Abstract
Bacterial vaginosis is a common condition associated with increased risk of sexually transmitted diseases, including human immunodeficiency virus infections. In contrast, vulvovaginal candidiasis has a much weaker association with sexually transmitted diseases. We found that vaginal lavage fluid from women with bacterial vaginosis is deficient in antimicrobial polypeptides and antimicrobial activity compared to fluid from healthy women or women with vulvovaginal candidiasis. Effective treatment normalized the concentrations of antimicrobial polypeptides in both bacterial vaginosis and in vulvovaginal candidiasis, suggesting that the abnormalities were a result of the diseases. Unlike in vulvovaginal candidiasis, the neutrophil attractant chemokine interleukin-8 (IL-8) was not increased in bacterial vaginosis, accounting for low concentrations of neutrophil-derived defensins in vaginal fluid. In organotypic cultures of human vaginal epithelium containing dendritic cells, treatment with Lactobacillus jensenii, a typical vaginal resident, induced the synthesis of IL-8 mRNA and the epithelial human β-defensin-2 mRNA, but a typical bacterial vaginosis pathogen, Gardnerella vaginalis, had no effect. When the two bacteria were combined, Gardnerella vaginalis did not interfere with the immunostimulatory effect of Lactobacillus jensenii. The loss of normal immunostimulatory flora in bacterial vaginosis is thus associated with a local deficiency of multiple innate immune factors, and this deficiency could predispose individuals to sexually transmitted diseases.
The human vagina is a microbe-selective environment that supports commensal flora while resisting colonization by exogenous microbes. Lactobacilli, the predominant vaginal microbes, compete with exogenous microbes for attachment sites and nutrients and suppress exogenous flora by maintaining a low pH and secreting antimicrobial substances (bacteriocins) (2, 25). Moreover, vaginal and cervical epithelia secrete antimicrobial peptides and proteins that can contribute to the prevention of invasion by exogenous microbes (38). Despite these mechanisms, as many as 75% of women may experience an occasional vaginal infection, and some (5 to 10%) women suffer from recurrent bacterial vaginosis (BV) or vulvovaginal candidiasis (VVC) (9, 34).
Epidemiologic and laboratory studies of BV indicate that this condition could alter the local host defense barrier to other infections, and, of most concern, the barrier to human immunodeficiency virus (HIV) infection. Among women in South Africa (23), sex workers in Kenya (20), and pregnant women in Malawi (35), women with BV were at two to three times greater risk of subsequently acquiring HIV than were women without BV, and this difference persisted even after adjustment for potential confounding factors. Several cross-sectional studies also reported the association of BV and HIV infection (6, 12, 21, 30). Interestingly, evidence that VVC may increase susceptibility to HIV is much less convincing (10). Although the relationship between BV and susceptibility to HIV is undoubtedly complex, we explored the specific hypothesis that BV may decrease the concentration of innate antimicrobial substances in vaginal fluid.
The ability of vaginal fluid to selectively support resident microbes and to inhibit exogenous ones is dependent on its content of antimicrobial substances, including lactic acid and antimicrobial polypeptides (AMP) (38). Among the AMP found in vaginal fluid, the α-defensins human neutrophil peptides (HNP) are present only in neutrophils, and their presence would reflect an acute inflammatory response. The α-defensin HD-5 and the human β-defensins HBD-1 and HBD-2 originate from epithelial cells, and the synthesis and release of HBD-2 and HD-5 are induced by inflammatory stimuli (7, 28, 39). Lysozyme is released by neutrophils, as well as secreted from (predominantly cervical) epithelia, and is known to act synergistically with other AMP (8, 17, 33). Calprotectin is released from squamous epithelia but is also a major cytosolic protein in neutrophils and macrophages. It is antimicrobial and fungistatic and can prevent invading microbes from binding to mucosal epithelial cells (4, 22, 24). The secretory leukoprotease inhibitor (SLPI), secreted by epithelial cells, inhibits neutrophil elastase and cathepsin G, contributes to protection of the tissues in an inflammatory response (3), exerts antimicrobial activity (11), and interferes with HIV infection (19). Recent studies suggest that the activity of vaginal fluid against HIV may depend on the synergistic activity of multiple cationic polypeptides (40).
In the present study, we examined the interactions between pathological alterations of vaginal flora and the composition and antimicrobial properties of vaginal fluid.
MATERIALS AND METHODS
Study population.
Vaginal lavage fluid (VLF) samples from 64 women (mean age, 24.7 ± 4.5 years [standard deviation]) were collected by clinicians at the UCLA Arthur Ashe Student Health and Wellness Center during the initial examination and, when appropriate, during a return visit after treatment, in conformity with an approved IRB protocol (98-11-049-12). The women were either healthy (H; n = 19) or presented with BV (n = 16, 12 returns), VVC (n = 24, 20 returns), or both BV and VVC (n = 7, 6 returns), as determined by standard clinical diagnostic criteria. Women with BV had at least three of the four Amsel criteria (1): typical adherent homogeneous discharge, a vaginal pH of >4.5, amine odor after the addition of 10% KOH to the vaginal specimen, and clue cells on the wet smear. Women with VVC had inflammatory symptoms such as pain or itching, and yeasts were visible on the wet smear. We confirmed the diagnosis of VVC by microscopic inspection of lavage fluids for yeast (24 of 24 VLF samples from patients with clinically diagnosed VVC contained visible yeast) and by culture (23 of 24 grew Candida albicans, as analyzed by the UCLA Clinical Laboratory). Patients were excluded from the study if they had a sexually transmitted disease, were pregnant, or were trying to become pregnant. One sample was excluded because it contained chlamydia. Patients with either BV or VVC were treated according to standard medical protocols: Treatment for BV could be one of the following: (i) 0.75% metronidazole gel, applied vaginally, one or two times a day for 5 days (no alcohol or intercourse during treatment); (ii) 750-mg tabs of Flagyl at one tab every day for 7 days; or (iii) 2% Cleocin cream, with one applicator inserted vaginally for 3 to 7 nights. Treatment for VVC consisted of one of the following: (i) Terazol 7 (terconazole) for 7 days vaginally; (ii) Diflucan given as 150 mg orally; or (iii) use of an over-the-counter antifungal preparation (e.g., Monostat). Subjects were asked to return 1 month later to have another sample collected (3 to 4 weeks posttreatment). Samples from patients who had both BV and VVC were collected and analyzed but not reported in the present study because the effects were dependent on the relative impact of BV versus VVC on each patient.
