-DB6ɛ. The DNA sequence of the insert in each expression plasmid was confirmed by sequencing with an ABI310 automated DNA sequencer. The sequence of each insert was identical to that reported for 3D7 var2CSA (PFL0030c; GenBank accession no. NP_701371). Mammalian 293T cells were transfected as described below to express the DBL domains (DBL1X to DBL6ɛ) on the surface. Transfected cells were tested for binding to CSA as described previously (34). CSA-binding assays were performed in the presence of anti-rDBL3γ mouse sera and preimmune sera to test the abilities of sera to block binding.
Mammalian cell culture, transfection, and immunofluorescence assays.
293T cells were cultured in Dulbecco's modified Eagle's medium (Invitrogen) with 10% heat-inactivated fetal calf serum in a humidified CO2 (5%) incubator at 37°C. Fresh monolayers of 40 to 60% confluent 293T cells growing in 35-mm-diameter wells were transfected with 2 μg of plasmid DNA using Lipofectamine Plus reagent (Invitrogen) as indicated by the manufacturer. Transfected cells were used for immunofluorescence and binding assays 36 to 40 h after transfection (34). Immunofluorescence assays using mouse monoclonal antibody DL6 (kindly provided by Roselyn Eisenberg and Gary Cohen), which reacts with amino acids 272 to 279 (14) of HSV gD, or anti-rDBL3γ mouse sera were performed as described earlier (12) to detect the expression of the fusion proteins. The binding of Bio-CSA to transfected 293T cells was tested as previously described (34).
Binding of refolded and purified rDBL3γ to biotinylated CSA immobilized on streptavidin-coated microwells by ELISA.
One hundred microliters of Bio-CSA, Bio-CSB, or Bio-CSC was immobilized on each well of streptavidin-coated microwell plate (Stretawell-96; Roche Applied Science) as indicated by the manufacturer. Five hundred nanograms of refolded and purified rDBL3γ was incubated overnight at 4°C, and unbound rDBL3γ was removed by washing four times with PBST. Residual free sites in the wells were saturated with 3% BSA in PBST for 1 h at 37°C, and the wells were washed four times with PBST. One hundred microliters of anti-penta-His antibody (Sigma) diluted to 1:5,000 was added to each well, and the wells were incubated at 37°C for 1 h, followed by four washes with PBST. One hundred microliters of Extravidin peroxidase (Sigma) (1:5,000 dilution) was incubated in each well for 1 h at 37°C. Bound immunocomplexes were detected with OPD (Sigma). Absorbance was measured at 490 nm using an ELISA reader (Molecular Devices).
Inhibition of binding of rDBL3γ-CSA binding by anti-rDBL3γ sera raised in mice.
Anti-rDBL3γ mouse sera were tested for inhibition of the rDBL3γ-CSA interaction. Microtiter plate wells were coated with 100 μl of rDBL3γ (5 μg ml−1). Plates were incubated at 4°C overnight and blocked with 3% BSA as described above. Microtiter plate wells were incubated with different dilutions of mouse antibodies raised against rDBL3γ. Preimmune sera were used as controls. Five hundred nanograms of Bio-CSA was incubated in each well at 37°C for 1 h. After being washed with PBST four times, the well was incubated with Extravidin peroxidase (Sigma) for 1 h at 37°C. Results were expressed as the percentages of binding observed with respect to the values for controls.
RESULTS
Purity, homogeneity, and functional activity of rDBL3γ.
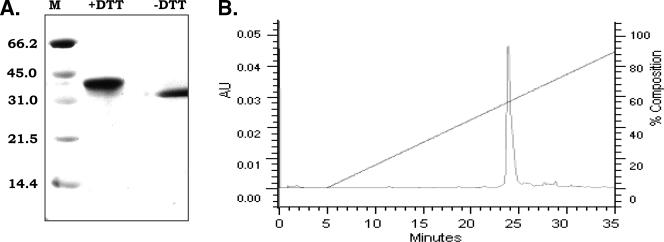
The DBL3γ domain of var1CSA derived from P. falciparum FCR3 was expressed in E. coli, purified from inclusion bodies under denaturing conditions by metal affinity chromatography, refolded by rapid dilution, and purified to homogeneity by ion-exchange and gel filtration chromatography. Refolded and purified rDBL3γ was analyzed for purity, homogeneity, and functional activity. A single band of the expected size (∼38 kDa) was detected on silver-stained SDS-PAGE gels (Fig. 1). rDBL3γ migrated slower on SDS-PAGE gels after reduction with dithiothreitol, indicating that the refolded protein contained disulfide linkages (Fig. 1). The free thiol content in rDBL3γ, which contains 10 cysteines, was measured using Ellman's method (22). The detection limit for free thiols by this assay was 30 μM. No free thiols were detected in rDBL3γ at a protein concentration of 50 μM, indicating that >94% of cysteines were disulfide linked. The homogeneity of refolded rDBL3γ was analyzed by reverse-phase chromatography. rDBL3γ eluted from a C8 column as a single symmetric peak, suggesting that refolded rDBL3γ was conformationally homogeneous (Fig. 1).
FIG. 1.
Characterization of refolded and purified DBL3γ domain (rDBL3γ) of FCR3 var1CSA. (A) Mobility of rDBLγ as determined by SDS-PAGE. Refolded rDBLγ has lower mobility in SDS-PAGE after reduction with dithiothreitol (+DTT), indicating the presence of disulfide linkages. Molecular mass markers (M) in kDa are shown. (B) Reverse-phase chromatography profile of rDBL3γ. Refolded DBL3γ elutes as a single, symmetric peak upon reverse-phase chromatography on a C8 column, indicating that it is conformationally homogenous. AU, absorbance units (280 nm).
