Abstract
Trichomonas vaginalis is one of the most common nonviral sexually transmitted human infections and, worldwide, has been linked to increased incidence of human immunodeficiency virus type 1 transmission, preterm delivery, low birth weight, cervical cancer, and vaginitis. The molecular pathways that are important in initiating host inflammatory and immune responses to T. vaginalis are poorly understood. Here we report interactions of human cervicovaginal epithelial cells with the most abundant cell surface glycoconjugate of the parasite, the T. vaginalis lipophosphoglycan (LPG). Purified LPG mediated the adhesion of parasites to human vaginal epithelial cells in a dose-dependent manner. Furthermore, T. vaginalis LPG (but not LPG from Tritrichomonas foetus, the causative agent of bovine trichomoniasis) induced a selective upregulation of chemotactic cytokines by human endocervical, ectocervical, and vaginal epithelial cells, which do not express Toll-like receptor 4/MD2. The T. vaginalis LPG triggered interleukin 8 (IL-8), which promotes the adhesion and transmigration of neutrophils across the endothelium, and macrophage inflammatory protein 3α, which is a chemoattractant for immune cells and is essential for dendritic cell maturation. These effects were dose dependent and sustained in the absence of cytotoxicity and IL-1β release and utilized, at least in part, a signaling pathway independent from the Toll-like/IL-1 receptor adaptor protein MyD88.
Human trichomoniasis, caused by Trichomonas vaginalis, is one of the most common nonviral sexually transmitted infections; over 180 million people worldwide, including 8 to 10 million Americans, become infected annually (29, 33, 48). In addition to being a cause of serious discomfort and the leading cause of vaginitis, trichomoniasis has been linked to preterm delivery (in ∼40% of cases), low birth weight (7, 44), infertility (17), and cervical cancer (47, 49). T. vaginalis infection also predisposes women to human immunodeficiency virus type 1 (HIV-1) infection (8, 44) and other viral infections (16). Several reports suggest that T. vaginalis infection increases HIV-1 shedding in the female genital tract (5, 23, 25), a development which correlates with increased sexual and perinatal transmission of HIV (21, 45).
Although T. vaginalis is the most studied trichomonad and much is known about trichomoniasis, its pathogenic mechanisms are not well defined. T. vaginalis is an extracellular parasitic protozoan; it adheres to and damages vaginal epithelial cells (15, 43) and lives in the vaginas of women and the urethras of men. Although women may develop specific antibodies to T. vaginalis antigens, the infections do not provide lasting immunity and reinfections are common (29, 33). In men the infection is usually asymptomatic, although there may be an irritating urethritis or prostatitis. In women, the disease is associated with a wide spectrum of symptoms, ranging from a relatively asymptomatic state to severe inflammation (22, 29). Knowledge of the molecular factors that initiate or modulate host inflammatory responses is still in its infancy.
It is known that parasitic protozoans contain a variety of complex carbohydrates on their surfaces, e.g., glycolipids, glycoproteins, and lipid-anchored glycosylated phosphatidylinositol (GPI). These glycoconjugates have been reported to play important roles in host cell invasion and evasion of host immune responses by several protozoa (9, 18, 46). We have demonstrated that T. vaginalis and the related bovine parasite, Tritrichomonas foetus, express lipophosphoglycans (LPGs) (2 × 106 to 3 × 106 copies/parasite) anchored on the cell surface via an inositol-phosphoceramide (36, 37). These molecules are analogous to LPGs from other parasites but clearly distinct from them, as indicated by their unusual monosaccharide compositions (for a review, see reference 18). The high density of LPGs on the parasite surface suggests that they have important roles in parasite biology and in the infections they cause. Indeed, we previously showed that pretreatment of trichomonads with periodate abolished adhesion of specific parasites to their respective host cells, implying the involvement of carbohydrate molecules in these processes (15, 39). This is, therefore, of fundamental importance to further our understanding of the pathobiology of trichomoniasis and to define the role that this molecular component(s) plays in the infection of host tissues, in particular vaginal epithelial cells.
For many years, significant evidence has accumulated to suggest the involvement of a T. vaginalis product(s) in host inflammatory responses during infection (32, 35). In the present study, we demonstrate that LPG, the main cell surface glycoconjugate of T. vaginalis, initiates secretion of cytokines by host cells.
MATERIALS AND METHODS
Parasites and LPG.
T. vaginalis isolates (UR1) obtained from a symptomatic patient were cultured in Diamond's Trypticase-yeast extract medium (pH 6.0) with 10% heat-inactivated horse serum (HyClone Laboratory) at 37°C, as reported previously (43). Parasites were harvested in late log phase (24 h) by centrifugation and washed twice with phosphate-buffered saline (PBS) (pH 7.4) and suspended in the appropriate medium for experiments. LPGs from T. vaginalis and T. foetus were isolated and purified on an octyl-Sepharose column, as reported previously (36, 37).
Adhesion of T. vaginalis to human vaginal epithelial cells.
