Abstract
Sepsis is a considerable health problem and a burden on the health care system. Endotoxin, or lipopolysaccharide (LPS), present in the outer membrane of gram-negative bacteria, is responsible for more than 50% of the sepsis cases and is, therefore, a legitimate target for therapeutic approaches against sepsis. In this study, we selected and characterized a llama single-chain antibody fragment (VHH) directed to Neisseria meningitidis LPS. The VHH, designated VHH 5G, showed affinity to purified LPS as well as to LPS on the surfaces of the bacteria. Epitope mapping using a panel of N. meningitidis mutants revealed that VHH 5G recognizes an epitope in the inner core of LPS, and as expected, the VHH proved to have broad specificity for LPS from different bacteria. Furthermore, this VHH blocked binding of LPS to target cells of the immune system, resulting in the inhibition of LPS signaling in whole blood. Moreover, it was found to remove LPS efficiently from aqueous solutions, including serum. The selected anti-LPS VHH is a leading candidate for therapies against LPS-mediated sepsis.
Lipopolysaccharide (LPS) (or endotoxin), present exclusively in the outer leaflet of the outer membrane of gram-negative bacteria, is one of the major contributors to innate immunity (6). This nonspecific immune response is capable of destroying invading pathogens, but if overstimulated, it can also initiate toxic effects against the host. LPS contributes to toxicity when it is released into the blood circulation after a bacterial infection. This undesirable interaction between the host and the pathogen leads to the fatal syndrome known as sepsis (21). To exert its pathological effect at low concentrations, LPS interacts with high-affinity receptors present both on the surfaces of host cells and in plasma. Interaction of the LPS with these receptors results in an inflammatory response, occasionally with deleterious effects for the host.
The gram-negative bacterium Neisseria meningitidis is an important cause of meningitis and sepsis in children and young adults. Despite advances in supportive care, invasive meningococcal disease still forms a major health threat. There is an urgent requirement for the development of novel therapies against such disease, since an effective vaccine against serogroup B of N. meningitidis, which is responsible for most cases of meningitis and septicemia in industrialized countries, is currently not available. Moreover, development of an effective polysaccharide-based vaccine against serogroup B strains, in the same way as is done for the other serogroups of N. meningitidis (23), may prove unsuccessful, since the polysaccharides of the serogroup B capsule are also found on human neuronal-cell adhesion molecules and other surface-expressed molecules. These polysaccharides can be classified as autoantigens and may therefore cause a potential problem of autoimmunity when used as a vaccine (13, 14). Therefore, alternative approaches to fighting neisserial infections are sought. LPS is a major contributor to the health threat imposed by N. meningitidis, and therefore, therapeutic measures in the fight against the disease, and sepsis in general, are focused on this molecule.
Structures of diverse LPS molecules revealed that the lipid A moiety and the inner core region of these molecules are considerably conserved among the gram-negative bacteria. Antibodies raised against these conserved regions are predicted to provide protection against a broad range of gram-negative bacteria (4, 10). Twelve distinct immunotypes of meningococcal LPS (L1 to L12) can be defined by monoclonal antibody reactivity (24). This heterogeneity is caused by controlled gene expression of the genes involved in LPS biosynthesis and by phase variation of some of these genes (18). Genes involved in the biosynthesis of the inner core are less subjected to phase variation, which makes this structure relatively well conserved, except for some variations in the presence and the position of a phosphoethanolamine on one of the heptoses (HepII) in the backbone of the inner core (22).
Camelidae (camels and llamas) possess, in addition to the conventional immunoglobulins formed by the association between a heavy and a light chain, single-chain antibodies formed by a heavy chain only (15). These single-chain antibodies in general, and their antigen-binding domains (called VHHs) in particular, are interesting polypeptides with broad biotechnological applications (11, 12, 36). Using phage display, several VHHs that recognize antigens with diverse structure and characteristics have been selected in vitro (25). Furthermore, by proper design of the selection procedure, VHHs with specific properties, such as functioning in high detergent concentrations (11), inhibiting enzyme activities (20), or preventing bacteriophage infection (9), could be selected.
The aim of this study was to explore the possibilities of using anti-LPS VHHs as a therapy against sepsis. Such VHHs should have several properties: they should be insensitive to the variations present in the LPS molecules and should, therefore, recognize an epitope in the conserved inner core of the LPS molecule. Monoclonal antibodies directed against this region of the LPS molecule proved to be broadly cross-reactive, recognizing LPS from different phylogenetic strains (4, 7, 8, 10). Furthermore, the inner core of the LPS molecules was shown to be accessible in fully encapsulated intact N. meningitidis cells, even to the larger conventional antibodies (22). Additionally, successful anti-LPS VHHs should be able to compete with the high-affinity LPS-binding proteins present on different cells of the immune system and in plasma. In this paper, we describe the selection of such a VHH that shows high specificity to LPS molecules from different N. meningitidis serogroup B strains and additionally displays cross-reactivity to LPS from other gram-negative bacteria. Furthermore, we demonstrate the ability of this VHH to detoxify LPS in whole blood and to efficiently deplete LPS from solutions when immobilized to a solid support.
MATERIALS AND METHODS
Selection of anti-LPS VHHs.
A nonimmune VHH phage display library displaying a diversity of more than 109 different VHHs was generated from the peripheral blood lymphocytes of eight different young llamas and kindly provided by Unilever Research (Vlaardingen, The Netherlands). Phage display (17) was used to select phages that specifically bind to LPS as follows. Nunc Polysorb immunoplates were coated overnight at 4°C with various concentrations of LPS immunotype L3 from N. meningitidis strain H44/76 in phosphate-buffered saline (PBS). The wells were blocked with 2% bovine serum albumin (BSA) in PBS. Subsequently, phages were preincubated in 1% BSA, added to the wells, and incubated for 2 h at room temperature. After extensive washing with PBS containing 0.05% Tween 20 (PBST) and PBS, bound phages were eluted with 100 mM triethylamine by 15 min of incubation at room temperature. The eluted phages were directly neutralized by the addition of 1 M Tris-HCl, pH 7.5. Phages were rescued by infection of the Escherichia coli strain TG1 [supE hsdΔ5 thi Δ(lac-proAB) F′(traD36 proAB+ lacIq lacZΔM15)] and selected on agar plates containing ampicillin. For the production of phages, E. coli containing phagemids was superinfected with helper phage VCSM13 (Stratagene), and phage particles were produced overnight at 37°C. The selected phages were panned a second time on wells coated with LPS as described above, and phages from single colonies were tested for their binding specificities for LPS using an uncoated, empty plate as a negative control.
