Abstract
It is well-documented that infants born to smoking mothers weigh less at birth than infants born to nonsmoking mothers. The purpose of this study was to investigate the influence of prenatal smoking on the development of later infant obesity. Evidence suggests prenatally smoke-exposed infants catch up in weight by age 6 months, although results of this accelerated growth are inconsistent across the body of research literature. In this descriptive study of 630 infants, catch-up growth rate continued and smoke-affected infants were more likely to be obese than their nonsmoke-affected counterparts from age 6 to 14 months. The findings of this study provide insight about the potential effects of maternal prenatal smoking on the risk of early obesity. This paper also discusses the importance of assisting mothers to cease smoking while pregnant.
Keywords: infant, obesity, risk, mothers, mother-child relations, smoking
Introduction
The negative consequences of maternal prenatal smoking on infant prenatal growth and infant birth weight are well-documented. While there is a well-known, real health threat to infants born prematurely or with very low birth weights, conversely there is also awaiting smoke-affected infants the likelihood of a later chronic problem of excess weight and obesity. Obesity during infancy is not well-known as potentially associated with maternal prenatal smoking, and infant obesity is not recognized as serious, like the more life threatening effects associated with prematurity and low birth weight. Yet, the consequences of obesity include long-term problems that develop later in life and are generally seen in adults.
… awaiting these smoke-affected infants [is] the likelihood of a later chronic problem of excess weight and obesity.
The significance of this study emerges from the link between infant, childhood, and adult obesity. Obese infants can become obese children, and obese children can become obese adults (Wells, Stanley, Laidlaw, Day, & Davies, 1996). Persistence of obesity during infancy and childhood increases the risk of obesity during adolescence. Although adolescent obesity accounts for only 30% to 40% of adult obesity, adults who were obese as adolescents constitute a majority of the heaviest adults. Furthermore, the relationship between obesity and risk of hypertension, cancer, diabetes, cardiovascular disease, arthritis, atherosclerosis, and diminished physical abilities later in life impact on the significance of this study on the development of infant obesity
Origins and Prevalence of Obesity
Infant obesity was identified as a public health problem more than 2 decades ago. The prevalence of obesity among children increased 54% in the years between the first National Health and Nutrition Examination Survey (1963-1970) and the second survey (1976-1980) (Gortmaker, Dietz, Sobol, & Wehler, 1987). In the same period, the prevalence of “superobesity” among children increased 98% (Gortmaker et al., 1987). Data from the third National Health and Nutrition Examination Survey (1988-1991) showed another increase in prevalence of overweight in all groups of children: More than 1 in 5 adolescents (20% for males, 22% for females) were found to be overweight (United States Department of Agriculture, 1995). This statistic lead the Department of Agriculture to estimate that 10 million U.S. children are overweight—a number that has doubled in the past 20 years, placing more Americans at risk for an array of health and emotional problems (Webb, 1998).
With extensive data from studies on twins and on families, as well as the more recent studies identifying a gene for obesity, researchers have established that obesity is influenced by genetic factors (Bouchard, 1997). However, genetic makeup alone cannot explain the increasing prevalence of obesity in developed countries. While genetic influences largely determine whether or not a person can become obese, psychosocial and sociocultural influences in the environment may determine whether such a person does or does not become obese and, if so, the extent of that obesity (Antonella et al., 1994; Hayman, Meininger, Coates, & Gallagher, 1995).
Obesity is commonly considered a multifactorial problem and the phenotypic expression of complex interactions between a variety of genetic and environmental influences. Genetics may account for the differences in predisposition to the effects of environmental factors. Environmental factors such as family characteristics and child rearing patterns can minimize or maximize genetic potential.