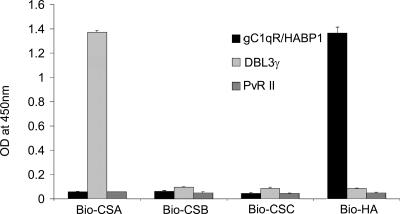
The functional activity of rDBL3γ was examined by testing the binding of rDBL3γ with its receptor, CSA. Biotinylated receptors, namely, Bio-CSA, Bio-CSB, Bio-CSC, and Bio-HA, were immobilized on streptavidin-coated ELISA plate wells and incubated with refolded rDBL3γ to allow binding. Bound rDBL3γ was detected using a mouse monoclonal antibody against the C-terminal penta-histidine tag. Binding of rDBL3γ was observed only for wells coated with Bio-CSA (Fig. 2). The lack of binding to CSB, CSC, and HA indicates that rDBL3γ bound CSA with specificity. The human endothelial receptor gC1qR/HABP1, which binds the globular head of complement component C1q as well as HA (17), and the receptor-binding domain, region II (PvRII), of P. vivax Duffy binding protein (12), were used as control ligands. PvRII did not bind CSA, CSB, CSC, or HA (Fig. 2). Recombinant gC1qR/HABP1 bound HA as expected but not CSA, CSB, or CSC (Fig. 2).
FIG. 2.
Binding of rDBL3γ to CSA. Binding of rDBL3γ to Bio-CSA, Bio-CSB, Bio-CSC, and Bio-HA immobilized on streptavidin-coated microwells was detected using a mouse monoclonal antibody against penta-His. Recombinant PvRII, the binding domain of PvDBP, and recombinant gC1qR/HABP1, which binds HA, were used as control ligands. rDBL3γ binds CSA but not CSB, CSC, or HA. gC1qR/HABP1 binds HA but not CSA, CSB, or CSC. PvRII does not bind any of the receptors tested. OD, optical density.
Mouse sera raised against rDBL3γ recognize diverse CSA-binding P. falciparum laboratory strains and placental field isolates.
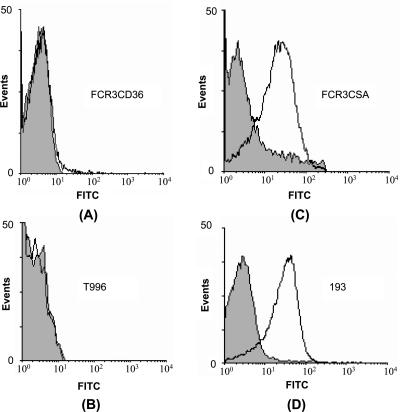
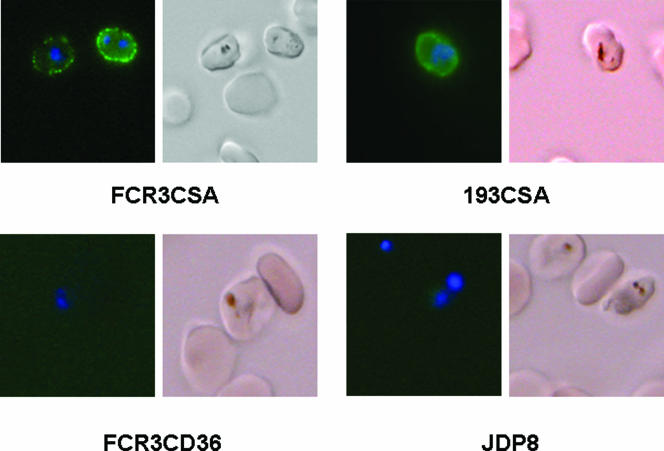
Pooled sera from five mice immunized with rDBL3γ detected rDBL3γ by ELISA up to a dilution of 1:100,000 and blocked the binding of rDBL3γ to CSA in an ELISA-based binding assay with 50% inhibition at a dilution of 1:500. Anti-rDBL3γ mouse sera were tested for recognition of diverse P. falciparum laboratory strains and field isolates by FACS and L-IFA. The P. falciparum laboratory strains and field isolates were first tested for binding to SBEC C2 cells, which display CSA on their surface (26). P. falciparum placental isolates bound SBEC C2 cells (Table 1) . The addition of CSA (100 μg ml−1) inhibited the binding of placental isolates and CSA-binding laboratory strains to SBEC C2 cells, confirming that these parasites used CSA as a receptor for adhesion to SBEC C2 cells (Table 1). Preincubation of SBEC C2 cells with rDBL3γ also inhibited the adhesion of IEs (Table 1). Anti-rDBL3γ mouse sera recognized CSA-binding P. falciparum laboratory strains and placental isolates by FACS as well as L-IFA (Fig. 3 and 4; Table 1). In contrast, anti-rDBL3γ mouse sera did not recognize P. falciparum laboratory strains or field isolates that did not bind CSA (Fig. 3; Table 1). In the FACS studies, specific shifts characteristic of Alexafluor-stained IEs were observed in the cases of CSA-binding laboratory strains, such as FCR3CSA, and placental isolates, such as 193-CSA (Fig. 3). The fraction of erythrocytes with specific shifts was higher for CSA-binding isolates than for laboratory isolates such as FCR3CD36 and T996 and peripheral isolates RAJ68, RAJ104, and JDP8, which did not bind CSA (Fig. 3; Table 1). The pattern of recognition of CSA-binding IEs by anti-rDBL3γ sera by L-IFA clearly indicated reactivity with the surface of IEs (Fig. 4). Anti-rBL3γ sera thus recognized common conserved epitopes that are shared by diverse CSA-binding P. falciparum isolates. Preimmune sera collected from mice prior to immunization with rDBL3γ showed no reactivity with any of the parasites when tested by FACS or L-IFA. While reactivity with FCR3CSA was observed by FACS when anti-mouse IgG goat serum coupled to fluorescein isothiocyanate was used for secondary staining with anti-rBL3γ mouse sera for primary staining, no reactivity was observed when fluorescein isothiocyanate-coupled anti-mouse IgM goat serum was used for secondary staining (data not shown). This rules out the possibility that the reactivity of anti-rBL3γ mouse sera with CSA-binding isolates was due to nonspecific binding of mouse IgM.
TABLE 1.
