Abstract
Amastin surface proteins belong to a large family of developmentally regulated proteins comprising up to 45 members that have recently been discovered in the genus Leishmania and are highly similar to the amastin proteins in Trypanosoma cruzi. All members of the amastin gene family contain a highly conserved 11-amino-acid (aa) signature at the N terminus, which is unique to the amastin proteins and to the Trypanosomatidae family. Recent studies have demonstrated that this region is highly protective in a mouse model. The goal of the present study was to evaluate the potential of the 50-aa N-terminal domain of amastin proteins harboring the conserved 11-aa amastin signature peptide as a relevant immune biomarker of cutaneous leishmaniasis (CL) and visceral leishmaniasis (VL). We report here the amastin-binding total immunoglobulins (IgG) and/or IgG subclasses in the sera of patients at different stages of CL (n = 90) and VL (n = 41). In CL cases, there is no significant difference in seroreactivities between active, recovered, and nonhealed cases. However, the amastin peptide-reactive antibodies were present at high titers in 19 of 20 sera collected from patients with active VL compared to sera from patients recovered from VL and asymptomatic cases of VL. These data suggest that the amastin signature peptide could represent a relevant biomarker for the serodiagnosis of VL and, most importantly, that it could permit differentiation among the different stages of the disease.
Leishmania protozoa are the causative agents of human leishmaniasis in nearly 90 countries, threatening over 350 million people, more than 12 million of whom are infected (1). Clinical manifestations of this disease range from self-healing cutaneous leishmaniasis (CL) to debilitating mucocutaneous leishmaniasis to life-threatening visceral leishmaniasis (VL). A measure of the seriousness of this parasitic disease is the two million new cases of human leishmaniasis that appear annually throughout the developing world (28). Leishmania organisms in the form of metacyclic promastigotes are transmitted to humans through the bite of an infected female sandfly. Within a short time, the promastigotes are taken by professional phagocytes (neutrophils, monocytes, and macrophages), as well as dendritic cells and fibroblasts. During this process, the promastigote loses its flagella and transforms into the nonflagellated amastigote form, in which it multiplies exclusively in the phagolysosomal compartment of macrophages (14).
At present, most diagnostic tools from PCR to antigen-based enzyme-linked immunosorbent assays (ELISAs) are not suitable for field conditions, and the diagnosis of VL still relies on bone marrow puncture (30). A better knowledge of Leishmania proteins should allow us to improve the diagnostic markers. The goal of the present study is to evaluate the serum reactivities of different stages of CL and VL cases to the conserved extracellular domain of newly identified surface antigens in Leishmania harboring the amastin signature sequence (29). Amastins belong to a large family of surface proteins that have recently been discovered in the genus Leishmania (21) and show high similarity to the amastin proteins in Trypanosoma cruzi. The members of the amastin gene family, approximately 45 in all, are dispersed all through the genome of Leishmania. Amastin genes usually code for small proteins of about 200 amino acids (aa) (21). Amastins are unique to kinetoplastids and are expressed specifically in the amastigote stage of the parasite (21, 29). Amastin gene expression is upregulated by a decrease in the pH (one of the properties of the intraphagolysosomal space). Due to its relative hydrophobic sequence and its localization in the membrane, it is believed that the amastin family plays a role in proton or ion traffic across the membrane to adjust the cytoplasmic pH. Nonetheless, the role of the amastin gene family in the virulence of Leishmania is still unclear (21). All members of the amastin gene family possess two predicted extracellular domains. The first domain, located between transmembrane helices 1 and 2, is 55 to 60 aa long and contains a highly conserved sequence of 11 aa at positions 52 to 62 that is present in all of the Leishmania and Trypanosoma amastin homologs and corresponds to the amastin signature sequence. This well-defined domain was not found in any other protein, which suggests that amastin surface proteins are probably unique to trypanosomatid protozoa. Although the putative function of the amastin signature sequence remains elusive, a recent report by Stober et al. showed that the N terminus of amastin proteins (aa 1 to 63) that harbors the 11-aa (CITLWGLKTDC) amastin signature sequence is highly immunogenic and induces protection in mice (27). In addition, Salotra et al. showed an upregulation of class III amastins (29) in post-kala-azar dermal leishmaniasis (PKDL) by comparing the PKDL with kala-azar parasites using microarrays (23). However, the role of amastin proteins in persistence and reactivation in PKDL remains to be characterized.
In the present study, we evaluated different stages of Iranian CL and VL antibody responses to the amastin signature peptide. In Iran, both zoonotic and anthroponotic CL caused by L. major and L. tropica, respectively, are endemic in different regions, especially in the cities of Esfahan and Mashhad. Kala-azar is another form of leishmaniasis which is endemic in Iran, with major foci in the northwest, south, and southwest. The causative agent is L. infantum and the main reservoir is infected dogs. The disease is more common among the rural population and children, aged 1 to 10 years, are the targets of disease. By using ELISA and Western blotting analyses, we show that the amastin signature peptide could be a valuable diagnostic tool for serodiagnosis of the active stage of visceral leishmaniasis.