Smoking and Infant Weight Gain
Studies of the effects of maternal smoking on infant growth are consistent in that there is widespread agreement that infants born to smoking mothers weigh less at birth compared to infants born to nonsmoking mothers (Cliver et al., 1995; Hellerstedt, Himes, Story, Alton, & Edwards, 1997; Horta, Victora, Menezes, Halpern, & Barros, 1997; Kramer, Platt, Yang, McNamara, & Usher, 1999; Muscati, Koski, & Gray-Donald, 1996; Wang, Tager, VanVunakis, Speizer, & Hanrahan, 1997; Zaren, Cnattingius, & Lindmark, 1997). This fetal growth retardation is followed by an accelerated “catch-up” growth rate in the early months following birth. “Catch-up” growth is the period of recovery that occurs when a stage of growth retardation ends and favorable conditions are restored (Strauss & Dietz, 1998). The literature on the results of this postnatal catch-up growth is less consistent. Some investigators found that smoke-affected infants, experiencing an initial accelerated growth rate, caught up with their nonsmoke-affected peers by 4 to 6 months of age but remained lighter even into the toddler years (Conter, Cortinovis, Rogari, & Riva, 1995; Fox, Sexton, & Hebel, 1990; Olsen, 1992; Schulte-Hobein, Schwartz-Bickenbach, Abt, Plum, & Nau, 1992). Others found smoke-affected infants to be significantly heavier than their nonsmoke-affected peers by 1 year of age and even into the toddler years (Little, Lambert, Worthington-Roberts, & Ervin, 1994; Vik, Jacobsen, Vatten, & Bakketeig, 1996).
In summary: Although infant obesity was identified as a public health problem more than 2 decades ago, little is known about the factors that influence the development of obesity during infancy, including exposure to prenatal smoking. The purpose of this paper is to facilitate an understanding of the influence of maternal prenatal smoking on infant growth and the development of obesity.
Theoretical Framework
Based on the epidemiologic concepts of risk, susceptibility, and causality, the Web of Causation Model was the theoretical framework for this study (Clark, 1999). Risk is the probability that an individual will develop a specific condition and is affected by a variety of influences including physical, emotional, environmental, and lifestyle factors. The basis for risk lies in susceptibility, the ability to be affected by factors contributing to a particular health condition. In this model, factors are explored in terms of their interplay, and both direct and indirect causes of a problem like obesity are identified (Clark, 1999; Freidman, 1994). The web encompasses the interrelationships between a multitude of factors, some known and some unknown, but all bearing ultimately on risk (Valanis, 1992). Exposure to multiple causal factors may have an additive or multiplicative effect.
The Web of Causation Model allows the mapping of interrelationships among contributing factors and assists in determining areas where control efforts will be most feasible and effective. Infant obesity was viewed as the result of multiple interacting factors. Understanding these factors will lead to earlier identification of infants at risk for obesity. This model can assist with exploring whether or not, through professional nursing practice, some factors affecting infant growth can be modified to facilitate positive infant growth outcomes.
Research Design
The material for this paper is from a study that explored obesity in infants in collaboration with a National Institutes of Health funded prospective study (1988–1996) of infant growth (Marilyn Stember, PI). With an interest in understanding infant growth failure, these infants—recruited at age 2 weeks—and their families were followed on a monthly basis through 14 months of age. The focus of this obesity study was on infants who were obese at birth or became obese during infancy.
A longitudinal, prospective design was used to identify the influence of maternal prenatal smoking along with additional variables on infant growth, as listed below under “Follow-up Data Collection.” This design had the advantage of obtaining data on the same individuals over time, as well as observing variables as they were changing.
Method
Sample
This study of infant obesity included 630 infants (54% boys, 46% girls) and their families. The racial composition of the sample was 71% Caucasian, 16% Hispanic, 11% African-American, and 2% Asian. The number of subjects declined over time from 630 infants at birth to 622 at 1 month, 543 at 4 months, 496 at 7 months, 470 at 10 months, and 433 at 14 months of age. The rate of attrition ranged from a low of 1.3% between birth and 1 month of age to a high of 12.7% between months 1 and 4.
Criteria for inclusion in the study were an essentially normal infant at birth (i.e., no organic etiology) and a mother able to communicate in English. Preterm infants (gestational age less than 37 weeks) and infants with intrauterine growth retardation (birth weight less than 2,500 grams) were excluded from this study because of their different associated growth patterns in the postnatal period.
At the time of initial data collection, most parents (65%) were married and more than 77% of fathers lived in the home. Seventeen percent of families had a grandmother living in the home. Household sizes ranged from as few as 2 to as many as 12 members. Mothers reported educational attainment ranging from 7 years to as much as 24 years. Mothers' ages varied from 14 to 43 years. Almost one-third (30.3%) of mothers were smoking during pregnancy. The usual weight of mothers ranged from 80 pounds to 277 pounds, with an average of 136 pounds. The average weight gain during pregnancy was 34 pounds.