Purification of anti-LPS VHHs.
VHHs were produced in E. coli in the presence of 1 mM isopropyl-β-d-thiogalactopyranoside for 4 to 5 h at 37°C. Subsequently, VHHs were purified from the periplasmic fraction after spheroplasting of the cells (39). The periplasmic fractions were applied on BD Talon metal-affinity resin (BD Biosciences), and bound His-tagged VHHs were washed and eluted according to the manufacturer's protocol. The eluted anti-LPS VHHs were extensively dialyzed against PBS and stored at −20°C.
To obtain highly purified VHHs that do not show any priming activity in whole blood, His-tagged VHHs were produced in the yeast Saccharomyces cerevisiae and purified from the extracellular medium using metal affinity chromatography. VHHs, which were eluted with 500 mM imidazole in PBS, were dialyzed against 25 mM sodium acetate buffer, pH 5.0, and loaded on Unosphere Q (Bio-Rad). VHHs were found in the flowthrough, whereas many contaminating proteins remained bound to the column. The VHHs were concentrated by binding to Unosphere S (Bio-Rad) after the pH was calibrated to 3.8 using acetic acid. Bound VHHs were eluted with 500 mM NaCl in the same buffer. Finally the buffer was exchanged with PBS by dialysis.
LPS immunoprecipitation.
About 4 μg of the anti-LPS VHHs 7F, 5G, 7H, and 12H and of an irrelevant VHH (C) were bound to 25 μl of Talon beads (50% slurry) for 20 min at room temperature in 250 μl PBS. The beads were washed with PBS, and subsequently, 500 μl of the different LPS solutions (2 μg/ml) were added and incubated for 1 h at room temperature. The beads were washed three times with PBS, and ∼1/3 of the bound LPS was analyzed using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (15% separating gels) and silver staining (32). The amounts of LPS bound to the beads were deduced by comparison with 1/3 of the total amount of LPS used in the experiment (standard [std]). L3, L8, and galE LPS were isolated from the wild-type N. meningitidis strain H44/76 of L3 (16) and L8 (24) immunotypes and from the galE mutant strain (19), respectively. Bordetella pertussis LPS was a generous gift from J. Geurtsen. B4:O111 LPS from E. coli and Re LPS from Salmonella enterica serovar Typhimurium were purchased from Sigma-Aldrich.
LPS depletion.
The VHHs were coupled to Talon beads as described above. Subsequently, 100 μl of L3 LPS (2 μg/ml) was added and incubated for 1 h at room temperature. Talon beads were collected by centrifugation, and the supernatant fractions, which contained the unbound LPS, were assayed for priming of human whole blood (31).
Various VHHs were used as negative controls in the different assays depending on how these VHHs were produced and purified and whether they were suitable for the desired experiment. These VHHs were selected against different antigens, and sequence analysis confirmed the presence of the VHH. Furthermore, these VHHs were functional in binding of their corresponding antigens.
Whole-cell enzyme-linked immunosorbent assay (ELISA).
Wells of Nunc Maxisorp plates were coated overnight at 37°C with 100 μl of heat-inactivated bacteria (optical density at 600 nm of 0.1). The wild-type N. meningitidis strain H44/76 (immunotype L3) (16) and its galE (19), rfaC (28), icsA (33), and lpxA (26) mutants were utilized to determine the epitope recognized by the isolated VHHs. Plates were blocked with 2% BSA in PBS, washed with PBS, and incubated with different dilutions of the VHHs (1 mg/ml) for 1 to 2 h at room temperature. Plates were washed with PBST and PBS prior to the incubation with rabbit anti-llama heavy-chain serum for 1 h at room temperature. Subsequently, plates were washed with PBST and PBS and incubated with peroxidase-conjugated goat anti-rabbit immunoglobulin Gs for 1 h at room temperature. Peroxidase activity was developed with o-phenylenediamine in the presence of H2O2. Absorptions at 490 nm were measured using a microtiter plate reader.
LPS priming of human whole blood.
LPS priming was measured using the oxidative-burst assay (31). Appropriate LPS dilutions (10 μl) in PBS containing 5 mM glucose (PBS-G) were incubated with VHHs or buffer (both 10 μl) for about 10 min at room temperature. Subsequently, EDTA-anticoagulated blood obtained from healthy volunteers was diluted 10-fold with PBS-G, and 90 μl was added to the LPS samples. The mixtures were incubated for 30 min at 37°C under constant agitation. Enhanced-chemiluminescence responses were measured using a Centro LB960 luminometer (Berthold Technology, Bald Wildbad, Germany) in 10 μl of the reaction mixtures after a 10-fold dilution with PBS-G. N-formyl-methionyl-leucyl-phenylalanine (fMLP) (1 μM) and 180 μM luminol in Hanks balanced salt solution were injected automatically into the reaction mixture, and the emitted photons were measured over a time period of 20 min. Curves were obtained for all samples presenting the chemiluminescence response in counts per minute versus time. Absolute counts were obtained by calculating the area under the curve of the chemiluminescence for 20 min. A monoclonal antibody directed to CD14, monoclonal antibody (MAb) 60bca, was used as an inhibitor in the assay (34).
LPS depletion with VHHs coupled to Talon beads was also studied using the oxidative-burst assay. VHHs coupled to Talon beads by means of their His tag were used to capture LPS from a 100-μl solution (2 μg/ml) during 20 min at room temperature. The amount of LPS in the unbound fraction was measured in the oxidative-burst assay. The chemiluminescence responses were compared with those induced with equivalent volumes of untreated LPS solutions. Since VHHs were stripped from Talon in the presence of plasma, VHH 5G was coupled to N-hydroxysuccinimide-activated Sepharose (Amersham Biosciences) according to the manufacturer's protocol to investigate sequestration of LPS by the VHHs from serum.
Binding of FITC-labeled LPS to human monocytes.
