Abstract
Pneumococcal neuraminidase, NanA, is a pneumococcal vaccine candidate. Prior culture-confirmed pneumococcal contacts were shown to induce serum anti-NanA antibodies during the first 2 years of life. The antibody concentrations at neither 12 nor 18 months were significantly associated with the risk of subsequent pneumococcal carriage or acute otitis media.
Streptococcus pneumoniae (pneumococcus) causes significant morbidity and mortality worldwide. The two types of pneumococcal vaccines currently available are the 23-valent polysaccharide vaccine and the heptavalent conjugate vaccine. Although generally safe and efficacious, these vaccine formulations possess certain shortcomings, and the search for alternative vaccines and vaccine components continues. One vaccine candidate is NanA, a major pneumococcal neuraminidase produced by all clinical isolates of pneumococcus. NanA is a cell surface-associated enzyme with sialidase activity (5, 17). By cleaving the terminal sialic acid residues from a wide variety of glycoproteins, glycolipids, and oligosaccharides, NanA has the potential to cause great damage to the host (20, 21, 24).
The precise role of NanA in pathogenesis has not been established, but it is suggested to play a role in pneumococcal carriage and acute otitis media (AOM). NanA has been hypothesized to enhance pneumococcal colonization by exposing new receptors for adherence (1, 9, 10, 24), damaging host defense proteins (7), decreasing the viscosity of mucus (20), and modifying the cell surfaces of competing bacteria within the nasopharynx (21). A recent study demonstrated the importance of NanA in the development of both upper and lower respiratory tract infections and sepsis in mice (14). An increase in the expression and activity of NanA has been demonstrated for transparent pneumococcal colony variants predominating during pneumococcal carriage (3, 7, 27). Treatment of chinchilla tracheas with neuraminidase in vitro increases pneumococcal adherence (25), and the disruption of the nanA gene significantly reduces the extent and duration of nasopharyngeal colonization in vivo as well as the survival and persistence of pneumococci in the middle ear (23). Immunization with purified NanA confers a limited degree of protection in mice against intranasal challenge with virulent pneumococci (11). In a chinchilla model, immunization with NanA resulted in a significant reduction in nasopharyngeal colonization as well as in the incidence of otitis media with effusion (12). The exact mechanism of vaccine-induced anti-NanA immunity is not known.
The Finnish Otitis Media (FinOM) Cohort Study (6, 22) gave us the unique possibility of investigating the development of serum anti-NanA antibodies in children during their first 2 years of life in relation to prior culture-confirmed pneumococcal contacts. In addition, we determined whether anti-NanA concentrations at 12 and 18 months were associated with the risk of subsequent pneumococcal carriage or AOM. In short, 329 FinOM Cohort Study children were followed from 2 to 24 months for pneumococcal carriage and AOM by taking bacterial cultures of nasopharyngeal and middle ear fluid samples during scheduled and unscheduled (sick patient) visits to the study clinic. Serum samples for antibody measurements were collected at 6, 12, 18, and 24 months.
We measured the concentrations of anti-NanA immunoglobulin G (IgG) antibodies in serum samples of 50 randomly selected children, from whom samples were taken at 6, 12, 18, and 24 months, and 45 adults by enzyme immunoassay as described previously (19) with minor modifications. Microtiter plates from Greiner (Frickenhausen, Germany) and a dilution buffer of 10% fetal bovine serum (Gibco, BRL, Karlsruhe, Germany) in phosphate-buffered saline were used. To evaluate the association of anti-NanA with subsequent pneumococcal carriage and AOM, we measured the anti-NanA antibodies in serum samples collected at the ages of 12 and 18 months from the total cohort. The samples were available from 287 and 260 children, respectively. The models for calculations of risks have been described earlier by Rapola et al. (18).
