Abstract
Several human mucosal fluids are known to possess an innate ability to inhibit human immunodeficiency virus type 1 (HIV-1) infection and replication in vitro. This study compared the HIV-1 inhibitory activities of several mucosal fluids, whole, submandibular/sublingual (sm/sl), and parotid saliva, breast milk, colostrum, seminal plasma, and cervicovaginal secretions, from HIV-1-seronegative donors by using a 3-day microtiter infection assay. A wide range of HIV-1 inhibitory activity was exhibited in all mucosal fluids tested, with some donors exhibiting high levels of activity while others showed significantly lower levels. Colostrum, whole milk, and whole saliva possessed the highest levels of anti-HIV-1 activity, seminal fluid, cervicovaginal secretions, and sm/sl exhibited moderate levels, and parotid saliva consistently demonstrated the lowest levels of HIV-1 inhibition. Fast protein liquid chromatography gel filtration studies revealed the presence of at least three distinct peaks of inhibitory activity against HIV-1 in saliva and breast milk. Incubation of unfractionated and fractionated whole saliva with antibodies raised against human lactoferrin (hLf), secretory leukocyte protease inhibitor (SLPI), and, to a lesser extent, MG2 (high-molecular-weight mucinous glycoprotein) reduced the HIV-1 inhibitory activity significantly. The results suggest that hLf and SLPI are two key components responsible for HIV-1 inhibitory activity in different mucosal secretions. The variation in HIV inhibitory activity between the fluids and between individuals suggests that there may be major differences in susceptibility to HIV infection depending both on the individual and on the mucosal fluid involved.
Novel intervention strategies to reduce mucosal transmission of human immunodeficiency virus type 1 (HIV-1) are becoming increasingly important as 90% of new infections worldwide result from sexual or perinatal transmission (25). In recent years, one such strategy has been to identify endogenous human factors that possess potent antiviral activities which could ultimately be used in active microbicidal formulations in order to prevent HIV-1 transmission.
Endogenous anti-HIV-1 activity has been demonstrated in whole, parotid, and submandibular/sublingual (sm/sl) saliva, colostrum, whole milk, and seminal plasma (1, 11, 13, 24, 29, 32, 38, 45, 46, 51) but not in cerebrospinal fluid or urine (38). The incidence of oral HIV-1 transmission is very low and can be attributed both to endogenous salivary factors that prevent oral excretion of transmissible levels of virus (45, 47, 48) and to lysis of HIV-infected cells due to the hypotonicity of saliva (2, 3). Anti-HIV-1 activity has been detected consistently in whole saliva. As initially reported by Fultz (11), whole saliva from humans and chimpanzees potently inhibited infection of peripheral blood mononuclear cells by HIV-1. Filtration of saliva prior to testing resulted in a partial decrease in HIV-1 inhibitory activity, indicating that saliva contains both filterable and nonfilterable antiviral factors (64). The filterable component was shown to be high-molecular-mass mucins, such as MG2 (150 to 200 kDa), which act by aggregating the virus, thus reducing titers of HIV-1 in saliva (24, 30, 57). Nagashunmugam et al. (30) also demonstrated virus aggregation and stripping of the envelope glycoprotein gp120 from the virus by inhibitory components in sm/sl saliva.
A filterable, low-molecular-mass protein that has been extensively investigated is secretory leukocyte protease inhibitor (SLPI), an 11.7-kDa protein present in oral, respiratory, and genital secretions (8, 14, 20, 26, 45-48, 52, 54, 62). Many of these studies have suggested an important role for SLPI in inhibiting HIV-1 activity, while others have indicated either no role (59) or a variable effect (17) of SLPI. However, there appears to be a correlation between elevated levels of salivary SLPI and an increased HIV-1 inhibitory effect of whole saliva (54). The mechanism by which SLPI inhibits HIV-1 infections is thought to involve the host cell target rather than direct binding of SLPI to the virus (26, 27, 59), and recently, annexin II, which is a cofactor for macrophage HIV-1 infection, has been identified as a host ligand for SLPI (23).