Collection of vaginal lavage fluid.
The vaginal wall was washed with 10 ml of sterile water, and the recovered VLF was promptly cleared of cells and bacteria by centrifugation and sterile filtration through a 0.2-μm-pore-size syringe filter (Pall Corp., Ann Arbor, MI). The VLF was divided into aliquots and stored frozen at −80°C until use. An equal volume of phosphate-buffered saline containing 10% glycerol was added to the cell pellet. A portion of the cells was transferred to a microscope slide by using a Cytospin centrifuge (Shandon Lipshaw, Inc., Pittsburgh, PA) and then observed after differential staining (Quik-Stain; Dade Behring, Newark, DE) to confirm the diagnosis. The remaining cell pellet was frozen and cultured for common vaginal microbes (lactobacilli, Gardnerella, Prevotella, and Candida) at the UCLA Clinical Laboratory.
Dot blot immunoassay.
A volume of 4 μl of VLF or standard (diluted in 0.05% bovine serum albumin in 0.1% acetic acid) was dotted onto Immobilon-P polyvinylidene difluoride membrane (Millipore Corp., Danvers, MA) after wetting with methanol, followed by a rinse in Tris-buffered saline (TBS). The membrane was fixed for 20 min in TBS containing 0.05% glutaraldehyde (Sigma, St. Louis, MO), blocked for 30 min in Superblock (Pierce, Rockford, IL) at 37°C, and then incubated overnight in antibody diluted in 30% Superblock-0.05% Tween 20-TBS (pH 7.5). Primary antibodies were diluted 1:2,000 except for rabbit anti-lysozyme antibody (Dako, Glostrup, Denmark), which was diluted 1:1,000. Rabbit anti-HNP, anti-HBD-1, anti-HBD-2, and anti-HD-5 antibodies were made in our laboratory (27, 32, 39). Rabbit anti-calprotectin antibodies (MRP-8 and MRP-10) were a generous gift from Kenneth Miyasaki (UCLA Department of Dentistry). Dot blots were washed in 0.05% Tween 20-TBS (pH 5) with 0.1% bovine serum albumin (A-7305; Sigma) and then incubated for 1 h with horseradish peroxidase-conjugated second antibody diluted 1:20,000 in 30% Superblock-0.05% Tween 20-TBS (pH 7.5). After a washing step as described above, the blots were placed in SuperSignal West Pico chemiluminescent substrate (Pierce) for 5 min and then developed by using the Chemidoc system (Bio-Rad, Hercules, CA). Dot blots were analyzed by using the QuantOne software program developed by Bio-Rad. The intensity of the sample dot luminescence was compared to those of a standard curve. The samples were analyzed at multiple dilutions to minimize blocking of the signal by other proteins.
SLPI.
SLPI was detected by using enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions.
Protein.
Total protein was determined via the bicinchoninic acid assay (Pierce) using bovine serum albumin (A-7305; Sigma) for the standard curve.
CFU assay.
The CFU assay was performed as follows. Escherichia coli 8739 (a fecal isolate obtained from the American Type Culture Collection [ATCC], Rockville, MD) was grown to exponential phase in Trypticase soy broth (TSB) washed in genital tract (GT) buffer (20 mM KPO4, 60 mM NaCl [pH 4.5]) containing 0.3% TSB by weight and then brought to a concentration of 108 cells/ml in the same solution. Vaginal fluid (27 μl) was divided into aliquots in sterile microfuge tubes, and 3 μl of E. coli was added to the vaginal fluid or a control tube containing 27 μl of GT buffer. CFU counts were determined as previously described (26). In brief, input CFU was determined by adding 2.2 μl of control to 200 μl of GT buffer in two of the top wells of a 96-well plate (Costar, Corning, NY), and this was then serially diluted 1:6 down the lane of the plate for a total of seven dilutions. A volume of 6 μl of bacteria was plated in duplicate onto a TSB-agar plate (BioMerieux, Lombard, IL) using a multichannel pipette (four data sets for each sample). Plates were incubated at room temperature (∼24°C) overnight. The remaining samples were incubated for 2 h in an environmental shaker at 37°C, after which the samples were diluted and plated onto TSB-agar plates and incubated as described above. Colonies were counted in two consecutive dilutions whenever possible and then multiplied by the dilution of the well to determine the CFU. The assays were repeated for each specimen at least once, and the log CFU values were averaged for each specimen.
Lactate.
Total lactate content was determined by a colorimetric endpoint assay using a diagnostic kit from Sigma Corp. Briefly, lactate oxidase converts lactate to pyruvate and peroxide (H2O2). Peroxidase uses H2O2 to catalyze the conversion of chromogen precursors to produce a colored dye with an absorbance at 540 nm that is directly proportional to the amount of lactate present in the sample.
Extraction of cationic peptides from cell pellets.
A volume of 500 μl of 5% acetic acid was added to 0.1 g of frozen cell pellet, followed by sonication for two 10-s bursts on ice, and then incubated at 4°C with rotation overnight. Cell debris was removed by centrifugation at 14,000 × g. The supernatant containing cationic proteins was used for analysis on acid urea and 16.5% sodium dodecyl sulfate (SDS)-Tricine-polyacrylamide gel electrophoresis (PAGE) (Gradiapore, New South Wales, Australia).
Cytokine analysis.
Human interleukin-1α (IL-1α), IL-1β, IL-1 receptor antagonist (IL-1ra), and IL-8 were analyzed by using a solid-phase sandwich enzyme-linked immunosorbent assay (Biosource, Camarillo, CA) according to the manufacturer's instructions. The values were converted to pg/ml (IL-1β) or ng/ml (IL-1ra) using a standard curve that was generated in parallel to the test samples for reference. The lower limit of sensitivity was 1 pg/ml for IL-1β and IL-1α, 4 pg/ml for IL-1ra, and 0.7 pg/ml for IL-8.
Organotypic vaginal epithelial culture.