Several trends over time are apparent. The number of married parents participating in the study increased from 65% at 1 month to 73% at 14 months. This trend was repeated for the percentage of fathers living in the same home with the infant: 77% at 1 month to 83% at 14 months. The number of grandmothers living in the home of infants was consistent every time at 17%.
Operational Definitions
Body mass index (BMI) is increasingly being used as an indicator of excess weight and obesity. BMI is the relationship between weight and stature and is most commonly used as an indicator of fatness in the form of weight in kilograms divided by height in meters squared (kg/m2). For this study, obesity in infants was defined as BMI greater than the gender- and age-specific 84th percentile (1 SD above the mean) based on within study reference norms.
Maternal smoking was defined as the cigarette smoking habits of the infant's mother during pregnancy. Mothers were classified smoker for smoking any number of cigarettes during the first, second, and/or third trimesters. Of the mothers who smoked during pregnancy (n = 119), 81% smoked 5 or more cigarettes a day. The number of smoking mothers decreased in the second trimester (n = 134, 70%) and again in the third trimester (n = 131, 69%). Information about maternal smoking was obtained upon entry into the study from a researcher-developed Demographic and Perinatal Data Questionnaire, along with family demographics.
Follow-up Data Collection
Data collection used multiple methods and sources of data. Anthropometric measures (weight, length, head circumference, upper arm circumference, triceps skinfold thickness) were obtained monthly for 14 months to correspond with the infant's birthday using a precise measurement protocol described by Lohman, Roche, and Martorell (1988). Measures deviating from the infant's monthly target date were adjusted. At the home visits, which were conducted at 1, 4, 7, 10, and 14 months, additional measures were made, including maternal characteristics, family demographic information, family socioeconomic status, family life stress, infant sleep pattern, infant nutrition, infant temperament and activity.
Data Analysis
Data analysis included comparing obese with not-obese infants and also comparing smoke affected with nonsmoke affected infants regardless of their obesity status. Logistic regression analysis was used to assess the effects of smoking on infant obesity, while holding selected variables constant. The resulting odds ratio (OR) in this study approximates how likely or unlikely it is for obesity to be present given certain conditions.
A forced-entry procedure, using three separate blocks of variables, was used to evaluate the effect of individually significant predictor variables on infant obesity. Model 1 included maternal prenatal smoking and other psychosocial and sociocultural variables, as listed under “Follow-up Data Collection.” Infant gender and race/ethnicity were entered in Model 2. Finally, Model 3, birth BMI, and BMI from the previous study month were entered into the logistic regression analyses.
Results
Comparison of Obese and Not-Obese Infants
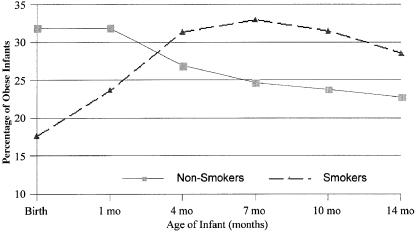
The percentage of obese infants whose mothers smoked prenatally increased from 18% at birth to a high of 33% at 7 months, decreasing to 29% by 14 months (see Figure 1). When obese and not-obese infant groups were compared, significant differences in the percentages of the obese and not-obese infant groups were evident relative to maternal prenatal smoking. At 1 month of age, fewer prenatal smoke-exposed infants were obese (t=2.02, p=.03). Conversely, at 14 months of age, more prenatal smoke-exposed infants were obese (t=−2.29, p=.02). The obese and not-obese groups differed, but not significantly at 4, 7, or 10 months of age as the two groups each reversed their pattern of weight gain.
When obese and not-obese infant groups were compared, significant differences in the percentages of the obese and not-obese infant groups were evident relative to maternal prenatal smoking.
Percentage of infants of prenatally smoking and nonsmoking mothers who were obese at birth, 1, 4, 7, 10, and 14 months of age.