MATERIALS AND METHODS
Parasite growth.
The following strains of L. major and L. infantum were used, respectively; MHRO/IR/75/ER and MCAN/98/LLM-877. Amastigotes of L. major were maintained by continuous passage in the BALB/c and, for L. infantum, Syrian hamsters were used. The infected spleens were isolated, homogenized, and cultured in NNN media in the presence of 100 μg of gentamicin/ml. Promastigotes were collected by centrifugation (270 × g, 10 min, 4°C), washed three times in phosphate-buffered saline (8 mM Na2HPO4, 1.74 mM KH2PO4, 0.25 mM KCl, 137 mM NaCl), and resuspended at a concentration of 2 × 108 parasites/ml. This preparation was frozen and thawed 10 times using liquid N2 and a 37°C water bath, and the protein concentration was determined by using bicinchoninic acid reagent (BCA; Pierce Chemical Co., Rockford, Ill.). Protein preparation was kept at −70°C until use.
Synthesis of the N-terminal domain of an L. major amastin member displaying conserved amino acid sequences with other L. major members and with one L. infantum amastin member.
The amastin signature peptide of L. major LmjF08.0810 consisting of 52 amino acids with the sequence PIDMFRPHNTSRIGNTPCLTLWGYKSECYSTKYDVRSDDLWANCTDRLLQFR was selected for the present study. This sequence shares 48 to 100% homology to seven other L. major amastin homologs (LmjF08.0850, LmjF08.0800, LmjF08.0840, LmjF08.0830, LmjF08.0820, LmjF08.0970, and LmjF08.0960), as well as 53% homology to L. infantum (LinJ34.840). The amastin peptide was chemically synthesized (University of Lausanne, Lausanne, Switzerland) by solid-phase Fmoc (9-fluorenylmethoxy carbonyl) chemistry as described previously (22) and purified to homogeneity under the good laboratory practice condition. Amino acid analysis, analytical high-pressure liquid chromatography, and mass spectrometry were used to determine the purity of the final product. The purity has been analyzed by high-pressure liquid chromatography and been shown to be >95%.
Origin and collection of sera.
All CL samples were collected from the city of Mashhad, in eastern Iran. The VL samples were collected from different villages around the Meshkinshahr area, northwest of Iran, where there have been no reports of CL. Three different groups were selected corresponding to active, recovered, and nonhealed CL cases. For the active CL group, 30 newly diagnosed patients with CL without prior treatment were chosen. Patients were subjected to parasitologic investigation and confirmed by parasite detection in the smear and/or NNN culture. All were negative for the leishmanin skin test (LST). The LST was carried out by interadermal injection of 0.1 ml of leishmanin (Pasteur Institute of Iran) (induration < 5 mm). Thirty recovered individuals with 100% positive LSTs (induration > 5 mm) and scarring were selected. The mean average after their healing is 3.2 ± 1.5 years. The nonhealed cases were selected among those who have had the active lesion for more than 3 years, and 30 individuals were selected (90% negative for LST). Three different groups of active, recovered, and asymptomatic individuals were selected, and 21 serum samples were obtained from active VL cases ranging in age from 1 to 3 years and were diagnosed by the detection of Giemsa-stained amastigotes in bone marrow aspirates and by clinical evaluation (fever, splenomegaly), together with an indirect immunofluorescence assay with whole parasites as the antigen. The recovered cases consisted of 10 individuals ranging from 5 to 15 years old. These subjects had been diagnosed with VL based on criteria similar to those for the active cases, and standard intravenous antimony treatments were applied. All had positive LST results. The third group consisted of asymptomatic individuals without any disease living in the same area and with a positive LST. Ten healthy individuals living in areas where leishmaniasis is not endemic and who had negative LST results were selected as a control group (normal cases).
Western blotting.
Promastigote parasite antigens (3 × 108 promastigotes subjected to 10 cycles of alternate freeze-thawing) were obtained at a concentration of 1 mg/ml of total proteins (BCA Chemical, Rockford, Ill.). This preparation (2 μg/lane) or the amastin signature peptide (1 μg/lane) was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis on either 12 or 15% polyacrylamide in an equal volume of sample buffer (125 mM Tris-HCl [pH 6.8], 6% sodium dodecyl sulfate, 20% glycerol, 10% 2-mercaptoethanol, 0.05% bromophenol blue). The proteins were then transferred by Western blotting (Mini-Protean II; Bio-Rad) to a nitrocellulose membrane at 90 V for 1 h. The membrane was further treated with 1:100 dilutions of six active VL sera. Peroxidase conjugates of direct human poly antibody (poly Ab) (immunoglobulin M [IgM], IgG, and IgA) were used at a 1:1,000 dilution.
ELISA.