Another secretory factor with anti-HIV-1 properties is human lactoferrin (hLf) (9), an iron-binding glycoprotein of the transferrin family (4, 61). This 80-kDa glycoprotein exhibits bacteriostatic and bactericidal activity against diverse pathogenic microorganisms (34, 44, 58, 63). The bovine and human milk forms have also been reported to have antiviral activities against a number of viruses, including HIV-1 (5, 13, 21, 22, 31).
Thus, it is probable that the anti-HIV-1 activity in mucosal fluids, particularly saliva, may arise from several endogenous factors that work in synergy. However, despite numerous investigations into the HIV-1 inhibitory activity of individual mucosal secretions, little comparative information exists that describes the relative anti-HIV-1 properties inherent in a variety of mucosal fluids. Therefore, we have compared the anti-HIV-1 activities in saliva (whole, parotid, and sm/sl), colostrum, whole milk, seminal plasma, and cervicovaginal secretions to identify common key components that inhibit HIV-1 activity.
MATERIALS AND METHODS
Mucosal samples.
A total of 65 mucosal fluid samples were collected from 45 volunteers. These included 10 samples of breast milk, 5 samples of colostrum, 10 samples of cervicovaginal secretions from females, and 10 samples of seminal plasma from males. Ten subjects (five males and five females) donated matched whole, parotid, and sm/sl saliva. All samples were collected in accordance with appropriate ethical approval guidelines obtained from the ethics committee of Guy's and St. Thomas' Hospitals. All subjects were of unknown HIV-1 infection status but were all healthy individuals from low-risk groups for HIV-1. The subjects ranged from 21 to 65 years of age, and both male and female subjects were recruited. All human mucosal fluids were tested at physiological concentrations (i.e., undiluted) unless specified otherwise. All samples were stored in aliquots at −20°C until tested for HIV-1 inhibitory activity.
Unstimulated whole saliva (5 ml) was collected by expectoration into a sterile chilled container. Saliva was clarified by centrifugation at 8,000 × g for 30 min at 4°C and then stored at −20°C. Repeated freeze-thawing of the sample was avoided, as this could have resulted in a loss of HIV-1 inhibitory activity. Parotid saliva was collected using Lashley cups placed directly over one parotid duct and held in place with gentle suction (19); salivary flow was stimulated with 100 μl of 5% citric acid at 2-min intervals for a total of 10 min, and the secretion was collected into a sterile chilled container. sm/sl saliva was obtained using a universally fitting sm/sl collector (kindly provided by the Department of Dental Materials at King's College London Dental Institute). The collector was fitted onto the floor of the oral cavity and held in place by gentle suction, and sm/sl saliva was stimulated with 5% citric acid as described above. In order to remove any contaminating citric acid that might interfere with the assays, sm/sl saliva, once collected, was dialyzed against 0.25% phosphate-buffered saline (PBS) and lyophilized prior to storage. Prior to use, the lyophilized saliva was resuspended in PBS to its original volume and clarified by centrifugation at 5,000 × g for 5 min at 4°C.
Colostrum and breast whole-milk samples were expressed into sterile containers and stored at −20°C immediately. Prior to use, samples were centrifuged at 6,000 × g for 30 min at 4°C to remove the lipid fraction. Colostrum samples were collected during the first 3 days postpartum and were distinguished by an intense yellow color relative to that of breast milk. Samples were filter sterilized prior to use. Semen was collected from donors at the Assisted Conception Unit of St. Thomas' Hospital, London, United Kingdom, by ejaculation into a sterile container. Samples (2 ml) were centrifuged at 4,000 × g for 10 min at 4°C and the supernatant stored at −20°C.
Cervicovaginal secretion samples were collected based on the methods described by O'Shea et al. (35). Briefly, tampons were inserted intravaginally for 10 h overnight and removed the next day and placed into a sterile tube. Sterile ammonium bicarbonate (10 ml) was added and placed on a rolling drum for 20 min at room temperature. The eluate was extracted by squeezing through a 50-ml syringe and was lyophilized and stored at −4°C. Prior to use, the lyophilized sample was resuspended in RPMI 1640 culture medium to approximate physiological concentrations and centrifuged at 3,000 × g for 10 min at 4°C.
Virus and cell lines.