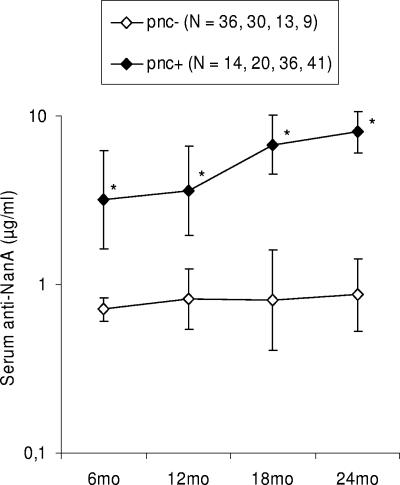
All sera from the 50 children and 45 adults contained a detectable concentration (>0.49 μg/ml) of anti-NanA IgG antibodies. The geometric mean concentrations (GMC) of anti-NanA antibodies increased with age (Table 1). The GMC of the anti-NanA concentration was significantly higher in adults than in children at 6 and 12 months (P < 0.001), while the GMC of anti-NanA at 24 months was comparable to that in adults. The increase in anti-NanA concentration by age was strongly associated with prior culture-confirmed pneumococcal contacts (Fig. 1). Those children without confirmed pneumococcal contacts (pnc− children) had no age-dependent increase in the GMC of anti-NanA, while those with prior pneumococcal contacts (pnc+ children) had significantly higher GMCs at all ages (P < 0.001).
TABLE 1.
GMC with 95% CIs of serum anti-NanA IgG in children at the ages of 6, 12, 18, and 24 months and in adults
| Age | No. of subjects | Anti-NanA GMC (95% CI) |
|---|---|---|
| 6 mo | 50 | 1.08 (0.817-1.43)a |
| 12 mo | 50 | 1.486 (1.003-2.201)b |
| 18 mo | 49 | 3.858 (2.500-5.954) |
| 24 mo | 50 | 5.385 (3.814-7.605) |
| Adults | 45 | 5.581 (4.648-6.703) |
There was a significant difference in the GMC between infants and adults (P < 0.001, Student's t test).
There was a significant difference in the GMC between infants and adults (P < 0.001, Student's t test).
FIG. 1.
Development of serum anti-NanA IgG antibodies during the first 24 months of life in pnc− and pnc+ children. Numbers in parentheses indicate the numbers of samples analyzed at each age point. During the follow-up, the proportion of pnc+ children increased, while the proportion of pnc− children decreased. Geometric mean concentrations with 95% confidence intervals are given. *, P < 0.001 for pnc+ versus pnc− children (Student's t test).
We used a logistic regression model to evaluate the log-transformed anti-NanA concentrations at 12 (n = 287) and 18 (n = 260) months of age as risk factors for subsequent asymptomatic pneumococcal carriage (nasopharyngeal swabs taken at the ages of 18 and 24 months). No indication of a statistically significant association of the anti-NanA concentration and pneumococcal carriage 6 months later was found: the odds ratios for an increase of 1 log unit in anti-NanA concentration at 12 and 18 months were 1.15 (95% confidence interval [CI], 0.93 to 1.42) and 1.24 (95% CI, 0.98 to 1.57), respectively.
We used an extended version of the Cox proportional-hazard model to estimate the risk of subsequent pneumococcal AOM during the age intervals from 12 to 18 and from 18 to 24 months in relation to log-transformed anti-NanA concentrations at the beginning of the age interval. At 12 months, the higher anti-NanA concentrations were not associated with the risk of subsequent pneumococcal AOM (relative risk, 1.01; 95% CI, 0.81 to 1.28). At 18 months, higher anti-NanA concentrations tended to predict a slightly reduced risk of pneumococcal AOM, although the reduction was not statistically significant (relative risk, 0.85; 95% CI, 0.64 to 1.12).
All serum samples tested were positive for anti-NanA already at 6 months of age, which may indicate the cross-reactivity of NanA with the neuraminidases produced by other streptococci (8). The concentrations of serum anti-NanA were not significantly associated with subsequent pneumococcal carriage or AOM, which is well in line with our previous findings on the antibodies against several pneumococcal virulence proteins: pneumococcal surface adhesin A (PsaA) (18), pneumococcal BVH proteins (4), and putative pneumococcal proteinase maturation protein A (PpmA) (2). The concentrations of serum antibodies against NanA and other pneumococcal proteins may be too low for protection against pneumococcal carriage and AOM, while higher concentrations could be reached by vaccination. The effects of immunity to NanA on colonization may also be partially masked if immunity to other pneumococcal antigens has an effect on the risk of pneumococcal carriage and AOM. Similarly with the data from the human colonization study (15), we have previously shown that the presence of salivary antibodies to pneumococcal surface protein A (PspA) is associated with a decreased risk of subsequent pneumococcal AOM at the age of 18 months (B. Simell, M. Melin, T. Jaakkola, K. Jousimies, M. Lahdenhari, T. M. Kilpi, D. Briles, S. Hollingshead, and H. Käyhty, Abstra. 5th Int. Symp. Pneumococci Pneumococcal Dis. abstr. P012.02, 2006). On the other hand, recent studies of animal models suggest that CD4+ T cells rather than antibodies function as an effector mechanism at least against pneumococcal colonization (13, 16, 26).