Microtiter plates (Maxisorb plate; Nunc) were coated with freeze-thawed (F/T) parasite antigens of either L. major or L. infantum at concentrations of 10 μg/ml. The amastin signature synthetic peptide was used at concentration of 20 μg/ml. The plates were incubated overnight at 4°C and then blocked with 1× phosphate-buffered saline-1% bovine serum albumin at 37°C for 2 h to prevent nonspecific binding. Sera were added (1:50), followed by incubation for 2 h at 37°C. As a secondary antibody, horseradish peroxidase-labeled goat anti-human poly Ab (1:1,000) or biotin-labeled total IgG and its subclasses (total IgG at 1:3,000, IgG1 at 1:1,000, IgG2 at 1:2,000, IgG3 at 1:4,000, and IgG4 at 1:10,000 dilutions) were used with incubation at 37°C. The plates were washed three times and, in the cases of biotin-labeled antibodies, an extra step was taken for incubation with streptavidin-horseradish peroxidase (BRL, Gaithersburg, MD) for 1 h at room temperature. Binding of conjugates was visualized with O-phenylenediamine in 0.1 M sodium citrate (pH 4.5) containing 0.03% H2O2. The reaction was stopped by adding 4 M H2SO4, and the absorbance value was measured at 492 nm in an automatic micro-ELISA reader (Techan). The absorbance is expressed as the mean optical density at 492 nm ± the standard deviation (SD) in all groups. The cutoff value is determined as 5 SDs above the mean absorbance of normal sera.
Statistical analyses.
All statistical analyses were carried out by using SPSS for Windows version 13.0 (SPSS, Inc., Chicago, Ill.). The differences were determined by one-way analysis of variance and/or by the general linear Tukey model, and they were considered statistically significant when the P value was <0.05.
RESULTS
Sequence alignment of the N-terminal domain of four L. major amastin members and one L. infantum amastin member.
The alignment of the predicted amino acid sequence within the first conserved extracellular domain of several amastin homologs harboring the amastin signature sequence is shown in Fig. 1. Homologs within the L. major chromosomes 8 (LmjF08.0800; six identical copies) and 34 (LmjF34.0960 and LmjF34.0970) and the L. infantum amastin homolog LinJ34.0840 were compared. Sequence homology within the amastin signature region ranges from 48 to 100% among different L. major amastin homologs and is at 53% between the L. major (LmjF08.810) and L. infantum (LinJ35.840) homologs.
FIG. 1.
Sequence alignments of the N-terminal domain of four L. major amastin members and one L. infantum amastin member. The L. major amastin homologs present on chromosomes 8 and 34 (LmjF08.0800 and LmjF08.0820; four more identical copies, LmjF34.0960, and LmjF34.0970) and the L. infantum homolog LinJ34.0860 (previously reported as LinJ34.0860) (21) are shown in this alignment. The 11-aa amastin signature sequence is underlined. An asterisk means that amino acids are identical in all sequences shown in the alignment. A colon means that conserved substitutions are observed, and a period means that semiconserved substitutions are observed.
Detection of amastin-binding antibodies in sera from patients with active VL.
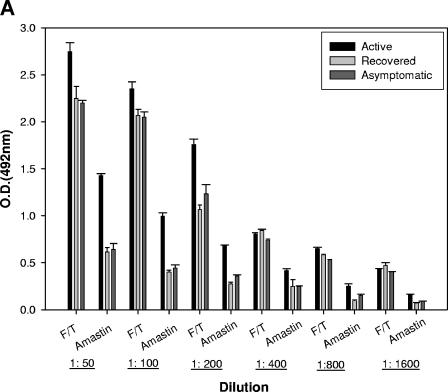
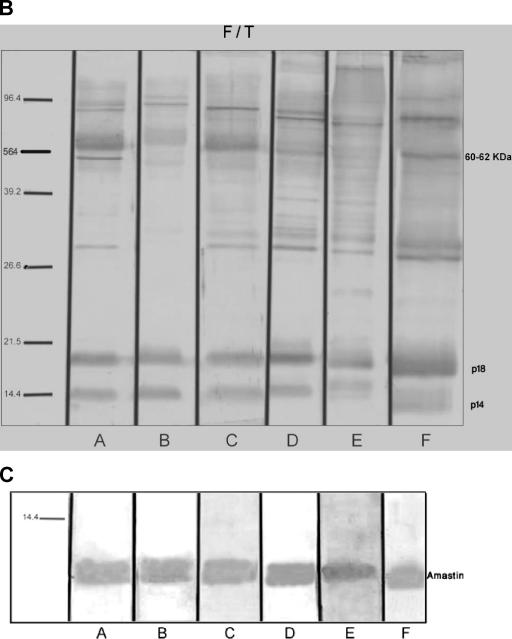
To evaluate the antigenicity of the amastin signature peptide, a mixture of 10 active sera isolated from patients with VL was analyzed by ELISA using a highly pure synthetic peptide harboring the amastin signature sequence and crude lysates (F/T antigen) from L. infantum promastigotes. A titration curve was determined, and the optimal concentrations were found to be 10 μg/ml for F/T antigens and 20 μg/ml for the amastin signature peptide. A strong reactivity of pooled sera from six patients with VL was observed toward promastigote crude antigen preparation, with no significant differences between different dilutions and between the three groups of active, asymptomatic, and recovered VL (Fig. 2A). In contrast, the sera from active patients with VL demonstrate a strong antibody response against the amastin signature peptide, which is significantly different from that observed with asymptomatic and recovered VL groups (Fig. 2A). The reactivity against the crude antigen preparation from L. infantum promastigotes (F/T antigen) and the amastin signature was also confirmed by Western blotting using sera from six patients with active VL (A to F) (Fig. 2B and C). As we have previously reported, there are several antigens in the F/T lysate that were strongly revealed by sera from active VL cases (20). These bands are mainly p14 and p18 and proteins with molecular masses of 60 to 62 kDa. As shown in Fig. 2C, all sera were also reactive with the amastin signature peptide (the upper part of the sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel does not contain any band).