Primary human vaginal epithelial cells (HVECs) were derived from normal discarded tissues in accordance with an Institutional Review Board-approved procedure and cultured as reported previously (15). For adhesion studies, HVECs were plated in 24-well plates containing Williams' medium (15, 43) and allowed to approach confluence (∼80%). HVECs were equilibrated with fresh medium containing Williams'-Diamond's medium (2:1) at 37°C and 5% CO2 in air for 30 min before the start of the experiments. This medium supports both HVECs and parasites. Parasites were radiolabeled with [35S]cysteine-methionine (∼1 to 1.5 mCi; American Radio Labeled Chemicals) for 14 h prior to harvest and washed with PBS (2×) followed by Williams'-Diamond's medium and suspended in a desired volume. Known amounts of labeled parasites (∼8 × 105) were added to HVECs (∼5 × 104) (quadruplicate wells for each condition) and incubated for 30 min (37°C, 5% CO2). At the end of the incubation period, the wells were washed five times with 1 ml PBS. The HVECs were solubilized with 1 N NaOH, and the adherent radioactivity was determined by liquid scintillation counting. For competition binding experiments, various amounts of T. vaginalis LPG (5 to 100 μg) were added to HVECs and equilibrated for 15 min before the addition of parasites. Each experiment was repeated three times, and the means of the data are presented.
T. vaginalis LPG.
For composition analysis, LPG was isolated and purified as described previously (36). Our preliminary analyses (36) showed the presence of mannose (Man) in LPG, as would be expected for a GPI anchor. However, we later determined by two different methods that LPG does not contain Man but does contain xylase (Xyl) (38, 41). The monosaccharide composition of LPG was determined by high-performance anion exchange-pulse amperometric detector (HPAE-PAD) and gas-liquid chromatography-mass spectrometry (GLC-MS) analyses. For HPAE-PAD analysis, LPG was hydrolyzed with 4 M trifluoroacetic acid at 125°C for 1 h and the monosaccharides were separated on a PA1 column (Dionex Corp., CA) preequilibrated with 10 mM NaOH for 4 min and eluted with H2O for 55 min. For GLC-MS analysis, LPG was hydrolyzed with 0.5 N HCl in dry methanol (65°C, overnight) followed by acetylation and trimethylsylation.
Enzymatic treatments.
β-Galactosidase (jack bean), β-N-acetylhexosaminidase (jack bean), β-mannosidase (Helix pomatia), α′-mannosidase (jack bean), and endo-β-galactosidase (Bacteroides fragilis) were obtained from Oxford GlycoSystems (New York, NY). Incubation conditions were as described by the manufacturer in a total volume of 0.2 ml. Saccharides released upon endo-β-galactosidase treatment of LPG were separated on a C18 Sep-Pak column (Waters Corp., Milford, MA) equilibrated with 0.1% trifluoroacetic acid and analyzed by HPAE-PAD and/or Glyko-FACE (fluorophore-assisted carbohydrate electrophoresis) analysis.
Cytokine assays.
Previously established and well-characterized HPV16/E6E7-immortalized vaginal (Vk2/E6E7), ectocervical (Ect1/E6E7), and endocervical (End1/E6E7) epithelial cell lines were cultured in keratinocyte serum-free medium (Invitrogen, Carlsbad, CA), supplemented with 50 μg/ml bovine pituitary extract, 0.1 ng/ml epidermal growth factor, 100 units/ml penicillin, 100 μg/ml streptomycin, and CaCl2 to a final calcium concentration of 0.4 mM (12). In vitro constructs of squamous nonkeratinizing stratified epithelial tissues generated from normal primary ectocervical epithelial cells were purchased from Mattek, Ashland, Mass. (2), and equilibrated at 37°C in 5% CO2 in culture medium provided by the manufacturer for 24 h before stimulation. Stimulation with various concentrations of purified T. vaginalis LPG and T. foetus LPG was performed for 6 to 24 h, and cell culture supernatants were collected for cytokine determination by the electro-chemiluminescence multiplex system Sector 2400 imager from Meso Scale Discovery (Gaithersburg, MD). Critical findings were confirmed by traditional enzyme-linked immunosorbent assay (QuantiGlo IL-8 and Quantikine macrophage inflammatory protein 3α (MIP-3α) from R & D Systems, Minneapolis, MN).
Cell viability assays.
A CellTiter96 3-(4,5-dimethylthiazol-2,5-diphenyltetrazolium bromide (MTT) assay (Promega, Madison, WI) and a Roche lactate dehydrogenase (LDH) cytotoxicity detection kit (Fisher Scientific, Pittsburgh, PA) were used to assess cytotoxicity after T. vaginalis LPG stimulation. The MTT assay was performed as previously described in detail (13). For the LDH assay, cell culture supernatants (50 μl) were mixed with an equal volume of LDH reaction mixture and incubated for 30 min at room temperature. Absorbances were measured at 490 nm and 650 nm. The colorimetric reactions were read using a multilabel microplate counter with Victor 2 and Wallac 1420 software 2.01 (Perkin Elmer Life Sciences, Boston, MA).
Transfection and luciferase assay.
