Abstract
The OspC protein of Borrelia burgdorferi is an immunodominant antigen. Here we demonstrate that the loop 5 domain of type A OspC is surface exposed, elicits bactericidal antibody in mice, and is antigenic in humans. The data suggest that loop 5 may be suitable for inclusion in a polyvalent, chimeric OspC vaccinogen.
Outer surface protein C (OspC) of the Lyme disease spirochetes is a 22-kDa immunodominant (10) antigen that is expressed upon tick feeding and during early stages of infection (27). Although a strong antibody response to OspC is mounted during natural infection, the response does not lead to bacterial clearance because OspC production is turned off shortly after the establishment of infection (27). OspC has emerged as an important virulence factor and a potential candidate for Lyme disease vaccine development. However, efforts to develop an OspC-based vaccine have been hampered by its heterogeneity among strains (29, 32, 33). Although vaccination with OspC elicits a highly protective response, most studies have reported only strain-specific protection (3, 11, 20, 22, 25, 26). Recent analyses have provided significant insight into our understanding of the antigenic structure of OspC and the basis of strain-specific protection. Twenty-one OspC types, designated A through U, have been defined (18, 28, 30). By infecting mice with clonal populations of Borrelia burgdorferi that produce specific OspC types, Earnhart et al. demonstrated that the antibody response during early infection is largely OspC type specific (6). This suggests that the dominant epitopes presented during early infection are likely to reside within the type-specific domains of OspC. While earlier studies suggested that only 4 of the 21 OspC types are associated with invasive infection (28), recent studies have demonstrated that isolates producing additional OspC types can also establish invasive infection (1, 6). However, type A OspC appears to predominate in strains that cause invasive infections in humans. Epitope-mapping analyses of type A OspC revealed that one of the dominant linear epitopes that elicits a response in mice resides within the loop 5 domain (6). The loop 5 domain is highly variable at the intertype level but conserved within sequences of a given type (6). In the present study, we refine the location of the epitope, demonstrate its surface exposure on intact bacteria, and demonstrate that it elicits bactericidal antibody.
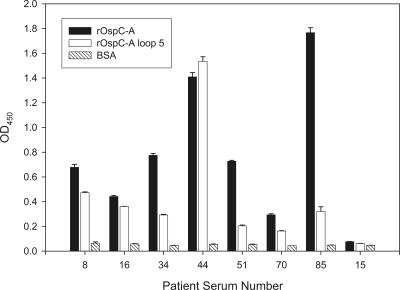
Most studies that have sought to define the immunodominant epitopes of OspC have been conducted with mice (3, 11, 20, 23). However, it has been demonstrated that the antibody responses to some epitopes differ for humans versus mice and other mammals (19). The first objective of the present study was to determine whether the loop 5 domain of OspC is recognized by antibody elicited during infection in humans. Ideally, these analyses would be conducted with serum collected from individuals infected with a clonal population of a type A-producing strain. Since one cannot determine with absolute certainty whether an individual is infected with a heterogenous or a homogenous population, we sought to identify patient sera that exhibit a response to type A-specific sequences. To accomplish this, a panel of serum samples collected from patients with erythema migrans (early-stage Lyme disease) were screened by enzyme-linked immunosorbent assay (ELISA). Recombinant type (r-type) A OspC and an r-type A OspC subfragment containing loop 5 residues 130 to 150 were used to coat 96-well plates (250 ng of r-protein/well; 0.1 M Na2HPO4; 4°C overnight). The plates were blocked (10% nonfat dry milk in phosphate-buffered saline, 0.5% Tween 20; 37°C for 2 h) and washed, and human Lyme disease patient serum (diluted 1:400) was added to each well (37°C; 1 h). Horseradish peroxidase-conjugated goat anti-human immunoglobulin G (IgG; Sigma) (50 μl of a 1:40,000 dilution) was added (1 h; 37°C), followed by TMB substrate (3,3′,5,5′-tetramethylbenzidine) as instructed by the supplier (Sigma). The optical density values at 450 nm were determined by using a plate reader. Additional wells were coated with bovine serum albumin to serve as negative controls. All assays were performed in triplicate. The mean A450 value is presented with standard deviations. As shown in Fig. 1, several serum samples were found to have a strong IgG response to both the full-length type A OspC and the loop 5 fragment. Serum samples 8 and 44 displayed the strongest immunoreactivity with the loop 5 fragment and hence were selected for further analysis.
FIG. 1.