FIG.2.
Detection and concentration of amastin-binding antibodies in sera from patients with active VL. (A) Microtiter plates were coated with either 10 or 20 μg of L. infantum lysates or the amastin signature peptide/ml, respectively. Six sera of active VL cases were pooled, and serial dilutions were made. Anti-human peroxidase-conjugated antibody at a 1:1,000 dilution was used. The results are given as the mean optical density (O.D.) ± the SD at the indicated dilutions. (B and C) Western blotting analysis was performed by using 2 μg of amastin signature peptide and 1 μg of F/T lysates of L. infantum/lane and six sera samples (A to F) diluted to 1:100. Low-molecular-mass markers are indicated in kilodaltons on the left.
FIG. 2—
Continued.
The amastin-reactive antibodies are present at higher titers in sera collected from patients with active VL and are potentially relevant immune signatures for detecting VL.
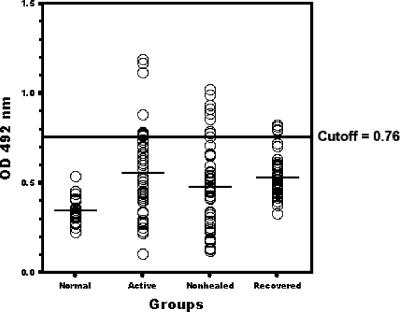
In order to determine whether the amastin signature synthetic peptide could be considered a valuable tool for the serodiagnosis of CL and/or VL, we analyzed the reactivity of 90 CL and 40 VL sera against this peptide. According to the clinical features of each donor, sera were separated into three groups for both CL and VL patients. For the CL patients, the first group was composed of sera from 30 individuals with recent active lesion without any treatment (all LST negative). The second group consisted of nonhealed cases and was composed of sera from 30 individuals who had had the lesion for more than 3 years (90% LST negative). The third group included 30 individuals who had recovered completely from disease, and all had positive LSTs. We used 10 normal sera that have been collected from individuals who live where the disease is not endemic and the LST was negative. Figure 3 shows the mean reactivity values for each group of CL sera against the amastin signature peptide. The CL active sera reacted with a mean absorbance value of 0.55 (SD = 0.24), nonhealed sera reacted with a mean absorbance value of 0.49 (SD = 0.24), and recovered sera reacted with a mean absorbance value of 0.52 (SD = 0.12). We found that 20% of sera from active CL patients, 8% of sera from nonhealed individuals, and 16% of recovered sera were higher than the cutoff value (mean of normal serum reactivity + 5 SD), which is 0.76. The difference between all three CL groups and normal individuals is highly significant (P < 0.05). In contrast, the serum reactivities between these three groups are not significant (P > 0.05). Therefore, in CL cases, it is not possible to differentiate between disease stages using the amastin signature peptide. The serum reactivity toward the L. major F/T antigen preparation in all three groups is significantly higher than in normal cases, and it is also higher than the response against the amastin signature peptide (Fig. 3). Thus, in the case of CL patients, we were not able to differentiate between the different stages of the disease using either the crude antigen preparation or the amastin peptide.
FIG. 3.
Reactivity of sera from patients with CL and healthy controls with amastin signature peptide. An ELISA was performed with sera diluted 1:50 from all patients at different stages. The horizontal line represents cutoff values that were calculated by adding 5 SD values to the mean optical density (OD) of normal sera.