Cervical (Ect1/E6E7) and vaginal (End1/E6E7) epithelial cells were plated at 1 × 104 cells/well overnight at 37°C in 96-well cell culture plates (Falcon, Franklin Lakes, NJ). Cells were transiently cotransfected with the p-NF-κB luciferase reporter plasmid (Clontech, Palo Alto, CA) and the Renilla pRL-TK vector (Promega, Madison, WI) with or without dominant-negative (dn) mutant pDeNy-hMyD88 (InvivoGen, San Diego, CA) using 0.16 μg DNA/well and 3 μl/μg DNA Gene Juice transfection reagent (Novagen, Madison, WI). The total amount of DNA in each transfection was kept constant by adding pDeNy-mcs plasmid (InvivoGen). After 16 to 24 h, the cells were stimulated for 2 to 24 h with various doses of T. vaginalis LPG, T. foetus LPG, or recombinant interleukin 1β (IL-1β) as a positive control. At the end of each stimulation period, cells were lysed with the buffer provided by the manufacturer and the lysates were processed with luciferase substrate (Promega, Madison, WI) to measure luciferase activity using a single-sample TD20/20 luminometer from Turner BioSystems (Sunnyvale, CA). The results were presented as a ratio of firefly (Photinus pyralis) and Renilla reniformis luciferase activity as the pRL-TK vector serves as an internal control for transfection efficiency.
Statistical analysis.
One-way analysis of variance and Dunett's multiple comparison test (GraphPad Prizm, v. 3.0) were used to compare differences in cell responses between treatment conditions. A P value of <0.05 was considered significant.
RESULTS
LPG composition.
We previously demonstrated that LPG plays a role in parasite-host cell biology. We therefore undertook the following studies. The monosaccharide composition of LPG was determined by HPAE-PAD and GLC-MS as described in Materials and Methods. Purified LPG contains Glc, Gal, GlcN, GalN, rhamnose (Rha), and Xyl in an approximate molar ratio of 1:4.2:15.7:1:12.5:6.2. It is interesting that although LPG is bound to the parasite surface through a GPI anchor (36, 37), we found no evidence of Man. However, significant amounts of glucosamine (GlcN), Rha, and Xyl were observed. Earlier work showed that LPG binds strongly to the lectin RCA-1, indicating the presence of terminal β1,4-linked Gal residue(s) (37). Treatment of LPG with β-galactosidase and β-N-acetylhexosaminidase individually resulted in release of Gal and HexNAc, which were verified by HPAE-PAD and Glyko-FACE analyses (data not shown). The detailed nature of the LPG structure is under active investigation, and these results should shed light on its function in regulating immune responses.
LPG role in parasite-host adhesion.
A direct binding assay was developed to examine the role of T. vaginalis LPG in host-parasite adhesion. As shown in Fig. 1A, the binding of parasites to HVECs is saturable and is inhibited up to 73% by intact T. vaginalis LPG. Similarly, as shown in Fig. 1B, coincubation of unlabeled parasites (100×) with labeled parasites displaced binding up to 90%. The effect of T. vaginalis LPG on host-parasite adhesion is abolished by mild acid treatment. LPGs from T. foetus and Leishmania donovani and bacterial lipopolysaccharides (LPSs) (Salmonella) are ineffective at displacing parasites in the assay (data not shown). These results demonstrate that T. vaginalis LPG exhibits specificity in host-parasite adhesion (data not shown).
FIG. 1.
A. Inhibition of T. vaginalis adhesion with T. vaginalis LPG. Approximately 8 × 105 32S-labeled parasites were added to confluent HVECs (5 × 104) containing different concentrations of LPG in a total volume of 0.6 ml and incubated for 30 min at 37°C (5% CO2). B. Inhibition of T. vaginalis adhesion to HVECs with various densities of unlabeled parasites. Unlabeled parasites were coincubated with 8 × 105 32S-labeled parasites and HVECs in 1.2 ml for 30 min at 37°C (5% CO2). Binding was determined as described in the text.
LPG and cytokine induction.
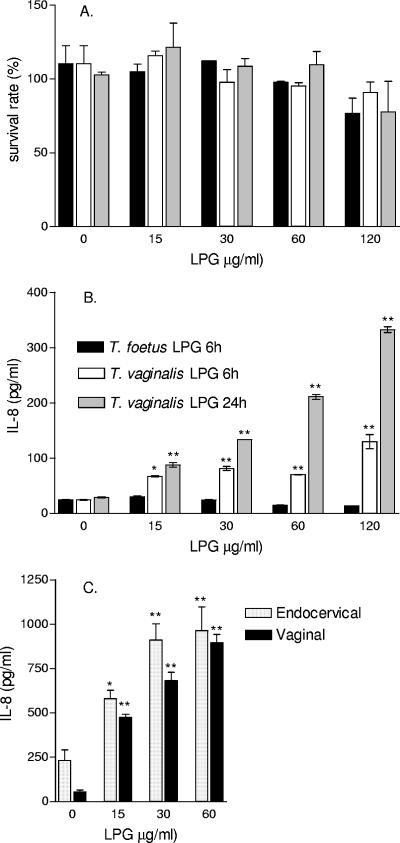
The stimulatory activity of T. vaginalis LPG on host immune responses was assessed by cytokine production by epithelial cells derived from normal human vaginal, ectocervical, and endocervical tissues. We and others have previously demonstrated that these cells closely resemble the phenotypes of the primary cells and tissues of origin and do not express the major LPS signaling molecules Toll-like receptor 4 (TLR4) and MD2 (10-12, 14). Purified LPG from both T. vaginalis and T. foetus was nontoxic to these cells at doses of ≤60 μg/ml/24 h (Fig. 2A).