In the series of three preliminary logistic regression analyses, maternal smoking was statistically significant for the total group at 1, 10, and 14 months of age, and for boys at 7, 10, and 14 months of age. In the final logistic regression models, maternal smoking was associated with a 50% decrease in the risk of infant obesity at age 1 month, before catch-up had begun (OR=0.5, p<.05). Once catch-up began, the association of prenatal smoking became evident by prenatal maternal smoking being associated with an 80% increase (OR=1.8, p<.05) and a 70% increase (OR=1.7, p<.10) in the risk of infant obesity at age 7 and 14 months, respectively.
Additional Comparison of Smoke-Affected and Nonsmoke-Affected Infants
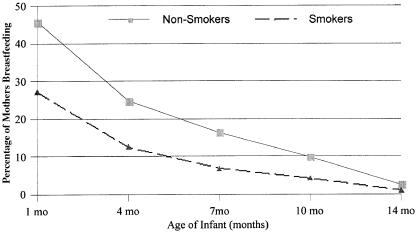
When infants of mothers who smoked were compared with infants of mothers who did not smoke prenatally, several trends were noted. Mothers who smoked prenatally were younger on average by 2 years (25.6 yrs. vs. 27.5 yrs.) than mothers who did not smoke prenatally. In addition, the reported maternal prepreganant body weight was an average of 2 pounds less (134.1 lbs. vs. 136.2 lbs.), but weight gain during pregnancy averaged 2 pounds more (35.5 lbs. vs. 33.4 lbs.) than for mothers who did not smoke during pregnancy. Additional differences included a higher percentage of fathers not living in the same home with the mothers who smoked prenatally (35% vs. 18%). Also, a higher percentage of the smoking mothers (76%) were Caucasian. Fewer smoking mothers breastfed their infants at 1 month (27%) compared to nonsmoking mothers (46%) (see Figure 2). This trend continued through 14 months of age with 2 to 2.5 times as many nonsmoking mothers sustaining breastfeeding compared to smoking mothers; although, few mothers in either group continued breastfeeding as long as 14 months.
Percentage of prenatally smoking and nonsmoking mothers who breastfed their infants at 1, 4, 7, 10, and 14 months of age.
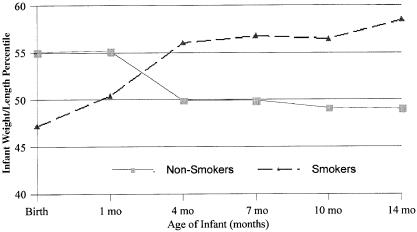
When smoke-affected and nonsmoke-affected infant groups were compared, significant differences in the means (smoke affected = 3.45 months, nonsmoke affected = 3.89 months) in the groups were evident with age of introduction of solid food (t=3.94, p<.001, df 409.9). Additionally, significant differences in the groups were evident with weight-for-length percentile birth (t=2.78, p<.01), 4 months (t=−2.28, p<.05), 7 months (t=−2.42, p<.05), 10 months (t=−2.36, p<.05), and 14 months (t=−2.90, p<.01). The anthropometric difference between groups is illustrated in Figure 3.
Mean weight-for-length percentile of infants of smoking and nonsmoking mothers at birth, 1, 4, 7, 10, and 14 months of age.
Limitations
The analysis measured prenatal smoking and breastfeeding as dichotomous (yes-or-no) variables and, thus, did not consider the amount of prenatal smoking or breast milk intakes. It did not take into consideration postnatal nicotine exposure through either the air or breast milk. Breastfeeding and abstaining from smoking are both choices related to the health consciousness of mothers. The obesity group pattern of infants of nonsmoking mothers is similar to the weight gain pattern of breastfed infants. Additionally, the group obesity pattern of the prenatally smoke-exposed infants is similar to the weight gain of formula-fed infants.
Discussion
The empirical results of this study support some findings published in the research literature while contradicting others. While infants of smoking mothers began at birth with BMIs lower than infants of nonsmoking mothers, this deficit was quickly overcome. The catch-up growth rate continued and these smoke-affected infants were more likely to be obese than their nonsmoke-affected counterparts from 6 months through 14 months of age. While this finding supports the role of maternal prenatal smoking in infant obesity reported by Little et al. (1994) as well as Vik et al. (1996), it contradicts the findings of Conter et al. (1995), Fox et al. (1990), Olsen (1992), and Schulte-Hobein et al. (1992).