Organotypic cultures of vaginal epithelium (VE) containing normal human VE and dendritic cells were purchased from MatTek Corp. (Ashland, MA). VE cells were placed in culture overnight (37°C, 5% CO2) with an air-liquid interface using the Mattek media. Heat-inactivated bacteria was prepared as follows. Lactobacillus jensenii (ATCC 25258) was grown overnight at 37°C in MRS broth (Difco/Becton Dickinson, Sparks, MD). Dilutions were made with MRS broth to bring the lactobacillus to an optical density at 620 nm (OD620) of 1.0, 0.1, 0.01, or 0.001. A clinical isolate of Gardnerella vaginalis (obtained from Elizabeth Wagar at the UCLA Clinical Laboratory) was grown for approximately 1 week in BHI medium (Difco/Becton Dickinson), 4% sonicated horse blood (BBL/Becton Dickinson) at 37°C, and 5% CO2 and then diluted with the same medium to OD620 values as described above. Then, 200-μl aliquots of the bacteria or fresh bacterial media were boiled for 5 min to heat inactivate them, and then they were stored at −80°C until use. The tissue culture medium was changed prior to adding 20 μl of heat-inactivated bacteria or medium (MRS or BHI) to the apical surface. IL-1β (20 ng/ml) was added to the medium (basal surface) as a positive control for HBD-2 induction. Organotypic cultures were incubated 24 h after the addition of bacteria prior to tissue processing. In a separate set of experiments, combinations of L. jensenii and G. vaginalis were added to the tissue surface to determine whether there were any synergistic or inhibitory interactions between these two microbes. A 20-μl volume of each heat-inactivated bacterial sample (described above) was added to the apical surface in an array of concentrations. An OD620 of zero represents the bacterium-free medium used for the culture of each bacterium.
Quantitative real-time reverse transcription-PCR.
Membranes with VE were placed in TRIzol reagent (Invitrogen, Carlsbad, CA) and then homogenized for 30 s with a hand-held biohomogenizer (PRO Scientific, Oxford, CT). RNA was purified according to the manufacturers' instructions. The iScript cDNA synthesis kit (Bio-Rad) was used to make cDNA from RNA according to the manufacturer's instructions. Real-time PCRs were made with the Bio-Rad iQ SYBR Green Supermix kit using primers for HBD-2 (5′-CCTGTTACCTGCCTTAAGAGTG-3′ and 5′-GAATCCGCATCAGCCACAG-3′), IL-1 (5′-TGACCTGAGCACCTTCTTTC-3′ and 5′-CGCAGGACAGGTACAGATT-3′), IL-8 (5′-AAGGAACCATCTCACTGTGTGTAAAC-3′ and 5′-ATCAGGAAGGCTGCCAAGAG-3′), and the control G3PDH (5′-TGGTATCGTGGAAGGACTC-3′ and 5′-AGTAGAGGCAGGGATGATG-3′). A two-step PCR (40 cycles of 95°C for 30 s and 58°C for 30 s) was used in the Bio-Rad iCycler, and iCycler iQ software was used to analyze the data.
Statistical tests.
The demographic characteristics reported by women were analyzed using SAS. Sigma Stat software was used for analyses of the biological data. Only predetermined hypotheses were tested, and therefore no adjustment was made for multiple comparisons.
RESULTS
Donor characteristics.
Table 1 lists the characteristics of the donors, who were all college-age, generally healthy women.
TABLE 1.
Study population
| Parameter | Diagnosis groupa
|
|||
|---|---|---|---|---|
| All diagnoses (n = 52) | BV (n = 13) | VVC (n = 20) | H (n = 19) | |
| Age (±SD) | 24.7 (4.5)b | 25.1 (2.5) | 23.6 (3.9) | 25.9 (5.8) |
| No. of subjects (%) | ||||
| White | 30 (58) | 4 (30) | 11 (55) | 15 (79) |
| African American | 1 (2) | 0 (0) | 0 (0) | 1 (5) |
| Hispanic | 10 (19) | 6 (46) | 4 (20) | 0 (0) |
| Asian/Pacific Islander | 8 (15) | 3 (23) | 3 (15) | 2 (11) |
| Other | 3 (6) | 0 (0) | 2 (10) | 1 (5) |
n, number of subjects.
One subject did not provide her age.
Lactic acid and pH.
As would be expected, VLF samples from women with BV had a significantly (according to the Mann-Whitney rank sum test) lower total lactate and higher pH than those from healthy women or women with VVC (Table 2). Comparing pre- and posttreatment values in each patient with BV, the pH decreased and lactate increased significantly after treatment (Wilcoxon sign rank test < 0.001), both reaching the range seen in healthy women or women with VVC.
TABLE 2.
Lactate and pH content of vaginal lavage fluids
| Diagnosis group | Median (range)
|
P (compared to H)a | No. of subjects | |
|---|---|---|---|---|
| pH | Lactate concn (mM) | |||
| Healthy women | 4.0 (3.7-4.2) | 1.1 (0.6-1.8) | 19 | |
| BV-affected women | ||||
| Before treatment | 4.9 (4.5-5.7) | 0.4 (0.3-0.5) | <0.001 | 16 |
| After treatment | 4.1 (3.9-4.4) | 1.8 (1.2-2.9) | 0.87 | 12 |
| VVC-affected women | ||||
| Before treatment | 4.0 (3.8-4.2) | 1.7 (0.8-2.5) | 0.43 | 24 |
| After treatment | 3.9 (3.6-4.0) | 1.5 (0.9-1.7) | 0.25 | 20 |
As determined by the Mann-Whitney rank sum test.
AMP analysis.