LPS was isolated from wild-type N. meningitidis strain H44/76 by the hot-phenol extraction method (38). Labeling with fluorescein isothiocyanate (FITC-LPS) was performed as described previously (30). Peripheral blood mononuclear cells (PBMC) and neutrophils were isolated from heparin-anticoagulated blood obtained from healthy volunteers using Histopaque-Ficoll-Paque gradient centrifugation (30). The binding experiments and analysis of the fluorescence-activated cell sorting data were done essentially as described previously (37). Briefly, FITC-L3 LPS from N. meningitidis or FITC-Re595 LPS from Salmonella enterica serovar Minnesota was incubated in the presence of 0, 2, or 10 μg VHHs with a PBMC-neutrophil mixture (107 cells/ml) and 2% human pooled serum in a final volume of 70 μl for 30 min with gentle shaking at 37°C in RPMI 1640 (Cambrex) supplemented with 0.05% human serum albumin (RPMI/HSA). Subsequently, 1 ml of RPMI/HSA was added and the cells were centrifuged for 10 min at 300 × g to remove unbound FITC-LPS. The CD14-positive monocytes were labeled afterwards with a nonblocking monoclonal antibody directed against CD14 (LeuM3 coupled to phycoerythrin [LeuM3-PE]) (BD Biosciences). Unbound LeuM3-PE was removed by the addition of 1 ml of RPMI/HSA and centrifugation for 10 min at 300 × g. The cells were resuspended in 250 μl of RPMI/HSA, and binding of FITC-LPS to monocytes was analyzed on a FACSCalibur instrument (Becton Dickinson). Monocytes were distinguished on the basis of double labeling with LeuM3-PE.
RESULTS
Selection of llama VHHs against LPS.
To obtain llama VHHs directed to N. meningitidis LPS, phage display selection was performed on purified L3 LPS, coated directly into Nunc Polysorb wells, using a VHH phage display library generated from nonimmunized llamas. After two rounds of selection, a 20- to 30-fold enrichment compared with empty wells was reached. Monoclonal phages were subsequently tested for binding to the LPS compared with binding to empty wells using ELISA. Sequencing of several of the clones that showed a positive signal in ELISA yielded four different VHHs with unique sequences. Whereas the lengths of CDR3 complementarity-determining regions varied between the different VHHs, they all contained a high number of positively charged and aromatic amino acids (not shown). Strikingly, both these amino acid forms are frequently found in LPS-binding proteins (5).
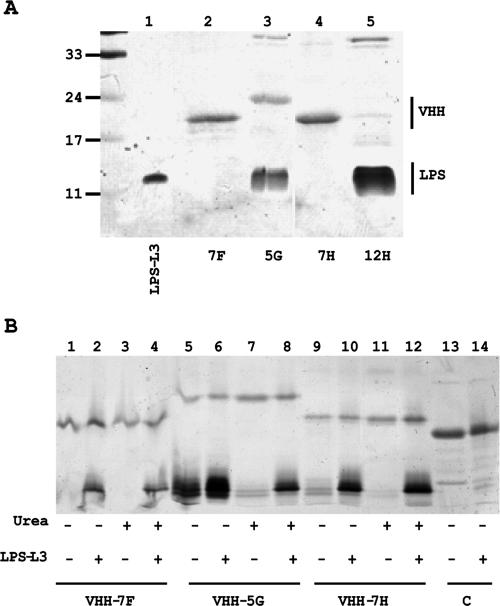
To study the function of these VHHs in more detail, their expression in E. coli was induced with isopropyl-β-d-thiogalactopyranoside and the proteins produced were isolated from the periplasmic fraction and purified by affinity chromatography on Talon beads. Except for that of VHH 12H, the yields of the VHHs were satisfactory (Fig. 1A). Interestingly, the VHH 5G and 12H samples contained large amounts of contaminating LPS (Fig. 1A, lanes 3 and 5). Treatment of these samples with phenol resulted in the fractionation of the LPS in a pure form into the water phase. Analysis of these phenol extracts by mass spectrometry confirmed the presence of monophosphoryl-hexa-acylated lipid A from E. coli (results not shown). Apparently, VHH 5G and VHH 12H bind to the noncognate LPS from E. coli and may therefore represent antibodies that recognize a broad spectrum of LPS substrates. The other VHHs contained amounts of LPS that were barely detectable by SDS-PAGE, suggesting that these VHHs are more specific to the LPS from N. meningitidis and that they have no or only low affinity to the E. coli LPS.
FIG. 1.
Characterization of the selected anti-LPS VHHs. (A) SDS-PAGE gel showing the copurification of E. coli LPS with VHHs. Purified VHHs were loaded on a 15% gel, and after electrophoresis the gel was stained first with silver for the presence of LPS and then with Coomassie brilliant blue to visualize proteins. Molecular mass marker proteins (in kDa) are indicated on the left, and L3 LPS from N. meningitidis was loaded in lane 1. (B) Binding of LPS after denaturation and renaturation of the VHHs. The purified VHHs indicated (lanes 1, 5, and 9) were denatured with urea (lanes 3, 7, and 11) to study the loss of bound E. coli LPS. The nondenatured VHHs (lanes 2, 6, and 10) and the denatured VHHs, which were allowed to renature in PBS (lanes 4, 8, and 12), were incubated with N. meningitidis L3 LPS. After the incubation, the VHH-Talon beads were pelleted and washed, and the LPS bound to the beads was analyzed by SDS-PAGE. Binding of LPS to beads containing an irrelevant VHH was also analyzed (lanes 13 and 14). The gels were stained as described for panel A.
To confirm the specific association of the E. coli LPS with the selected VHHs, the VHHs were denatured by incubation with 8 M urea at room temperature while they were coupled to Talon beads. After removal of the urea and resuspension of the beads in PBS, the VHHs were analyzed with SDS-PAGE (Fig. 1B, odd-number lanes). Most of the contaminating E. coli LPS was lost from the VHH 5G sample after treatment with urea (Fig. 1B, compare lanes 5 and 7). Similar results were obtained with VHH 12H (result not shown). This observation indicates that E. coli LPS was specifically bound by the VHHs and that this binding was lost after denaturation of the VHH with urea. To investigate whether denatured VHHs renatured correctly after removal of urea, N. meningitidis L3 LPS was added to the VHHs and the amounts of LPS bound were analyzed (Fig. 1B, even-number lanes). The amounts of LPS precipitated by the renatured VHHs were similar to those immunoprecipitated by equivalent amounts of native VHHs (Fig. 1B, compare lanes 2 and 4, lanes 6 and 8, and lanes 10 and 12 for VHH 7F, VHH 5G, and VHH 7H, respectively), indicating that the VHHs regained their full activity after denaturation and renaturation. It should be noted that even in the presence of contaminating E. coli LPS, VHH 5G bound additional L3 LPS (Fig. 1B, compare lanes 5 and 6), suggesting that VHH 5G has a high capacity to bind LPS. An aspecific VHH (C) did not bind any LPS in its native form (Fig. 1B, lanes 13 and 14), nor after denaturation and renaturation (not shown).