FIG. 2.
Effects of T. vaginalis LPG on cell viability and IL-8 production. A. LPG doses of <60 μg/ml were nontoxic to the ectocervical (Ect1/E6E7) epithelial cells. Similar results were obtained for the vaginal and endocervical epithelial cell lines. B. T. vaginalis but not T. foetus LPG stimulates IL-8 production in ectocervical (Ect1/E6E7) epithelial cells. C. IL-8 production adjusted per viable cell counts in endocervical (End1/E6E7) and vaginal (Vk2/E6E7) epithelial cells. Results represent means and standard errors of the means from triplicate cultures in one of two independent experiments with each cell line. *, P < 0.05; **, P < 0.01.
For cytokine measurement, cells were exposed for 6 to 24 h to nontoxic doses of purified LPG. T. vaginalis LPG induced in the vaginal, ectocervical, and endocervical epithelial cells a marked dose-dependent upregulation of the chemokines IL-8 and MIP-3α (Fig. 2B and C, 3, and 4A and B). The chemokine responses to LPG occurred over a broad dose range and in the absence of cytotoxicity, as demonstrated by parallel MTT viability tests (Fig. 2A). In the same cell cultures, only a moderate increase of IL-6 was detected, and the IL-1β and tumor necrosis factor alpha (TNF-α) levels remained at baseline (Fig. 3). In contrast, the LPG purified from the bovine parasite T. foetus showed no effect on cytokine production, indicating a species specificity of the chemokine-triggering mechanism (Fig. 2B). Similarly, a nontoxic dose of T. vaginalis LPG but not T. foetus LPG induced IL-8 and MIP-3α when applied to the apical surface of three-dimensional ectocervical epithelial tissues (VEC100) reconstructed from primary nontransformed epithelial cells (data not shown).
FIG. 3.
Multiplex cytokine analysis of ectocervical (Ect1/E6E7) cultures stimulated with T. vaginalis LPG for 24 h. Cytokine concentrations were measured by a Meso-Scale 2400 imager. LPG induced IL-6, IL-8, and MIP-3α but not IL-10, IL-1β, and TNF-α release. Results represent means and standard errors of the means from triplicate cultures.
FIG. 4.
Inhibition of LPG signaling with dnMyD88. A. MIP-3α concentrations measured by enzyme-linked immunosorbent assay in supernatants from Vk2/E6E7 cells collected 24 h after stimulation with T. vaginalis LPG and IL-1β. B. IL-8 measured in the same culture supernatants. C. NF-κB activation (presented as firefly/Renilla luciferase ratio) in Ect1/E6E7 cells transfected with control plasmid pDeNy-mcs or with dnMyD88 and stimulated for 4 h with IL-1β (20 ng/ml) or 15 μg/ml of LPG. The bars represent means and standard errors of the means from triplicate cultures. **, P < 0.01, different from nontreated (medium) control; n.s., no significant difference from medium control.
The Toll/interleukin 1 receptor adaptor protein MyD88 has been implicated in the selectivity of cytokine induction in response to LPS (50). To determine the relative importance of this pathway for the LPG-induced chemokine responses, MyD88 signaling in the epithelial cells was blocked by transient transfection with dn pDNMyD88 plasmid. The cells were then stimulated with LPG for 6 to 24 h, and supernatants were harvested for cytokine measurement. In some experiments, the epithelial cells were cotransfected with pNF-κB-luciferase reporter plasmid.
The transient transfection with the dnMyD88 mutant led to a significant reduction of IL-1β-induced MIP-3α (P < 0.01) and IL-8 (P < 0.001) production by the vaginal epithelial cells (Fig. 4A and B). The cotransfection of NF-κB-luciferase reporter with dnMyD88 abolished the IL-1β- and LPG-triggered NF-κB activation.
DISCUSSION
Our findings confirm the role of surface carbohydrates in the adhesion of T. vaginalis to host epithelial cells in the lower female genital tract and strengthen the evidence of LPG involvement in this process. Earlier studies showed that periodate treatment of parasites abolished the binding of trichomonads to human and bovine vaginal epithelial cells, suggesting the involvement of a carbohydrate-containing molecule(s) in the adhesion processes (15, 39). Furthermore, a direct binding assay directly implicated T. foetus LPG in adhesion of T. foetus to bovine vaginal epithelial cells (39). The same phenomenon was observed in the present study with T. vaginalis LPG and human vaginal primary cells. Importantly, each of the LPG molecules (T. foetus and T. vaginalis) exhibits species specificity in displacement binding assays, which is in total agreement with other experiments displaying species specificity (40, 43). Our direct binding assay results are in agreement with the results of a recent study (3) investigating the effect of T. vaginalis LPG mutants mediating the adherence of parasites to cervical epithelial cells. Bastida-Corcuera et al. (3) demonstrated that T. vaginalis LPG mutants reduced adherence to the same immortalized human ectocervical epithelial cell line that was used for our cytokine stimulation experiments (Ect1/E6E7). These results provide strong evidence that T. vaginalis LPG is involved in adhesion of parasites to host cells.