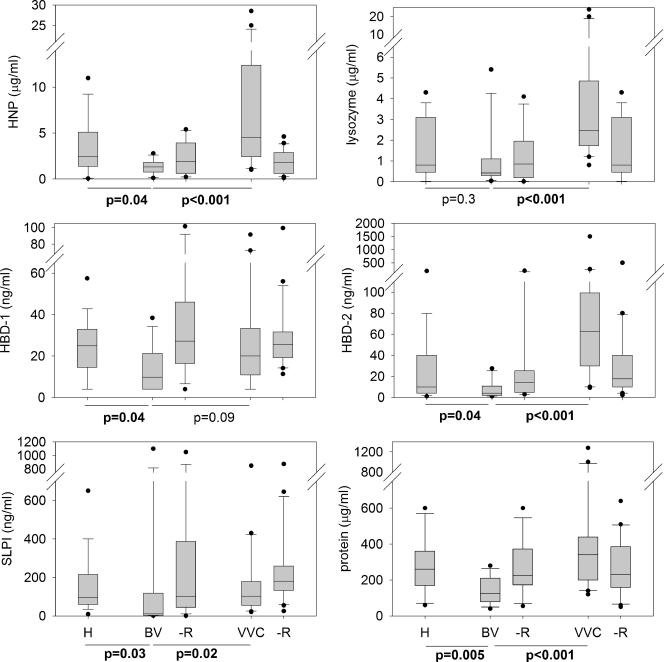
VLF samples from healthy women and those with BV or VVC at 3 weeks posttreatment were analyzed for AMP (Fig. 1) . The concentration in VLF of most AMP, as well as total protein, was reduced in BV group compared to H or VVC. The difference between BV and H was statistically significant (P < 0.05) for the defensins HNP, HBD-1, and HBD-2 and for SLPI and total protein (Fig. 1). The difference in lysozyme did not reach statistical significance (Fig. 1). The differences were even more striking when BV was compared to VVC and reached statistical significance (P < 0.05) for HNP-1, HBD-2, SLPI, lysozyme, and total protein but not for HBD-1. There was no difference in peptide concentration between the healthy, BV, or VVC VLF samples for HD-5 and calprotectin. The median values (interquartile ranges) were as follows for HD-5: H, 12 ng/ml (7 to 25 ng/ml); BV, 12 ng/ml (7 to 19 ng/ml); and VVC, 16 ng/ml (5 to 25 ng/ml). The median values (interquartile ranges) were as follows for calprotectin: H, 10 μg/ml (5 to 14 μg/ml); BV, 6 μg/ml (2 to 12 μg/ml); and VVC, 8 μg/ml (7 to 15 μg/ml).
FIG. 1.
AMP in vaginal infections. VLF samples from healthy women (H) and those with BV or VVC before and after (-R) treatment were analyzed by immunoassay. Total protein was analyzed by the bicinchoninic acid assay. The box-whisker plot shows median values and the 25 to 75% interquartile range as lines within the boxes, the 10 to 90% range as whiskers, and values outside this range as dots. The Mann-Whitney rank-sum test was used to compare BV to H and BV to VVC, and P values of ≤0.05 are indicated in boldface. BV, n = 19; BVR, n = 16; BVR, n = 12; VVC, n = 24; VVCR, n = 20. The values after treatment (BVR and VVCR) are shown here for reference. The analysis of the effects of treatment was performed separately using paired before-and-after samples for each donor (Fig. 2).
To analyze the effect of treatment on individual patients with BV or VVC, the fold change for each protein concentration after treatment compared to before treatment was calculated (Fig. 2). As can be seen in the top panel, the median concentrations of all AMP except HD-5 in BV-VLF increased after treatments (Fig. 2). For defensins HBD-1 and HBD-2 and total protein the change was statistically significant for the entire group (P < 0.05 [Wilcoxon signed rank statistic]). In contrast to BV, most AMP in VVC samples were decreased after treatment (lower panel). For HBD-2, HNP, lysozyme, SLPI, and total protein the decrease after treatment was statistically significant for the group (P < 0.05). HBD-1, HD-5, and calprotectin concentrations were essentially unchanged. In general, treatment led to a correction of abnormal polypeptide patterns in both BV and VVC (Fig. 3), although a much larger sample would be needed to determine whether residual defects may be persist after treatment.
FIG. 2.
Effects of treatment on vaginal AMP. For each protein, we calculated the ratio of the concentration in VLF after treatment to that before treatment. The individual ratios and group median and interquartile range are shown. BV, n = 12; VVC, n = 20. The Wilcoxon signed-rank statistic was used to compare the values before and after treatment, and the P values are shown above the horizontal axis, with P values of <0.05 indicated in boldface.
FIG. 3.
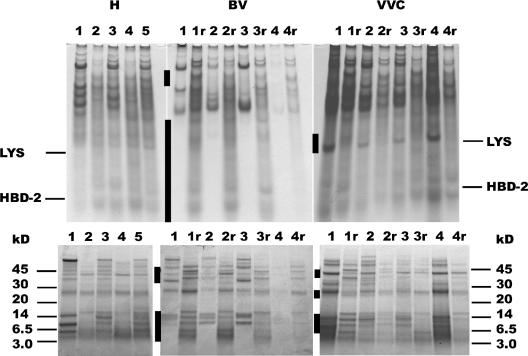
Differences in the protein composition of VLF in healthy women (H) and women with vaginal infection (BV and VVC) before and after treatment. The top panel shows acid-urea-PAGE in which the mobility of each protein species depends on its cationic charge and inversely on its size. The bottom panel is an SDS-Tricine-PAGE, which separates the proteins by size. The migration of lysozyme and HBD-2 standards is indicated in the upper panel, whereas molecular mass markers are indicated in the lower panel (in kilodaltons). For patients with BV or VVC, paired samples are shown before and after (r) treatment. Areas of marked differences are indicated by bars on the left side of each gel.
Median and interquartile ranges for lactate (in mM) are shown in Table 2. Based on normal values for vaginal fluid lactate 10 to 20 mM, we estimated that the sampled VLF is diluted approximately 10- to 20-fold compared to vaginal fluid in situ. As expected, BV fluid contained the lowest concentration of lactate.
Changes in the polypeptide composition of VLF.
Further evidence that the protein composition of VLF is changed during vaginal infections was obtained by electrophoretic analysis of the samples (Fig. 3). In BV there was a selective depletion of small cationic polypeptides, as well as of several larger polypeptides, but the pattern became more normal after treatment. To examine the possibility that the missing proteins were adsorbed by the large numbers of bacteria present in BV, we extracted the cellular fraction of each sample with 5% acetic acid, a treatment that dissociates bound cationic polypeptides from anionic biological surfaces. We then analyzed the extract by AU-PAGE and SDS-Tricine-PAGE (Fig. 4). The extracts contained proteins that migrated similarly to proteins in their respective lavage fluids. In particular, the pellets from BV samples did not contain the proteins that were deficient in the corresponding BV lavage fluids.