Recognition of P. falciparum IEs by anti-DBL3γ mouse sera by L-IFA and FACS and inhibition of adhesion of P. falciparum IEs to SBEC C2 cells by CSA and recombinant DBL3γ
| Parasite straina | Source | % Inhibition of binding to SBEC C2 cells under static conditions (avg. ± SD) byb:
|
% Recognition byc:
|
||
|---|---|---|---|---|---|
| rDBL3γ | CSA | L-IFA | FACS | ||
| FCR3CSA | Lab strain | 94 ± 2 | 96 ± 1 | 65 | 46.1 |
| BC-1-CSA | Lab strain | 89 ± 2 | 94 ± 1 | 32 | 24.3 |
| D10 | Lab strain | NT | NT | 0 | 2.83 |
| T996 | Lab strain | NT | NT | 0 | 0.86 |
| FCR3CD36 | Lab strain | NT | NT | 0 | 3.57 |
| 24-CSA | Placental isolate | 84 ± 3 | 92 ± 2 | 59 | 44.2 |
| 42-CSA | Placental isolate | 87 ± 5 | 90 ± 3 | 87 | 72.9 |
| 42DJ-CSA | Placental isolate | 83 ± 3 | 92 ± 2 | 39 | 44.1 |
| 193-CSA | Placental isolate | 90 ± 2 | 95 ± 1 | 94 | 87.1 |
| 938-CSA | Placental isolate | 88 ± 3 | 93 ± 3 | 42 | 21.9 |
| 939-CSA | Placental isolate | 86 ± 2 | 94 ± 2 | 51 | 32.23 |
| RAJ-68 | Peripheral isolate | NT | NT | 0 | 2.67 |
| RAJ-104 | Peripheral isolate | NT | NT | 0 | 3.54 |
| JDP8 | Peripheral isolate | NT | NT | 0 | 2.3 |
P. falciparum peripheral and placental field isolates as well as P. falciparum laboratory strains were tested for binding to SBEC C2 cells. P. falciparum laboratory (Lab) strains FCR3CD36, D10, and T996 and field isolates RAJ-68, RAJ-104, and JDP8 collected from peripheral blood of nonpregnant donors do not bind SBEC C2 cells and were not tested (NT) for inhibition of binding with CSA and rDBL3γ.
Number of IEs bound to SBEC C2 cells in control wells was in the range of 90 to 125 bound IEs per field. Results represent inhibition efficiencies (average± standard deviation) determined from three independent experiments. Each assay was performed in duplicate wells in each experiment.
Mouse sera raised against rDBL3γ of FCR3 var1CSA were tested for recognition of P. falciparum laboratory strains and field isolates by L-IFA and FACS. The percentages of IEs that reacted with anti-rDBL3γ sera by L-IFA and FACS are reported.
FIG. 3.
Recognition of diverse P. falciparum isolates by mouse sera raised against rDBL3γ of FCR3 var1CSA by use of flow cytometry. Mouse sera raised against rDBL3γ were tested for recognition of P. falciparum FCR3CD36 (A) and T996 (B), which do not bind CSA, and of FCR3CSA (C) and 193 (D), which bind CSA. Gray areas represent signals from preimmune sera.
FIG. 4.
Recognition of surfaces of P. falciparum IEs by anti-rDBL3γ mouse sera using L-IFA. Mouse sera raised against rDBL3γ of FCR3 var1CSA react with the IE surface of P. falciparum laboratory strain FCR3CSA and that of placental isolate 193CSA, which bind CSA, but not with that of P. falciparum laboratory strain FCR3CD36 or that of peripheral field isolate JDP8, which do not bind CSA. Parasites were stained with DNA-intercalating dye DAPI (blue) to identify IEs and with anti-rDBL3γ mouse sera followed by Alexafluor 488-conjugated chicken anti-mouse IgG (green).
Mouse sera raised against rDBL3γ block adhesion of diverse CSA-binding P. falciparum laboratory strains and field isolates to Saimiri monkey brain endothelial cells and placental cryosections.
Anti-rDBL3γ mouse sera and preimmune mouse sera were tested for inhibition of binding of IEs to SBEC C2 cells and placental cryosections. Anti-rDBL3γ sera inhibited the binding of diverse CSA-binding P. falciparum laboratory strains and placental isolates to SBEC C2 cells under static conditions (Table 2). Anti-rDBL3γ sera also blocked the adhesion of diverse CSA-binding parasite isolates to placental cryosections under flow conditions (Table 2). rDBL3γ was thus immunogenic and elicited cross-reactive antibodies that blocked the cytoadherence of diverse CSA-binding P. falciparum laboratory strains and placental isolates under both static and flow conditions.
TABLE 2.
Inhibition of adhesion of P. falciparum isolates to SBEC C2 cells under static conditions and to placental cryosections under flow conditions by anti-rDBL3γ mouse sera
| Parasite strain | % Inhibition by anti-rDBL3 γ mouse sera (avg ± SD) of IE binding to:
|
|
|---|---|---|
| SBEC C2 (static)a | Placenta (flow)b | |
| FCR3CSA | 86 ± 1 | 76 ± 5 |
| BC1-1CSA | 90 ± 3 | 82 ± 5 |
| 24-CSA | 86 ± 3 | 88 ± 2 |
| 42DJ-CSA | 86 ± 4 | 79 ± 9 |
| 42-CSA | 90 ± 5 | 93 ± 3 |
| 193-CSA | 91 ± 1 | 93 ± 9 |
| 938-CSA | 88 ± 3 | 90 ± 4 |
| 939-CSA | 89 ± 3 | 83 ± 4 |
Anti-rDBL3γ mouse sera (1:20 dilution) were tested for inhibition of binding of IEs to SBEC C2 cells in static binding assays. Binding in the presence of preimmune mouse sera (1:20 dilution) was used as a control to determine inhibition efficiencies of anti-rDBL3γ sera. The number of IEs bound to SBEC C2 cells was scored in five random fields in each well by Giemsa staining. Results represent inhibition efficiencies (average ± standard deviation) determined from three independent experiments. Each assay was performed in duplicate wells in each experiment. The number of IEs bound to SBEC C2 cells in control wells was in the range of 90 to 125 bound IEs per field.
