Abstract
The safety, immunogenicity, and efficacy of DNA and modified vaccinia virus Ankara (MVA) prime-boost regimes were assessed by using either thrombospondin-related adhesion protein (TRAP) with a multiple-epitope string ME (ME-TRAP) or the circumsporozoite protein (CS) of Plasmodium falciparum. Sixteen healthy subjects who never had malaria (malaria-naive subjects) received two priming vaccinations with DNA, followed by one boosting immunization with MVA, with either ME-TRAP or CS as the antigen. Immunogenicity was assessed by ex vivo gamma interferon (IFN-γ) enzyme-linked immunospot assay (ELISPOT) and antibody assay. Two weeks after the final vaccination, the subjects underwent P. falciparum sporozoite challenge, with six unvaccinated controls. The vaccines were well tolerated and immunogenic, with the DDM-ME TRAP regimen producing stronger ex vivo IFN-γ ELISPOT responses than DDM-CS. One of eight subjects receiving the DDM-ME TRAP regimen was completely protected against malaria challenge, with this group as a whole showing significant delay to parasitemia compared to controls (P = 0.045). The peak ex vivo IFN-γ ELISPOT response in this group correlated strongly with the number of days to parasitemia (P = 0.033). No protection was observed in the DDM-CS group. Prime-boost vaccination with DNA and MVA encoding ME-TRAP but not CS resulted in partial protection against P. falciparum sporozoite challenge in the present study.
Malaria remains one of the world's major killers of children (26), and a vaccine is urgently required. There are several lines of evidence that implicate T cells in the control of pre-erythrocytic malaria infection in mice (18, 38) and humans (16, 17). Recent years have seen the development of vaccine strategies aiming to induce significant levels of cellular responses.
DNA vaccines alone have shown efficacy in both murine (15, 40) and nonhuman primate (6, 52) studies in controlling malaria, many other infectious diseases, cancers, and autoimmune diseases (10). Clinical trials have confirmed the safety of DNA vaccines used alone encoding either the pre-erythrocytic malaria circumsporozoite (CS) protein (13, 24) or the thrombospondin-related adhesion protein (TRAP) (30), but the magnitude of the cellular response induced in humans by DNA vaccines alone has been considerably lower than in animal studies (52, 53) and insufficient to be protective against experimental malaria challenge. Heterologous prime-boost immunization strategies, using sequential administration of different antigen delivery systems encoding the same epitopes or antigen, have been shown to induce enhanced and persistent levels of CD8+ T cells and Th1-type CD4+ T cells, which are protective against murine models of malaria (25, 38, 41). This approach has also been applied to animal models for a range of intracellular diseases including human immunodeficiency virus infection (1, 4, 21), Ebola virus infection (47), hepatitis B (33), and tuberculosis (28). The aim of the present study was to evaluate two DNA-MVA heterologous prime-boost regimens, encoding either CS or TRAP.
A recent clinical trial (27) assessed a heterologous prime-boost regimen in healthy human volunteers that have never had malaria (malaria naive). Three sequential DNA priming vaccinations, followed by two modified vaccinia virus Ankara (MVA) boosting vaccinations encoding TRAP (DDDMM-ME TRAP), induced high levels of T cells and subjects showed a significant (P = 0.013) delay to parasitemia after experimental sporozoite challenge with a different strain of P. falciparum compared to subjects receiving homologous regimens and unvaccinated control subjects. Analysis of the immunogenicity suggested an abbreviated regimen of two DNA-ME TRAP vaccinations and one MVA-ME TRAP vaccination (DDM-ME TRAP) would yield similar results, and this abbreviated regimen was adopted as group 1 of the present study. Group 2 was an identical regimen of two DNA priming vaccinations and one MVA boost but encoding the CS antigen (DDM-CS).
RTS,S/AS02A is a CS-based protein-in-adjuvant malaria vaccine which protected 42% of malaria-naive human subjects against sporozoite challenge (22) and 34% of semi-immune adults against natural infection during 15 weeks follow-up (8). More recently, it has shown efficacy against the first clinical episode of malaria of 29.9% in children in Mozambique (3), lasting at least 18 months (2). Other recent CS-based approaches include the development of a virus-like particle expressing CSP-derived B and T-cell epitopes (31), which has entered clinical trials (32, 50), and heterologous prime-boost strategies using RTS,S/AS02A as a boost following DNA (12, 54) or as a prime or boost with MVA (11) vaccines encoding CS. Particulate vaccines generate high levels of antibodies to the NANP repeat region of the CS, which may be their key mechanism of action. Th1-type CD4+ T cells (23) and CS peptide-specific CD8+ T cells detectable by intracellular staining (ICS) (48) are induced by RTS,S/AS02A, but the amount and duration of protection may be increased by higher levels of cellular immunity. We therefore sought to induce enhanced levels of effector T cells to CS and then assess the efficacy against sporozoite challenge in comparison to a similar regimen encoding TRAP.
MATERIALS AND METHODS
Healthy male and female volunteers aged 18 to 45 years were recruited from the Oxford area and underwent medical screening as previously described (56). Exclusion criteria included a prior history of malaria; immunosuppression; epilepsy; infection with hepatitis B virus, hepatitis C virus, or human immunodeficiency virus; pregnancy; an anti-nuclear antibody titer of >1:80; drug or alcohol abuse; significant psychiatric disorder; or other significant illness. The study received ethical approval from the Oxfordshire Research Ethics Committee and an Independent Local Safety Monitor was appointed in Oxford. The protocol for the present study was also approved by the Naval Medical Research Center (NMRC) Institutional Review Board and the U.S. Navy Surgeon General in accordance with U.S. Navy regulations (SECNAVINST 3900.39B) and in compliance with all applicable U.S. Federal regulations governing the use of human subjects. All subjects gave written, informed consent prior to participation. The trial was conducted according to Good Clinical Practice, was externally monitored, and a clinical trial (DDX) application was reviewed by the UK Medicines and Healthcare Products Regulatory Agency. The primary endpoint of the study was to assess whether vaccinated volunteers were protected wholly or partially against malaria infection in a sporozoite challenge model, as determined by the number of subjects developing malaria infection and the time in days between exposure and parasitemia as detected by thick-film blood smear and compared to controls. Other trial endpoints included the safety and immunogenicity of the vaccine regimens.
Sixteen subjects were enrolled into the vaccination study with an additional six unvaccinated control subjects for the challenge study. Subjects were randomly assigned to either group 1 or group 2 using a random-number program (Microsoft Excel software). Group 1 subjects (DDM-ME TRAP) received two intramuscular vaccinations of 2 mg of DNA-ME TRAP 1 month apart, followed by one intradermal vaccination of 1.5 × 108 PFU MVA-ME TRAP 1 month later, divided between three sites within a 5-cm radius. ME TRAP is a multiple epitope string including 14 CD8+ T-cell epitopes, 1 CD4+ T-cell epitope, and 2 B-cell epitopes from six pre-erythrocytic P. falciparum antigens fused to the N terminus of TRAP as previously described (27). Group 2 subjects (DDM-CS) received two intramuscular vaccinations of 2.5 mg of DNA-CS 1 month apart, followed by one intradermal vaccination of 108 PFU MVA-CS 1 month later, divided between two sites within a 5-cm radius. The DNA-CS was manufactured and supplied by Vical, Inc., San Diego, CA, and has also been referred to as the PfCSP DNA vaccine, as well as VCL-2510, in previous publications (12, 13, 24, 52-54). The other vaccines were manufactured by contract manufacturers (DNA-ME TRAP [QIAGEN, Germany] and MVA-ME TRAP and MVA-CS [IDT, Germany]). The two MVA vaccines and DNA-ME TRAP were developed by Oxford University; DNA-CS was codeveloped by the U.S. Navy and Vical, Inc. The vaccines were stored at −20°C and allowed to thaw prior to administration. All vaccinations were administered to the nondominant arm on days 0, 28, and 56.