FIG. 4.
Acid-urea-PAGE of the cellular (c) and fluid (f) components of VLF in BV. Paired samples are shown before and after (r) treatment for BV. The migration of HNP (defensin)-1, lysozyme, and HBD-2 standards is as indicated.
Antimicrobial activity.
Escherichia coli is part of the normal flora of the gastrointestinal tract and is easily spread to the vagina and bladder. E. coli 8739, a fecal isolate obtained from the ATCC, was chosen for the VLF CFU assay because it is a relevant exogenous microbe that is not part of the abnormal flora in BV. Tenfold-concentrated E. coli in exponential phase was added to VLF (final concentration of 107 bacteria/ml), followed by incubation for 2 h at 37°C. The time zero and 2-h CFU counts were determined, and the log change was calculated. Figure 5 shows the median and interquartile range for the log change in CFU. Values above zero represent growth, and values below zero represent bacterial killing after 2 h of incubation. Healthy, VVC, and VVCR (i.e., VVC after treatment) VLF samples were toxic to E. coli, whereas the bacteria were able to grow in all of the BV VLF samples. VLF from bacterial vaginosis after treatment (BVR) demonstrated increased but variable killing ability. The killing ability (log change CFU) of BVR VLF was correlated with the completeness of recovery, as assessed by the concentration of lactic acid in the VLF (correlation coefficient of −0.8 [P = 0.002]).
FIG. 6.
Cytokine concentrations in VLF determined by enzyme-linked immunosorbent assay. The box-whisker plot shows median values and the 25 to 75% interquartile range as lines within the boxes, the 10 to 90% range as whiskers, and values outside this range as dots. Median BV values were compared to H and VVC by using the rank-sum test. Before- and after-treatment values (BV versus BV-R and VVC versus VVC-R, respectively) were compared by the paired Student t test with P values of ≤0.05 in boldface.
Cytokine analysis.
IL-1 (IL-1α and IL-1β) is an acute-phase cytokine and is induced by inflammatory stimuli. As seen in Fig. 6, IL-1 was elevated in the BV and VVC VLF samples compared to normal. However, IL-1ra, a competitive inhibitor of IL-1, was also elevated in the BVR and VVC VLF. BV samples had the lowest ratio of IL-1/IL-1ra, i.e., potentially more IL-1 activity. IL-1 is a major inducer of the neutrophil-attractant chemokine IL-8. Paradoxically, in BV VLF, IL-1 was elevated, but IL-8 was reduced or unchanged.
Induction of HBD-2, IL-1, and IL-8 by heat-killed bacteria in organotypic VE cultures.
Organotypic tissue that contained normal human-derived VE and dendritic cells was placed in culture (37°C, 5% CO2) at an air-liquid interface. Heat-inactivated bacteria or media (MRS or BHI) were added to the air-exposed apical surface. IL-1β was added to the media (basal surface) as a positive control for HBD-2 and IL-8 induction. Organotypic cultures were incubated 24 h after the addition of bacteria prior to tissue processing and real-time PCR analysis. As can be seen in Fig. 7A, L. jensenii stimulated HBD-2, IL-1, and IL-8 in a dose-dependent manner, whereas G. vaginalis had no effect. To determine whether there were any synergistic or antagonistic interactions between these two microbes, combinations of L. jensenii and G. vaginalis were added to the tissue surface. In Fig. 7B, all of the plots are essentially superimposable. This indicates that heat-inactivated G. vaginalis exerts neither a stimulatory nor an inhibitory effect on the ability of L. jensenii to induce the expression of HBD-2, IL-1, and IL-8.
FIG. 7.
Induction of HBD-2, IL-1, and IL-8 by L. jensenii but not G. vaginalis. Bacteria were grown to saturation and then brought to an OD620 of 1.0, 0.1, 0.01, or 0.001 with their respective culture medium (MRS for L. jensenii or BHI plus 4% lysed horse blood for G. vaginalis). Aliquots were boiled for 5 min to heat inactivate the bacteria. (A) A total of 20 μl of heat-inactivated or culture medium (M) bacteria was placed onto the top surface of the VE tissue (MatTek), or IL-1β (20 ng/ml) was added to the culture medium. Control (C) tissue was untreated. After 24 h of incubation, tissues were analyzed by quantitative reverse transcription-PCR. Concentrations of cytokine mRNAs are shown relative to the G3PDH mRNA as differences in threshold cycles (ΔCT). Closed and open symbols indicate L. jensenii and G. vaginalis, respectively. Differences significant at a P of <0.05 between samples treated with the two different bacteria are marked by asterisks. (B) Combinations of L. jensenii and G. vaginalis (20 μl each at an OD620 of 1.0, 0.1, 0.01, 0.001, or 0 with their respective culture medium) were placed on the top surface of the VE tissue, and the mRNA was analyzed 24 h later. Concentrations of cytokine mRNAs are shown relative to G3PDH mRNA as differences in threshold cycles (ΔCT). The OD620 values of the added G. vaginalis were as follows: 0, •; 0.001, ○; 0.01, ▾; 0.1, ▿; and 1.0, ▪. Representative error bars are shown. L. jensenii (LbJ) stimulated mRNA expression of HBD-2, IL-1, and IL-8 in a dose-dependent manner. G. vaginalis had no detectable effect on stimulation by L. jensenii.
DISCUSSION
We report that VLF from women with BV is deficient in AMP and antimicrobial activity compared to VLF from healthy women or women with VVC. The deficiency is not merely due to dilution but reflects altered composition with reduced concentrations of small cationic peptides that migrate rapidly in both acid-urea-PAGE (Fig. 3A) and SDS-PAGE (Fig. 3B). Quantitative analysis corroborates these results (Fig. 1), where nearly all of the AMP were two- to fourfold lower in BV fluid compared to H and VVC fluids. The vaginal AMP originates from different sites and cell types. The α-defensins 1 to 3 (HNP) originate from neutrophils, the β-defensin HBD-2 is induced by inflammation in epithelial cells, and SLPI is predominantly secreted into cervical mucus. Our observation of decreases in all three components suggests that BV is associated with a local impairment of multiple innate immune pathways. The antimicrobial activity of BV VLF is also much reduced (Fig. 5). Based on our previous studies (38), the antimicrobial activity of vaginal fluid depends predominantly on its lactic acid content with a smaller contribution from AMP, and both of these factors are reduced in BV. Reduced cationic polypeptide concentrations, including SLPI, would also be expected to decrease the resistance to HIV transmission (19, 40).