Anti-rDBL3γ pooled mouse sera were tested at a dilution of 1:20 for the inhibition of adhesion of IEs to placental cryosections under flow conditions. Binding in the presence of preimmune mouse sera (1:20 dilution) was used as a reference to determine the inhibition efficiencies of anti-rDBL3γ sera. Results represent inhibition efficiencies (average ± standard deviation) determined from two independent experiments. Assays were performed with two different placental cryosections, each of which was mounted in duplicate flow cells for each experiment. The number of bound IEs was scored in 10 fields per flow cell in both experiments. The number of bound IEs in the presence of preimmune serum was used as a reference. The number of bound IEs in control wells was in the range of 213 to 328 per field.
Anti-rDBL3γ mouse sera recognize DBL3X of var2CSA and block its binding to CSA.
Initial studies indicated that var1CSA is responsible for the CSA-binding phenotype of FCR3CSA (9). However, subsequent studies demonstrated that transcription of another var gene, var2CSA, is upregulated in CSA-binding parasites (42, 56). Here, we have expressed the DBL domains of var2CSA from P. falciparum 3D7 (DBL1X, DBL2X, DBL3X, DBL4ɛ, DBL5ɛ, and DBL6ɛ) on the surface of mammalian 293T cells and tested them for binding to Bio-CSA. In control experiments, the DBL domains were tested for binding to Bio-CSB and Bio-CSC. The DBL3γ domain of var1CSA was used as a positive control in the binding studies. The DBL2X and DBL3X domains of var2CSA specifically bound Bio-CSA in this study (Table 3). Soluble CSA, but not CSB or CSC, inhibited the binding of DBL2X, DBL3X, and DBL3γ to Bio-CSA, confirming that these domains bound CSA with specificity (Table 4) . Mouse sera raised against DBL3γ of var1CSA recognized DBL3γ as well as DBL3X expressed on the surface of 293T cells (Table 3). Importantly, anti-rDBL3γ mouse sera completely blocked the binding of both DBL3γ and DBL3X domains with CSA at a dilution of 1:20 (Table 4). The DBL3γ domain of var1CSA thus shares cross-reactive epitopes with the DBL3X domain of var2CSA. Antisera elicited against DBL3γ did not recognize DBL2X of var2CSA and did not block its binding to CSA (Tables 3 and 4). Anti-rBL3γ sera also did not recognize any of the other DBL domains of var2CSA (Table 3).
TABLE 3.
Binding of DBL domains of 3D7 var2CSA to CSA and recognition by mouse sera raised against DBL3γ of FCR3 var1CSA
| Construct | Frequency of reactivity (%)a
|
Binding efficiency (%)b
|
|||
|---|---|---|---|---|---|
| DL6 | Anti-rDBL3γ antibodies | CSA | CSB | CSC | |
| pRE4-DBL3γ | 74 ± 4 | 63 ± 4 | 72 ± 5 | 0 | 0 |
| pRE4-DBL1X | 70 ± 8 | 0 | 0 | 0 | 0 |
| pRE4-DBL2X | 60 ± 6 | 0 | 58 ± 6 | 0 | 0 |
| pRE4-DBL3X | 66 ± 8 | 57 ± 3 | 60 ± 10 | 0 | 0 |
| pRE4-DBL4ɛ | 58 ± 4 | 0 | 0 | 0 | 0 |
| pRE4-DBL5ɛ | 63 ± 7 | 0 | 0 | 0 | 0 |
| pRE4-DBL6ɛ | 54 ± 6 | 0 | 0 | 0 | 0 |
| pRE4 | 83 ± 4 | 0 | 0 | 0 | 0 |
The DBL3γ domain of FCR3 var1CSA and all the DBL domains (DBL1X to DBL6ɛ) of 3D7 var2CSA were expressed on the surfaces of mammalian 293T cells as fusions to HSV gD by use of the transfection vector pRE4. Mouse monoclonal antibody DL6 directed against HSV gD sequences in the fusion proteins and mouse polyclonal sera raised against DBL3γ of FCR3 var1CSA were used to detect expression of DBL domains on 293T cell surfaces. Preimmune mouse serum does not react with any cells transfected with any of the DBL constructs tested. Around 1,000 to 1,500 293T cells were scored for recognition by DL6 and anti-rDBL3γ mouse sera in immunofluorescence assays, and the frequencies of reactivity were determined for both antibodies. Results represent the average and standard deviation of three independent experiments.
Transfected 293T cells were incubated with Bio-CSA, Bio-CSB, and Bio-CSC to allow binding followed by incubation with anti-biotin sera raised in mice. Anti-mouse IgG chicken IgY conjugated with Alexafluor 488 (Molecular Probes) was used to detect binding of biotinylated CSA, CSB, and CSC. Around 1,000 to 1500 293T cells stained with DAPI were scored for staining with Alexafluor 488. The binding efficiency was calculated as follows: binding efficiency (%) = [(number of Alexafluor 488-stained 293T cells)/(number of DAPI-stained 293T cells] × 100. Where the binding efficiency is reported as zero, no Alexafluor 488-stained cells were seen in the entire well. Results reported are average ± standard deviation from three independent experiments.
TABLE 4.