For a long time, the cytopathic consequences of parasite adherence to genital tract epithelia have been regarded as the primary cause of the inflammatory reaction to T. vaginalis infection. Adhesion of T. vaginalis is cytotoxic to epithelial cells in vitro (15, 31). The importance of this observation is borne out by the finding that, a few days after infection, there is a degeneration of the vaginal epithelium, typically followed by leukocytic neutrophil infiltration that is associated with abundant vaginal discharge and intensely inflamed vaginal tissues. However, little is known about the early mechanism of how neutrophils accumulate or how epithelial cells mediate the initial inflammatory response upon T. vaginalis infection. Our study provides evidence for the early involvement of chemokine production by epithelial cells that occurs in response to nontoxic doses of LPG and precedes cytotoxic effects.
IL-8 is the major chemokine responsible for neutrophil recruitment to sites of tissue insult and inflammation (1, 26). Shaio et al. (34, 35) reported the presence of IL-8 in the vaginal discharge from patients with symptomatic trichomoniasis, providing evidence for involvement of IL-8 in the inflammatory response to T. vaginalis. Production of IL-8 by monocytes and neutrophils stimulated by live trophozoites (32) or undefined T. vaginalis membrane fragments has been reported (35). Here we report for the first time species-specific IL-8 production by human vaginal, ectocervical, and endocervical epithelial cells in response to a chemically defined T. vaginalis constituent, purified T. vaginalis LPG.
Increased IL-8 levels in genital tract secretions and amniotic fluid have been correlated with upper genital tract infections and preterm labor (19), suggesting that T. vaginalis IL-8 upregulation may underlie the mechanisms linking trichomoniasis with preterm labor. On the other hand, IL-8 has been shown to activate HIV-1 replication (27). Via this mechanism, as well as via recruitment of HIV-1 host cells to the site of infection, increased levels of IL-8 provide a plausible pathway for increased shedding of HIV-1 and predisposition to HIV-1 infection, both of which have been associated with trichomoniasis (5, 23, 25).
It has been hypothesized that the production of IL-8 upon T. vaginalis infection may be secondary to the cytopathic effects and the release of the early response proinflammatory cytokines TNF-α and IL-1β by damaged epithelial cells. We previously demonstrated that IL-8 is secreted as it is produced by the cervical and vaginal epithelial cells, with no storage of significant IL-8 amounts in the epithelial cytoplasm (13). On the other hand, large intracellular stores of IL-1 and some small amounts of TNF-α are present in these cells and released upon membrane rupture and cell death (13). TNF-α and IL-1β induce NF-κB activation via TNF receptor I and IL-1 receptor I, respectively, and also may act as Toll-like receptor agonists in the reproductive tract epithelial cells (28, 42). The notion of the primary role of IL-1β and TNF-α in the T. vaginalis-induced inflammatory response has found indirect support in the evidence of increased cervicovaginal levels of these cytokines under pathological conditions commonly associated with trichomoniasis, e.g., vaginitis, bacterial vaginosis, HIV-1 infection, and preterm labor (6, 30).
Contradicting this notion, our results demonstrate that LPG stimulates a significantly increased IL-8 production in the absence of cell toxicity and at low baseline levels of endogenous IL-1β and TNF-α. These findings suggest a direct recognition rather than a secondary cytopathic or host factor-mediated signaling effect of T. vaginalis LPG and support our hypothesis that the chemokine response to T. vaginalis may be initiated via LPG interaction with epithelial cell surface receptors. Nevertheless, although the results presented in this study clearly indicate that LPG can trigger responses directly in vaginal epithelial cells, this effect does not preclude LPG interaction with leukocytes, such as macrophages, during the subsequent inflammatory response that may occur by other mechanisms and have a potent contribution to the pathogenesis of trichomoniasis.
The sustained production of MIP-3α, which is a novel finding of our study, may represent an important role of the cervicovaginal epithelium in the immune response to T. vaginalis. MIP-3α is known to recruit Langerhans-like CD34+ progenitor dendritic cells (DCs) to the site of inflammation, specifically by guiding them as they traverse the lamina propria on their way to the epithelial surface, a journey which precedes their maturation (4). DCs are potent antigen-presenting cells that likely play multiple roles in HIV-1 infection by becoming directly infected or by capturing and transmitting the virus to T cells in draining lymph nodes (24). An important finding of this study is that LPG-induced IL-8 and MIP-3α production by the vaginal epithelial cells is at least partially MyD88 independent. The cytoplasmic adaptor protein MyD88 is essential for rapid NF-κB activation and cytokine production in response to multiple TLR ligands including LPS (24). The MyD88-independent activation via TLR is important for DC maturation (20). On the other hand, activation of DCs in the absence of MyD88 activation may lead to Th2-skewed immune responses (21a). Although no definitive in vivo data are available to substantiate the above findings, it is intriguing to speculate that MyD88-independent TLR activation by LPG may represent a plausible mechanism for evading the host immune function. The molecular pathways involved in the LPG-induced chemokine activation remain to be further elucidated and require further experimental and clinical studies to determine their role in host immune responses.