Each subject was observed for at least 30 min after vaccination and underwent clinical review 2, 7, and 28 days after each vaccination in order to report solicited and unsolicited adverse events. Up to 80 ml of blood was drawn at days 0, 7, 28, 35, 56, and 63, the day of challenge (day 70), the challenge day plus 7 days (i.e., day 77), day 86, and day 90 postchallenge (i.e., day 160 approximately) for full blood count, biochemistry (renal and hepatic function), and immunogenicity determinations. Anti-nuclear antibodies were measured at screening and at days 28, 56, and 86.
Immunogenicity analysis.
Fresh ex vivo gamma interferon (IFN-γ) enzyme-linked immunospot (ELISPOT) assays were performed as described elsewhere (49). Peripheral blood mononuclear cells (PBMC) were stimulated for 18 h with pools of 15mer peptides for TRAP, the multiple epitope string (ME), and CS, overlapping by 10 amino acids at concentrations of 25 μg/ml per peptide. The results were calculated as the average number of spots in the duplicate stimulant wells (cells plus stimulant) minus the average number of spots in the duplicate background wells (cells plus culture media) and expressed as spot-forming units (SFU) per million PBMC. Cultured ELISPOT assays were performed as follows. Frozen PBMC were thawed and stimulated with one pool of 57 TRAP peptides (group 1) or 48 CS peptides (group 2) for 10 days, after which an ELISPOT assay against three pools of TRAP/CS was done in duplicate using 50,000 originally cultured cells per well (20). Anti-TRAP and anti-CS repeat region R32LR (a protein repeat-based molecule containing the sequence [NVDP(NANP)32]2) antibody concentrations were measured by specific immunoglobulin G enzyme-linked immunosorbent assay to recombinant CS or TRAP (kindly supplied by GlaxoSmithKline Biologicals) protein and were expressed as a titer. Briefly, serial threefold dilutions of serum were added to microtiter plates coated with recombinant capture CS antigen at 10 μg/ml, and bound antibodies were detected by using alkaline-phosphatase-conjugated antibodies specific for whole human IgG (Pharmingen). The results were expressed as endpoint titers calculated by regression of the straight part of a curve of optical density versus serum dilution to a cutoff of two standard deviations above background control values. Preimmunization serum samples for each individual were used as the background control.
P. falciparum challenge.
To assess the efficacy of the vaccines, the 16 vaccinated subjects and 6 unvaccinated infectivity control subjects underwent experimental challenge with P. falciparum at Imperial College, London, United Kingdom, 14 days after the final vaccination. Laboratory-reared Anopheles stephensi mosquitoes were infected with the chloroquine-sensitive 3D7 strain of P. falciparum parasites in an adapted model (9) as described previously (27) to assess the efficacy of the vaccines. From the evening of day 6 subjects attended clinic twice daily for a review of symptoms, vital signs monitoring (pulse, blood pressure, and oral temperature) and withdrawal of 3 ml of blood for thick film and PCR analysis. Field's stain films were examined immediately by experienced microscopists for the appearance of parasitized erythrocytes. A total of 200 high-power fields were examined before a subject was declared slide negative. Subjects who reached day 15 without blood film evidence of malaria infection were monitored daily until day 21. All subjects were treated immediately with Riamet (artemether [20 mg], lumefantrine [120 mg]; Novartis) upon diagnosis of malaria. Subjects returned to clinic on two consecutive days for negative blood films posttreatment. Subjects who reached day 21 without evidence of P. falciparum infection were considered fully protected by the vaccines but received Riamet therapy to avoid continued intensive follow-up. During the challenge follow-up period, blood samples were analyzed by PCR in real time (the method of PCR detection of P. falciparum parasites is discussed in detail elsewhere [5]), although the clinicians assessing the subjects were blinded to the results.
Statistical analysis.
Statistical analysis for the study was performed using Microsoft Excel, SPSS for Windows 11.5, and Sigmaplot software. All data are reported for the total cohort, except the immunogenicity data that are reported for the total cohort without outliers, as defined by responses ±3 standard deviations from the mean of two replicates for that time point. Ex vivo IFN-γ ELISPOT responses were expressed as geometric and arithmetic means and were represented graphically. The significance of any changes in ex vivo IFN-γ ELISPOT responses was assessed by paired Student t testing of log-transformed data. Antibody responses were expressed as geometric mean titers relative to baseline (day 0). Comparison of time point responses was accomplished by using a paired Student t test of log-transformed data. The correlation between the ex vivo IFN-γ ELISPOT response and the number of days to parasitemia was analyzed by the Spearman's rank correlation coefficient with a two-tailed test of significance. Vaccine efficacy was displayed by Kaplan-Meier analysis with the statistical significance of any differences observed analyzed by the log-rank test.
RESULTS
Safety.
All 16 subjects received the complete course of three vaccinations, and the vaccines were shown to be safe and well tolerated in the present study. Vaccine-related (graded by the investigator as “probably” or “definitely” vaccine related) adverse events that were reported are shown in Table 1. No serious or severe vaccine-related adverse events occurred. The two DNA vaccines had a low incidence of side effects. Local redness and induration were seen after all doses of the two MVA vaccines. For general adverse events postvaccination, symptoms judged to be vaccine related were reported by all eight subjects receiving MVA-ME TRAP, including three fevers (>37.5°C). The pain and general symptoms typically resolved within 48 h and always within 7 days. At the final follow-up visit approximately 14 weeks after the MVA vaccine, seven of eight DDM-ME TRAP subjects reviewed had no visible signs of vaccination, and the remaining subject had 1 mm of skin discoloration at the vaccination site. Of six DDM-CS subjects reviewed 14 weeks after the MVA vaccine (two subjects were lost to final follow-up), four of six had no visible signs of vaccination, and the remaining two subjects had 2 and 4 mm of skin discoloration at the vaccination site. There was one grade 3 (severe) general adverse event during the study, which was unrelated to vaccination (knee dislocation after a fall). Minor unsolicited adverse events not considered related to vaccination were common, including viral upper respiratory tract and gastrointestinal infections, nonspecific headaches, and fatigue.
TABLE 1.
Vaccine-related adverse events (events graded as “probably” or “definitely” related)
| Vaccine | No. of events: type(s) of events
|
|||
|---|---|---|---|---|
| Group 1 ME TRAP (n = 8 for each dose)
|
Group 2 CS (n = 8 for each dose)
|
|||
| Local | General | Local | General | |
| DNA-ME TRAP/DNA-CS | Six events: three redness (1 to 4 mm); three pain; one muscle fasciculation | None | Four events: two redness (1 to 3 mm), one induration (2 mm); one pain | Five events: one malaise; one headache; two myalgia; one diarrhea |
| DNA-ME TRAP/DNA-CS | Four events: three redness (1 to 4 mm); two pain | None | Three events: one redness (1 mm); two pain | None |
| MVA-ME TRAP/MVA-CS | Eight events: eight redness (28 to 94 mm [six at grade 2]); eight induration (8 to 60 mm); seven pain (two at grade 2) | Eight vaccine-related events: three fevers (>37.5°C); six feverishness (one at grade 2); seven malaise; two arthralgia; four headache; six myalgia; two nausea; five fatigue | Eight events: eight redness (18 to 52 mm [2 at grade 2]); eight induration (8 to 23 mm); seven pain | Three vaccine-related events: zero fevers (>37.5°C); one feverishness (at grade 2); two malaise; one headache; three fatigue |
All events are grade 1 (mild) unless otherwise stated.
In terms of hematological and biochemistry monitoring, one subject had a low neutrophil count 7 days after receiving the third vaccine, MVA-ME TRAP, that coincided with an influenza-like illness. The neutrophil count returned to the normal range the following week, and this transient neutropenia was judged to be related to a viral infection, which is a well-known association (45). Four subjects had transient low platelet counts during parasitemia, which is an established phenomenon (39). One subject had abnormalities of liver function tests after malaria infection which resolved on follow-up. Finally, two subjects had raised anti-nuclear antibody level of 1:320 after malaria infection (1:80 at screening, 1:160 at day 56 after two vaccinations but prior to challenge), which were down to 1:160 at the subsequent follow-up visit. This was not considered clinically significant (19).
Immunogenicity.