Inhibition of binding of CSA to DBL3γ domain of FCR3 var1CSA and DBLX domains of 3D7 var2CSA with mouse sera raised against rDBL3γ
| Construct | Transfection efficiency (%) | Efficiency of Bio-CSA binding (%) after preincubation witha:
|
||||
|---|---|---|---|---|---|---|
| Preimmune sera | Anti- rDBL3γ sera | CSA | CSB | CSC | ||
| pRE4-DBL3γ | 79 ± 3 | 81 ± 3 | 0 | 0 | 76 ± 12 | 72 ± 6 |
| pRE4-DBL1X | 68 ± 5 | 0 | 0 | 0 | 0 | 0 |
| pRE4-DBL2X | 61 ± 6 | 57 ± 4 | 55 ± 6 | 0 | 54 ± 6 | 64 ± 5 |
| pRE4-DBL3X | 66 ± 6 | 62 ± 3 | 0 | 0 | 68 ± 9 | 63 ± 8 |
| pRE4-DBL4ɛ | 58 ± 4 | 0 | 0 | 0 | 0 | 0 |
| pRE4-DBL5ɛ | 60 ± 6 | 0 | 0 | 0 | 0 | 0 |
| pRE4-DBL6ɛ | 51 ± 5 | 0 | 0 | 0 | 0 | 0 |
Transfected 293T cells were preincubated with preimmune mouse sera (1:20 dilution), anti-rDBL3γ mouse sera (1:20 dilution), CSA (200 μg ml−1), CSB (200 μg ml−1), and CSC (200 μg ml−1) prior to incubation with Bio-CSA. The efficiency of Bio-CSA binding was determined as described for Table 3. Results reported are average± standard deviation of three independent experiments. Where the binding efficiency is reported as zero, no Alexafluor 488-stained cells were seen in the entire well.
DISCUSSION
The epidemiology of naturally acquired immunity against placental malaria suggests that multigravid women who have experienced placental malaria rapidly develop antibodies that cross-react with a wide range of placental isolates. This suggests that placental isolates may use a limited number of CSA-binding domains that share common B-cell epitopes. The first parasite-derived domain shown to bind CSA was the DBL3γ domain of var1CSA from the CSA-binding laboratory strain FCR3CSA (9). Later studies indicated that the expression of var1CSA is not restricted to placental isolates and that the expression of another gene, var2CSA, is upregulated in FCR3CSA and other CSA-binding placental and laboratory isolates (44, 45, 56). However, antibodies raised against rDBL3γ of var1CSA were shown to cross-react with a wide range of CSA-binding placental and laboratory isolates (15, 32). This result was paradoxical, given that CSA-binding parasites primarily express var2CSA, which does not contain any DBLγ domains. Here, we have produced recombinant DBL3γ from FCR3 var1CSA in its functional form and reexamined its immunogenicity. Characterization of rDBL3γ confirmed that it was pure, homogenous, and functional in that it bound CSA with specificity. We demonstrate here that the immunization of mice with rDBL3γ of FCR3 var1CSA elicited antibodies that recognize the homologous parasite FCR3CSA as well as a wide range of heterologous P. falciparum laboratory strains and placental field isolates that bind CSA (Fig. 3 and 4; Table 1). In contrast, anti-rBL3γ sera did not recognize the IE surfaces of P. falciparum peripheral isolates and strains that do not bind CSA (Fig. 3 and Table 1). Given the questions raised about the role of var1CSA in CSA binding and placental malaria, our confirmation of previous results demonstrating the cross-reactivity of anti-rBL3γ sera with placental isolates is important and reassuring.
However, how does one explain the observed cross-reactivity of anti-rBL3γ sera with CSA-binding placental isolates and laboratory strains, given that they primarily express var2CSA, which does not contain any DBLγ domains? In order to investigate this paradox, we expressed the DBL domains of var2CSA derived from P. falciparum 3D7 on the surface of 293T cells and tested them for binding to CSA and recognition by anti-rBL3γ sera. The DBL2X and DBL3X domains of var2CSA bound CSA in these assays. DNA sequencing confirmed that the sequences of the inserts in the different var2CSA expression constructs used here were identical to the published 3D7 var2CSA sequence. A previous study has reported that the DBL2X and DBL6ɛ domains of 3D7 var2CSA have CSA-binding activity (27). The differences observed between the CSA-binding activity of DBL domains of var2CSA here and that found in the previous study may be due to differences in the domain boundaries of the DBL constructs or differences in the expression systems and cells used for transfection in the two studies. Importantly, we found that anti-rBL3γ sera cross-reacted with the DBL3X domain of var2CSA and blocked the binding of DBL3X with CSA. Anti-rDBL3γ sera did not recognize any of the other DBL domains of var2CSA. The recognition of DBL3X by anti-rDBL3γ sera indicates the presence of common cross-reactive B-cell epitopes in these two diverse CSA-binding DBL domains and explains the paradoxical observations made here and earlier (11, 15, 32) that anti-rDBL3γ sera recognize CSA-binding parasites. We have demonstrated that in addition to recognizing a wide range of placental parasites, anti-rDBL3γ sera block adhesion of IEs to placental cryosections under physiologically relevant flow conditions. This observation indicates that it may be possible to develop prophylactic strategies that block placental sequestration of malaria parasites.
The B-cell epitopes that are recognized by protective antibodies from sera of multigravid women have not yet been identified. Following the identification of var2CSA as the expressed var gene in CSA-binding laboratory strains and placental isolates, recent efforts to identify targets of antibodies that protect against PAM have focused on CSA-binding DBL domains of var2CSA. Here, we have demonstrated that the CSA-binding DBL domains of var1CSA and var2CSA, namely, DBL3γ and DBL3X, respectively, share common B-cell epitopes. Such conserved epitopes that are shared by diverse CSA-binding DBL domains may serve as targets for protective antibodies and form the basis for development of a vaccine against PAM.
Acknowledgments
We thank Artur Scherf, Institut Pasteur, Paris, France, for providing a plasmid with FCR3 var1CSA gene; Gary Cohen and Roselyn Eisenberg, University of Pennsylvania, Philadelphia, Pennsylvania, for providing plasmid pRE4 and monoclonal antibody DL6; Anup Biswas and Kasturi Datta for providing recombinant gC1qR/HABP1; C. R. Pillai for providing P. falciparum field isolates RAJ68, RAJ104, and JDP8; Iréne Juhan-Vague, Laboratoire d'Hématologie, Marseille, France, for access to FACS; and Catherine Lepolard and Bruno Pouvelle, Université de la Méditerranée, for parasite cultures.