We conclude that pneumococcal contacts induce the development of serum anti-NanA IgG antibodies in early childhood. A role for human anti-NanA remains to be determined.
Acknowledgments
We thank Esa Ruokokoski and Eeva Koskenniemi for experienced data management. Elja Herva and Tarja Kaijalainen are acknowledged for the bacteriologic work. We also thank the study personnel and all parents and children who volunteered to participate in the FinOM Cohort Study.
The FinOM Studies were supported by Merck & Co. Inc., Sanofi Pasteur, Wyeth-Lederle Vaccines and Pediatrics, and the World Health Organization (GPV/VRD contract V23/181/116).
Footnotes
Published ahead of print on 23 August 2006.
REFERENCES
- 1.Andersson, B., J. Dahmen, T. Frejd, H. Leffler, G. Magnusson, G. Noori, and C. S. Eden. 1983. Identification of an active disaccharide unit of a glycoconjugate receptor for pneumococci attaching to human pharyngeal epithelial cells. J. Exp. Med. 158:559-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bogaert, D., E. Holmlund, M. Lahdenkari, R. de Groot, T. Kilpi, P. W. Hermans, and H. Kayhty. 2006. Development of antibodies against the putative proteinase maturation protein A in relation to pneumococcal carriage and otitis media. FEMS Immunol. Med. Microbiol. 46:166-168. [DOI] [PubMed] [Google Scholar]
- 3.Briles, D. E., L. Novak, M. Hotomi, F. W. van Ginkel, and J. King. 2005. Nasal colonization with Streptococcus pneumoniae includes subpopulations of surface and invasive pneumococci. Infect. Immun. 73:6945-6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holmlund, E., T. Jaakkola, L. Saarinen, M. Lahdenkarin, J. Hamel, B. Brodeur, T. M. Kilpin and H. Käyhty. 2004. Abstr. 4th Int. Symp. on Pneumococci Pneumococcal Dis., abstr. IMM-26.
- 5.Kelly, R. T., S. Farmer, and D. Greiff. 1967. Neuraminidase activities of clinical isolates of Diplococcus pneumoniae. J. Bacteriol. 94:272-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kilpi, T., E. Herva, T. Kaijalainen, R. Syrjanen, and A. K. Takala. 2001. Bacteriology of acute otitis media in a cohort of Finnish children followed for the first two years of life. Pediatr. Infect. Dis. J. 20:654-662. [DOI] [PubMed] [Google Scholar]
- 7.King, S. J., K. R. Hippe, J. M. Gould, D. Bae, S. Peterson, R. T. Cline, C. Fasching, E. N. Janoff, and J. N. Weiser. 2004. Phase variable desialylation of host proteins that bind to Streptococcus pneumoniae in vivo and protect the airway. Mol. Microbiol. 54:159-171. [DOI] [PubMed] [Google Scholar]
- 8.King, S. J., A. M. Whatmore, and C. G. Dowson. 2005. NanA, a neuraminidase from Streptococcus pneumoniae, shows high levels of sequence diversity, at least in part through recombination with Streptococcus oralis. J. Bacteriol. 187:5376-5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linder, T. E., R. L. Daniels, D. J. Lim, and T. F. DeMaria. 1994. Effect of intranasal inoculation of Streptococcus pneumoniae on the structure of the surface carbohydrates of the chinchilla eustachian tube and middle ear mucosa. Microb. Pathog. 16:435-441. [DOI] [PubMed] [Google Scholar]
- 10.Linder, T. E., D. J. Lim, and T. F. DeMaria. 1992. Changes in the structure of the cell surface carbohydrates of the chinchilla tubotympanum following Streptococcus pneumoniae-induced otitis media. Microb. Pathog. 13:293-303. [DOI] [PubMed] [Google Scholar]
- 11.Lock, R. A., J. C. Paton, and D. Hansman. 1988. Comparative efficacy of pneumococcal neuraminidase and pneumolysin as immunogens protective against Streptococcus pneumoniae. Microb. Pathog. 5:461-467. [DOI] [PubMed] [Google Scholar]