The laboratory strain HIV-1 RF was obtained from the MRC AIDS Reagent Project (37). Virus titer was quantified by end point dilution in H9 lymphoblastoid cell cultures and expressed as the 50% tissue culture infective dose (TCID50). Stock virus was stored in liquid nitrogen, and 50 TCID50s were used in all assays. The human T-lymphoblastoid cell line C8166 was obtained from the MRC AIDS Reagent Project (41). Cells were cultured in growth medium supplemented with 10% (vol/vol) heat-inactivated fetal calf serum, penicillin-gentamicin (15 μg/ml), and nystatin (100 U/ml) and incubated at 37°C in 5% CO2.
Direct cytotoxicity of mucosal fluids.
Mucosal fluids (50 μl) were incubated in 96-well microtiter plates with C8166 cells at a concentration of 4 × 104 cells in 300 μl of growth medium per well. Cultures were incubated in 5% CO2 at 37°C for 72 h. The controls were C8166 cells incubated with medium only. All cultures were examined microscopically for viability using the trypan blue staining method.
Assessment of antiviral activity.
The 3-day microtiter-based infection assay described by O'Connor et al. (32) was adapted as follows. Poly-l-lysine hydrobromide (50 μl of 50 μg/ml; Sigma) was used to coat 96-well microtiter plates at room temperature for 1 h. HIV-1RF (50 TCID50s in 25 μl of growth medium) was added to each well and incubated for 1 h at room temperature to allow HIV-1 binding. Each mucosal fluid sample (50 μl) was added in duplicate and incubated at 37°C in 5% CO2 for 1 h. All seminal fluid samples were assessed for anti-HIV-1 activity at a dilution of 1:50 with growth medium to avoid cytotoxicity (32). C8166 cells were then added at a concentration of 4 × 104 cells in 300 μl growth medium and incubated at 37°C in 5% CO2 for 72 h. Plates were washed three times with PBS between all stages. HIV-1 infection was assessed by quantitating the p24 antigen in culture supernatants, using an enzyme-linked immunosorbent assay (Abbott Diagnostics). The results were expressed as the percentage of p24 antigen (pg/ml) in mucosal fluid-treated cells compared with that in cells treated with virus alone. All mucosal fluid samples were assessed for anti-HIV-1 activity in three independent assays and the mean levels determined. The coefficient of variation for a pooled saliva sample tested five times on different days was 26.7%.
FPLC gel filtration of whole saliva and breast milk.
The positions of the marker proteins, MG2 (250 kDa; Sigma), sheep breast milk lactoferrin (80 kDa), and recombinant SLPI (14.7 kDa; R&D Systems, United Kingdom), were calibrated on a Superose 12 gel chromatography column (Pharmacia). Whole saliva and breast milk demonstrated high levels of HIV-1 inhibitory activity and were selected for fractionation by fast protein liquid chromatography (FPLC) gel filtration in order to identify the active components. Pooled breast milk (four subjects) was centrifuged at 6,000 × g for 30 min at 4°C to remove lipids. Pooled whole saliva (four subjects) was centrifuged at 8,000 × g for 30 min at 4°C to remove particulate material. An aliquot (500 μl) of each was filtered (0.2 μm), applied to the column, and eluted with 50 mM Tris containing 150 mM NaCl (pH 7.0). Fractions (1 ml) were collected, concentrated using centrifugal filters (Millipore), and filter sterilized (0.2 μm) prior to being tested in the 3-day infection assay. All fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, using 12% polyacrylamide gels according to the method of Laemmli et al. (18). The gels were either silver stained or transferred to nitrocellulose and analyzed by Western blotting, using polyclonal immunoglobulin G (IgG) anti-human lactoferrin antibody (1:250 in PBS containing 1% bovine serum albumin; Dako, Denmark) and polyclonal IgG human anti-SLPI antibody (1:1,000 in PBS containing 1% bovine serum albumin; R&D Systems, United Kingdom).
Effect of SLPI, hLf, and MG2 antibodies on anti-HIV-1 activity in saliva.