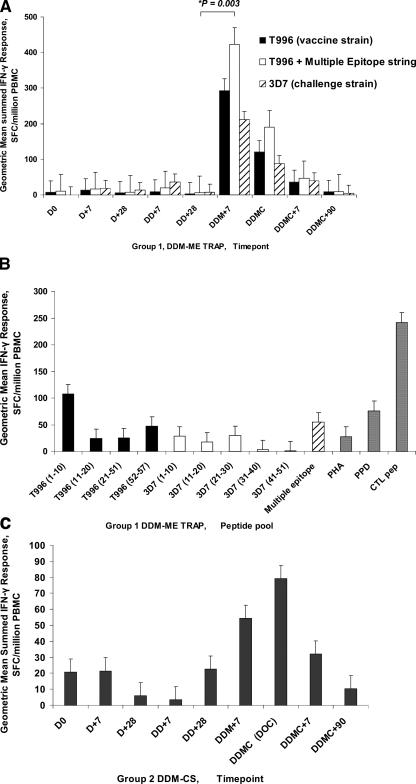
Effector T cells, as measured by ex vivo IFN-γ ELISPOT assay, were induced in subjects receiving both vaccine regimens (Table 2 and Fig. 1). One subject from group 1 was excluded from the analysis because of an exceptionally high background response at one time point. In group 1 subjects (DDM-ME TRAP, Fig. 1A), each of the two DNA-ME TRAP doses induced only low levels of responses that were not statistically significant from the baseline. A strong boosting effect was seen, however, after the administration of MVA-ME TRAP. Geometric mean total summed responses to the ME and T996 TRAP pools prior to the MVA-ME TRAP boost (DD+28) were 6 SFU/106 PBMC and rose to 423 SFU/106 PBMC after the boost (DDM+7, P = 0.003). Previous studies have shown that MVA alone does not produce such high responses (27). On the day of challenge 2 weeks after the MVA-ME TRAP vaccination, the geometric mean responses remained high at 190 SFU/106 PBMC. Comparison of peak responses to the vaccine strain T996 of TRAP, and the heterologous challenge strain 3D7 of TRAP (Table 2 and Fig. 1A) provides evidence of cross-reactivity. The responses to individual TRAP peptide pools at the DDM+7 time point were spread across the four pools used, indicating a broad response (Fig. 1B).
TABLE 2.
Ex vivo IFN-γ ELISPOT responses for DDM-ME TRAP and DDM-CS
| Time pointb | Results for group 1
|
Results for group 2 (all peptides in vaccines [3D7 CS])
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All peptides in vaccines (ME + T996 TRAP)
|
Vaccine strain (T996 TRAP)
|
Challenge strain (3D7 TRAP)
|
||||||||||
| AM | SEM | GM | AM | SEM | GM | AM | SEM | GM | AM | SEM | GM | |
| D0 | 17 | 6.7 | 10 | 11 | 4.1 | 8 | 23 | 12.3 | 8 | 55 | 19.7 | 21 |
| D+7 | 25 | 6.1 | 17 | 19 | 3.8 | 14 | 23 | 5.4 | 18 | 38 | 15.6 | 22 |
| D+28 | 12 | 3.7 | 8 | 8 | 2.0 | 6 | 16 | 4.0 | 13 | 24 | 8.4 | 6 |
| DD+7 | 32 | 11.6 | 19 | 24 | 9.3 | 9 | 62 | 19.6 | 37 | 14 | 5.6 | 4 |
| DD+28 | 25 | 14.8 | 6 | 15 | 10.6 | 2 | 18 | 10.5 | 8 | 27 | 5.7 | 23 |
| DDM+7 | 609 | 174.5 | 423 | 428 | 138.0 | 294 | 376 | 146.8 | 211 | 99 | 35.8 | 54 |
| DDMC | 323 | 96.2 | 190 | 240 | 83.0 | 120 | 171 | 54.4 | 88 | 129 | 39.4 | 79 |
| DDMC+7 | 120 | 44.0 | 47 | 90 | 33.7 | 37 | 74 | 20.8 | 39 | 60 | 23.7 | 32 |
| DDMC+90 | 19 | 9.2 | 10 | 16 | 8.1 | 8 | 14 | 7.3 | 5 | 14 | 4.8 | 10 |
The geometric mean (GM), arithmetic mean (AM), and standard error of the mean are shown for each time point in group 1 (DDM-ME TRAP) for all of the peptides in the vaccine (multiple epitope string [ME] and T996 TRAP), for T996 TRAP (vaccine strain), and for the 3D7 TRAP (challenge strain) and for each time point in group 2 (DDM-CS) for the CS peptides.
D0 = day 0, D+7 = first DNA vaccination plus 7 days, D+28 = first DNA vaccination plus 28 days (same as the day of the second vaccination), DD+7 = second DNA vaccination plus 7 days, DD+28 = second DNA vaccination plus 28 days (same as the day of the MVA vaccination), DDM+7 = MVA vaccination plus 7 days, DDMC = day of challenge (same as the day of MVA vaccination plus 14 days), DDMC+7 = day of challenge plus 7 days, and DDMC+90 = day of challenge plus 90 days.
FIG. 1.
Geometric mean ex vivo ELISPOT responses to DDME-TRAP (A and B) and DDM-CS (C) for groups 1 and 2. Panel A shows the geometric mean summed ex vivo ELISPOT responses, in SFC per million PBMC, to TRAP for group 1 subjects receiving DDM-ME TRAP. D0 = day 0, D+7 = first DNA vaccination plus 7 days, D+28 = first DNA vaccination plus 28 days (i.e., the day of the second vaccination), DD+7 = second DNA vaccination + 7 days, DD+28 = second DNA vaccination plus 28 days (i.e., the day of the MVA vaccination), DDM+7 = MVA vaccination plus 7 days, DDMC = day of challenge (i.e., the day of the MVA vaccination plus 14 days), DDMC+7 = day of challenge plus 7 days, and DDMC+90 = day of challenge plus 90 days. Error bars are one standard error of the mean.
In group 2 (DDM-CS, Fig. 1C) administration of MVA-CS is followed by an increase in mean summed ex vivo response to CS, which did not reach statistical significance (the DD-CS+28 geometric mean total summed responses to CS peptides were 23 SFU/106 PBMC, rising to 54 SFU/106 PBMC at DDM-CS+7 [P = 0.14]). On the day of challenge, the geometric mean summed responses were higher at 79 SFU/106 PBMC, which is significantly higher than preboost at DD-CS+28 (P = 0.045). The responses to individual CS peptide pools at the DDM+7 time point (not shown) were spread across the peptide pools with much lower responses to the heterologous 7G8 strain.
Unvaccinated control subjects showed geometric mean total summed responses to ME and T996 TRAP peptides of 13, 36, and 2 SFU/106 PBMC at the day-of-challenge, challenge-plus-7-day, and challenge-plus-90-day time points, respectively, and the geometric mean total summed responses to CS peptides of 33, 15, and 7 SFU/106 PBMC at the day-of-challenge, challenge-plus-7-day, and challenge-plus-90-day time points, respectively.
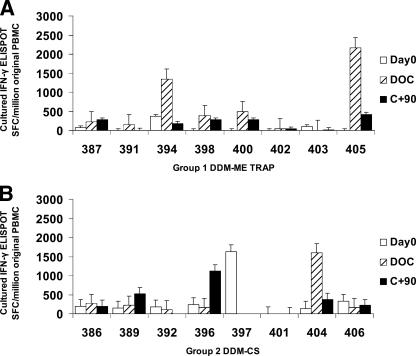
Cultured ELISPOT responses were present in some subjects on the day of challenge and 90 days later and are shown in Fig. 2. Several subjects showed high levels of responses by cultured IFN-γ ELISPOT after vaccination with either regimen, a finding indicative of the presence of memory T cells. The greatest response was seen for the day of challenge in subject 405, who showed complete protection against parasitemia.
FIG. 2.
Cultured ex vivo ELISPOT responses. Cultured ELISPOT responses in SFC per million PBMC are shown for each subject at day 0, day of challenge (DOC; 14 days after final vaccination), and 90 days after challenge (C+90). Responses to TRAP (group 1 [A]) or to CS (group 2 [B]) were measured after a 9-day culture period. No data are shown for the following time points due the lack of samples for analysis: subject 392, C+90, subject 397, DOC and C+90; and subject 401, DOC. Error bars are one standard error of the mean.