ELISAs: identification of serum samples harboring type A OspC targeting antibody. r-type A full-length OspC, r-type A loop 5, and bovine serum albumin were used to coat the wells of ELISA plates. The wells were screened with serum from human Lyme disease patients. All assays were performed in triplicate, and the mean is presented along with the standard deviation. All methods were as described in the text. Serum from patient 15 which was determined to be IgG negative for antibody to B. burgdorferi B31MI served as a negative control.
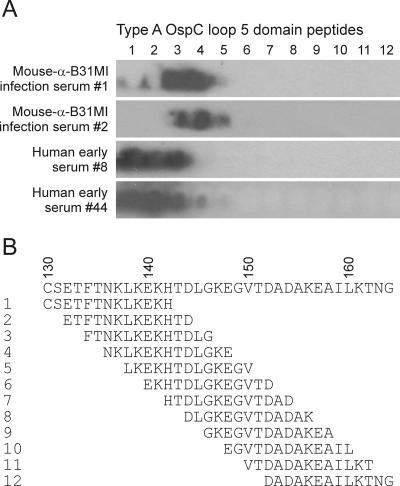
To more accurately define the residues within the loop 5 domain that are recognized by infection-induced antibody, PepSpot arrays were screened with the sera from patients 8 and 44 and with serum from mice infected with a clonal population of the type A OspC-producing strain B31MI (6). The PepSpot arrays consisted of 12- to 13-residue overlapping peptides (two-amino-acid step) spanning the loop 5 domain of type A OspC spotted onto Whatman 50 cellulose membrane (∼150 nmol/cm2; JPT Peptide Technologies GmbH, Berlin, Germany). The PepSpot membranes were blocked (5% nonfat dry milk in Tris-buffered saline-0.5% Tween 20), washed, and screened with mouse and human serum samples (diluted 1:1,000 and 1:400 in blocking solution, respectively), and antibody binding was detected with species-specific anti-IgG antiserum. Although the specific residues that make up the immunoreactive domain differed slightly in mice and humans, the major epitopes localized within residues 130 to 146 (Fig. 2). In type A OspC sequences, this region encompasses the C-terminal region of alpha helix 3 and the N-terminal portion of loop 5.
FIG. 2.
Identification of the specific residues that comprise the type A OspC loop 5 epitopes through PepSpot analysis. Overlapping peptides that span the loop 5 domain were generated and spotted onto nitrocellulose. The immobilized peptides were then screened with serum from mice infected with a clonal population of a type A OspC-producing strain (B31 MI) or with serum from human Lyme disease patients (as indicated). (A) Immunoblotting results for loop 5 domain; (B) peptide sequences.
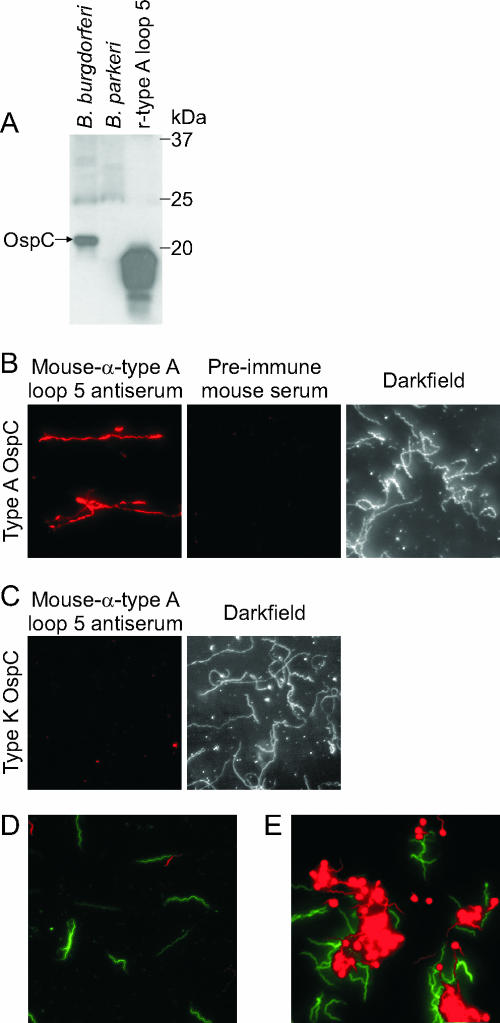
The crystal structures of OspC spatially place loop 5 on a prominent bend of the protein (7, 17). This loop has been postulated to be part of a potential ligand-binding pocket (17). To determine whether loop 5 is displayed at the cell surface and is accessible to antibody in in vitro grown spirochetes, immunofluorescence assays (IFAs) were performed by using anti-loop 5 antiserum. Immunoblot analyses with whole-cell lysates of B. burgdorferi B31 MI (type A OspC), B. parkeri, and S-tagged r-type A loop 5 demonstrated that the loop 5 antiserum is specific, establishing the suitability of this antiserum for IFAs (Fig. 3A). The strains analyzed by IFA consisted of B. burgdorferi B31MI (type A OspC) and LDP74 (type K OspC). The spirochetes were grown at 33°C and transferred to 37°C for 3 days to stimulate OspC expression. IFAs were conducted with permeabilized cells (acetone fixed), nonpermeabilized