In summary, our study provides evidence for the major role of T. vaginalis LPG in the pathogenesis of the mucosal inflammatory reaction and suggests its modulatory role in the innate host immune response through selective species-specific cytokine upregulation and multiple signaling pathways.
Acknowledgments
This work was partially supported by Connors Interdisciplinary Grant for Gender Biology, Brigham and Women's Hospital (R.N.F.), and NIH grants from NIAID AI47334 (B.N.S.) and NCRR P41 RR10888 (C.E.C.).
We have no conflicting financial interests.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Atta-ur-Rahman, K. Harvey, and R. A. Siddiqui. 1999. Interleukin-8: an autocrine inflammatory mediator. Curr. Pharm. Des. 5:241-253. [PubMed] [Google Scholar]
- 2.Ayehunie, S., C. Cannon, S. Lamore, J. Kubilus, D. J. Anderson, J. Pudney, and M. Klausner. 2005. Organotypic human vaginal-ectocervical tissue model for irritation studies of spermicides, microbicides, and feminine-care products. Toxicol. In Vitro 205:689-698. [DOI] [PubMed] [Google Scholar]
- 3.Bastida-Corcuera, F. D., C. Y. Okumura, A. Colocoussi, and P. J. Johnson. 2005. Trichomonas vaginalis lipophosphoglycan mutants have reduced adherence and cytotoxicity to human ectocervical cells. Eukaryot. Cell 4:1951-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caux, C., B. Vanbervliet, C. Massacrier, S. Ait-Yahia, C. Vaure, K. Chemin, M. C. Dieu-Nosjean, and A. Vicari. 2002. Regulation of dendritic cell recruitment by chemokines. Transplantation 73:S7-S11. [DOI] [PubMed] [Google Scholar]
- 5.Cohen, M. S. 2000. HIV transmission—AIDS researchers look at Africa. N. Engl. J. Med. 342:970-973.10738058 [Google Scholar]
- 6.Coombs, R. W., P. S. Reichelderfer, and A. L. Landay. 2003. Recent observations on HIV type-1 infection in the genital tract of men and women. AIDS 17:455-480. [DOI] [PubMed] [Google Scholar]
- 7.Cotch, M. F., J. G. Pastorek II, R. P. Nugent, S. L. Hillier, R. S. Gibbs, D. H. Martin, D. A. Eschenbach, R. Edelman, J. C. Carey, J. A. Regan, M. A. Krohn, M. A. Klebanoff, A. V. Rao, G. G. Rhoads, et al. 1997. Trichomonas vaginalis associated with low birth weight and preterm delivery. Sex. Transm. Dis. 24:353-360. [DOI] [PubMed] [Google Scholar]
- 8.Draper, D., W. Donohoe, L. Mortimer, and R. P. Heine. 1998. Cysteine proteases of Trichomonas vaginalis degrade secretory leukocyte protease inhibitor. J. Infect. Dis. 178:815-819. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson, M. A. 1999. The structure, biosynthesis and functions of glycosylphosphatidylinositol anchors, and the contributions of trypanosome research. J. Cell Sci. 112:2799-2809. [DOI] [PubMed] [Google Scholar]
- 10.Fichorova, R. N., and D. J. Anderson. 1999. Differential expression of immunobiological mediators by immortalized human cervical and vaginal epithelial cells. Biol. Reprod. 60:508-514. [DOI] [PubMed] [Google Scholar]
- 11.Fichorova, R. N., A. O. Cronin, E. Lien, D. J. Anderson, and R. R. Ingalls. 2002. Response to Neisseria gonorrhoeae by cervicovaginal epithelial cells occurs in the absence of toll-like receptor 4-mediated signaling. J. Immunol. 168:2424-2432. [DOI] [PubMed] [Google Scholar]
- 12.Fichorova, R. N., J. G. Rheinwald, and D. J. Anderson. 1997. Generation of papillomavirus-immortalized cell lines from normal human ectocervical, endocervical, and vaginal epithelium that maintain expression of tissue-specific differentiation proteins. Biol. Reprod. 57:847-855. [DOI] [PubMed] [Google Scholar]
- 13.Fichorova, R. N., L. D. Tucker, and D. J. Anderson. 2001. The molecular basis of nonoxynol-9-induced vaginal inflammation and its possible relevance to human immunodeficiency virus type 1 transmission. J. Infect. Dis. 184:418-428. [DOI] [PubMed] [Google Scholar]