Specificities of the VHHs.
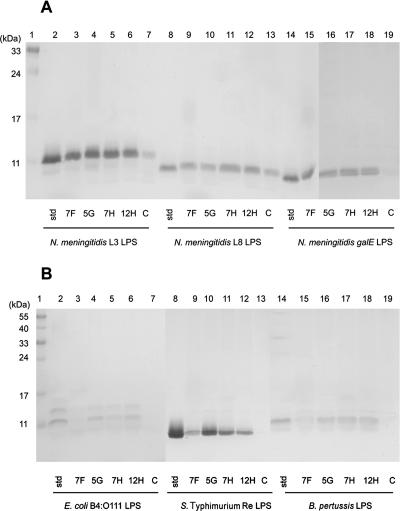
VHHs produced in E. coli contained contaminating LPS with the same size as N. meningitidis L3 LPS, which thwarted the interpretation of the results of the immunoprecipitations to some extent. Furthermore, contaminating LPS may compromise the function of the VHHs in binding to other LPS molecules. To eliminate these difficulties, the selected VHHs were cloned into yeast-compatible vectors and transferred into S. cerevisiae, and the proteins produced were isolated from the culture supernatant and purified using affinity chromatography on Talon beads. VHHs coupled to Talon beads were used to immunoprecipitate L3, L8, and galE LPS forms from N. meningitidis, LPS from E. coli B4:O111, Re LPS from Salmonella serovar Typhimurium, and LPS from B. pertussis. All four VHHs precipitated the different LPS forms from N. meningitidis very efficiently (Fig. 2A). The intensities of the LPS bands that were immunoprecipitated with the four anti-LPS VHHs were almost identical to that of the std band, indicating that all the LPS used in the experiment was immunoprecipitated (Fig. 2A). Since the VHHs also recognized the shorter L8 and galE LPS molecules (see Fig. 3F for the schematic structure of the different LPS forms from N. meningitidis), they were probably directed against an epitope in the conserved inner core region of the LPS. Therefore, these VHHs may bind a broader range of LPS molecules. Consistently, VHHs 5G, 7H, and 12H also precipitated LPS from E. coli B4:O111, Re LPS from Salmonella serovar Typhimurium, and LPS from B. pertussis (Fig. 2B). VHH 7F, on the other hand, barely showed binding to LPS molecules other than those of N. meningitidis (Fig. 2B, lanes 3, 9, and 15), suggesting that this VHH is more specific to N. meningitidis LPS. Although the irrelevant VHH (C) showed considerable binding of N. meningitidis L8 LPS (Fig. 2A, lane 13), it showed no or barely any binding of the other LPS species tested.
FIG. 2.
Immunoprecipitation of free LPS. SDS-PAGE gel showing the amounts of LPS that were immunoprecipitated by VHHs immobilized to Talon beads. (A) Three LPS derivatives from N. meningitidis H44/76 (L3, L8, and galE) were efficiently precipitated by all four anti-LPS VHHs. (B) LPS from E. coli B4:O111, Re LPS from Salmonella serovar Typhimurium, and LPS from B. pertussis were precipitated with the VHHs 5G, 7H, and 12H but not with VHH 7F. In both panels, the lanes marked std represent input LPS, i.e., the maximal amount of LPS that could be immunoprecipitated. The lanes marked C show LPS that was precipitated with an irrelevant VHH. Lane 1 contains the molecular mass standard in kDa (indicated at the left). In panel B, the larger part of the gel is shown to include the O antigen containing high-molecular-mass LPS bands of E. coli B4:O111.
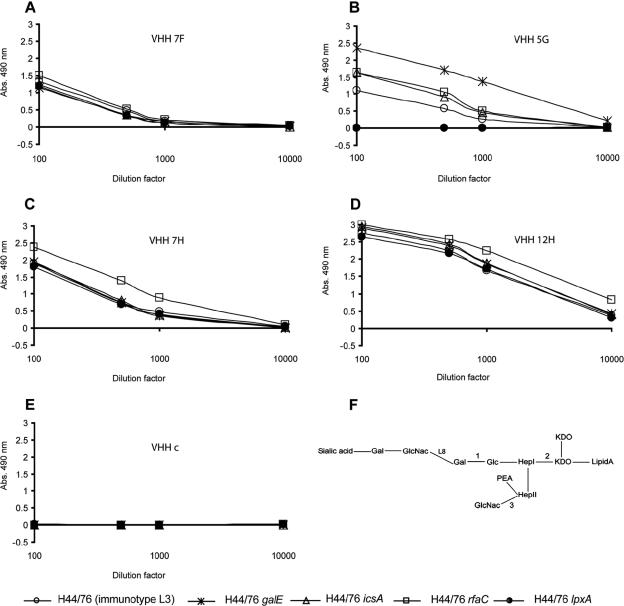
FIG. 3.
Epitope mapping of the selected VHHs. Wild-type N. meningitidis strain H44/76 expressing L3 LPS (schematically represented in panel F) and its galE, rfaC, and icsA mutant derivatives (expressing LPS molecules, truncated at positions 1, 2, and 3, respectively; panel F) and an lpxA mutant strain producing no LPS at all were coated on microtiter plates. Serial dilutions of the different VHHs were added to the wells, and bound VHHs were detected by successive incubation with rabbit anti-llama heavy-chain serum and a goat anti-rabbit antibody coupled to a peroxidase. Peroxidase activity was developed, and the absorptions (Abs.) measured at 490 nm are plotted. (F) Schematic structure of the N. meningitidis L3 LPS. Gal, galactose; GlcNac, N-acetylglucosamine; Glc, glucose; KDO, 2-keto-3-deoxyoctulosonic acid; Hep, heptose; PEA, phosphoethanolamine. L8 denotes where the structure of the L8 LPS ends.
Epitope mapping.