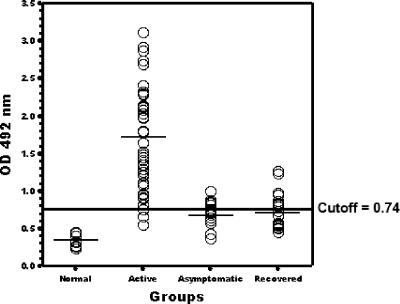
In the case of individuals with VL, the first group consisted of 21 clinically confirmed active cases prior to any treatment (all LST negative). The second group consisted of 10 individuals with no symptoms of VL but with a positive LST, and the third group was composed of 10 individuals who had recovered from VL by treatment and had positive LSTs. In Fig. 4, the specific total IgG reactivities against the amastin signature peptide are shown. Clearly, the active VL sera reacted with a mean absorbance value of 1.71, much higher than the cutoff value at 0.74. Both asymptomatic and recovered VL sera showed much lower mean absorbance values that are closer to the cutoff value. Of 21 sera collected from patients with active VL, 95.2% reacted with the amastin signature synthetic peptide (mean absorbance ± SD = 1.71 ± 0.63), whereas 100% were reactive with F/T antigen of L. infantum (mean absorbance ± SD = 2.9 ± 0.15) (Table 1) . The difference between mean absorbance values of the active VL group and normal cases is highly significant (P < 0.05). The mean absorbance values against the amastin signature peptide for the asymptomatic and recovered individuals are at 0.71 (SD = 0.24) and 0.74 (SD = 0.56), respectively, and there is no significant difference between these two groups (asymptomatic and recovered cases). In contrast, there is a significant difference between active VL and these two groups. Similar results were obtained with the L. infantum amastin signature peptide (data not shown). Overall, these data suggest that the amastin signature peptide represents a potentially relevant immune signature for detecting active VL, and it could distinguish between active and other stages of visceral leishmaniasis.
FIG. 4.
Reactivity of sera (total IgG) from patients with VL and healthy controls with amastin signature peptide. An ELISA was performed with sera diluted 1:50 from all patients at different stages. The horizontal line represents cutoff values that were calculated by adding 5 SD values to the mean optical density (OD) of normal sera.
TABLE 1.
Percentage of serum reactivities toward amastin signature peptide in normal and VL cases
| VL status (no. of cases) | F/T (poly Ab; IgM, IgG, and IgA)
|
Amastin signature (poly Ab; IgM, IgG, and IgA)
|
||
|---|---|---|---|---|
| Mean absorbance ± SD | % Reactivity | Mean absorbance ± SD | % Reactivity | |
| Active (21) | 2.99 ± 0.15 | 100.0 | 1.71 ± 0.69 | 95.2 |
| Asymptomatic (10) | 2.18 ± 0.81 | 90.0 | 0.69 ± 0.15 | 25.0 |
| Recovered (10) | 2.15 ± 0.77 | 90.0 | 0.73 ± 0.23 | 30.0 |
Profile of total IgG and IgG subclasses against the amastin signature peptide in VL patients.
In the present study, we attempted to define whether the total IgG and/or IgG subclasses against the amastin signature peptide were also able to differentiate between disease stages of VL. We therefore tested the IgG subclasses (IgG1, IgG2, IgG3, and IgG4) against the amastin signature in different VL groups. Our data showed no significant difference (one-way analysis of variance) in serum reactivities between the different VL disease stages toward each IgG subclass (Table 2). In contrast, significant differences were observed between total IgG in different VL stages. The patients with active stage showed significantly higher reactivity toward amastin signature peptide than asymptomatic and recovered VL individuals. It has been shown that classes and subclasses of antibody response are sometimes instrumental because each isotype has a distinct biological function (10). Hence, it is important to determine the level of each subclass in response to the amastin signature peptide. It is important to note that the level of IgG3 is significantly higher than for the other subclasses (IgG3 ≫ IgG2 > IgG1 > IgG4) in all three VL groups (active, asymptomatic, and recovered cases). The mean absorbance value of each IgG subclass in all three VL groups is significantly higher than for normal cases (P < 0.05).
TABLE 2.
Total IgG and IgG subclasses against amastin signature peptide in the sera isolated from VL patients and normal healthy individuals
| Subject type (no. of cases) | Mean absorbance ± SD
|
||||
|---|---|---|---|---|---|
| Total IgG | IgG1 | IgG2 | IgG3 | IgG4 | |
| Normal (10) | 0.22 ± 0.07 | 0.12 ± 0.018 | 0.22 ± 0.15 | 0.28 ± 0.04 | 0.23 ± 0.03 |
| Active (21) | 1.71 ± 0.63 | 0.61 ± 0.24 | 0.69 ± 0.21 | 1.31 ± 0.52 | 0.63 ± 0.23 |
| Asymptomatic (10) | 0.71 ± 0.24 | 0.45 ± 0.14 | 0.15 ± 0.13 | 1.54 ± 0.29 | 0.46 ± 0.13 |
| Recovered (10) | 0.74 ± 0.56 | 0.56 ± 0.23 | 0.69 ± 0.09 | 1.39 ± 0.23 | 0.48 ± 0.07 |
DISCUSSION
The diagnosis of VL is still based on parasite detection in aspirates from lymph node, bone marrow, and/or spleen. This parasitological method is not sensitive and involves an invasive procedure. Some VL patients are very anemic, have coagulation disorders, and are not fit for these invasive aspirations. In addition, the sensitivity of the parasitological method on aspirates of bone marrow and lymph node is also much lower, and up to 50% of cases of visceral leishmaniasis are expected to remain untreated, if the treatment is conditional on diagnosis confirmation (4). Among the various serological tests that have been described, the complement fixation test, the indirect immunofluorescence test, ELISA, and the direct agglutination test are the most frequently used. Each of these assays has its advantages and disadvantages. The antigen used in these assays is usually derived from Leishmania promastigotes cultured in vitro (8). In recent years, the direct agglutination test has been the preferred technique in many studies because the test is easier to use under field conditions and does not require any equipment; variation in antigen quantity among batches has, however, been a substantial limitation to its use (6). The rapid development of molecular biology techniques in the last decade has opened the way for the use of highly purified recombinant specific antigens. Synthetic peptides have proven to be valuable tools in the diagnosis of a variety of diseases (16).