- 12.Long, J. P., H. H. Tong, and T. F. DeMaria. 2004. Immunization with native or recombinant Streptococcus pneumoniae neuraminidase affords protection in the chinchilla otitis media model. Infect. Immun. 72:4309-4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malley, R., K. Trzcinski, A. Srivastava, C. M. Thompson, P. W. Anderson, and M. Lipsitch. 2005. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc. Natl. Acad. Sci. USA 102:4848-4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manco, S., F. Hernon, H. Yesilkaya, J. C. Paton, P. W. Andrew, and A. Kadioglu. 2006. Pneumococcal neuraminidases A and B both have essential roles during infection of the respiratory tract and sepsis. Infect. Immun. 74:4014-4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCool, T. L., T. R. Cate, G. Moy, and J. N. Weiser. 2002. The immune response to pneumococcal proteins during experimental human carriage. J. Exp. Med. 195:359-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCool, T. L., and J. N. Weiser. 2004. Limited role of antibody in clearance of Streptococcus pneumoniae in a murine model of colonization. Infect. Immun. 72:5807-5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Toole, R. D., L. Goode, and C. Howe. 1971. Neuraminidase activity in bacterial meningitis. J. Clin. Investig. 50:979-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rapola, S., V. Jantti, M. Eerola, P. H. Makela, H. Kayhty, and T. Kilpi. 2003. Anti-PsaA and the risk of pneumococcal AOM and carriage. Vaccine 21:3608-3613. [DOI] [PubMed] [Google Scholar]
- 19.Rapola, S., V. Jantti, R. Haikala, R. Syrjanen, G. M. Carlone, J. S. Sampson, D. E. Briles, J. C. Paton, A. K. Takala, T. M. Kilpi, and H. Kayhty. 2000. Natural development of antibodies to pneumococcal surface protein A, pneumococcal surface adhesin A, and pneumolysin in relation to pneumococcal carriage and acute otitis media. J. Infect. Dis. 182:1146-1152. [DOI] [PubMed] [Google Scholar]
- 20.Scanlon, K. L., W. F. Diven, and R. H. Glew. 1989. Purification and properties of Streptococcus pneumoniae neuraminidase. Enzyme 41:143-150. [DOI] [PubMed] [Google Scholar]
- 21.Shakhnovich, E. A., S. J. King, and J. N. Weiser. 2002. Neuraminidase expressed by Streptococcus pneumoniae desialylates the lipopolysaccharide of Neisseria meningitidis and Haemophilus influenzae: a paradigm for interbacterial competition among pathogens of the human respiratory tract. Infect. Immun. 70:7161-7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Syrjanen, R. K., T. M. Kilpi, T. H. Kaijalainen, E. E. Herva, and A. K. Takala. 2001. Nasopharyngeal carriage of Streptococcus pneumoniae in Finnish children younger than 2 years old. J. Infect. Dis. 184:451-459. [DOI] [PubMed] [Google Scholar]
- 23.Tong, H. H., L. E. Blue, M. A. James, and T. F. DeMaria. 2000. Evaluation of the virulence of a Streptococcus pneumoniae neuraminidase-deficient mutant in nasopharyngeal colonization and development of otitis media in the chinchilla model. Infect. Immun. 68:921-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tong, H. H., M. James, I. Grants, X. Liu, G. Shi, and T. F. DeMaria. 2001. Comparison of structural changes of cell surface carbohydrates in the eustachian tube epithelium of chinchillas infected with a Streptococcus pneumoniae neuraminidase-deficient mutant or its isogenic parent strain. Microb. Pathog. 31:309-317. [DOI] [PubMed] [Google Scholar]
- 25.Tong, H. H., M. A. McIver, L. M. Fisher, and T. F. DeMaria. 1999. Effect of lacto-N-neotetraose, asialoganglioside-GM1 and neuraminidase on adherence of otitis media-associated serotypes of Streptococcus pneumoniae to chinchilla tracheal epithelium. Microb. Pathog. 26:111-119. [DOI] [PubMed] [Google Scholar]
- 26.Trzcinski, K., C. Thompson, R. Malley, and M. Lipsitch. 2005. Antibodies to conserved pneumococcal antigens correlate with, but are not required for, protection against pneumococcal colonization induced by prior exposure in a mouse model. Infect. Immun. 73:7043-7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiser, J. N., D. Bae, H. Epino, S. B. Gordon, M. Kapoor, L. A. Zenewicz, and M. Shchepetov. 2001. Changes in availability of oxygen accentuate differences in capsular polysaccharide expression by phenotypic variants and clinical isolates of Streptococcus pneumoniae. Infect. Immun. 69:5430-5439. [DOI] [PMC free article] [PubMed] [Google Scholar]