cells (air dried), and standard methods as previously described (24). The slides were screened with a 1:1,000 dilution of mouse α-loop 5 antiserum, mouse preimmune serum, or rabbit-α-flagellin antiserum. Detection was achieved by using Alexa Fluor 568-conjugated goat α-mouse IgG or Alexa Fluor 488-conjugated goat α-rabbit IgG (10 μg ml−1 in blocking buffer). Slides were visualized on an Olympus BX51 fluorescence scope using a rhodamine or fluorescein filter set, as appropriate, or by dark-field microscopy, and photographed by using an Olympus MagnaFIRE camera. The labeling observed by IFA was highly specific and consistent with the immunoblot analyses; the type A-producing isolate was surface labeled (Fig. 3B), while the B. burgdorferi LDP74 type K OspC was not (Fig. 3C). In addition, consistent with the upregulation of OspC at elevated temperature, IFAs revealed markedly greater surface labeling of spirochetes grown at 37°C than cells grown at 33°C. The α-FlaB antiserum, which recognizes an inner-membrane-anchored, periplasmic protein, did not label nonpermeabilized cells but readily labeled cells permeabilized with acetone (data not shown). This control demonstrates that the loop 5 epitope is in fact surface exposed and that the experimental conditions used in the IFA did not disrupt cell integrity and thereby artificially expose epitopes that are not naturally presented on the surface of the bacteria.
FIG. 3.
Demonstration that loop 5 is surface exposed and that antibody to loop 5 is bactericidal. The IFAs and bactericidal assays were conducted with antiserum generated against type A loop 5. (A) The results demonstrate the specificity of the anti-loop 5 antiserum. Whole-cell lysates of B. burgdorferi B31 MI, B. parkeri, and r-type A loop 5 fragment were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, immunoblotted, and screened with anti-type A loop 5 antiserum (1:1,000). Molecular masses of the protein markers are to the right of the figure. To assess the surface exposure of loop 5 on intact cells, IFAs were performed. (B and C) B. burgdorferi strains B31MI (type A [B]) and LDP74 (type K [C]) were grown at 37°C, washed with PBS, and fixed to slides by air drying. The slides were blocked and probed with a 1:1,000 dilution of mouse α-loop 5 antiserum or mouse preimmune serum as indicated. Secondary detection was by Alexa Fluor 568-conjugated goat α-mouse IgG (Molecular Probes) at 10 μg ml−1. Magnification, ×400. Dark-field images for the mouse preimmune serum and the anti-type A loop 5 antiserum are shown in panels B and C. The loop 5 antiserum specifically labels only the type A cells. (D and E) To assess the potential bactericidal activity of the anti-loop 5 antiserum, bactericidal assays were performed. B. burgdorferi B31MI (type A) was incubated with preimmune sera (D) or mouse α-loop 5 antiserum in the presence of guinea pig complement (E). Staining was then performed by using the BacLight LIVE/DEAD assay system (Molecular Probes). Live cells stain green, whereas dead or damaged cells stain red. All procedures were performed as described in the text.