In the present study, we used a highly pure 52-aa synthetic peptide corresponding to the first extracellular domain of Leishmania amastin surface proteins. This peptide contains a highly conserved sequence of 11 aa, which is present in all of the Leishmania and Trypanosoma amastin homologs and represents the amastin signature sequence (21). This well-defined domain was not found in any other protein in the databases, which suggests that the family of amastin surface proteins is probably unique to trypanosomatid protozoa. Since the expression of amastins is specific to the amastigote stage and this stage is associated with pathology in humans, we were interested in examining the antibody response against the highly pure amastin signature peptide. We showed that the amastin antibody response was specific for the active stage of VL patients. Using serial dilutions in the ELISA, we showed that the seroreactivity toward amastin peptide is similar to that obtained with the crude promastigote lysates (F/T antigen). By comparing different stages of VL, we showed that the amastin-reactive antibodies are present at high titers in 20 of 21 sera collected from patients with active VL (95% reactivity) and thus could be used as a stage-specific disease marker. This is an important finding since there are not too many antigens which could show stage specificity for VL. During the last few years, many purified and recombinant antigens, such as soluble leishmanial antigen (13), gp63 (25), H2A (26), KMP11 (18), rPSA (3), rCPB (20), rK39 (2), and A2 (5), have been used in serological tests. It has been reported that, during the acute phase of the VL disease, the host may produce specific antibodies, including the A2 and K39 antigens (15). Therefore, amastin is another antigen that acts similarly, and it would be possible to make a diagnostic kit consisting of these three antigens. Although the use of peptide or recombinant protein mixtures requires a rigorous standardization and close monitoring of the solid phase during the antigen adsorption step in order to warrant reproducible performance, the fusion of different antigenic peptides into a single stable molecule represents an interesting alternative that permits a uniform adsorption without the loss of the individual peptides.
In the present study, we also assessed the level of IgG subclasses toward amastin signature peptide and showed that IgG3 has the highest absorbance in all three stages. Although in our study there are no significant differences between the IgG3 level at different stages of VL patients, there are two other studies from Sudan and Brazil in which the researchers monitored each patient before and after treatment (7, 19). Elassad et al. determined IgG subclasses longitudinally in 28 VL patients before treatment and after 1 month of treatment with Pentostam. Those authors noted a significant decrease in antibody levels for subclasses IgG1 and IgG3 after treatment. This may be due to immune regulation at the helper cell level, and treatment might have lowered the parasite load and allowed the immune response to switch from a Th2 to Th1 cytokine response (7). In a study from Brazil, Pedras et al. showed that IgG subclass antibody detection constitutes a valuable alternative to increase the efficiency of the serological diagnosis of mucosal leishmaniasis. Those authors found that the anti-Leishmania braziliensis IgG3 is the first antibody that decreases in serum 90 days after treatment (19). IgG3 is one of the key components of FcγR-mediated effector response (antibody-dependent cell-mediated cytotoxicity, immune phagocytosis) and can neutralize pathogens at their entry into the body (17). FcγRs are largely expressed by many cells, such as neutrophils, monocytes, macrophages, and NK cells (11). It has been shown that in malaria cases, IgG3, which represents a small percentage of total IgGs and has a significantly shorter half-life than other IgG subclasses, can be more efficient than IgG1 in clearing malaria infections, and it is more protective (9). Furthermore, it has recently been shown that IgG3 is more potent than other subclasses in neutralizing human immunodeficiency virus type 1 (24). Therefore, it is highly crucial to have the most appropriate secondary antibody response in infected individuals, since this type of response has the best chance of clearing the infection and/or controlling disease (10). This could be fundamental for vaccine strategies, and for this reason we consider amastin to be a good vaccine candidate against leishmaniasis, as has recently been suggested by the work of Stober et al. with mice (27).
In CL cases, the percentage of seroreactivity against the amastin signature region in active stage is only 20%, and it is not as strong as for VL active individuals. This may be due to pathophysiological differences that are present in these two different forms of leishmaniasis (15). The level of amastin antibody production is much higher in VL than in cutaneous forms of the disease. This could also be explained by differences in the expression of these amastin homologs between L. major and L. infantum. Our recent data suggest that there are important differences, at least at the mRNA expression levels, between several amastin homologs of L. major and L. infantum (21; unpublished data).