- 14.Fisette, P. L., S. Ram, J. M. Andersen, W. Guo, and R. R. Ingalls. 2003. The Lip lipoprotein from Neisseria gonorrhoeae stimulates cytokine release and NF-kappaB activation in epithelial cells in a Toll-like receptor 2-dependent manner. J. Biol. Chem. 278:46252-46260. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert, R. O., G. Elia, D. H. Beach, S. Klaessig, and B. N. Singh. 2000. Cytopathogenic effect of Trichomonas vaginalis on human vaginal epithelial cells cultured in vitro. Infect. Immun. 68:4200-4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gram, I. T., M. Macaluso, J. Churchill, and H. Stalsberg. 1992. Trichomonas vaginalis (TV) and human papillomavirus (HPV) infection and the incidence of cervical intraepithelial neoplasia (CIN) grade III. Cancer Causes Control 3:231-236. [DOI] [PubMed] [Google Scholar]
- 17.Grodstein, F., M. B. Goldman, and D. W. Cramer. 1993. Relation of tubal infertility to history of sexually transmitted diseases. Am. J. Epidemiol. 137:577-584. [DOI] [PubMed] [Google Scholar]
- 18.Guha-Niyogi, A., D. R. Sullivan, and S. J. Turco. 2001. Glycoconjugate structures of parasitic protozoa. Glycobiology 11:45R-59R. [DOI] [PubMed] [Google Scholar]
- 19.Hitti, J., S. L. Hillier, K. J. Agnew, M. A. Krohn, D. P. Reisner, and D. A. Eschenback. 2001. Vaginal indicators of amniotic fluid infection in preterm labor. Obstet. Gynecol. 97:211-219. [DOI] [PubMed] [Google Scholar]
- 20.Hoebe, K., E. M. Janssen, S. O. Kim, L. Alexopoulou, R. A. Flavell, J. Han, and B. Beutler. 2003. Upregulation of costimulatory molecules induced by lipopolysaccharide and double-stranded RNA occurs by Trif-dependent and Trif-independent pathways. Nat. Immunol. 4:1223-1229. [DOI] [PubMed] [Google Scholar]
- 21.John, C. C., R. W. Nduatti, and D. A. Mbori-Ngacha. 2001. Correlates of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission: association with maternal plasma HIV-1 RNA load, genital HIV-1 DNA shedding and breast infections. J. Infect. Dis. 183:206-212. [DOI] [PubMed] [Google Scholar]
- 21a.Kaisho, T., K. Hoshino, T. Iwabe, O. Takeuchi, T. Yasui, and S. Akira. 2002. Endotoxin can induce MyD88-deficient dendritic cells to support Th2 cell differentiation. Int. Immunol. 14:695-700. [DOI] [PubMed] [Google Scholar]
- 22.Krieger, J. N. 1981. Urologic aspects of trichomoniasis. Investig. Urol. 18:411-417. [PubMed] [Google Scholar]
- 23.Laga, M., A. Manoka, M. Kivuvu, B. Malele, M. Tuliza, N. Nzila, J. Goeman, F. Behets, V. Batter, M. Alary, et al. 1993. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS 7:95-102. [DOI] [PubMed] [Google Scholar]
- 24.Lekkerkerker, A. N., Y. van Kooyk, and T. B. Geijtenbeek. 2006. Viral piracy: HIV-1 targets dendritic cells for transmission. Curr. HIV Res. 4:169-176. [DOI] [PubMed] [Google Scholar]
- 25.Magnus, M., R. Clark, L. Myers, T. Farley, and P. J. Kissinger. 2003. Trichomonas vaginalis among HIV-infected women: are immune status or protease inhibitor use associated with subsequent T. vaginalis positivity? Sex. Transm. Dis. 30:839-843. [DOI] [PubMed] [Google Scholar]
- 26.Mukaida, N. 2000. Interleukin-8: an expanding universe beyond neutrophil chemotaxis and activation. Int. J. Hematol. 72:391-398. [PubMed] [Google Scholar]
- 27.Narimatsu, R., D. Wolday, and B. K. Patterson. 2005. IL-8 increases transmission of HIV type 1 in cervical explant tissue. AIDS Res. Hum. Retrovir. 21:228-233. [DOI] [PubMed] [Google Scholar]
- 28.O'Neil, L. A., and C. Greene. 1998. Signal transduction pathways activated by the IL-1 receptor family: ancient signaling machinery in mammals, insects and plants. J. Leukoc. Biol. 63:650-657. [PubMed] [Google Scholar]
- 29.Petrin, D., K. Delgaty, R. Bhatt, and G. Garber. 1998. Clinical and microbiological aspects of Trichomonas vaginalis. Clin. Microbiol. Rev. 11:300-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Platz-Christensen, J. J., I. Mattsby-Baltzer, P. Thomsen, and N. Wiqvist. 1993. Endotoxin and interleukin-1 alpha in the cervical mucus and vaginal fluid of pregnant women with bacterial vaginosis. Am. J. Obstet. Gynecol. 169:1161-1166. [DOI] [PubMed] [Google Scholar]
- 31.Rasmussen, S. E., M. H. Nielsen, I. Lind, and J. M. Rhodes. 1986. Morphological studies of the cytotoxicity of Trichomonas vaginalis to normal human vaginal epithelial cells in vitro. Genitourin. Med. 62:240-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryu, J. S., J. H. Kang, S. Y. Jung, M. H. Shin, J. M. Kim, H. Park, and D. Y. Min. 2004. Production of interleukin-8 by human neutrophils stimulated with Trichomonas vaginalis. Infect. Immun. 72:1326-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwebke, J. R., and D. Burgess. 2004. Trichomoniasis. Clin. Microbiol. Rev. 17:794-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaio, M. F., P. R. Lin, J. Y. Liu, and K. D. Tang. 1994. Monocyte-derived interleukin-8 involved in the recruitment of neutrophils induced by Trichomonas vaginalis infection. J. Infect. Dis. 170:1638-1640. [DOI] [PubMed] [Google Scholar]