The ability of the loop 5 antiserum to efficiently bind to OspC at the cell surface raised the possibility that the interaction could be bactericidal, as has been demonstrated for antibody to full-length OspC (3, 14, 15, 19, 25). To determine whether antibody targeting loop 5 also exhibits bactericidal activity, killing assays were conducted with B. burgdorferi isolates B31MI and LDP74 cultivated at 33°C or temperature shifted to 37°C. The spirochetes were harvested by centrifugation, washed, and adjusted to 5 × 105 cells per 500 μl (in BSK-H medium), and 12.5 μl was transferred into a sterile 0.65-ml microcentrifuge tube. Then, 10 μl of heat-inactivated (56°C; 30 min) loop 5 serum was added with or without guinea pig complement (7.5 μl; Sigma Chemical, St. Louis, Mo.), the components were mixed and incubated at 33 or 37°C for 8 h. A total of 70 μl of H2O was added, and spirochetes were stained with the Live/Dead BacLight stain (Molecular Probes, Eugene, Oreg.) according to the manufacturer's instructions. In brief, two stains are added to the cells; SYTO 9 and propidium iodide. These dyes can distinguish live bacteria (i.e., with intact membranes) from bacteria with compromised membranes. Live bacteria fluoresce green due to staining with SYTO 9, whereas dead or damaged bacteria fluoresce red due to staining with propidium iodide. The baseline level of cells with disrupted membranes observed upon treatment with preimmune heat inactivated serum (with or without complement) was ∼25% (Fig. 3D). In contrast, ∼70% of the cells exposed to the α-loop 5 antiserum displayed membrane disruption (Fig. 3E). The bactericidal activity was determined to be complement dependent. The blebbing effect seen here upon treatment with anti-loop 5 antibody is consistent with that reported with other anti-OspC antibodies (3, 8). It is also important to note that, consistent with the upregulation of OspC at elevated temperature, the percentage of dead cells was consistently higher in spirochetes grown at 37°C than that in bacteria grown at 33°C (data not shown). It is clear from the data presented that anti-loop 5 antibody is bactericidal.
Several reports have outlined the clear and strong justification for the development of Lyme disease vaccines (reviewed in reference 12). However, at the present time, no vaccine is commercially available. In an effort to develop a broadly protective Lyme disease vaccine, Baxter pursued a strategy of generating a vaccine cocktail of 14 different full-length r-OspC proteins (12). However, the cocktail was deemed unacceptably reactigenic. The reactigenicity may have resulted from the large amount of protein that was required to elicit a sufficient response to the unique protective epitopes of each OspC type protein in the cocktail. A potential problem with cocktail vaccines that use multiple full-length proteins is the potential for misdirection of the antibody response to conserved, irrelevant, nonprotective epitopes. It may be possible to overcome this problem through the development of a chimeric, r-vaccinogen composed of the naturally presented immunodominant linear epitopes of each of the dominant OspC types. This general concept has its origins in efforts to develop malarial vaccines using epitopes from proteins expressed at different stages of infection (12). The same concept has been applied in the development of a hexavalent M protein vaccine for group A streptococci (5) and in the development of vaccines against several other pathogens with excellent success (2, 4, 9, 13, 16, 21, 31). With new insights into the physical and antigenic structure of OspC, it may now be possible to develop an effective, r-polyvalent, chimeric, OspC vaccine. The newly identified loop 5 domain appears to be ideally suited for inclusion in such a vaccine.
Acknowledgments
This study was supported in part by grants from NIAID, NIH (R.T.M.), and the American Heart Association (C.G.E.) and by a minority supplement from NIAID, NIH (E.L.B.).
REFERENCES
- 1.Alghaferi, M. Y., J. M. Anderson, J. Park, P. G. Auwaerter, J. N. Aucott, D. E. Norris, and J. S. Dumler. 2005. Borrelia burgdorferi ospC heterogeneity among human and murine isolates from a defined region of Northern Maryland and Southern Pennsylvania: lack of correlation with invasive and non-invasive genotypes. J. Clin. Microbiol. 43:1879-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apta, D., K. Raviprakashb, A. Brinkman, A. Semyonov, S. Yang, C. Skinnera, L. Diehl, R. Lyons, K. Porter, and J. Punnonen. 2006. Tetravalent neutralizing antibody response against four dengue serotypes by a single chimeric dengue envelope antigen. Vaccine 24:335-344. [DOI] [PubMed]