This work was supported by an International Senior Research Fellowship to C.E.C. from the Wellcome Trust, United Kingdom, and by grants to J.G. from BIOMALPAR program, an FP6 funded network of excellence; PAL +2002 of the MENRT; ACI program, INSERM MIC no. 0318; and the Malaria Antigen Discovery Program (MADP) Malaria in Pregnancy Initiative, Bill and Melinda Gates Foundation, no. 29202.
Editor: J. L. Flynn
REFERENCES
- 1.Achur, R. N., M. Vialyavetti, A. Alkhalil, C. F. Ockenhouse, and D. C. Gowda. 2000. Characterization of proteoglycans of human placenta and identification of unique chondroitin sulfate proteoglycans of the intervillous spaces that mediate the adherence of Plasmodium falciparum-infected erythrocytes to the placenta. J. Biol. Chem. 275:40344-40356. [DOI] [PubMed] [Google Scholar]
- 2.Avril, M., B. Traore, F. T. M. Costa, C. Lepolard, and J. Gysin. 2004. Placenta cryosection for study of the adhesion of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate A in flow conditions. Microbes Infect. 6:249-255. [DOI] [PubMed] [Google Scholar]
- 3.Baruch, D. I., B. L. Pasloske, H. B. Singh, X. Bi, X. C. Ma, M. Feldman, T. F. Taraschi, and R. J. Taraschi. 1997. Cloning the Plasmodium falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell 82:77-87. [DOI] [PubMed] [Google Scholar]
- 4.Beeson, J. G., G. V. Brown, M. E. Molyneix, C. Mhango, F. Dzinjalamala, and S. Rogerson. 1999. Plasmodium falciparum isolates from infected pregnant women and children are associated with distinct adhesive and antigenic properties. J. Infect. Dis. 180:464-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beeson, J. G., S. J. Rogerson, B. M. Cooke, J. C. Reeder, W. Chai, A. M. Lawson, M. E. Molyneux, and G. V. Brown. 2000. Adhesion of Plasmodium falciparum-infected erythrocytes to hyaluronic acid in placental malaria. Nat. Med. 6:86-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beeson, J. G., and G. V. Brown. 2004. Plasmodium falciparum-infected erythrocytes demonstrate dual specificity for adhesion to hyaluronic acid and chondroitin sulfate A and have distinct adhesive properties. J. Infect. Dis. 189:169-179. [DOI] [PubMed] [Google Scholar]
- 7.Brabin, B. J. 1983. An analysis of malaria in pregnancy in Africa. Bull. W. H. O. 61:1005-1016. [PMC free article] [PubMed] [Google Scholar]
- 8.Brabin, B. J., M. Hakimi, and D. Pelletier. 2001. An analysis of anemia and pregnancy-related maternal mortality. J. Nutr. 131:604S-615S. [DOI] [PubMed] [Google Scholar]
- 9.Buffet, P. A., B. Gamain, C. Scheidig, D. Baruch, J. D. Smith, and R. Hernandez-Rivas. 1999. Plasmodium falciparum domain mediating adhesion to chondroitin sulfate A: a receptor for human placental infection. Proc. Natl. Acad. Sci. USA 96:12743-12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bull, P. C., B. S. Lowe, M. Kortok, C. S. Molyneux, C. I. Newbold, and K. Marsh. 1998. Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat. Med. 4:358-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chia, Y. S., C. Badaut, N. G. Tuikue Ndam, A. Khattab, S. Igonet, N. Fievet, G. A. Bentley, P. Deloron, and M. Q. Klinkert. 2005. Functional and immunological characterization of a Duffy-binding-like gamma domain from Plasmodium falciparum erythrocyte membrane protein-1 expressed by a placental isolate. J Infect. Dis. 192:1284-1293. [DOI] [PubMed] [Google Scholar]
- 12.Chitnis, C. E., and L. H. Miller. 1994. Identification of the erythrocyte binding domains of Plasmodium vivax and Plasmodium knowlesi proteins involved in erythrocyte invasion. J. Exp. Med. 180:497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chitnis, C. E., P. Sinnis, and L. H. Miller. 1999. The sporozoite, the merozoite and the infected red cell: parasite ligands and host receptors, p. 249-285. In P. Perlmann and M. Wahlgren (ed.), Malaria: molecular and clinical aspects. Harwood Academic Publishers, Amsterdam, The Netherlands.
- 14.Cohen, G. H., W. C. Wilcox, D. L. Sodora, D. Long, J. Z. Levin, and R. J. Eisenberg. 1988. Expression of herpes simplex virus type 1 glycoprotein D deletion mutants in mammalian cells. J. Virol. 62:1932-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costa, F. T. M., T. Fusai, D. Parzy, Y. Sterkers, M. Torrentino, J. B. Douki, B. Traore, S. Petres, A. Scherf, and J. Gysin. 2003. Immunization with recombinant duffy binding-like-γ3 induces pan-reactive and adhesion blocking antibodies against placental chondroitin sulfate A-binding Plasmodium falciparum parasites. J. Infect. Dis. 188:153-164. [DOI] [PubMed] [Google Scholar]
- 16.Creasey, A. M., T. Staalsoe, A. Raza, D. E. Arnot, and J. A. Rowe. 2003. Nonspecific immunoglobulin M binding and chondroitin sulfate A binding are linked phenotypes of Plasmodium falciparum isolates implicated in malaria during pregnancy. Infect. Immun. 71:4767-4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deb, T. B., and K. Datta. 1996. Molecular cloning of human fibroblast hyaluronic acid binding protein confirms its identity with P-32, a protein co-purified with splicing factor SF2. Hyaluronic acid binding protein as P-32 protein, co-purified with splicing factor SF2. J. Biol. Chem. 271:2206-2212. [DOI] [PubMed] [Google Scholar]
- 18.Dorman, E. K., C. E. Shulman, J. Kingdom, J. N. Bulmer, J. Mwendwa, N. Peshu, and K. Marsh. 2002. Impaired uteroplacental blood flow in pregnancies complicated by falciparum malaria. Ultrasound Obstet. Gynecol. 19:165-170. [DOI] [PubMed] [Google Scholar]
- 19.Duffy, M. F., T. J. Byrne, S. R Elliot, D. W. Wilson, S. J. Rogerson, J. G. Beeson, R. Noviyanti, and G. V. Brown. 2005. Broad analysis reveals a consistent pattern of var gene transcription in Plasmodium falciparum repeatedly selected for a defined adhesion phenotype. Mol. Microbiol. 56:774-788. [DOI] [PubMed] [Google Scholar]