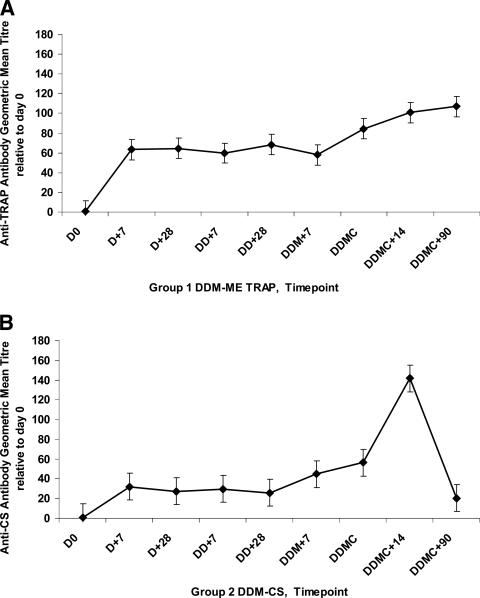
Levels of antibodies to TRAP and CS peptide pools for groups 1 and 2, respectively, are shown in Fig. 3. Anti-TRAP antibodies were induced in group 1 subjects 7 days after the first vaccination (P = 0.01); the antibody levels appeared to begin to increase further 2 weeks after the MVA-ME TRAP boost (DDMC) (although this did not reach statistical significance) and were still elevated 3 months after the final vaccination (DOC+90). The range of geometric mean titers at the time point 14 days after the challenge, relative to day 0, for the eight subjects receiving DDM-ME TRAP was 46 to 399. Anti-CS antibodies were induced in group 2 subjects 7 days after the first vaccination (P = 0.01) and were boosted by the challenge (the geometric mean titers on the day of challenge and 14 days later were 56 and 142, respectively [P = 0.05]). The range of titers 14 days after the challenge for the eight subjects receiving DDM-CS was 14 to 681. No such induction of antibodies or boost by challenge was seen for the control subjects (data not shown).
FIG. 3.
Anti-TRAP and anti-CS antibody titers. The levels of anti-TRAP antibodies for group 1 subjects (A) and anti-CS repeat antibodies for group 2 subjects (B) are shown and are expressed as geometric mean titers relative to day 0. D0 = day 0, D+7 = first DNA vaccination plus 7 days, D+28 = first DNA vaccination plus 28 days (i.e., the day of the second vaccination), DD+7 = second DNA vaccination plus 7 days, DD+28 = second DNA vaccination plus 28 days (i.e., the day of the MVA vaccination), DDM+7 = MVA vaccination plus 7 days, DDMC = day of challenge (i.e., the day of the MVA vaccination plus 14 days), DDMC+7 = day of challenge plus 7 days, and DDMC+90 = day of challenge plus 90 days. Error bars are one standard error of the mean.
Protective efficacy.
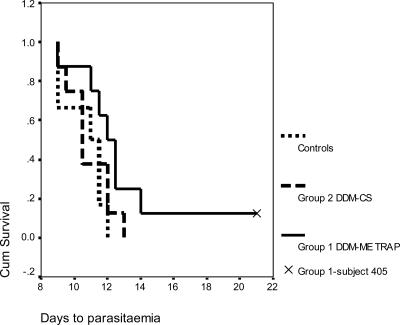
Parasitemia was detected in six of six control subjects at a mean of 10.7 days (95% confidence interval [CI] = 9.3 to 12.1). Seven of eight group 1 “DDM-ME TRAP” subjects tested positive at a mean of 11.8 days (95% CI = 10.4 to 13.2). One subject (subject 405) reached day 21 without a diagnosis of malaria and was considered fully protected. In addition to having negative blood films, this subject had consistently negative PCR assays and, interestingly, showed the highest peak ex vivo ELISPOT response to ME and TRAP at 1496 SFC/106 PBMC, as well as the highest cultured ELISPOT response. There was a significant delay to parasitemia for the DDM-ME TRAP subjects compared to control subjects (P = 0.045 [log-rank test]). Eight of eight group 2 “DDM-CS” subjects were diagnosed with malaria at a mean of 10.9 days (95% CI = 9.7 to 12.0). There was no significant difference between the DDM-CS group and the controls, thus showing no evidence of efficacy for this regimen. The Kaplan-Meier survival plot for all subjects is shown in Fig. 4.
FIG. 4.
Kaplan-Meier survival curves postchallenge. The survival curves for each group are shown, with days to parasitemia representing the number of days after experimental P. falciparum challenge that each subject received a diagnosis of malaria. Six of six control subjects, seven of eight group 1 DDM-ME TRAP subjects, and eight of eight group 2 DDM-CS subjects were diagnosed with malaria. One group 1 subject, subject 405, did not develop malaria. There was a significant delay to parasitemia for the DDM-ME TRAP group compared to control subjects (P = 0.045 [log-rank test]) but not for the DDM-CS group and the controls.
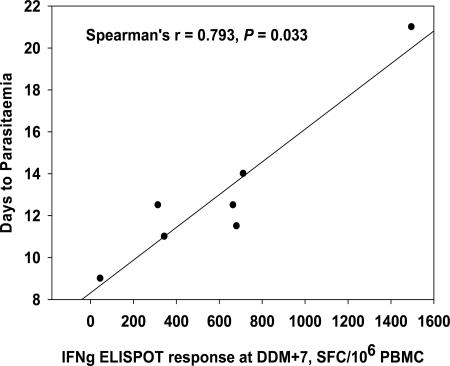
There was a significant correlation between the ex vivo IFN-γ ELISPOT responses for DDM-ME TRAP subjects and the number of days to parasitemia as measured by Spearman's rank correlation (r = 0.793, two tailed, P = 0.033, Fig. 5). This correlation was not seen for the DDM-CS subjects or the control group. No correlation was seen between antibody levels and time to parasitemia.
FIG. 5.
Correlation between ex vivo IFN-γ ELISPOT responses at DDM+7 and days to parasitemia for group 1 (DDM-ME TRAP). The summed ex vivo IFN-γ ELISPOT response to ME TRAP 7 days after the final vaccination (DDM+7) for each group 1 subject correlates with the number of days to parasitemia as measured by Spearman's rank correlation coefficient (r = 0.793, two tailed, P = 0.033). One subject had been excluded from all immunogenicity analysis because of a very high background at one time point.
DISCUSSION
Aim.
We sought to compare two well-described P. falciparum liver-stage specific antigens delivered by heterologous prime-boost regimens. Protection against experimental malaria challenge and immunogenicity were assessed. The first regimen, DDM-ME TRAP, has since been used in a semi-immune population in The Gambia (29) and is similar to a regimen (DDDMM-ME TRAP) previously used in malaria-naive subjects (27). The second is a new regimen assessing prime-boost delivery of vaccines encoding the CS antigen.
Safety.
The vaccines were safe and well tolerated. As shown in previous studies in similar populations (13, 24, 30), few local adverse events were reported after either the DNA-ME TRAP or the DNA-CS vaccines. Any solicited general adverse events reported were assessed by the investigator as being only “possibly” related, at maximum. After administration of MVA-ME TRAP or MVA-CS, local redness, induration, and pain, as well as systemic symptoms, were reported. The general symptoms were typically mild and short-lived. The spectrum of symptoms seen was similar to our previous experience with poxvirus vaccines (11, 30, 51, 56).
Immunogenicity.
Strong effector T-cell responses, as measured by ex vivo IFN-γ ELISPOT assay, were induced by the DDM-ME TRAP regimen, with a marked boost effect after the MVA vaccination. The strain of P. falciparum used for challenge, 3D7, differs in amino acid sequence from the strain used for the vaccines, T996, by 6.1%, which is more variation than typically found between isolates from Africa (37), and there was evidence of cross-reactivity as responses to 3D7 approached responses to T996. The peak responses 7 days after administration of DDM-ME TRAP (arithmetic mean and geometric mean of 609 and 423 SFC/106 PBMC, respectively) are in the middle of the range of ex vivo IFN-γ ELISPOT responses in Oxford malaria-naive subjects to prime-boost regimens using the DNA, MVA, and FP9 vaccines encoding ME TRAP reported previously by our group (27, 49). These studies showed arithmetic means of between 158 and 1,609 SFC/106 PBMC and geometric means (previously unpublished data) of 65 to 703 SFC/106 PBMC. The same DDM-ME TRAP regimen in semi-immune adults in The Gambia resulted in geometric mean responses of 255 SFC/106 PBMC (29).