- 3.Bockenstedt, L. K., E. Hodzic, S. Feng, K. W. Bourrel, A. de Silva, R. R. Montgomery, E. Fikrig, J. D. Radolf, and S. W. Barthold. 1997. Borrelia burgdorferi strain-specific OspC-mediated immunity in mice. Infect. Immun. 65:4661-4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caro-Aguilar, I., S. Lapp, J. Pohl, M. R. Galinski, and A. Moreno. 2005. Chimeric epitopes delivered by polymeric synthetic linear peptides induce protective immunity to malaria. Microbes Infect. 7:1324-1337. [DOI] [PubMed]
- 5.Dale, J. B. 1999. Mutlivalent group A streptococcal vaccine designed to optimize the immunogenicity of six tandem M protein fragments. Vaccine 17:193-200. [DOI] [PubMed] [Google Scholar]
- 6.Earnhart, C. G., E. L. Buckles, J. S. Dumler, and R. T. Marconi. 2005. Demonstration of OspC type diversity in invasive human Lyme disease isolates and identification of previously uncharacterized epitopes that define the specificity of the OspC antibody response. Infect. Immun. 73:7869-7877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eicken, C., C. Sharma, T. Klabunde, R. T. Owens, D. S. Pikas, M. Hook, and J. C. Sacchettini. 2001. Crystal structure of Lyme disease antigen outer surface protein C from Borrelia burgdorferi. J. Biol. Chem. 276:10010-10015. [DOI] [PubMed] [Google Scholar]
- 8.Escudero, R., M. Halluska, P. Backenson, J. Coleman, and J. Benach. 1997. Characterization of the physiological requirements for the bactericidal effects of a monoclonal antibody to OspB of Borrelia burgdorferi by confocal microscopy. Infect. Immun. 65:1908-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan, C. F., and X. G. Mei. 2005. Co-immunization of BALB/c mice with recombinant immunogens containing G protein fragment and chimeric CTL epitope of respiratory syncytial virus induces enhanced cellular immunity and high level of antibody response. Vaccine 23:4453-4461. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs, R., S. Jauris, F. Lottspeich, V. Preac-Mursic, B. Wilske, and E. Soutschek. 1992. Molecular analysis and expression of a Borrelia burgdorferi gene encoding a 22-kDa protein (pC) in Escherichia coli. Mol. Microbiol. 6:503-509. [DOI] [PubMed] [Google Scholar]
- 11.Gilmore, R. D., K. J. Kappel, M. C. Dolan, T. R. Burkot, and B. J. B. Johnson. 1996. Outer surface protein C (OspC) but not P39 is a protection immunogen against a tick-transmitted Borrelia burgdorferi challenge: evidence for a conformational protective epitope in OspC. Infect. Immun. 64:2234-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanson, M. S., and R. Edelman. 2004. Vaccines against Lyme disease, p. 487-498. In M. Levine, J. B. Kaper, R. Rappuoli, M. A. Liu, and M. F. Good (ed.), New generation vaccines, vol. 3. Marcel Dekker AG, New York, N.Y. [Google Scholar]
- 13.Horvath, A., L. Karpati, H. K. Sun, M. Good, and I. Toth. 2005. Toward the development of a synthetic group a streptococcal vaccine of high purity and broad protective coverage. J. Med. Chem. 47:4100-4104. [DOI] [PubMed] [Google Scholar]
- 14.Ikushima, M., K. Matsui, F. Yamada, S. Kawahashi, and A. Nishikawa. 2000. Specific immune response to a synthetic peptide derived from outer surface protein C of Borrelia burgdorferi predicts protective borreliacidal antibodies. FEMS Immunol. Med. Microbiol. 29:15-21. [DOI] [PubMed] [Google Scholar]
- 15.Jobe, D. A., S. D. Lovrich, R. F. Schell, and S. M. Callister. 2003. C-terminal region of outer surface protein C binds borreliacidal antibodies in sera from patients with Lyme disease. Clin. Diagn. Lab. Immunol. 10:573-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotloff, K. L., M. Coretti, K. Palmer, J. D. Campbell, M. A. Reddish, M. C. Hu, S. S. Wasserman, and J. B. Dale. 2005. Safety and immunogenicity of a recombinant multivalent group A streptococcal vaccine in healthy adults: phase 1 trial. JAMA 292:738-739. [DOI] [PubMed] [Google Scholar]