- 20.Duffy, P. E., and M. Fried. 2003. Antibodies that inhibit Plasmodium falciparum adhesion to chondroitin sulfate A are associated with increased birth weight and the gestational age of newborns. Infect. Immun. 71:6620-6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elliot, S. R., M. F. Duffy, T. J. Byrne, J. G. Beeson, E. J. Mann, D. W. Wilson, S. J. Rogerson, and G. V. Brown. 2005. Cross-reactive surface epitopes on chondroitin sulfate A-adherent Plasmodium falciparum-infected erythrocytes are associated with transcription of var2csa. Infect. Immun. 73:2848-2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellman, G. L. 1959. Tissue sulfhydril groups. Arch. Biochem. Biophys. 82:70-77. [DOI] [PubMed] [Google Scholar]
- 23.Flick, K., C. Scholander, Q. Chen, V. Fernandez, B. Pouvelle, J. Gysin, and M. Wahlgren. 2001. Role of nonimmune IgG bound to PfEMP1 in placental malaria. Science 293:2098-2100. [DOI] [PubMed] [Google Scholar]
- 24.Fried, M., and P. E. Duffy. 1996. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science 272:1502-1504. [DOI] [PubMed] [Google Scholar]
- 25.Fried, M., F. Nosten, A. Brockman, B. J. Brabin, and P. E. Duffy. 1998. Maternal antibodies block malaria. Nature 395:851-852. [DOI] [PubMed] [Google Scholar]
- 26.Fusai, T., D. Parzy, D. Spillmann, F. Eustacchio, B. Pouvelle, C. Lepolard, A. Scherf, and J. Gysin. 2000. Characterisation of the chondroitin sulphate of Saimiri brain microvascular endothelial cells involved in Plasmodium falciparum cytoadhesion. Mol. Biochem. Parasitol. 108:25-37. [DOI] [PubMed] [Google Scholar]
- 27.Gamain, B., A. R. Trimnell, C. Scheidig, A. Scherf, L. H. Miller, and J. D. Smith. 2005. Identification of multiple chondroitin sulfate A (CSA)-binding domains in the var2CSA gene transcribed in CSA-binding parasites. J. Infect. Dis. 191:1010-1013. [DOI] [PubMed] [Google Scholar]
- 28.Garner, P., and A. M. Gulmezoglu. 2003. Drugs for preventing malaria-related illness in pregnant women and death in the newborn. Cochrane Database Syst. Rev. 1:CD000169. [DOI] [PubMed] [Google Scholar]
- 29.Gysin, J., B Pouvelle, N. Fievet, C. Lepolard, and A. Scherf. 1999. Ex vivo desequestration of Plasmodium falciparum-infected erythrocytes from human placenta by chondroitin sulfate A. Infect. Immun. 67:6596-6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kyes, S. A., Z. Christodoulou, A. Raza, P. Horrocks, R. Pinches, J. A. Rowe, and C. I. Newbold. 2003. A well-conserved Plasmodium falciparum var gene shows an unusual stage-specific transcript pattern. Mol. Microbiol. 48:1339-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.le Cessie, S. F., H. Verhoeff, G. Mengistie, P. Kazembe, R. Broadhead, and B. J. Brabin. 2002. Changes in haemoglobin levels in infants in Malawi: effect of low birth weight and fetal anaemia. Arch. Dis. Child. Fet. Neonat. Ed. 86:F182-F187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lekana Douki, J. B., B. Traore, F. T. M. Costa, T. Fusaï, B. Pouvelle, Y. Sterkers, A. Scherf, and J. Gysin. 2002. Sequestration of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate A, a receptor for maternal malaria: monoclonal antibodies against the native parasite ligand reveal pan-reactive epitopes in placental isolates. Blood 100:1478-1484. [DOI] [PubMed] [Google Scholar]
- 33.Marsh, K., and R. J. Howard. 1986. Antigens induced on erythrocytes by P. falciparum: expression of diverse and conserved determinants. Science 231:150-153. [DOI] [PubMed] [Google Scholar]
- 34.Mayor, A., N. Bir, R. Sawhney, S. Singh, P. Pattnaik, S. Singh, A. Sharma, and C. E. Chitnis. 2004. Receptor-binding residues lie in central regions of Duffy-binding-like domains involved in red cell invasion and cytoadherence by malaria parasites. Blood 105:2557-2563. [DOI] [PubMed] [Google Scholar]
- 35.McGregor, I. A., M. E. Wilson, and W. Z. Billewicz. 1983. Malaria infection of the placenta in The Gambia, West Africa; its incidence and relationship to stillbirth, birthweight and placental weight. Trans. R. Soc. Trop. Med. Hyg. 77:232-244. [DOI] [PubMed] [Google Scholar]
- 36.McGregor, I. A. 1984. Epidemiology, malaria and pregnancy. Am. J. Trop. Med. Hyg. 33:517-525. [DOI] [PubMed] [Google Scholar]
- 37.Pandey, K. C., S. Singh, P. Pattnaik, C. R. Pillai, U. Pillai, A. Lynn, S. K. Jain, and C. E. Chitnis. 2002. Bacterially expressed and refolded receptor binding domain of Plasmodium falciparum EBA-175 elicits invasion inhibitory antibodies. Mol. Biochem. Parasitol. 123:23-33. [DOI] [PubMed] [Google Scholar]
- 38.Ranjan, A., and C. E. Chitnis. 1999. Mapping regions containing binding residues within functional domains of Plasmodium vivax and Plasmodium knowlesi erythrocyte-binding proteins. Proc. Natl. Acad. Sci. USA 96:14067-14072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reeder, J. C., A. F. Cowman, K. M. Dvern, J. G. Beeson, J. K. Thompson, S. J. Rogerson, and G. V. Brown. 1999. The adhesion of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate A is mediated by P. falciparum erythrocyte membrane protein 1. Proc. Natl. Acad. Sci. USA 96:5198-5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reeder, J. C., A. N. Holder, J. G. Beeson, and G. V. Brown. 2000. Identification of glycosaminoglycan binding domains in Plasmodium falciparum erythrocyte membrane protein 1 of a chondroitin sulfate A-adherent parasite. Infect. Immun. 68:3923-3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ricke, C. H., T. Staalsoe, K. Koram, B. D. Akanmori, E. M. Riley, T. G. Theander, and L. Hviid. 2000. Plasma antibodies from malaria-exposed pregnant women recognize various surface antigens of Plasmodium falciparum-infected erythrocytes in a parity-dependent manner and block parasite adhesion to chondroitin sulfate A. J. Immunol. 165:3309-3316. [DOI] [PubMed] [Google Scholar]
- 42.Riley, E. M., L. Hviid, and T. G. Theander. 1994. Malaria, p. 119-143. In F. Kierszenbaum (ed.), Parasitic infections and the immune system. Academic Press, New York, N.Y.