The ex vivo IFN-γ ELISPOT response induced by the DDM-CS regimen was modest compared to the ME TRAP regimen and peaked 1 week later than the ME TRAP regimen, 14 days after the final vaccination (i.e., the day of challenge), with evidence of a prime-boost effect. A previous clinical trial of three doses of the same DNA-CS vaccine (53) yielded responses using a different ex vivo ELISPOT protocol with a longer incubation time (geometric mean of 24.5 SFC/106 PBMC) which are likely to be in a similar range as 28 days after two doses of DNA-CS in the present study (geometric mean of 23 SFC/106 PBMC).
As expected, control subjects showed insignificant T-cell responses to both CS and TRAP peptide pools 7 and 90 days after challenge. There was no evidence of boosting of the ex vivo IFN-γ ELISPOT response by sporozoite challenge in either vaccination group or in the control subjects. This is compatible with previous challenge studies by our group (49, 51), although boosting by sporozoite challenge has been seen after the lower responses induced by DNA vaccination-only regimens (55). This is an interesting issue, since boosting of vaccine responses by natural exposure is highly desirable in the field. However, natural exposure does not appear to be a powerful stimulus for T-cell responses measured by ex vivo IFN-γ ELISPOT assay, since the responses to TRAP or CS in adults in areas of endemicity are low compared to the levels obtained in the present study (14, 29, 36). One week prior to the challenge ex vivo ELISPOT responses for the DDM-ME TRAP regimen peaked at a geometric mean of 423 SFC/106 PBMC (range, 46 to 1,496), and a week after challenge they had fallen to geometric mean of 47 SFC/106 PBMC (range, 1 to 318). The dynamics of the decline in effector T cells from the peak response is not fully known, and we do not have an unchallenged vaccinated group for comparison. The challenge may be having an impact on the rate of decline of the response but is not powerful enough to boost such high levels. It seems that we are trying to induce levels of T-cell responses that “beat nature,” and perhaps the ex vivo ELISPOT responses achieved by vaccination with prime-boost strategies exceed the levels that can show a benefit from a single episode of parasitemia. Nevertheless, the contribution of the challenge to functional subsets of T cells remains to be explored.
The presence of cultured ELISPOT responses in some subjects from both regimens 3 months after challenge suggests the induction of T-cell memory. Unvaccinated control subjects in previous studies using TRAP did not show such increases in cultured ELISPOT responses postchallenge (20).
The pattern of antibody induction differed for TRAP and CS. The DDM-ME TRAP regimen induced anti-TRAP antibodies, whose levels remained high 90 days after the challenge. Anti-CS repeat region antibodies were boosted by the challenge, peaking 14 days later, and no such induction of antibodies or boost by challenge was seen for the control subjects. There was no correlation between the antibody level on the day of challenge and the number of days to parasitemia. Although direct comparison with assays reported by other groups has not been possible, these antibody titers are likely to be low in comparison to the levels induced by predominantly antibody-inducing vaccines such as RTS,S/AS02A (22).
Efficacy.
The level of peak ex vivo IFN-γ ELISPOT responses to TRAP at the DDM+7 time point correlated with the number of days to parasitemia. One subject out of eight was completely protected against sporozoite challenge. To our knowledge, this is the first case of a regimen using a DNA vaccine resulting in complete protection in an individual against infectious challenge. A prime-boost regimen encoding the same ME-TRAP insert but utilizing two doses of fowlpox strain 9 ME-TRAP boosted by one dose of MVA ME-TRAP has previously resulted in complete protection against malaria in a similar protocol in Oxford, United Kingdom (57). In the present study the DDM-ME TRAP group showed a delay to parasitemia compared to the controls, as has previously been reported for an extended regimen of the same vaccines, DDDMM-ME TRAP (27). Such a delay represents evidence of a reduction in parasites emerging from the liver (7, 42). However, a recent, double-blind field efficacy trial in semi-immune adults in The Gambia of DDM-ME TRAP (29) did not show significant efficacy against parasitemia, and the relationship between exposure to infectious bites, parasitemia, and clinical endpoints are ongoing topics of exploration (43, 44).
The lack of efficacy seen in the DDM-CS group was disappointing. This may be because an insufficient magnitude of T-cell response was generated, and in that respect our aim to evaluate efficacy in the face of strong cellular immunity to the CS was not achieved. Likewise, the vaccinations did not result in the induction of anti-CS repeat antibodies at the levels achievable by RTS,S/AS02A (22, 46), and therefore, without evidence of either a major cellular or humoral response, the lack of efficacy might be predicted. This is in contrast to the evaluation of similar regimens in mouse models, where a heterologous prime-boost with the CS antigen results in the induction of T-cell responses, antibodies, and efficacy against challenge (25, 38).
It is unclear why in the present study CS appeared to be an inferior antigen to TRAP for the induction of cellular immunity and protection. The MVA-CS vaccine was administered at two-thirds of the dose of MVA-ME TRAP for historical reasons (11), and this may have resulted in insufficient boosting. However, a recent series of clinical trials in subjects receiving two priming doses of fowlpox strain 9 (FP9) encoding CS, followed by the same MVA-CS vaccine used in the present study, showed little improvement in immunogenicity when the MVA-CS was administered at a dose of 5 × 108 PFU compared to 108 PFU (51). High levels of anti-CS repeat antibody responses are induced by RTS,S/AS02A, where the presence of the hepatitis B surface antigen and the adjuvant ASO2A are likely to contribute to the successful induction of anti-CS antibodies. Although CD4+ (23) and CD8+ (48) T-cell responses have been reported after RTS,S/AS02A immunization, the cellular response after RTS,S/AS02A is lower than that induced by prime-boost regimens encoding TRAP (27). It is possible that the CS antigen induces significant regulatory T-cell activity that limits the effector response. For example, interleukin-10-mediated immunosuppression induced by the polymorphic immunodominant CD4+ T-cell epitope region, ThTR, of CS has been described (34).
Future directions.
The goal remains to assess efficacy of these vaccines against malaria challenge in human subjects with strong T-cell responses to CS. Future directions therefore include looking into ways of improving cellular immunogenicity, including dose increases, variations in the dosing interval, and the use of different vectors. In addition, the DDM-CS regimen, with an increased interval between DNA and MVA immunization, will be assessed in a semi-immune population in Ghana; natural priming may result in higher final responses (P. Bejon and A. V. S. Hill, unpublished data). The efficacy demonstrated here with the DDM-ME TRAP regimen needs to be built upon. Assessing regimens delivering multiple major malaria proteins is the next step, and such studies are under way (35). Ultimately, the simultaneous induction of high levels of cellular immunity and antibodies is the most attractive approach to achieving the goal of improving the efficacy of vaccines against malaria.
Acknowledgments
This study was supported by a grant from the Wellcome Trust and by the Military Infectious Diseases Research Program STO F 63002A.810.F.A0011 and by ONR-ATD0603792N.01889.135.A0039. The external monitoring was funded by The Malaria Vaccine Initiative at PATH.
We are grateful to all of the subjects who volunteered to participate in this study. We thank Angela Hunt-Cooke (Oxford University) and Simon Correa (MRC, The Gambia) for reading the blood films, Nicole Freydberg and Chris Dacosta at the NMRC for administrative support, Frank Williams at the NMRC for statistical advice, Jacqui Mendoza for assistance rearing mosquitoes at Imperial College, David Warrell (Oxford University) for his role as Local Safety Monitor, and Tony Hope (Oxford University) for his role as advisor on ethical issues.