Overall, we show here for the first time that the amastin conserved signature sequence at the N-terminal extracellular domain is highly immunogenic in both CL and VL. Due to the unique feature of amastins, which are present only in trypanosomatids, the possibilities for a cross-reactivity with other common diseases, such as malaria and tuberculosis, in areas where Leishmania is endemic are almost nonexistent. The amastin signature sequence is an interesting target for further investigation of VL caused by L. donovani, especially the rare cases of PKDL. There is a recent report using microarrays showing that, among PKDL cases, there is specific upregulation of class III amastins (12). At present, we are focusing on L. donovani-infected individuals to further investigate the potential of amastin peptides as sensitive and stage-specific diagnostic tools against this fatal disease.
Acknowledgments
We thank Zohereh Babaloo from the Medical School of the University of Tabriz and Seyed Ali Akbar Shamsian from the Department of Parasitology of the Medical School of Mashhad University for providing some of the VL and CL sera.
A.R. is the recipient of a Fonds de la Recherche en Santé du Québec doctoral award. B.P. is a Burroughs Wellcome Fund New Investigator in Molecular Parasitology and a member of a Canadian Institutes of Health research group (GR-14500) on host-pathogen interactions.
REFERENCES
- 1.Ashford, R. W. 2000. The leishmaniasis as emerging and reemerging zoonoses. Int. J. Parasitol. 30:1269-1281. [DOI] [PubMed] [Google Scholar]
- 2.Badaro, R., D. Benson, M. C. Eulalio, M. Freire, S. Cunha, E. M. Netto, D. Pedral-Sampaio, C. Madureira, J. M. Burns, R. L. Houghton, J. R. David, and S. G. Reed. 1996. rK39: a cloned antigen of Leishmania chagasi that predicts active visceral leishmaniasis. J. Infect. Dis. 173:758-761. [DOI] [PubMed] [Google Scholar]
- 3.Boceta, C., C. Alonso, and J. Ruiz. 2000. Leucine-rich repeats are the main epitopes in Leishmania infantum PSA during canine and human visceral leishmaniasis. Parasite Immunol. 22:55-62. [DOI] [PubMed] [Google Scholar]
- 4.Boelaret, M. 1999. Cost-effectiveness of competing diagnostic-therapeutic strategies for visceral leishmaniasis. Bull. W. H. O. 177:667-674. [PMC free article] [PubMed] [Google Scholar]
- 5.Carvalho, F. A., H. Charest, C. A. Tavares, G. Matlashewski, E. P. Valente, A. Rabello, R. T. Gazzinelli, and A. P. Fernandes. 2002. Diagnosis of American visceral leishmaniasis in humans and dogs using the recombinant Leishmania donovani A2 antigen. Diagn. Microbiol. Infect. Dis. 43:289-295. [DOI] [PubMed] [Google Scholar]
- 6.Davies, C. R., P. Kaye, S. L. Croft, and S. Sundar. 2003. Leishmaniasis: new approaches to disease control. BMJ 326:377-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elassad, A. M. S., S. A. Younis, A. Siddig, E. Grayson, and H. W. Ghalib. 1994. The significance of blood levels of IgM, IgA, IgG, and IgG subclasses in Sudanese visceral leishmaniasis patients. Clin. Exp. Immunol. 95:294-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fargeas, C., M. Hommel, R. Maingon, C. Dourado, M. Monsigny, and R. Mayer. 1996. Synthetic peptide-based enzyme-linked immunosorbent assay for serodiagnosis of visceral leishmaniasis. J. Clin. Microbiol. 34:241-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garraud, O., S. Mahanty, and R. Parraut. 2003. Malaria-specific antibody subclasses in immune individuals: a key source of information for vaccine design. Trends Immunol. 24:30-35. [DOI] [PubMed] [Google Scholar]
- 10.Garraud, O., R. Perraut, G. Riveau, and T. B. Nutman. 2003. Class and subclass selection in parasite-specific antibody responses. Trends Parasitol. 19:300-304. [DOI] [PubMed] [Google Scholar]
- 11.Gessner, J. E., H. Heiken, A. Tamm, and R. E. Schmidt. 1998. The IgG Fc receptor family. Ann. Hematol. 76:231-248. [DOI] [PubMed] [Google Scholar]
- 12.Guerin, P. J., P. Olloaro, S. Sundar, M. Boelaert, S. L. Croft, P. Desjeux, M. K. Wasunna, and A. D. Bryceson. 2002. Visceral leishmaniasis: current statues of control, diagnosis, and treatment, and a proposed research and development agenda. Lancet Infect. Dis. 2:494-501. [DOI] [PubMed] [Google Scholar]
- 13.Ho, M., J. Leeuwenburg, G. Mbugua, A. Wamachi, and A. Voller. 1983. An enzyme-linked immunosorbent assay for field diagnosis of visceral leishmaniasis. Am. J. Trop. Med. Hyg. 32:943-946. [DOI] [PubMed] [Google Scholar]