To determine the binding sites of the different VHHs in the LPS in more detail, various strains of N. meningitidis which express LPS molecules with defined truncations in the oligosaccharide moiety (see Fig. 3F) were used in a whole-cell ELISA. As a control, the lpxA mutant strain, which totally lacks LPS, was used (26). All four VHHs recognized the wild-type N. meningitidis strain H44/76 expressing L3 LPS, as well as the mutant strains expressing shorter LPS molecules (Fig. 3). Since VHH 5G bound the rfaC mutant strain, which expresses an LPS molecule consisting of lipid A and 2-keto-3-deoxyoctulosonic acid (KDO) only, but failed to bind the LPS-deficient lpxA mutant strain, the data indicate that VHH 5G is an LPS-specific antibody and that its epitope must reside in the lipid A and KDO regions. The lack of binding of VHH 5G with the lpxA mutant was not due to shielding by capsule, since VHH 5G also did not react with the lpxA cps double mutant (3), which lacks both LPS and capsule (results not shown). Interestingly, this VHH reacted better with N. meningitidis strains expressing shorter LPS molecules than with the wild-type strain, suggesting that the full-length LPS shields the epitope to some extent. Remarkably, the VHHs 7F, 7H, and 12H reacted with the LPS deletion strain, suggesting that they interact with other antigens in addition to LPS or that they interact indirectly with LPS through unidentified components. The binding of the different VHHs to the N. meningitidis strains was specific, since an irrelevant VHH did not show any interaction with the different N. meningitidis strains studied (Fig. 3E).
The three VHHs that reacted with the lpxA mutant strain in a whole-cell ELISA can perfectly sequester LPS and detoxify it (see below). However, since it is not clear whether these VHHs bind directly to LPS or bind LPS through an unidentified component, additional studies are required to fully characterize these VHHs before using them for any application. For these reasons, only VHH 5G was used in the following studies.
Inhibition of LPS binding to human monocytes.
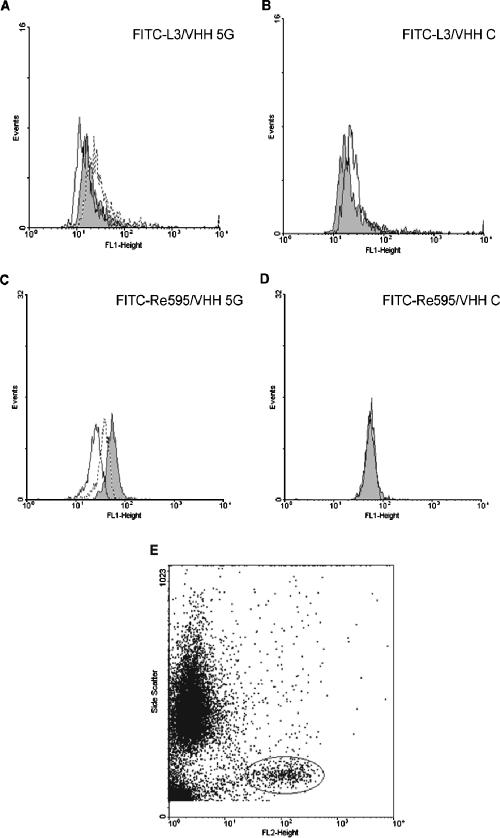
LPS interacts with high-affinity receptors present on immune cells and in plasma. To investigate whether anti-LPS VHH can compete with these high-affinity receptors, the binding of FITC-labeled L3 LPS from N. meningitidis and Re595 LPS from Salmonella serovar Minnesota to PBMCs was studied in the absence or presence of VHH 5G. The FITC fluorescence that was associated with the monocytes was displaced in the presence of VHH 5G (Fig. 4). Both FITC-labeled L3 LPS from N. meningitidis and Re595 LPS from Salmonella serovar Minnesota were displaced by VHH 5G but not by the irrelevant VHH (Fig. 4, compare A with B and C with D, respectively). Inhibition of LPS binding to CD14-expressing cells was dose dependent. This was clearly shown in the case of Salmonella serovar Minnesota LPS (Fig. 4C). Inhibition of the LPS binding did not result from shielding of the LPS receptor CD14 by the VHH, since the labeled CD14-specific MAb LeuM3-PE could still bind to the cell, whereas monocytes incubated with the anti-CD14 MAb 60bca did not bind any LeuM3-PE afterwards (result not shown). CD14-specific MAb LeuM3-PE was used to gate the cells that bind LPS (Fig. 4E). These results indicate that the anti-LPS VHH is able to block binding of LPS to its high-affinity receptor present on PBMCs.
FIG. 4.
Inhibition of FITC-labeled LPS binding to monocytes. Fluorescence-activated cell sorting histograms showing the distribution of LPS-FITC associated with CD14-expressing cells. FITC-labeled L3 LPS from N. meningitidis (A) or FITC-labeled Re595 LPS from Salmonella serovar Minnesota (C) was incubated with isolated PBMCs in the absence of any VHHs (gray surfaces) or in the presence of 2 μg (stippled lines) or 10 μg (solid lines) of VHH 5G. The effect of an irrelevant VHH C (at 10 μg) on the distribution of the FITC-labeled L3 or Re595 LPS is depicted in panels B and D, respectively. Panel E shows the dot plot of PBMCs stained with the anti-CD14 MAb LeuM3-PE. Cells were gated via side scatter (y axis) and PE fluorescence.
Detoxification of LPS.
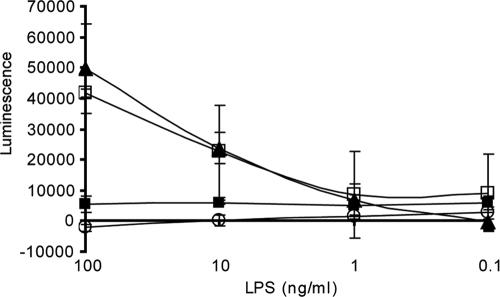
During sepsis, LPS stimulates the immune cells to massively produce inflammatory responses. The anti-LPS VHH was found to reduce binding of LPS to target cells efficiently. To study whether this inhibition prevents the response of these cells to LPS, priming of whole blood with N. meningitidis L3 LPS (34) to produce an oxidative burst in response to fMLP was studied in the presence or absence of VHH 5G. In contrast to an irrelevant VHH, VHH 5G inhibited LPS signaling very efficiently (Fig. 5). The inhibition exerted by the anti-LPS VHH was almost equal to that found in the presence of the anti-CD14 MAb 60bca, known for its blocking activity (34). However, MAb 60bca blocks the binding site of LPS on effector cells, whereas VHH binds to LPS and shows no interaction with the effector cells. This may explain the higher molar concentration needed for VHH than for MAb 60bca (Fig. 5).