The opinions and assertions herein are those of the authors and not to be construed as official or reflecting the views of the U.S. Navy, the U.S. Army, the Department of Defense, or the U.S. Government. J.E.E. and T.L.R. are military service members and D.J.C. was a military service member during the time when this research was conducted. This work was prepared as part of their official duties. With respect to the contributions of the U.S. government authors (J.E.E., T.L.R., and D.J.C.), Title 17 USC §105 provides that “copyright protection under this title is not available for any work of the United States Government.” Title 17 USC §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person's official duties. J.S. is an employee and cofounder of Oxxon Therapeutics, Ltd., which is developing prime-boost vaccines for therapeutic applications using MVA. A.V.S.H. is a cofounder of and consultant to Oxxon Therapeutics, Ltd.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Allen, T. M., T. U. Vogel, D. H. Fuller, B. R. Mothe, S. Steffen, J. E. Boyson, T. Shipley, J. Fuller, T. Hanke, A. Sette, J. D. Altman, B. Moss, A. J. McMichael, and D. I. Watkins. 2000. Induction of AIDS virus-specific CTL activity in fresh, unstimulated peripheral blood lymphocytes from rhesus macaques vaccinated with a DNA prime/modified vaccinia virus Ankara boost regimen. J. Immunol. 164:4968-4978. [DOI] [PubMed] [Google Scholar]
- 2.Alonso, P. L., J. Sacarlal, J. J. Aponte, A. Leach, E. Macete, P. Aide, B. Sigauque, J. Milman, I. Mandomando, Q. Bassat, C. Guinovart, M. Espasa, S. Corachan, M. Lievens, M. M. Navia, M. C. Dubois, C. Menendez, F. Dubovsky, J. Cohen, R. Thompson, and W. R. Ballou. 2005. Duration of protection with RTS,S/AS02A malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: single-blind extended follow-up of a randomised controlled trial. Lancet 366:2012-2018. [DOI] [PubMed] [Google Scholar]
- 3.Alonso, P. L., J. Sacarlal, J. J. Aponte, A. Leach, E. Macete, J. Milman, I. Mandomando, B. Spiessens, C. Guinovart, M. Espasa, Q. Bassat, P. Aide, O. Ofori-Anyinam, M. M. Navia, S. Corachan, M. Ceuppens, M. C. Dubois, M. A. Demoitie, F. Dubovsky, C. Menendez, N. Tornieporth, W. R. Ballou, R. Thompson, and J. Cohen. 2004. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet 364:1411-1420. [DOI] [PubMed] [Google Scholar]
- 4.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 5.Andrews, L., R. F. Andersen, D. Webster, S. Dunachie, R. M. Walther, P. Bejon, A. Hunt-Cooke, G. Bergson, F. Sanderson, A. V. Hill, and S. C. Gilbert. 2005. Quantitative real-time polymerase chain reaction for malaria diagnosis and its use in malaria vaccine clinical trials. Am. J. Trop. Med. Hyg. 73:191-198. [PubMed] [Google Scholar]
- 6.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, E. A. Emini, J. W. Shiver, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. [DOI] [PubMed] [Google Scholar]
- 7.Bejon, P., L. Andrews, R. F. Andersen, S. Dunachie, D. Webster, M. Walther, S. C. Gilbert, T. Peto, and A. V. Hill. 2005. Calculation of liver-to-blood inocula, parasite growth rates, and preerythrocytic vaccine efficacy, from serial quantitative polymerase chain reaction studies of volunteers challenged with malaria sporozoites. J. Infect. Dis. 191:619-626. [DOI] [PubMed] [Google Scholar]
- 8.Bojang, K. A., P. J. Milligan, M. Pinder, L. Vigneron, A. Alloueche, K. E. Kester, W. R. Ballou, D. J. Conway, W. H. Reece, P. Gothard, L. Yamuah, M. Delchambre, G. Voss, B. M. Greenwood, A. Hill, K. P. McAdam, N. Tornieporth, J. D. Cohen, and T. Doherty. 2001. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. Lancet 358:1927-1934. [DOI] [PubMed] [Google Scholar]
- 9.Chulay, J. D., I. Schneider, T. M. Cosgriff, S. L. Hoffman, W. R. Ballou, I. A. Quakyi, R. Carter, J. H. Trosper, and W. T. Hockmeyer. 1986. Malaria transmitted to humans by mosquitoes infected from cultured Plasmodium falciparum. Am. J. Trop. Med. Hyg. 35:66-68. [DOI] [PubMed] [Google Scholar]
- 10.Donnelly, J. J., J. B. Ulmer, J. W. Shiver, and M. A. Liu. 1997. DNA vaccines. Annu. Rev. Immunol. 15:617-648. [DOI] [PubMed] [Google Scholar]
- 11.Dunachie, S. J., M. Walther, J. M. Vuola, D. P. Webster, S. M. Keating, T. Berthoud, L. Andrews, P. Bejon, I. Poulton, G. Butcher, K. Watkins, R. E. Sinden, A. Leach, P. Moris, N. Tornieporth, J. Schneider, F. Dubovsky, E. Tierney, J. Williams, D. Gray Heppner, Jr., S. C. Gilbert, J. Cohen, and A. V. Hill. 2006. A clinical trial of prime-boost immunization with the candidate malaria vaccines RTS,S/AS02A and MVA-CS. Vaccine 24:2850-2859. [DOI] [PubMed] [Google Scholar]
- 12.Epstein, J. E., Y. Charoenvit, K. E. Kester, R. Wang, R. Newcomer, S. Fitzpatrick, T. L. Richie, N. Tornieporth, D. G. Heppner, C. Ockenhouse, V. Majam, C. Holland, E. Abot, H. Ganeshan, M. Berzins, T. Jones, C. N. Freydberg, J. Ng, J. Norman, D. J. Carucci, J. Cohen, and S. L. Hoffman. 2004. Safety, tolerability, and antibody responses in humans after sequential immunization with a PfCSP DNA vaccine followed by the recombinant protein vaccine RTS,S/AS02A. Vaccine 22:1592-1603. [DOI] [PubMed] [Google Scholar]
- 13.Epstein, J. E., E. J. Gorak, Y. Charoenvit, R. Wang, N. Freydberg, O. Osinowo, T. L. Richie, E. L. Stoltz, F. Trespalacios, J. Nerges, J. Ng, V. Fallarme-Majam, E. Abot, L. Goh, S. Parker, S. Kumar, R. C. Hedstrom, J. Norman, R. Stout, and S. L. Hoffman. 2002. Safety, tolerability, and lack of antibody responses after administration of a PfCSP DNA malaria vaccine via needle or needle-free jet injection, and comparison of intramuscular and combination intramuscular/intradermal routes. Hum. Gene Ther. 13:1551-1560. [DOI] [PubMed] [Google Scholar]
- 14.Flanagan, K. L., T. Mwangi, M. Plebanski, K. Odhiambo, A. Ross, E. Sheu, M. Kortok, B. Lowe, K. Marsh, and A. V. Hill. 2003. Ex vivo interferon-gamma immune response to thrombospondin-related adhesive protein in coastal Kenyans: longevity and risk of Plasmodium falciparum infection. Am. J. Trop. Med. Hyg. 68:421-430. [PubMed] [Google Scholar]
- 15.Gardner, M. J., D. L. Doolan, R. C. Hedstrom, R. Wang, M. Sedegah, R. A. Gramzinski, J. C. Aguiar, H. Wang, M. Margalith, P. Hobart, and S. L. Hoffman. 1996. DNA vaccines against malaria: immunogenicity and protection in a rodent model. J. Pharm. Sci. 85:1294-1300. [DOI] [PubMed] [Google Scholar]
- 16.Herrington, D., J. Davis, E. Nardin, M. Beier, J. Cortese, H. Eddy, G. Losonsky, M. Hollingdale, M. Sztein, M. Levine, et al. 1991. Successful immunization of humans with irradiated malaria sporozoites: humoral and cellular responses of the protected individuals. Am. J. Trop. Med. Hyg. 45:539-547. [DOI] [PubMed] [Google Scholar]