FIG. 5.
Bactericidal activity of VF fluid. Median and interquartile ranges of log changes in E. coli 8739 CFU are shown. Exponentially growing E. coli 8739 (ATCC) was added to VLF and incubated for 2 h at 37°C in an environmental shaker. Aliquots at time zero and 2 h were diluted, dotted onto Trypticase soy agar plates, and incubated overnight at room temperature. The number of colonies was multiplied by the dilution factor to determine the CFU. The log change CFU was calculated from the input CFU compared to the CFU at 2 h. A value of zero indicates no change, values above zero indicate bacterial growth, and values below zero indicate bactericidal activity.
We considered the possibility that decreased levels of AMP in women with BV reflected a genetic deficiency. However, the AMP levels observed in women with BV rose to the range observed in healthy women after successful medical treatment, suggesting that the women were genetically competent to produce normal amounts of AMP. VVC-VLF, in contrast, showed elevated protein and AMP compared to BV VLF, likely due to the proinflammatory effect of yeast infections. Although detailed comparison of H and VVC polypeptide concentrations was not one of the goals of the present study, it is notable that lysozyme and HBD-2 concentrations were significantly higher in VVC than in H samples (Fig. 1, P = 0.04 and 0.01, respectively). Because of within-group variations, comparisons of the H, BV, and VVC groups to each other are less sensitive to disease-related changes in AMP concentrations than paired pre- and posttreatment comparisons, where each patient serves as her own control. These analyses in VVC revealed significant posttreatment decreases of AMP (Fig. 2 and 3), as well as total protein levels, and the normalization of electrophoretic protein patterns after treatment. The increase in AMP during VVC is in sharp contrast to the decrease in AMP during BV and undoubtedly reflects the differential effects of the respective microbial agents on innate immunity.
BV is characterized by a microbial shift from predominantly lactobacilli to a mixed anaerobic milieu. These anaerobes are smaller in size and much more numerous than lactobacilli (34), leading to an increased bacterial surface area. Most AMP require a threshold concentration for effective disruption of microbial membranes and the resulting antimicrobial activity (31). We speculated that the cationic AMP could be removed from the fluid phase by binding to the increased surface area of the anionic bacterial membranes. To test this hypothesis, we extracted protein from VLF cell pellets, which contained bacteria and epithelial cells, but were unable to recover any low-molecular-weight cationic proteins (Fig. 4), indicating that the missing AMP were not trapped in the cellular fraction of BV VLF.
We next examined whether the change in the composition of VLF reflects differences in the modulatory effects of vaginal microbial flora on cytokine and AMP production. Other investigators have reported that BV generates a very low tissue inflammatory response so that 50% of women with BV are asymptomatic (14). Our analysis of local cytokine and chemokines responses found elevated IL-1β but not a concomitant elevation in IL-8, which is the primary chemotactic factor for neutrophils (Fig. 6). These findings are in agreement with previous reports (5). The low IL-8 concentration in the vaginal fluid of BV patients may explain the relatively low neutrophil influx in BV (5), as well as the low levels of the neutrophil-specific HNP defensins. Although the detailed mechanisms have not been identified, it has been suggested that factors secreted by the microbial flora in BV inhibit the IL-8 response despite the elevation of IL-1 concentrations (5).
The reasons for the deficiency of HBD-2 and other epithelially secreted polypeptides in BV appear to be more complex. In previous studies of epithelial production of HBD-2 in organotypic epidermal cultures, IL-1 was found to be a potent inducer of HBD-2 synthesis, as well as the predominant HBD-2-inducing component of the secretions of lipopolysaccharide-stimulated monocytes (15, 16). We therefore examined the possibility that the HBD-2 deficiency in BV samples was due to either a low concentration of IL-1 or a high concentration of its principal antagonist, IL-1ra. Surprisingly, IL-1ra was not induced in BV compared to H or VVC VLF, so that the ratio of IL-1ra to IL-1in BV VLF was lower than in the H or VVC samples (Fig. 6). We therefore used an in vitro model of VE to examine whether the bacterial flora can modify HBD-2 production independently of IL-1. In these experiments, L. jensenii or G. vaginalis was placed on the model VE surface. Although the complexity of microbial flora in healthy women and women with BV is well established (13), L. jensenii and G. vaginalis were chosen as model organisms because they are common and abundant in healthy women and women with BV, respectively: ca. 30 to 40% of healthy women are colonized by L. jensenii (37), and G. vaginalis is found in up to 94% of all cases of BV (18). The lactobacilli induced HBD-2, IL-1, and IL-8 (Fig. 6), but comparable densities of Gardnerella did not induce HBD-2 or IL-8 and only minimally induced IL-1. In addition, lactobacilli were more effective inducers of HBD-2 and IL-8 than was the relatively high concentration (20 ng/ml) of IL-1β. In another experiment, when excess of IL-1ra (200 ng/ml) was added to the culture medium before the addition of bacteria to the surface of the tissue, IL-1ra did not influence the bacterium-induced HBD-2 or IL-8 concentrations (data not shown). Taken together, these data suggest that the composition of microbial flora affects the concentrations of AMP through mechanisms distinct from the IL-1-dependent pathway.
Microbes can directly induce AMP and cytokines by interactions of their characteristic macromolecules (“patterns”) with Toll-like receptors and other “pattern” recognition systems (36). G. vaginalis is a gram-positive bacterium, as indicated by ultrastructural studies and the lack of lipopolysaccharide (29), but its peptidoglycan content is unusually low. These characteristics may contribute to its ability to avoid triggering the inflammatory response that is induced by other microbes that cause vaginal infections, such as Candida albicans. It has also been suggested that G. vaginalis may actively suppress inflammation, but the specific mechanisms involved are still speculative.