- 14.Killick-Kendrick, R. 1990. The life cycle of Leishmania in the sandfly with special reference to the form infective to the vertebrate host. Ann. Parasitol. Hum. Comp. 65(Suppl. 11):37-42. [DOI] [PubMed] [Google Scholar]
- 15.Kubar, J., and K. Fragaki. 2005. Recombinant DNA-derived Leishmania proteins: from the laboratory to the field. Lancet Infect. Dis. 5:107-114. [DOI] [PubMed] [Google Scholar]
- 16.Mabey, D., R. W. Peeling, A. Ustiasowski, and M. D. Perkins. 2004. Diagnostics for the developing world. Nat. Rev. Microbiol. 2:231-240. [DOI] [PubMed] [Google Scholar]
- 17.McLean, G. R., M. Torres, N. Elguezabal, A. Nakouzi, and A. Casadevall. 2002. Isotype can affect the fine specificity of an antibody for a polysaccharide antigen. J. Immunol. 169:1379-1386. [DOI] [PubMed] [Google Scholar]
- 18.Passos, S., L. P. Carvalho, G. Orge, S. M. Jeronimo, G. Bezerra, M. Soto, C. Alonso, and E. M. Carvalho. 2005. Recombinant Leishmania antigens for serodiagnosis of visceral leishmaniasis. Clin. Diagn. Lab. Immunol. 12:1164-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pedras, M. J., M. Orsini, M. Castro, V. M. A. Passos, and A. Rabello. 2003. Antibody subclass profile against Leishmania braziliensis and Leishmania amazonensis in the diagnosis and follow-up of mucosal leishmaniasis. Diagn. Microbiol. Infect. Dis. 47:477-485. [DOI] [PubMed] [Google Scholar]
- 20.Rafati, S., A. Nakhaee, T. Taheri, A. Ghashghaii, A. H. Salmanian, M. Jimenez, M. Mohebali, S. Masina, and N. Fazel. 2003. Expression of cysteine proteinase type I and II of Leishmania infantum and their recognition by sera during canine and human visceral leishmaniasis. Exp. Parasitol. 103:143-151. [DOI] [PubMed] [Google Scholar]
- 21.Rochette, A., F. McNicoll, J. Girard, M. Breton, E. Leblanc, M. G. Bergeron, and B. Papadopoulou. 2005. Characterization and developmental gene regulation of a large gene family encoding amastin surface proteins in Leishmania spp. Mol. Biochem. Parasitol. 140:205-220. [DOI] [PubMed] [Google Scholar]
- 22.Roggero, M. A., C. Servis, and G. Corradin. 1997. A simple and rapid procedure for the purification of synthetic polypeptides by a combination of affinity chromatography and methionine chemistry. FEBS Lett. 26:285-288. [DOI] [PubMed] [Google Scholar]
- 23.Salotra, P., R. C. Duncan, R. Singh, B. V. Subba Raju, G. Sreenvas, and H. L. Nakhasi. 2006. Upregulation of surface proteins in L. donovani isolated from patients of post kala-azar dermal leishmaniasis. Microbes Infect. 8:637-644. [DOI] [PubMed] [Google Scholar]
- 24.Scharf, O., H. Golding, L. R. King, N. Eller, D. Frazier, B. Golding, and D. E. Scott. 2001. Immunoglobulin G3 from polyclonal human immunodeficiency virus (HIV) immune globulin is more potent than other subclasses in neutralizing HIV type 1. J. Virol. 75:6558-6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shreffler, W. G., J. M. Burns, Jr., R. Badaro, H. W. Ghalib, L. L. Button, W. R. McMaster, and S. G. Reed. 1993. Antibody responses of visceral leishmaniasis patients to gp63, a major surface glycoprotein of Leishmania species. J. Infect. Dis. 167:426-430. [DOI] [PubMed] [Google Scholar]
- 26.Soto, M., J. M. Requena, L. Quijada, and C. Alonso. 1998. Multicomponent chimeric antigen for serodiagnosis of canine visceral leishmaniasis. J. Clin. Microbiol. 36:58-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stober, C. B., U. G. Lange, M. T. Roberts, B. Gilmartin, R. Francis, R. Almedia, S. C. Peacock, McCanns, and J. M. Blackwell. 2006. From genome to vaccines for leishmaniasis: screening 100 novel vaccine candidates against murine Leishmania major infection. Vaccine 24:2602-2616. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. 1999. Leishmaniasis: fourteenth program report, p. 1-6. UNDP/World Bank/Special Program for Research and Training in Tropical Diseases. World Health Organization, Geneva, Switzerland.
- 29.Wu, Y., E. Youssef, L. Fakhry, D. Sereno, T. Samira, and B. Papadopoulou. 2000. A new developmentally regulated gene family in Leishmania amastigotes encoding a homolog of amastin surface proteins. Mol. Biochem. Parasitol. 110:345-357. [DOI] [PubMed] [Google Scholar]
- 30.Zijlstra, E. E., M. S. Ali, A. M. el-Hassan, I. A. el-Toum, M. Satti, H. W. Ghalib, and P. A. Kager. 1992. Kala-azar: a comparative study of parasitological methods and the direct agglutination test in diagnosis. Trans. R. Soc. Trop. Med. Hyg. 86:505-507. [DOI] [PubMed] [Google Scholar]