FIG. 5.
Detoxification of solutions containing N. meningitidis L3 LPS by the VHH 5G. fMLP-induced oxidative burst was measured in whole blood after priming with the N. meningitidis L3 LPS at the indicated concentrations using chemiluminescence. Tenfold dilutions of whole blood were incubated with LPS (open squares) or with the same concentrations of LPS, which were preincubated with VHH 5G (filled squares) or a control VHH (filled triangles). The concentration of both VHHs was 100 μg/ml. The anti-CD14 MAb 60bca was used as a control inhibitor in the assay (open circles) at a concentration of 1 μg/ml. The experiment was repeated three times using blood from the same donor, and error bars are indicated.
Sequestration of LPS.
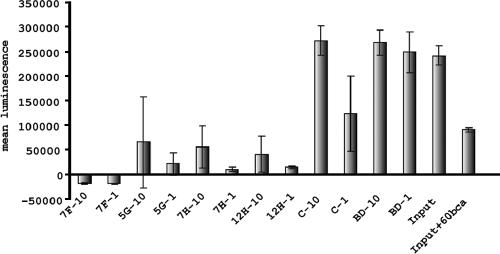
Different LPS forms were efficiently immunoprecipitated by the VHHs. To investigate whether this depletion is sufficient to detoxify a solution that was spiked with LPS, immobilized VHHs were used to bind LPS and the unbound fractions were used to prime whole blood. The oxidative burst produced by the solutions treated with the VHHs was compared with that produced by the untreated solution. To demonstrate the specificity of the VHHs, empty Talon beads and an irrelevant VHH were used. Furthermore, the inhibition of LPS signal by the anti-CD14 MAb 60bca was also measured. All VHHs depleted LPS very efficiently (Fig. 6). When the same volumes of preincubated solution as of the untreated solution were used in the assay, the mean luminescence was reduced by more than 90% (Fig. 6). Even when 10-fold more preincubated solution was used in the assay, the reduction in mean luminescence was higher than 70%. These results indicate that immobilized anti-LPS VHHs sequestered most of the LPS spiked into the PBS solution. The depletion of the LPS was not due to aspecific binding of LPS to Talon beads, since empty beads did not show any reduction of the amount of LPS in the solution (Fig. 6). The irrelevant VHH, on the other hand, depleted about 49% of the LPS (Fig. 6). Apparently, due to its hydrophobic nature, LPS shows aspecific interaction with different proteins. The specificity of the anti-LPS VHHs was clear when a 10-fold-larger volume was tested. Whereas anti-LPS VHHs still reduced the mean luminescence up to 70 to 80%, the irrelevant VHH did not prevent a strong response. This result indicates that LPS depletion by the anti-LPS VHHs is specific and that such depletion can be used to detoxify aqueous solutions.
FIG. 6.
Sequestration of the L3 LPS from an aqueous solution by VHHs. Graph showing the amounts of luminescence (y axis) produced by an LPS solution (2 μg/ml) before (input) and after incubation with the indicated anti-LPS VHHs, which were immobilized through the histidine tag to Talon beads. As controls, empty Talon beads (BD) and beads loaded with an irrelevant VHH (C) were used. Both equivalent amounts (−1) and 10-fold the amount (−10) of input were used in the assay. The inhibition of luminescence by the anti-CD14 MAb 60bca is also indicated. The experiment was repeated three times, and error bars are indicated.
The His-tagged VHHs dissociated from Talon beads in plasma and serum due to the anticoagulant (EDTA) used for the preparation of these fractions (not shown). In order to test the ability of the VHHs to sequester LPS from a complex solution, such as serum or plasma, VHH 5G was chemically coupled to Sepharose beads. VHH 5G coupled in this way was found to efficiently deplete LPS spiked into serum (not shown). Together, these results indicate that anti-LPS VHHs can be used to deplete LPS from different solutions, including plasma, a property that can be utilized in therapy in the fight against sepsis.
DISCUSSION
A llama single-chain antibody fragment recognizing LPS was selected from a VHH phage library derived from mRNA of peripheral blood lymphocytes of nonimmunized llamas. This VHH has the ability to capture LPS molecules from an aqueous solution very efficiently. It recognized LPS molecules present in bacteria, as well as free LPS molecules purified from different sources. The isolated VHH was also able to disturb binding of LPS to their targets and to disrupt LPS signaling that results in the generation of the effector molecules and the pathological condition of sepsis.
The presence of antibodies recognizing LPS in nonimmunized young llamas is not surprising, since even at a young age, llamas may encounter gram-negative bacterial infections. Immunization of llamas with purified LPS from N. meningitidis did not result in a detectable immune reaction, and attempts to select an anti-LPS VHH from a VHH phage library derived from mRNA of peripheral blood lymphocytes of an immunized llama failed (results not shown). Possibly purified LPS molecules are not immunogenic in llamas as reported earlier for other animals (2, 7).
Phage display is a powerful method for directing selection to a particular domain of the antigen. However, the phage display in E. coli may not be the best method of selecting for VHHs directed against LPS, since anti-LPS VHHs may interact with the endogenous LPS molecules, resulting in growth inhibition of the host and consequently in the loss of interesting anti-LPS VHHs. Alternative selection methods, such as yeast display, are currently under evaluation.
The selected VHH 5G was directed against an epitope deep in the LPS structure. Since it interacts with the LPS from an rfaC mutant strain of N. meningitidis, the epitope should be located in the lipid A or KDO residues. This part of the LPS is more conserved between gram-negative bacteria than the structures located more distally from lipid A. Accordingly, this VHH proved to bind to LPS from different bacteria. The ELISA signal for mutants expressing shorter LPS molecules was higher than that for the wild-type strain. This is probably due to the better exposure of the VHH epitope in these shorter LPS molecules.
LPS of E. coli strain TG1 cofractionated with the VHHs. It would be interesting to find out whether the LPS interacts with the VHHs during spheroplasting of E. coli or whether the interaction between VHH and LPS occurs earlier in LPS biogenesis, that is, during the transport of the LPS through the periplasm. Even though this interaction did not result in any detectable phenotype, these VHHs may still form interesting research tools for the study of LPS biogenesis.