The catch-up growth rate continued and these smoke-affected infants were more likely to be obese than their nonsmoke-affected counterparts from 6 months through 14 months of age.
The following explanation for this study's findings related to infant obesity and maternal smoking is conjecture. Because nicotine acts as an appetite suppressant, when infants are no longer exposed to prenatal nicotine, appetite may increase significantly and these infants might demand more feeding than they normally would without previous drug exposure. Alternately, smoking mothers may increase feeding to help their infants overcome the initial birth weight deficit. Further work in this area is needed to understand the role of maternal prenatal smoking on the development of obesity. Such study should be designed to separate the influence of breastfeeding on smoking and weight gain.
The findings of this study suggested that the strongest predictor of infant obesity was body mass index from the previous month studied. In addition, birth BMI was a significant predictor through 7 months of age. These findings support the existing research, which indicates heavy infants are more likely to remain heavy (Kumanyika, 1993; Zive et al., 1992). Other significant predictors of infant obesity during the first 14 months of life included nutrition and feeding practices, infant race/ethnicity and gender, infant temperament and activity, and additional maternal and family characteristics (Sowan & Stember, 2000; Sowan & Stember, in press).
Clinical Implications
Of particular interest to professional nurses and other health care professionals is the possible relationship between the risk variable of prenatal smoking and infant obesity. The findings of this study indicated that maternal prenatal smoking—a variable amenable to change—as well as factors that are intrinsic to the infant (gender, race/ethnicity) may play a significant role in the risk of obesity. Health promotion and prevention of infant obesity must come from efforts directed at community, family, and individuals. Perinatal educators can participate in the prevention of initial low birth weight and potentially later obesity through community efforts to improve prenatal care and prenatal nutrition and through community smoking cessation programs. Family and individual supportive interventions can be directed toward encouraging mothers who smoke to stop smoking during pregnancy in order to improve the birth outcomes and to help reduce the risk of later infant obesity. An example of such a preventive program was published in a previous issue of this journal (Rice, Fotouhi, Burn, Hoyer, & Ayers, 1997).
Perinatal educators can participate in the prevention of initial low birth weight and potentially later obesity through community efforts to improve prenatal care and prenatal nutrition and through community smoking cessation programs.
Conclusions
To summarize, the findings from this study of the impact of maternal prenatal smoking on the development of infant obesity supported some existing research litera- ture and contradicted some previous findings. Maternal prenatal smoking did not exert an influence at all ages studied. Further, maternal smoking exerted a lower weight-lowering influence at birth and an obesity influence after catch-up occured, suggesting the complexity studying infant obesity. Overall, the number of significant variables for explaining infant obesity decreased as the sample population got older, but these fewer number of variables explained more of the variance.
The complexity of infant obesity is difficult to capture. Health care professionals usually enter this complex arena after obesity is established. Early prevention and intervention are essential to affect a positive impact on the problem of obesity. The findings in this research have produced insight into and raised questions about the effect of maternal prenatal smoking on early obesity. Disappointing results in both individual and population interventions to treat childhood and adult obesity are in large part due to an incomplete understanding of the causes of obesity. Greater understanding of the complex relationships among the contributing factors of obesity is essential. The strength and direction of interactions between and among numerous contributing variables are not clear, and it may be that these relationships are the keys to unraveling the perplexities of obesity.
Funding
AAUW (N. Sowan, Fellow)
NRSA (F31 NR06830-01, N. Sowan, PI)
NIH, NINR (RO1 NR01670, M. Stember, PI)
Effects of Weight Loss in Overweight, Lactating Women on Infant Growth
A study in [the Volume 342, Number 7 issue of the] New England Journal of Medicine finds that weight loss of approximately 0.5 kg per week between 4–14 weeks postpartum in overweight women who are breastfeeding does not affect the growth of their infants. Though the women in the diet-and-exercise group lost more weight and fat mass, the gains in weight and length of the infants whose mothers were in the diet-and-exercise group were not significantly different from infants whose mothers were in the control group. According to the [journal's] editorial: “For the health and well-being of both mothers and infants, the nutritional aim should be to achieve balance in maternal weight and body composition over the entire reproductive cycle, not only in the early postpartum period.”