In vivo, BV flora appears to be less stimulatory than normal flora for the production of innate immune mediators, and this difference is replicated in vitro when organotypic vaginal epithelium is exposed to the BV agent G. vaginalis compared to the normally resident L. jensenii. By decreasing the stimulus to innate immunity, BV may produce a state of local immunosuppression that could increase susceptibility to HIV and other sexually transmitted diseases.
Acknowledgments
We thank Lynn Fukumoto, Jackie Nguyen, Jeannie Martinez, Marina Len, and Nancy Nguyen for help in sample procurement and analysis.
This study was supported by NIH grants PO1 AI 37945 (to Robert Lehrer) and R01 AI46514 (T.G.).
Editor: A. D. O'Brien
REFERENCES
- 1.Amsel, R., P. A. Totten, C. A. Spiegel, K. C. Chen, D. Eschenbach, and K. K. Holmes. 1983. Nonspecific vaginitis: diagnostic criteria and microbial and epidemiologic associations. Am. J. Med. 74:14-22. [DOI] [PubMed] [Google Scholar]
- 2.Aroutcheva, A. A., J. A. Simoes, and S. Faro. 2001. Antimicrobial protein produced by vaginal Lactobacillus acidophilus that inhibits Gardnerella vaginalis. Infect. Dis. Obstet. Gynecol. 9:33-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashcroft, G. S., K. Lei, W. Jin, G. Longenecker, A. B. Kulkarni, T. Greenwell-Wild, H. Hale-Donze, G. McGrady, X. Y. Song, and S. M. Wahl. 2000. Secretory leukocyte protease inhibitor mediates non-redundant functions necessary for normal wound healing. Nat. Med. 6:1147-1153. [DOI] [PubMed] [Google Scholar]
- 4.Brandtzaeg, P., T. O. Gabrielsen, I. Dale, F. Muller, M. Steinbakk, and M. K. Fagerhol. 1995. The leucocyte protein L1 (calprotectin): a putative nonspecific defense factor at epithelial surfaces. Adv. Exp. Med. Biol. 371A:201-206. [DOI] [PubMed] [Google Scholar]
- 5.Cauci, S., S. Guaschino, A. D. De, S. Driussi, S. D. De, P. Penacchioni, and F. Quadrifoglio. 2003. Interrelationships of interleukin-8 with interleukin-1beta and neutrophils in vaginal fluid of healthy and bacterial vaginosis positive women. Mol. Hum. Reprod. 9:53-58. [DOI] [PubMed] [Google Scholar]
- 6.Cohen, C. R., A. Duerr, N. Pruithithada, S. Rugpao, S. Hillier, P. Garcia, and K. Nelson. 1995. Bacterial vaginosis and HIV seroprevalence among female commercial sex workers in Chiang Mai, Thailand. AIDS 9:1093-1097. [DOI] [PubMed] [Google Scholar]
- 7.Dale, B. A., J. R. Kimball, S. Krisanaprakornkit, F. Roberts, M. Robinovitch, R. O'Neal, E. V. Valore, T. Ganz, G. M. Anderson, and A. Weinberg. 2001. Localized antimicrobial peptide expression in human gingiva. J. Periodontal Res. 36:285-294. [DOI] [PubMed] [Google Scholar]
- 8.Eggert-Kruse, W., I. Botz, S. Pohl, G. Rohr, and T. Strowitzki. 2000. Antimicrobial activity of human cervical mucus. Hum. Reprod. 15:778-784. [DOI] [PubMed] [Google Scholar]
- 9.Ferrer, J. 2000. Vaginal candidosis: epidemiological and etiological factors. Int. J. Gynaecol. Obstet. 71(Suppl. 1):S21-S27. [DOI] [PubMed] [Google Scholar]
- 10.Hester, R. A., and S. B. Kennedy. 2003. Candida infection as a risk factor for HIV transmission. J. Womens Health 12:487-494. [DOI] [PubMed] [Google Scholar]
- 11.Hiemstra, P. S., R. J. Maassen, J. Stolk, R. Heinzel-Wieland, G. J. Steffens, and J. H. Dijkman. 1996. Antibacterial activity of antileukoprotease. Infect. Immun. 64:4520-4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hillier, S. L. 1998. The vaginal microbial ecosystem and resistance to HIV. AIDS Res. Hum. Retrovir. 14(Suppl. 1):S17-S21. [PubMed] [Google Scholar]
- 13.Hillier, S. L. 2005. The complexity of microbial diversity in bacterial vaginosis. N. Engl. J. Med. 353:1886-1887. [DOI] [PubMed] [Google Scholar]
- 14.Klebanoff, M. A., J. R. Schwebke, J. Zhang, T. R. Nansel, K. F. Yu, and W. W. Andrews. 2004. Vulvovaginal symptoms in women with bacterial vaginosis. Obstet. Gynecol. 104:267-272. [DOI] [PubMed] [Google Scholar]
- 15.Liu, A. Y., D. Destoumieux, A. V. Wong, C. H. Park, E. V. Valore, L. Liu, and T. Ganz. 2002. Human beta-defensin-2 production in keratinocytes is regulated by interleukin-1, bacteria, and the state of differentiation. J. Investig. Dermatol. 118:275-281. [DOI] [PubMed] [Google Scholar]
- 16.Liu, L., A. A. Roberts, and T. Ganz. 2003. By IL-1 signaling, monocyte-derived cells dramatically enhance the epidermal antimicrobial response to lipopolysaccharide. J. Immunol. 170:575-580. [DOI] [PubMed] [Google Scholar]
- 17.Lollike, K., L. Kjeldsen, H. Sengelov, and N. Borregaard. 1995. Lysozyme in human neutrophils and plasma: a parameter of myelopoietic activity. Leukemia 9:159-164. [PubMed] [Google Scholar]
- 18.Luni, Y., S. Munim, R. Qureshi, and A. L. Tareen. 2005. Frequency and diagnosis of bacterial vaginosis. J. Coll. Physicians Surg. Pak. 15:270-272. [PubMed] [Google Scholar]