The selected VHH 5G was functional after a denaturation-renaturation cycle. This property of correct refolding, which is intrinsic to VHHs in general, may prove to be highly favorable for the applications of the anti-LPS VHHs in therapy, since a decontamination step might be required before its utilization in patients. The anti-LPS VHH selected is a leading candidate in the development of therapies against LPS-mediated sepsis. The binding properties of the anti-LPS VHH may be improved by in vitro evolution and protein engineering techniques to develop higher-affinity antibodies that will serve to capture LPS from the plasma of sepsis patients with greater efficiency than the current apheresis systems (1, 29). Nonselective apheresis, such as therapeutic plasma exchange, already shows a beneficial effect on the outcome of patients suffering from sepsis (27, 35). Redirection of the plasma through a column functionalized with anti-LPS VHHs may speed up the specific clearance of the LPS from the blood circulation and subsequently the recovery of the patient. Although most of the antisepsis therapies described in this paper are based on the utilization of only the VHH polypeptide, the utilization of the whole single-chain antibody, as well as the grafting of the selected antigen-binding domains on different immunoglobulins, is not discounted. Finally, anti-LPS VHHs can be produced at low cost on a large scale using microorganisms, resulting in an affordable therapy.
Acknowledgments
We thank J. Geurtsen for B. pertussis LPS, for assistance with LPS isolation, and for mass spectrometry analysis of lipid A, M. Bos and L. Steeghs for exchange of materials and strains, and J. van Strijp for critical discussions.
This work was supported with financial aid from SenterNovem (TSGE 3131).
Footnotes
Published ahead of print on 23 August 2006.
REFERENCES
- 1.Bengsch, S., K. S. Boos, D. Nagel, D. Seidel, and D. Inthorn. 2005. Extracorporeal plasma treatment for the removal of endotoxin in patients with sepsis: clinical results of a pilot study. Shock 23:494-500. [PubMed] [Google Scholar]
- 2.Bhattacharjee, A. K., S. M. Opal, R. Taylor, R. Naso, M. Semenuk, W. D. Zollinger, E. E. Moran, L. Young, C. Hammack, J. C. Sadoff, and A. S. Cross. 1996. A noncovalent complex vaccine prepared with detoxified Escherichia coli J5 (Rc chemotype) lipopolysaccharide and Neisseria meningitidis group B outer membrane protein produces protective antibodies against gram-negative bacteremia. J. Infect. Dis. 173:1157-1163. [DOI] [PubMed] [Google Scholar]
- 3.Bos, M. P., and J. Tommassen. 2005. Viability of a capsule- and lipopolysaccharide-deficient mutant of Neisseria meningitidis. Infect. Immun. 73:6194-6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braude, A. I., E. J. Ziegler, J. A. McCutchan, and H. Douglas. 1981. Immunization against nosocomial infection. Am. J. Med. 70:463-466. [DOI] [PubMed] [Google Scholar]
- 5.Chaby, R. 2004. Lipopolysaccharide-binding molecules: transporters, blockers and sensors. Cell Mol. Life Sci. 61:1697-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen, J. 2002. The immunopathogenesis of sepsis. Nature 420:885-891. [DOI] [PubMed] [Google Scholar]
- 7.Cross, A. S., S. Opal, P. Cook, J. Drabick, and A. Bhattacharjee. 2004. Development of an anti-core lipopolysaccharide vaccine for the prevention and treatment of sepsis. Vaccine 22:812-817. [DOI] [PubMed] [Google Scholar]
- 8.Cross, A. S., S. M. Opal, J. E. Palardy, J. J. Drabick, H. S. Warren, C. Huber, P. Cook, and A. K. Bhattacharjee. 2003. Phase I study of detoxified Escherichia coli J5 lipopolysaccharide (J5dLPS)/group B meningococcal outer membrane protein (OMP) complex vaccine in human subjects. Vaccine 21:4576-4587. [DOI] [PubMed] [Google Scholar]
- 9.De Haard, H. J., S. Bezemer, A. M. Ledeboer, W. H. Muller, P. J. Boender, S. Moineau, M. C. Coppelmans, A. J. Verkleij, L. G. Frenken, and C. T. Verrips. 2005. Llama antibodies against a lactococcal protein located at the tip of the phage tail prevent phage infection. J. Bacteriol. 187:4531-4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Padova, F. E., H. Brade, G. R. Barclay, I. R. Poxton, E. Liehl, E. Schuetze, H. P. Kocher, G. Ramsay, M. H. Schreier, D. B. McClelland, et al. 1993. A broadly cross-protective monoclonal antibody binding to Escherichia coli and Salmonella lipopolysaccharides. Infect. Immun. 61:3863-3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dolk, E., M. van der Vaart, D. Lutje Hulsik, G. Vriend, H. de Haard, S. Spinelli, C. Cambillau, L. Frenken, and T. Verrips. 2005. Isolation of llama antibody fragments for prevention of dandruff by phage display in shampoo. Appl. Environ. Microbiol. 71:442-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dumoulin, M., K. Conrath, A. Van Meirhaeghe, F. Meersman, K. Heremans, L. G. Frenken, S. Muyldermans, L. Wyns, and A. Matagne. 2002. Single-domain antibody fragments with high conformational stability. Protein Sci. 11:500-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finne, J., D. Bitter-Suermann, C. Goridis, and U. Finne. 1987. An IgG monoclonal antibody to group B meningococci cross-reacts with developmentally regulated polysialic acid units of glycoproteins in neural and extraneural tissues. J. Immunol. 138:4402-4407. [PubMed] [Google Scholar]
- 14.Finne, J., M. Leinonen, and P. H. Makela. 1983. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet ii:355-357. [DOI] [PubMed] [Google Scholar]
- 15.Hamers-Casterman, C., T. Atarhouch, S. Muyldermans, G. Robinson, C. Hamers, E. B. Songa, N. Bendahman, and R. Hamers. 1993. Naturally occurring antibodies devoid of light chains. Nature 363:446-448. [DOI] [PubMed] [Google Scholar]