Lovelady, Cheryl A., et al. (2000). The effect of weight loss in overweight, lactating women on the growth of their infants. The New England Journal of Medicine, 342(7), 449–453.
Butte, Nancy F. (2000). Dieting and exercise in overweight, lactating women. The New England Journal of Medicine, 342(7), 502–503.
A longer version of the above news brief appeared in the February 18, 2000, issue of MCH Alert, which is produced by the National Center for Education in Maternal and Child Health in Arlington, VA (http://www.ncemch.org/alert).
References
- Antonella E. P, Luca S, Emilia D, Rosaria P. M, Annarita C, Giuseppe C, Franco C, Giuliana V, Andriana F, Salvatore D, Armido R. Familial and environmental influences on body composition and body fat distribution in childhood in Southern Italy. International Journal of Obesity. 1994;18:596–601. [PubMed] [Google Scholar]
- Bouchard C. Human variation in body mass: Evidence of the role of genes. Nutrition Reviews. 1997;55(1 Pt 2):S21–30. doi: 10.1111/j.1753-4887.1997.tb06094.x. [DOI] [PubMed] [Google Scholar]
- Clark M. J. 1999. Nursing in the community (3rd ed.). Stamford, CT: Appleton & Lange. [Google Scholar]
- Cliver S. P, Goldenberg R. L, Cutter G. R, Hoffman H. J, Davis R. O, Nelson K. G. The effect of cigarette smoking on neonatal anthropometric measurements. Obstetrics & Gynecology. 1995;85(4):625–630. doi: 10.1016/0029-7844(94)00437-I. [DOI] [PubMed] [Google Scholar]
- Conter V, Cortinovis I, Rogari P, Riva L. Weight growth in infants born to mothers who smoked during pregnancy. British Medical Journal. 1995;310:768–771. doi: 10.1136/bmj.310.6982.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox N. L, Sexton M, Hebel R. J. Prenatal exposure to tobacco. I. Effects on physical growth at age three. International Journal of Epidemiology. 1990;19:66–71. doi: 10.1093/ije/19.1.66. [DOI] [PubMed] [Google Scholar]
- Freidman G. D. 1994. Primer of epidemiology. New York: McGraw-Hill. [Google Scholar]
- Gortmaker S. F, Dietz W. H, Sobol A. M, Wehler C. A. Increasing pediatric obesity in the United States. American Journal of Diseases of Children. 1987;141:535–540. doi: 10.1001/archpedi.1987.04460050077035. [DOI] [PubMed] [Google Scholar]
- Hayman L. L, Meininger J. C, Coates P. M, Gallagher P. R. Nongenetic influences of obesity on risk factors for cardiovascular disease during two phases of development. Nursing Research. 1995;44(5):277–282. [PubMed] [Google Scholar]
- Hellerstedt W. L, Himes J. H, Story M, Alton I. R, Edwards L. E. The effects of cigarette smoking and gestational weight change on birth outcomes in obese and normal-weight women. American Journal of Public Health. 1997;87(4):591–596. doi: 10.2105/ajph.87.4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horta B. L, Victora C. G, Menezes A. M, Halpern R, Barros F. C. Low birthweight, preterm births and intrauterine growth retardation in relation to maternal smoking. Pediatric Perinatal Epidemiology. 1997;11(2):140–151. doi: 10.1046/j.1365-3016.1997.d01-17.x. [DOI] [PubMed] [Google Scholar]
- Kramer M. S, Platt R, Yang H, McNamara H, Usher R. H. Are all growth-restricted newborns created equal(ly)? Pediatrics. 1999;103(3):599–602. doi: 10.1542/peds.103.3.599. [DOI] [PubMed] [Google Scholar]
- Kumanyika S. Ethnicity and obesity development in children. Prevention and Treatment of Childhood Obesity. 1993;669:81–92. doi: 10.1111/j.1749-6632.1993.tb18839.x. [DOI] [PubMed] [Google Scholar]