- 35.Shaio, M. F., P. R. Lin, J. Y. Liu, and K. D. Yang. 1995. Generation of interleukin-8 from human monocytes in response to Trichomonas vaginalis stimulation. Infect. Immun. 63:3864-3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh, B. N. 1993. Lipophosphoglycan-like glycoconjugate of Trichomonas foetus and Trichomonas vaginalis. Mol. Biochem. Parasitol. 57:281-294. [DOI] [PubMed] [Google Scholar]
- 37.Singh, B. N., D. H. Beach, D. G. Lindmark, and C. E. Costello. 1994. Identification of the lipid moiety and further characterization of the novel lipophosphoglycan-like glycoconjugates of Trichomonas vaginalis and Trichomonas foetus. Arch. Biochem. Biophys. 309:273-280. [DOI] [PubMed] [Google Scholar]
- 38.Singh, B. N., G. R. Hayes, J. J. Lucas, S. B. Levery, E. Mirgorodskaya, and C. E. Costello. 2000. Novel lipophosphoglycans of trichomonad parasites. Glycobiology 10:1127. (Abstract.) [Google Scholar]
- 39.Singh, B. N., J. J. Lucas, D. H. Beach, S. T. Shin, and R. O. Gilbert. 1999. Adhesion of Tritrichomonas foetus to bovine vaginal epithelial cells. Infect. Immun. 67:3847-3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh, B. N., J. J. Lucas, G. R. Hayes, I. Kumar, D. H. Beach, M. Frajblat, R. O. Gilbert, U. Sommer, and C. E. Costello. 2004. Tritrichomonas foetus induces apoptotic cell death in bovine vaginal epithelial cells. Infect. Immun. 72:4151-4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh, B. N., J. J. Lucas, G. R. Haynes, C. E. Costello, N. Viseux, and S. Levery. 2000. Characterization of novel trichomonad glycoconjugates. FASEB J. 14:1339. (Abstract.) [Google Scholar]
- 42.Soboll, G., L. Shen, and C. R. Wira. 2006. Expression of Toll like receptors (TLR) and responsiveness to TLR agonists by polarized mouse uterine epithelial cells in culture. Biol. Reprod. 75:131-139. [DOI] [PubMed] [Google Scholar]
- 43.Sommer, U., C. E. Costello, G. R. Hayes, D. H. Beach, R. O. Gilbert, J. J. Lucas, and B. N. Singh. 2005. Identification of Trichomonas vaginalis cysteine proteases that induce apoptosis in human vaginal epithelial cells. J. Biol. Chem. 280:23853-23860. [DOI] [PubMed] [Google Scholar]
- 44.Sorvillo, F., L. Smith, P. Kerndt, and L. Ash. 2001. Trichomonas vaginalis, HIV, and African-Americans. Emerg. Infect. Dis. 7:927-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tuomala, R. E., P. T. O'Driscoll, J. W. Bremer, C. Jennings, C. Xu, J. S. Read, E. Matzen, A. Landay, C. Zorrilla, W. Blattner, M. Charurat, and D. J. Anderson. 2003. Cell-associated genital tract virus and vertical transmission of human immunodeficiency virus type 1 in antiretroviral-experienced women. J. Infect. Dis. 187:375-384. [DOI] [PubMed] [Google Scholar]
- 46.Turco, S. J., and A. Descoteaux. 1992. The lipophosphoglycan of Leishmania parasites. Annu. Rev. Microbiol. 46:65-94. [DOI] [PubMed] [Google Scholar]
- 47.Viikki, M., E. Pukkala, P. Nieminen, and M. Hakama. 2000. Gynaecological infections as risk determinants of subsequent cervical neoplasia. Acta Oncol. 39:71-75. [DOI] [PubMed] [Google Scholar]
- 48.Weinstock, H., S. Berman, and W. Cates, Jr. 2004. Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspect. Sex. Reprod. Health 36:6-10. [DOI] [PubMed] [Google Scholar]
- 49.Yap, E. H., T. H. Ho, Y. C. Chan, T. W. Thong, G. C. Ng, L. C. Ho, and M. Singh. 1995. Serum antibodies to Trichomonas vaginalis in invasive cervical cancer patients. Genitourin. Med. 71:402-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zughaier, S. M., S. M. Zimmer, A. Datta, R. W. Carlson, and D. S. Stephens. 2005. Differential induction of the Toll-like receptor 4-MyD88-dependent and -independent signaling pathways by endotoxins. Infect. Immun. 73:2940-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]