- 16.Holten, E. 1979. Serotypes of Neisseria meningitidis isolated from patients in Norway during the first six months of 1978. J. Clin. Microbiol. 9:186-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoogenboom, H. R., A. P. de Bruine, S. E. Hufton, R. M. Hoet, J. W. Arends, and R. C. Roovers. 1998. Antibody phage display technology and its applications. Immunotechnology 4:1-20. [DOI] [PubMed] [Google Scholar]
- 18.Jennings, M. P., Y. N. Srikhanta, E. R. Moxon, M. Kramer, J. T. Poolman, B. Kuipers, and P. van der Ley. 1999. The genetic basis of the phase variation repertoire of lipopolysaccharide immunotypes in Neisseria meningitidis. Microbiology 145:3013-3021. [DOI] [PubMed] [Google Scholar]
- 19.Jennings, M. P., P. van der Ley, K. E. Wilks, D. J. Maskell, J. T. Poolman, and E. R. Moxon. 1993. Cloning and molecular analysis of the galE gene of Neisseria meningitidis and its role in lipopolysaccharide biosynthesis. Mol. Microbiol. 10:361-369. [PubMed] [Google Scholar]
- 20.Lauwereys, M., M. Arbabi Ghahroudi, A. Desmyter, J. Kinne, W. Holzer, E. De Genst, L. Wyns, and S. Muyldermans. 1998. Potent enzyme inhibitors derived from dromedary heavy-chain antibodies. EMBO J. 17:3512-3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin, G. S., D. M. Mannino, S. Eaton, and M. Moss. 2003. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 348:1546-1554. [DOI] [PubMed] [Google Scholar]
- 22.Plested, J. S., K. Makepeace, M. P. Jennings, M. A. Gidney, S. Lacelle, J. Brisson, A. D. Cox, A. Martin, A. G. Bird, C. M. Tang, F. M. Mackinnon, J. C. Richards, and E. R. Moxon. 1999. Conservation and accessibility of an inner core lipopolysaccharide epitope of Neisseria meningitidis. Infect. Immun. 67:5417-5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pollard, A. J., and E. R. Moxon. 2002. The meningococcus tamed? Arch. Dis. Child. 87:13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scholten, R. J., B. Kuipers, H. A. Valkenburg, J. Dankert, W. D. Zollinger, and J. T. Poolman. 1994. Lipo-oligosaccharide immunotyping of Neisseria meningitidis by a whole-cell ELISA with monoclonal antibodies. J. Med. Microbiol. 41:236-243. [DOI] [PubMed] [Google Scholar]
- 25.Spinelli, S., L. G. Frenken, P. Hermans, T. Verrips, K. Brown, M. Tegoni, and C. Cambillau. 2000. Camelid heavy-chain variable domains provide efficient combining sites to haptens. Biochemistry 39:1217-1222. [DOI] [PubMed] [Google Scholar]
- 26.Steeghs, L., R. den Hartog, A. den Boer, B. Zomer, P. Roholl, and P. van der Ley. 1998. Meningitis bacterium is viable without endotoxin. Nature 392:449-450. [DOI] [PubMed] [Google Scholar]
- 27.Stegmayr, B. G. 2000. Is there a future for adsorption techniques in sepsis? Blood Purif. 18:149-155. [DOI] [PubMed] [Google Scholar]
- 28.Stojiljkovic, I., V. Hwa, J. Larson, L. Lin, M. So, and X. Nassif. 1997. Cloning and characterization of the Neisseria meningitidis rfaC gene encoding alpha-1,5 heptosyltransferase I. FEMS Microbiol. Lett. 151:41-49. [DOI] [PubMed] [Google Scholar]
- 29.Teramoto, K., Y. Nakamoto, T. Kunitomo, H. Shoji, T. Tani, K. Hanazawa, and M. Kodama. 2002. Removal of endotoxin in blood by polymyxin B immobilized polystyrene-derivative fiber. Ther. Apheresis 6:103-108. [DOI] [PubMed] [Google Scholar]
- 30.Troelstra, A., P. Antal-Szalmas, L. A. de Graaf-Miltenburg, A. J. Weersink, J. Verhoef, K. P. Van Kessel, and J. A. Van Strijp. 1997. Saturable CD14-dependent binding of fluorescein-labeled lipopolysaccharide to human monocytes. Infect. Immun. 65:2272-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Troelstra, A., B. N. Giepmans, K. P. Van Kessel, H. S. Lichenstein, J. Verhoef, and J. A. Van Strijp. 1997. Dual effects of soluble CD14 on LPS priming of neutrophils. J. Leukoc. Biol. 61:173-178. [DOI] [PubMed] [Google Scholar]
- 32.Tsai, C. M., and C. E. Frasch. 1982. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119:115-119. [DOI] [PubMed] [Google Scholar]
- 33.van der Ley, P., M. Kramer, A. Martin, J. C. Richards, and J. T. Poolman. 1997. Analysis of the icsBA locus required for biosynthesis of the inner core region from Neisseria meningitidis lipopolysaccharide. FEMS Microbiol. Lett. 146:247-253. [DOI] [PubMed] [Google Scholar]
- 34.van Leeuwen, H. J., M. Van Der Tol, J. A. Van Strijp, J. Verhoef, and K. P. van Kessel. 2005. The role of tumour necrosis factor in the kinetics of lipopolysaccharide-mediated neutrophil priming in whole blood. Clin. Exp. Immunol. 140:65-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Venkataraman, R., S. Subramanian, and J. A. Kellum. 2003. Clinical review: extracorporeal blood purification in severe sepsis. Crit. Care 7:139-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verheesen, P., M. R. ten Haaft, N. Lindner, C. T. Verrips, and J. J. de Haard. 2003. Beneficial properties of single-domain antibody fragments for application in immunoaffinity purification and immuno-perfusion chromatography. Biochim. Biophys. Acta 1624:21-28. [DOI] [PubMed] [Google Scholar]
- 37.Weersink, A. J., K. P. Van Kessel, R. Torensma, J. A. Van Strijp, and J. Verhoef. 1990. Binding of rough lipopolysaccharides (LPS) to human leukocytes. Inhibition by anti-LPS monoclonal antibody. J. Immunol. 145:318-324. [PubMed] [Google Scholar]
- 38.Westphal, O., and J. K. Jann. 1965. Bacterial lipopolysaccharides extraction with water-phenol and further application of the procedure. Methods Carbohydr. Chem. 5:83-91. [Google Scholar]
- 39.Witholt, B., M. Boekhout, M. Brock, J. Kingma, H. V. Heerikhuizen, and L. D. Leij. 1976. An efficient and reproducible procedure for the formation of spheroplasts from variously grown Escherichia coli. Anal. Biochem. 74:160-170. [DOI] [PubMed] [Google Scholar]