Pooled whole saliva and salivary fractions obtained as described above were incubated for 1 h at 37°C in 5% CO2 with polyclonal antibodies to SLPI (1:2,000; R&D Systems), hLf (1:2,000; Sigma), and MG2 (1:1,000, MUC7 antibody which was raised against the C-terminal peptide; kindly provided by J. Bolscher, Academic Centre for Dentistry, Amsterdam, The Netherlands) or an irrelevant polyclonal IgG rabbit anti-human antibody (1:1,000; Dako, Denmark). The antibody-saliva mixture or antibody salivary fraction mixture was then tested for HIV-1 inhibitory activity as described above.
Statistics.
All statistical analysis was performed with analysis of variance by using the SPSS statistical analysis software package. A P value less than or equal to 0.05 was taken as significant.
RESULTS
Direct cytotoxicity of mucosal fluids.
All mucosal fluids were tested for direct cellular cytotoxicity on the C8166 T-cell line. No effect was observed except for seminal plasma samples, which caused extensive cell death within 48 h, as determined by trypan blue dye exclusion. Therefore, seminal fluid was used at a dilution of 1:50, which showed no cytotoxicity.
Anti-HIV-1 activity of mucosal fluids.
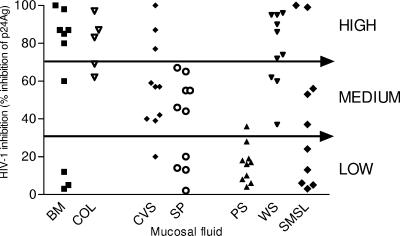
The seven types of mucosal fluids demonstrated various levels of inhibitory activity against HIV-1 (Fig. 1). A wide range of anti-HIV-1 activity was apparent between individuals in all mucosal fluid populations. Despite substantial intersubject differences, subjects tended to exhibit inhibitory levels within the same range (i.e., high, medium, or low anti-HIV-1 activity) for a given fluid. Whole saliva, colostrum, and breast milk demonstrated the highest mean levels of HIV-1 inhibitory activity, with generally over 70% of subjects showing medium to high activity (Fig. 1). Parotid saliva consistently demonstrated the lowest levels of anti-HIV-1 activity, with 80% of subjects exhibiting less than 20% inhibition. Seminal plasma and cervicovaginal secretions had medium levels of HIV-1 inhibitory activity. Of the genital secretions evaluated, cervicovaginal fluid appeared to possess the highest activity, as evidenced by 80% of cervicovaginal secretions having medium to high anti-HIV-1 activity compared with 60% of seminal fluid samples, which exhibited only medium activity. The levels of HIV-1 inhibitory activity shown by each fluid were not altered significantly following filtration (data not shown).
FIG. 1.
HIV-1 inhibitory activity of 65 mucosal fluid samples. Samples representing seven different human mucosal fluid types, namely, breast milk, colostrum, cervicovaginal secretions, seminal plasma, and whole, parotid, and submandibular/sublingual saliva (n = 5 for colostrum samples; n = 10 for all other fluid groups), were assayed in three independent studies (symbol denotes mean for each sample) for HIV-1 inhibition by quantification of p24 antigen (p24Ag) production. Values are expressed as percentages of reduction in p24 antigen production relative to the control with no inhibitor present. BM, breast milk; COL, colostrum; CVS, cervicovaginal secretions; SP, seminal plasma; PS, parotid saliva; WS, whole saliva; SMSL, submandibular/sublingual saliva.
Anti-HIV-1 activity of FPLC fractions from whole saliva and breast milk.