- 19.Ma, G., T. Greenwell-Wild, K. Lei, W. Jin, J. Swisher, N. Hardegen, C. T. Wild, and S. M. Wahl. 2004. Secretory leukocyte protease inhibitor binds to annexin II, a cofactor for macrophage HIV-1 infection. J. Exp. Med. 200:1337-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin, H. L., B. A. Richardson, P. M. Nyange, L. Lavreys, S. L. Hillier, B. Chohan, K. Mandaliya, J. O. Ndinya-Achola, J. Bwayo, and J. Kreiss. 1999. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J. Infect. Dis. 180:1863-1868. [DOI] [PubMed] [Google Scholar]
- 21.Moodley, P., C. Connolly, and A. W. Sturm. 2002. Interrelationships among human immunodeficiency virus type 1 infection, bacterial vaginosis, trichomoniasis, and the presence of yeasts. J. Infect. Dis. 185:69-73. [DOI] [PubMed] [Google Scholar]
- 22.Murthy, A. R., R. I. Lehrer, S. S. Harwig, and K. T. Miyasaki. 1993. In vitro candidastatic properties of the human neutrophil calprotectin complex. J. Immunol. 151:6291-6301. [PubMed] [Google Scholar]
- 23.Myer, L., L. Denny, R. Telerant, M. de Souza, T. C. Wright, Jr., and L. Kuhn. 2005. Bacterial vaginosis and susceptibility to HIV infection in South African women: a nested case-control study. J. Infect. Dis. 192:1372-1380. [DOI] [PubMed] [Google Scholar]
- 24.Nisapakultorn, K., K. F. Ross, and M. C. Herzberg. 2001. Calprotectin expression inhibits bacterial binding to mucosal epithelial cells. Infect. Immun. 69:3692-3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ocana, V. S., A. A. Pesce De Ruiz Holgado, and M. E. Nader-Macias. 1999. Characterization of a bacteriocin-like substance produced by a vaginal Lactobacillus salivarius strain. Appl. Environ. Microbiol. 65:5631-5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porter, E., H. Yang, S. Yavagal, G. C. Preza, O. Murillo, H. Lima, S. Greene, L. Mahoozi, M. Klein-Patel, G. Diamond, S. Gulati, T. Ganz, P. A. Rice, and A. J. Quayle. 2005. Distinct defensin profiles in Neisseria gonorrhoeae and Chlamydia trachomatis urethritis reveal novel epithelial cell-neutrophil interactions. Infect. Immun. 73:4823-4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Porter, E. M., L. Liu, A. Oren, P. A. Anton, and T. Ganz. 1997. Localization of human intestinal defensin 5 in Paneth cell granules. Infect. Immun. 65:2389-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quayle, A. J., E. M. Porter, A. A. Nussbaum, Y. M. Wang, C. Brabec, K. P. Yip, and S. C. Mok. 1998. Gene expression, immunolocalization, and secretion of human defensin-5 in human female reproductive tract. Am. J. Pathol. 152:1247-1258. [PMC free article] [PubMed] [Google Scholar]
- 29.Sadhu, K., P. A. Domingue, A. W. Chow, J. Nelligan, N. Cheng, and J. W. Costerton. 1989. Gardnerella vaginalis has a gram-positive cell-wall ultrastructure and lacks classical cell-wall lipopolysaccharide. J. Med. Microbiol. 29:229-235. [DOI] [PubMed] [Google Scholar]
- 30.Sewankambo, N., R. H. Gray, M. J. Wawer, L. Paxton, D. McNaim, F. Wabwire-Mangen, D. Serwadda, C. Li, N. Kiwanuka, S. L. Hillier, L. Rabe, C. A. Gaydos, T. C. Quinn, and J. Konde-Lule. 1997. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet 350:546-550. [DOI] [PubMed] [Google Scholar]
- 31.Shai, Y. 1999. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim. Biophys. Acta 1462:55-70. [DOI] [PubMed] [Google Scholar]
- 32.Singh, P. K., H. P. Jia, K. Wiles, J. Hesselberth, L. Liu, B. D. Conway, E. P. Greenberg, E. V. Valore, M. J. Welsh, T. Ganz, B. F. Tack, and P. B. J. McCray. 1998. Production of β-defensins by human airway epithelia. Proc. Natl. Acad. Sci. USA 95:14961-14966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh, P. K., B. F. Tack, P. B. McCray, Jr., and M. J. Welsh. 2000. Synergistic and additive killing by antimicrobial factors found in human airway surface liquid. Am. J. Physiol. Lung Cell Mol. Physiol. 279:L799-L805. [DOI] [PubMed] [Google Scholar]
- 34.Sobel, J. D. 2000. Bacterial vaginosis. Annu. Rev. Med. 51:349-356. [DOI] [PubMed] [Google Scholar]
- 35.Taha, T. E., D. R. Hoover, G. A. Dallabetta, N. I. Kumwenda, L. A. Mtimavalye, L. P. Yang, G. N. Liomba, R. L. Broadhead, J. D. Chiphangwi, and P. G. Miotti. 1998. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS 12:1699-1706. [DOI] [PubMed] [Google Scholar]
- 36.Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335-376. [DOI] [PubMed] [Google Scholar]
- 37.Vallor, A. C., M. A. Antonio, S. E. Hawes, and S. L. Hillier. 2001. Factors associated with acquisition of, or persistent colonization by, vaginal lactobacilli: role of hydrogen peroxide production. J. Infect. Dis. 184:1431-1436. [DOI] [PubMed] [Google Scholar]
- 38.Valore, E. V., C. H. Park, S. L. Igreti, and T. Ganz. 2002. Antimicrobial components of vaginal fluid. Am. J. Obstet. Gynecol. 187:561-568. [DOI] [PubMed] [Google Scholar]
- 39.Valore, E. V., C. H. Park, A. J. Quayle, K. R. Wiles, P. B. McCray, and T. Ganz. 1998. Human beta-defensin-1: an antimicrobial peptide of urogenital tissues. J. Clin. Investig. 101:1633-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Venkataraman, N., A. L. Cole, P. Svoboda, J. Pohl, and A. M. Cole. 2005. Cationic polypeptides are required for anti-HIV-1 activity of human vaginal fluid. J. Immunol. 175:7560-7567. [DOI] [PubMed] [Google Scholar]