- Little R. E, Lambert M. D, Worthington-Roberts B, Ervin C. H. Maternal smoking during lactation: Relation to infant size at one year of age. American Journal of Epidemiology. 1994;140:544–554. doi: 10.1093/oxfordjournals.aje.a117281. [DOI] [PubMed] [Google Scholar]
- Lohman T. G, Roche A. F, Martorell R. 1988. Anthropometric standardization reference manual. (Eds.) Champaign, IL: Human Kinetics Books. [Google Scholar]
- Muscati S. K, Koski K. G, Gray-Donald K. Increased energy intake in pregnant smokers does not prevent human fetal growth retardation. Journal of Nutrition. 1996;126(12):2984–2989. doi: 10.1093/jn/126.12.2984. [DOI] [PubMed] [Google Scholar]
- Olsen J. Cigarette smoking in pregnancy and fetal growth. Does the type of tobacco play a role. International Journal of Epidemiology. 1992;21:279–284. doi: 10.1093/ije/21.2.279. [DOI] [PubMed] [Google Scholar]
- Rice V. H, Fotouhi F, Burn E, Hoyer P, Ayers M. Exemplary program development: Hypermedia interactive smoking cessation intervention program for pregnant women. Journal of Perinatal Education. 1997;6(3):47–58. [Google Scholar]
- Schulte-Hobein B, Schwartz-Bickenbach D, Abt S, Plum C, Nau H. Cigarette smoke exposure and development of infants throughout the first year of life: Influence of passive smoking and nursing on cotinine levels in breast milk and infant's urine. Acta Paediatrica Scandivavica. 1992;81:550–557. doi: 10.1111/j.1651-2227.1992.tb12293.x. [DOI] [PubMed] [Google Scholar]
- Sowan N. A, Stember M. L. Factors influencing obesity in early infancy. The Clinical Letter for Nurse Practitioners. 2000;4(2):1–6. [Google Scholar]
- Sowan N. A, Stember M. L. Parental risk factors for infant obesity. in press. MCN: The American Journal of Maternal Child Health. [DOI] [PubMed]
- Strauss R. S, Dietz W. H. Growth and development of term children born with low birth weight: Effects of genetic and environmental factors. Journal of Pediatrics. 1998;133(1):67–72. doi: 10.1016/s0022-3476(98)70180-5. [DOI] [PubMed] [Google Scholar]
- United States Department of Agriculture. 1995. Third report on nutrition monitoring in the United States. (Executive Summary.) Washington, DC: U.S. Government Printing Office. [Google Scholar]
- Valanis B. 1992. Epidemiology in nursing and health care (2nd ed.). Norwalk, CT: Appleton & Lange. [Google Scholar]
- Vik T, Jacobsen G, Vatten L, Bakketeig L. S. Pre- and post-natal growth in children of women who smoked during pregnancy. Early Human Development. 1996;45:245–255. doi: 10.1016/0378-3782(96)01735-5. [DOI] [PubMed] [Google Scholar]
- Webb T. Child obesity ‘epidemic.’. 1998, October 28. Denver Post (Knight Ridder News Service article), p. A-08.
- Wang X, Tager I. B, VanVunakis H, Speizer F. E, Hanrahan J. P. Maternal smoking during pregnancy, urine cotinine concentrations, and birth outcomes. A prospective cohort study. International Journal of Epidemiology. 1997;26(5):978–988. doi: 10.1093/ije/26.5.978. [DOI] [PubMed] [Google Scholar]
- Wells J. C. K, Stanley M, Laidlaw A. S, Day J. E, Davies P. S. W. The relationship between components of infant energy expenditure and childhood body fatness. International Journal of Obesity. 1996;20:848–853. [PubMed] [Google Scholar]
- Zaren B, Cnattingius S, Lindmark G. Fetal growth impairment from smoking—Is it influenced by maternal anthropometry? Acta Obstetricia et Gynecologica Scandinavica—Supplement. 1997;76:30–34. [PubMed] [Google Scholar]
- Zive M. M, McKay H, Frank-Spohrer G. C, Broyles S. L, Nelson J. A, Nader P. R. Infant-feeding practices and adiposity in 4-year-old Anglo- and Mexican-Americans. American Journal of Clinical Nutrition. 1992;55:1104–1108. doi: 10.1093/ajcn/55.6.1104. [DOI] [PubMed] [Google Scholar]