- 17.Hill, A. V., C. E. Allsopp, D. Kwiatkowski, N. M. Anstey, P. Twumasi, P. A. Rowe, S. Bennett, D. Brewster, A. J. McMichael, and B. M. Greenwood. 1991. Common west African HLA antigens are associated with protection from severe malaria. Nature 352:595-600. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman, S. L., D. Isenbarger, G. W. Long, M. Sedegah, A. Szarfman, L. Waters, M. R. Hollingdale, P. H. van der Meide, D. S. Finbloom, and W. R. Ballou. 1989. Sporozoite vaccine induces genetically restricted T-cell elimination of malaria from hepatocytes. Science 244:1078-1081. [DOI] [PubMed] [Google Scholar]
- 19.Kavanaugh, A. F., and D. H. Solomon. 2002. Guidelines for immunologic laboratory testing in the rheumatic diseases: anti-DNA antibody tests. Arthritis Rheum. 47:546-555. [DOI] [PubMed] [Google Scholar]
- 20.Keating, S. M., P. Bejon, T. Berthoud, J. M. Vuola, S. Todryk, D. P. Webster, S. J. Dunachie, V. S. Moorthy, S. J. McConkey, S. C. Gilbert, and A. V. Hill. 2005. Durable human memory T cells quantifiable by cultured enzyme-linked immunospot assays are induced by heterologous prime boost immunization and correlate with protection against malaria. J. Immunol. 175:5675-5680. [DOI] [PubMed] [Google Scholar]
- 21.Kent, S. J., A. Zhao, S. J. Best, J. D. Chandler, D. B. Boyle, and I. A. Ramshaw. 1998. Enhanced T-cell immunogenicity and protective efficacy of a human immunodeficiency virus type 1 vaccine regimen consisting of consecutive priming with DNA and boosting with recombinant fowlpox virus. J. Virol. 72:10180-10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kester, K. E., D. A. McKinney, N. Tornieporth, C. F. Ockenhouse, D. G. Heppner, T. Hall, U. Krzych, M. Delchambre, G. Voss, M. G. Dowler, J. Palensky, J. Wittes, J. Cohen, and W. R. Ballou. 2001. Efficacy of recombinant circumsporozoite protein vaccine regimens against experimental Plasmodium falciparum malaria. J. Infect. Dis. 183:640-647. [DOI] [PubMed] [Google Scholar]
- 23.Lalvani, A., P. Moris, G. Voss, A. A. Pathan, K. E. Kester, R. Brookes, E. Lee, M. Koutsoukos, M. Plebanski, M. Delchambre, K. L. Flanagan, C. Carton, M. Slaoui, C. Van Hoecke, W. R. Ballou, A. V. Hill, and J. Cohen. 1999. Potent induction of focused Th1-type cellular and humoral immune responses by RTS,S/SBAS2, a recombinant Plasmodium falciparum malaria vaccine. J. Infect. Dis. 180:1656-1664. [DOI] [PubMed] [Google Scholar]
- 24.Le, T. P., K. M. Coonan, R. C. Hedstrom, Y. Charoenvit, M. Sedegah, J. E. Epstein, S. Kumar, R. Wang, D. L. Doolan, J. D. Maguire, S. E. Parker, P. Hobart, J. Norman, and S. L. Hoffman. 2000. Safety, tolerability and humoral immune responses after intramuscular administration of a malaria DNA vaccine to healthy adult volunteers. Vaccine 18:1893-1901. [DOI] [PubMed] [Google Scholar]
- 25.Li, S., M. Rodrigues, D. Rodriguez, J. R. Rodriguez, M. Esteban, P. Palese, R. S. Nussenzweig, and F. Zavala. 1993. Priming with recombinant influenza virus followed by administration of recombinant vaccinia virus induces CD8+ T-cell-mediated protective immunity against malaria. Proc. Natl. Acad. Sci. USA 90:5214-5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marshall, E. 2000. Malaria: a renewed assault on an old and deadly foe. Science 290:428-430. [DOI] [PubMed] [Google Scholar]
- 27.McConkey, S. J., W. H. Reece, V. S. Moorthy, D. Webster, S. Dunachie, G. Butcher, J. M. Vuola, T. J. Blanchard, P. Gothard, K. Watkins, C. M. Hannan, S. Everaere, K. Brown, K. E. Kester, J. Cummings, J. Williams, D. G. Heppner, A. Pathan, K. Flanagan, N. Arulanantham, M. T. Roberts, M. Roy, G. L. Smith, J. Schneider, T. Peto, R. E. Sinden, S. C. Gilbert, and A. V. Hill. 2003. Enhanced T-cell immunogenicity of plasmid DNA vaccines boosted by recombinant modified vaccinia virus Ankara in humans. Nat. Med. 9:729-735. [DOI] [PubMed] [Google Scholar]
- 28.McShane, H., R. Brookes, S. C. Gilbert, and A. V. Hill. 2001. Enhanced immunogenicity of CD4+ T-cell responses and protective efficacy of a DNA-modified vaccinia virus Ankara prime-boost vaccination regimen for murine tuberculosis. Infect. Immun. 69:681-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moorthy, V. S., E. B. Imoukhuede, P. Milligan, K. Bojang, S. Keating, P. Kaye, M. Pinder, S. C. Gilbert, G. Walraven, B. M. Greenwood, and A. S. Hill. 2004. A randomised, double-blind, controlled vaccine efficacy trial of DNA/MVA ME-TRAP against malaria infection in Gambian adults. PLOS Med. 1:e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moorthy, V. S., S. McConkey, M. Roberts, P. Gothard, N. Arulanantham, P. Degano, J. Schneider, C. Hannan, M. Roy, S. C. Gilbert, T. E. Peto, and A. V. Hill. 2003. Safety of DNA and modified vaccinia virus Ankara vaccines against liver-stage Plasmodium falciparum malaria in non-immune volunteers. Vaccine 21:2004-2011. [DOI] [PubMed] [Google Scholar]
- 31.Nardin, E. H., J. M. Calvo-Calle, G. A. Oliveira, R. S. Nussenzweig, M. Schneider, J. M. Tiercy, L. Loutan, D. Hochstrasser, and K. Rose. 2001. A totally synthetic polyoxime malaria vaccine containing Plasmodium falciparum B-cell and universal T-cell epitopes elicits immune responses in volunteers of diverse HLA types. J. Immunol. 166:481-489. [DOI] [PubMed] [Google Scholar]
- 32.Nardin, E. H., G. A. Oliveira, J. M. Calvo-Calle, K. Wetzel, C. Maier, A. J. Birkett, P. Sarpotdar, M. L. Corado, G. B. Thornton, and A. Schmidt. 2004. Phase I testing of a malaria vaccine composed of hepatitis B virus core particles expressing Plasmodium falciparum circumsporozoite epitopes. Infect. Immun. 72:6519-6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pancholi, P., D. H. Lee, Q. Liu, C. Tackney, P. Taylor, M. Perkus, L. Andrus, B. Brotman, and A. M. Prince. 2001. DNA prime/canarypox boost-based immunotherapy of chronic hepatitis B virus infection in a chimpanzee. Hepatology 33:448-454. [DOI] [PubMed] [Google Scholar]
- 34.Plebanski, M., K. L. Flanagan, E. A. Lee, W. H. Reece, K. Hart, C. Gelder, G. Gillespie, M. Pinder, and A. V. Hill. 1999. Interleukin 10-mediated immunosuppression by a variant CD4 T-cell epitope of Plasmodium falciparum. Immunity 10:651-660. [DOI] [PubMed] [Google Scholar]
- 35.Prieur, E., S. C. Gilbert, J. Schneider, A. C. Moore, E. G. Sheu, N. Goonetilleke, K. J. Robson, and A. V. Hill. 2004. A Plasmodium falciparum candidate vaccine based on a six-antigen polyprotein encoded by recombinant poxviruses. Proc. Natl. Acad. Sci. USA 101:290-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reece, W. H., M. Plebanski, P. Akinwunmi, P. Gothard, K. L. Flanagan, E. A. Lee, M. Cortina-Borja, A. V. Hill, and M. Pinder. 2002. Naturally exposed populations differ in their T1 and T2 responses to the circumsporozoite protein of Plasmodium falciparum. Infect. Immun. 70:1468-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robson, K. J., A. Dolo, I. R. Hackford, O. Doumbo, M. B. Richards, M. M. Keita, T. Sidibe, A. Bosman, D. Modiano, and A. Crisanti. 1998. Natural polymorphism in the thrombospondin-related adhesive protein of Plasmodium falciparum. Am. J. Trop. Med. Hyg. 58:81-89. [DOI] [PubMed] [Google Scholar]