- 43.Rogerson, S. J., S. C. Chaiyaroj, K. Ng, J. C. Reeder, and G. V. Brown. 1995. Chondroitin sulfate A is a cell surface receptor for Plasmodium falciparum-infected erythrocytes. J. Exp. Med. 1821:15-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salanti, A., T. Staalsoe, T. Lavstsen, A. T. R. Jensen, M. P. K. Sowa, D. E. Arnot, L. Hviid, and T. G. Theander. 2003. Selective upregulation of a single distinctly structured var gene in chondroitin sulfate A-adhering Plasmodium falciparum involved in pregnancy associated malaria. Mol. Microbiol. 49:179-191. [DOI] [PubMed] [Google Scholar]
- 45.Salanti, A., M. Dahlback, L. Turner, M. A. Nielsen, L. Barford, P. Magistrado, A. T. Jensen, T. Lavstsen, M. F. Ofori, K. Marsh, L. Hviid, and T. G. Theander. 2004. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J. Exp. Med. 200:1197-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharma, S. K., R. Chattopadhyay, K. Chakrabarti, S. Pati, V. K. Srivastava, P. Tyagi, S. Mahanty, S. K. Misra, T. Adak, B. S. Das, and C. E. Chitnis. 2004. Epidemiology of malaria transmission and development of natural immunity in a malaria endemic village, San Dulakudar, in Orissa state, India. Am. J. Trop. Med. Hyg. 71:457-465. [PubMed] [Google Scholar]
- 47.Singh, S., K. Pandey, R. Chattopadhayay, S. S. Yazdani, A. Lynn, A. Bharadwaj, A. Ranjan, and C. E. Chitnis. 2001. Biochemical, biophysical, and functional characterization of bacterially expressed and refolded receptor binding domain of Plasmodium vivax Duffy-binding protein. J. Biol. Chem. 276:17111-17116. [DOI] [PubMed] [Google Scholar]
- 48.Smith, J. D., C. E. Chitnis, A. G. Craig, D. J. Roberts, D. E. Hudson-Taylor, D. S. Peterson, R. Pinches, C. I. Newbold, and L. H. Miller. 1995. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell 82:101-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith, J. D., G. Subramanian, B. Gamain, D. I. Baruch, and L. H. Miller. 2000. Classification of adhesive domains in the Plasmodium falciparum erythrocyte membrane protein 1 family. Mol. Biochem. Parasitol. 110:293-310. [DOI] [PubMed] [Google Scholar]
- 50.Staalsoe, T., H. A. Giha, D. Dodoo, T. G. Theander, and L. Hviid. 1999. Detection of antibodies to various antigens of Plasmodium falciparum-infected erythrocytes by flow cytometry. Cytometry 35:329-336. [DOI] [PubMed] [Google Scholar]
- 51.Staalsoe, T., R. Megnekou, N. Fievet, C. H. Ricke, H. D. Zoring, R. Leke, D. W. Taylor, P. Deloron, and L. Hviid. 2001. Acquisition and decay of antibodies to pregnancy-associated variant antigens on the surface of Plasmodium falciparum-infected erythrocytes that protect against placental parasitemia. J. Infect. Dis. 184:618-626. [DOI] [PubMed] [Google Scholar]
- 52.Steketee, R. W., B. L. Nahlen, M. E. Parise, and C. Menendez. 2001. The burden of malaria in pregnancy in malaria-endemic areas. Am. J. Trop. Med. Hyg. 64:28-35. [DOI] [PubMed] [Google Scholar]
- 53.Su, X. Z., V. M. Heatwole, S. P. Wertheimer, F. Guinet, J. A. Herfeldt, D. S. Peterson, J. A. Ravetch, and T. E. Wellems. 1995. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum infected erythrocytes. Cell 82:89-100. [DOI] [PubMed] [Google Scholar]
- 54.Suguitan, A. L. J., R. J. Leke, G. Fouda, A. Zhou, L. Thuita, S. Metenou, J. Fogako, R. Megnekou, and D. W. Taylor. 2003. Changes in the levels of chemokines and cytokines in the placentas of women with Plasmodium falciparum malaria. J. Infect. Dis. 188:1074-1082. [DOI] [PubMed] [Google Scholar]
- 55.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 56.Tuikue Ndam, N. G., A. Salanti, G. Bertin, M. Dahlbeck, N. Fievet, L. Turner, et al. 2005. High level of var2csa transcription by Plasmodium falciparum isolated from the placenta. J. Infect. Dis. 192:331-335. [DOI] [PubMed] [Google Scholar]
- 57.Walter, P. R., Y. Garin, and P. Blot. 1982. Placental pathologic changes in malaria. A histologic and ultrastructural study. Am. J. Pathol. 109:330-342. [PMC free article] [PubMed] [Google Scholar]