- 17.Kumaran, D., S. Eswaramoorthy, B. J. Luft, S. Koide, J. J. Dunn, C. L. Lawson, and S. Swaminathan. 2001. Crystal structure of outer surface protein C (OspC) from the Lyme disease spirochete, Borrelia burgdorferi. EMBO J. 20:971-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lagal, V., D. Postic, E. Ruzic-Sabljic, and G. Baranton. 2003. Genetic diversity among Borrelia strains determine by single-stranded confromation polymorphism analysis of the ospC gene and its association with invasiveness. J. Clin. Microbiol. 41:5059-5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lovrich, S. D., D. A. Jobe, R. F. Schell, and S. M. Callister. 2005. Borreliacidal OspC antibodies specific for a highly conserved epitope are immunodominant in human Lyme disease and do not occur in mice or hamsters. Clin. Diagn. Lab. Immunol. 12:746-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mbow, M. L., R. D. Gilmore, Jr., and R. G. Titus. 1999. An OspC-specific monoclonal antibody passively protects mice from tick-transmitted infection by Borrelia burgdorferi B31. Infect. Immun. 67:5470-5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNeil, S. A., S. A. Halperin, J. M. Langley, B. Smith, A. Warren, G. P. Sharratt, D. M. Baxendale, M. A. Reddish, M. C. Hu, S. D. Stroop, J. Linden, L. F. Fries, P. E. Vink, and J. B. Dale. 2005. Safety and immunogenicity of 26-valent group a streptococcus vaccine in healthy adult volunteers. Clin. Infect. Dis. 41:1114-1122. [DOI] [PubMed] [Google Scholar]
- 22.Probert, W. S., M. Crawford, R. B. Cadiz, and R. B. LeFebre. 1997. Immunization with outer surface protein (Osp) A but not OspC provides cross-protection of mice challenged with North American isolates of Borrelia burgdorferi. J. Infect. Dis. 175:400-405. [DOI] [PubMed] [Google Scholar]
- 23.Probert, W. S., and R. B. LeFebvre. 1994. Protection of C3H/HeN mice from challenge with Borrelia burgdorferi through active immunization with OspA, OspB, or OspC but not with OspD or the 83-kilodalton antigen. Infect. Immun. 62:1920-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts, D., M. Caimano, J. McDowell, M. Theisen, A. Holm, E. Orff, D. Nelson, S. Wikel, J. Radolf, and R. Marconi. 2002. Environmental regulation and differential expression of members of the Bdr protein family of Borrelia burgdorferi. Infect. Immun. 70:7033-7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rousselle, J. C., S. M. Callister, R. F. Schell, S. D. Lovrich, D. A. Jobe, J. A. Marks, and C. A. Wienke. 1998. Borreliacidal antibody production against outer surface protein C of Borrelia burgdorferi. J. Infect. Dis. 178:733-741. [DOI] [PubMed] [Google Scholar]
- 26.Scheiblhofer, S., R. Weiss, H. Durnberger, S. Mostbock, M. Breitenbach, I. Livey, and J. Thalhamer. 2003. A DNA vaccine encoding the outer surface protein C from Borrelia burgdorferi is able to induce protective immune responses. Microbes Infect. 5:939-946. [DOI] [PubMed] [Google Scholar]
- 27.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 92:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seinost, G., D. E. Dykhuizen, R. J. Dattwyler, W. T. Golde, J. J. Dunn, N. Wang, G. P. Wormser, M. E. Schriefer, and B. J. Luft. 1999. Four clones of Borrelia burgdorferi sensu stricto cause invasive infection in humans. Infect. Immun. 67:3518-3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Theisen, M., B. Frederiksen, A.-M. Lebech, J. Vuust, and K. Hansen. 1993. Polymorphism in ospC gene of Borrelia burgdorferi and immunoreactivity of OspC protein: implications for taxonomy and for use of OspC protein as a diagnostic antigen. J. Clin. Microbiol. 31:2570-2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang, I. N., D. E. Dykhuizen, W. Qiu, J. J. Dunn, E. M. Bosler, and B. J. Luft. 1999. Genetic diversity of ospC in a local population of Borrelia burgdorferi sensu stricto. Genetics 151:15-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang, X. N., G. P. Zhang, J. Y. Zhou, C. H. Feng, Y. Y. Yang, Q. M. Li, J. Q. Guo, H. X. Qiao, J. Xi, D. Zhao, G. X. Xing, Z. L. Wang, S. H. Wang, Z. J. Xiao, X. W. Li, and R. G. Deng. 2005. Identification of neutralizing epitopes on the VP2 protein of infectious bursal disease virus by phage-displayed heptapeptide library screening and synthetic peptide mapping. Viral Immunol. 18:549-557. [DOI] [PubMed] [Google Scholar]
- 32.Wilske, B., U. Busch, V. Fingerle, S. Jauris-Heipke, V. Preac-Mursic, D. Robler, and G. Will. 1996. Immunological and molecular variability of OspA and OspC: implications for Borrelia vaccine development. Infection 24:208-212. [DOI] [PubMed] [Google Scholar]
- 33.Wilske, B., V. Preac-Mursic, S. Jauris, A. Hofmann, I. Pradel, E. Soutschek, E. Schwab, G. Will, and G. Wanner. 1993. Immunological and molecular polymorphisms of OspC, an immunodominant major outer surface protein of Borrelia burgdorferi. Infect. Immun. 61:2182-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]