- 38.Schneider, J., S. C. Gilbert, T. J. Blanchard, T. Hanke, K. J. Robson, C. M. Hannan, M. Becker, R. Sinden, G. L. Smith, and A. V. Hill. 1998. Enhanced immunogenicity for CD8+ T-cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat. Med. 4:397-402. [DOI] [PubMed] [Google Scholar]
- 39.Scott, C. S., D. Van Zyl, E. Ho, L. Ruivo, B. Mendelow, and T. L. Coetzer. 2002. Thrombocytopenia in patients with malaria: automated analysis of optical platelet counts and platelet clumps with the Cell Dyn CD4000 analyser. Clin. Lab. Hematol. 24:295-302. [DOI] [PubMed] [Google Scholar]
- 40.Sedegah, M., T. R. Jones, M. Kaur, R. Hedstrom, P. Hobart, J. A. Tine, and S. L. Hoffman. 1998. Boosting with recombinant vaccinia increases immunogenicity and protective efficacy of malaria DNA vaccine. Proc. Natl. Acad. Sci. USA 95:7648-7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sedegah, M., W. Weiss, J. B. Sacci, Jr., Y. Charoenvit, R. Hedstrom, K. Gowda, V. F. Majam, J. Tine, S. Kumar, P. Hobart, and S. L. Hoffman. 2000. Improving protective immunity induced by DNA-based immunization: priming with antigen and GM-CSF-encoding plasmid DNA and boosting with antigen-expressing recombinant poxvirus. J. Immunol. 164:5905-5912. [DOI] [PubMed] [Google Scholar]
- 42.Simpson, J. A., L. Aarons, W. E. Collins, G. M. Jeffery, and N. J. White. 2002. Population dynamics of untreated Plasmodium falciparum malaria within the adult human host during the expansion phase of the infection. Parasitology 124:247-263. [DOI] [PubMed] [Google Scholar]
- 43.Smith, D. L., J. Dushoff, R. W. Snow, and S. I. Hay. 2005. The entomological inoculation rate and Plasmodium falciparum infection in African children. Nature 438:492-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith, T., G. Killeen, C. Lengeler, and M. Tanner. 2004. Relationships between the outcome of Plasmodium falciparum infection and the intensity of transmission in Africa. Am. J. Trop. Med. Hyg. 71:80-86. [PubMed] [Google Scholar]
- 45.Stock, W., and R. Hoffman. 2000. White blood cells. 1. Non-malignant disorders. Lancet 355:1351-1357. [DOI] [PubMed] [Google Scholar]
- 46.Stoute, J. A., M. Slaoui, D. G. Heppner, P. Momin, K. E. Kester, P. Desmons, B. T. Wellde, N. Garcon, U. Krzych, M. Marchand, et al. 1997. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. N. Engl. J. Med. 336:86-91. [DOI] [PubMed] [Google Scholar]
- 47.Sullivan, N. J., A. Sanchez, P. E. Rollin, Z. Y. Yang, and G. J. Nabel. 2000. Development of a preventive vaccine for Ebola virus infection in primates. Nature 408:605-609. [DOI] [PubMed] [Google Scholar]
- 48.Sun, P., R. Schwenk, K. White, J. A. Stoute, J. Cohen, W. R. Ballou, G. Voss, K. E. Kester, D. G. Heppner, and U. Krzych. 2003. Protective immunity induced with malaria vaccine, RTS,S, is linked to Plasmodium falciparum circumsporozoite protein-specific CD4+ and CD8+ T cells producing IFN-gamma. J. Immunol. 171:6961-6967. [DOI] [PubMed] [Google Scholar]
- 49.Vuola, J. M., S. Keating, D. P. Webster, T. Berthoud, S. Dunachie, S. C. Gilbert, and A. V. Hill. 2005. Differential immunogenicity of various heterologous prime-boost vaccine regimens using DNA and viral vectors in healthy volunteers. J. Immunol. 174:449-455. [DOI] [PubMed] [Google Scholar]
- 50.Walther, M., S. Dunachie, S. Keating, J. M. Vuola, T. Berthoud, A. Schmidt, C. Maier, L. Andrews, R. F. Andersen, S. Gilbert, I. Poulton, D. Webster, F. Dubovsky, E. Tierney, P. Sarpotdar, S. Correa, A. Huntcooke, G. Butcher, J. Williams, R. E. Sinden, G. B. Thornton, and A. V. S. Hill. 2005. Safety, immunogenicity, and efficacy of a pre-erythrocytic malaria candidate vaccine, ICC-1132 formulated in Seppic ISA 720. Vaccine 23:857-864. [DOI] [PubMed] [Google Scholar]
- 51.Walther, M., F. M. Thompson, S. Dunachie, S. Keating, S. Todryk, T. Berthoud, L. Andrews, R. F. Andersen, A. Moore, S. C. Gilbert, I. Poulton, F. Dubovsky, E. Tierney, S. Correa, A. Huntcooke, G. Butcher, J. Williams, R. E. Sinden, and A. V. Hill. 2006. Safety, immunogenicity, and efficacy of prime-boost immunization with recombinant poxvirus FP9 and modified vaccinia virus Ankara encoding the full-length Plasmodium falciparum circumsporozoite protein. Infect. Immun. 74:2706-2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang, R., D. L. Doolan, T. P. Le, R. C. Hedstrom, K. M. Coonan, Y. Charoenvit, T. R. Jones, P. Hobart, M. Margalith, J. Ng, W. R. Weiss, M. Sedegah, C. de Taisne, J. A. Norman, and S. L. Hoffman. 1998. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science 282:476-480. [DOI] [PubMed] [Google Scholar]
- 53.Wang, R., J. Epstein, F. M. Baraceros, E. J. Gorak, Y. Charoenvit, D. J. Carucci, R. C. Hedstrom, N. Rahardjo, T. Gay, P. Hobart, R. Stout, T. R. Jones, T. L. Richie, S. E. Parker, D. L. Doolan, J. Norman, and S. L. Hoffman. 2001. Induction of CD4+ T cell-dependent CD8+ type 1 responses in humans by a malaria DNA vaccine. Proc. Natl. Acad. Sci. USA 98:10817-10822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang, R., J. Epstein, Y. Charoenvit, F. M. Baraceros, N. Rahardjo, T. Gay, J. G. Banania, R. Chattopadhyay, P. de la Vega, T. L. Richie, N. Tornieporth, D. L. Doolan, K. E. Kester, D. G. Heppner, J. Norman, D. J. Carucci, J. D. Cohen, and S. L. Hoffman. 2004. Induction in humans of CD8+ and CD4+ T-cell and antibody responses by sequential immunization with malaria DNA and recombinant protein. J. Immunol. 172:5561-5569. [DOI] [PubMed] [Google Scholar]
- 55.Wang, R., T. L. Richie, M. F. Baraceros, N. Rahardjo, T. Gay, J. G. Banania, Y. Charoenvit, J. E. Epstein, T. Luke, D. A. Freilich, J. Norman, and S. L. Hoffman. 2005. Boosting of DNA vaccine-elicited gamma interferon responses in humans by exposure to malaria parasites. Infect. Immun. 73:2863-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Webster, D. P., S. Dunachie, S. McConkey, I. Poulton, A. C. Moore, M. Walther, S. M. Laidlaw, T. Peto, M. A. Skinner, S. C. Gilbert, and A. V. Hill. 2006. Safety of recombinant fowlpox strain FP9 and modified vaccinia virus Ankara vaccines against liver-stage Plasmodium falciparum malaria in non-immune volunteers. Vaccine 24:3026-3034. [DOI] [PubMed] [Google Scholar]
- 57.Webster, D. P., S. Dunachie, J. M. Vuola, T. Berthoud, S. Keating, S. M. Laidlaw, S. J. McConkey, I. Poulton, L. Andrews, R. F. Andersen, P. Bejon, G. Butcher, R. Sinden, M. A. Skinner, S. C. Gilbert, and A. V. Hill. 2005. Enhanced T cell-mediated protection against malaria in human challenges by using the recombinant poxviruses FP9 and modified vaccinia virus Ankara. Proc. Natl. Acad. Sci. USA 102:4836-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]