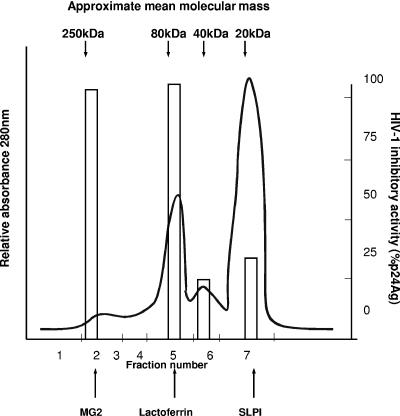
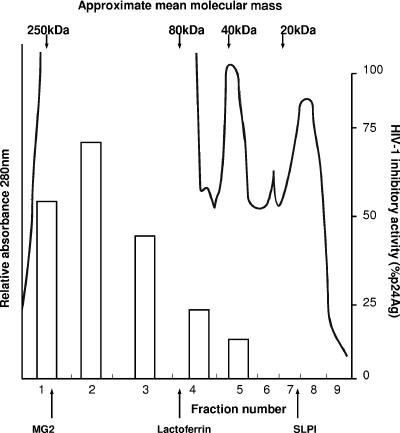
Since whole saliva and breast milk demonstrated the highest mean levels of HIV-1 inhibitory activity, these fluids were fractionated by FPLC and each fraction was tested for anti-HIV-1 activity (Fig. 2 and 3). The marker proteins MG2, hLf, and SLPI eluted at positions corresponding to 250 kDa, 80 kDa, and 14.7 kDa, respectively (Fig. 2 and 3). Whole saliva appeared to contain three or four different peaks of activity (Fig. 2). Fractions 2 and 4 exhibited the highest levels of anti-HIV-1 activity, corresponding to molecular masses from 150 to 250 kDa and 70 to 80 kDa, respectively. Activity was also found in peaks of 40 kDa and 10 to 20 kDa (Fig. 2). Breast milk showed the highest anti-HIV-1 activity in fractions 1 and 2, corresponding to higher-molecular-mass proteins (∼280 kDa and 200 kDa, respectively) (Fig. 3). Activity was present in decreasing amounts in fractions 3, 4, and 5, corresponding to molecular masses of ∼150, 80, and 40 kDa, respectively. No activity was observed in fractions corresponding to the molecular mass of SLPI.
FIG. 2.
FPLC gel filtration (size exclusion chromatography) of unstimulated pooled whole saliva. Unstimulated whole saliva was separated by gel filtration chromatography (FPLC) on a Superose 12 column (Pharmacia), with the elution of proteins monitored at 280 nm. The seven fractions were tested for HIV-1 inhibitory activity using the 3-day microtiter assay (as shown by the open bars), with levels based on percentages of reduction in HIV-1 p24 antigen (p24Ag) production (pg/ml). Standard marker proteins are denoted at the top, and the positions of recombinant MG2, sheep breast milk lactoferrin, and recombinant SLPI are shown at the bottom.
FIG. 3.
FPLC gel filtration chromatography of pooled breast milk. Pooled breast milk was separated by FPLC gel filtration chromatography on a Superose 12 column (Pharmacia), with the elution of proteins monitored at 280 nm, and then divided into nine main fractions. Each fraction was tested for HIV-1 inhibitory activity using the 3-day microtiter assay (as shown by the open bars), with levels based on percentages of reduction in HIV-1 p24 antigen (p24Ag) production (pg/ml). Standard marker proteins are denoted at the top, and the positions of recombinant MG2, sheep breast milk lactoferrin, and recombinant SLPI are shown at the bottom.
Antibody blocking of HIV inhibitory activity in whole saliva.
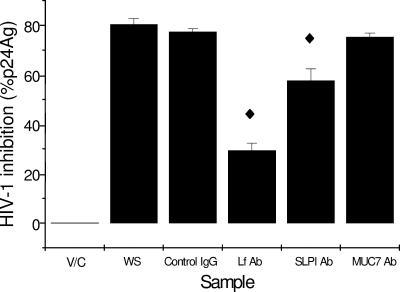
In an attempt to identify the specific contributions of hLf, SLPI, and MG2 to overall anti-HIV-1 activity, we preincubated pooled whole saliva with antibodies specific for these proteins prior to performing the microtiter assay (Fig. 4). Preincubation with the anti-hLf antibody reduced HIV-1 inhibitory activity by ∼65% compared to preincubation with the irrelevant IgG control (P < 0.01), and preincubation with the anti-SLPI antibody reduced inhibitory activity by ∼30% (P < 0.02). Incubation with the anti-MG2 antibody had no significant effect.
FIG. 4.
Effect of antilactoferrin, anti-SLPI, and anti-MG2 antibodies on HIV-1 inhibitory activity of pooled whole saliva from four donors. Pooled, filtered whole saliva was immunoprecipitated with goat polyclonal anti-SLPI, sheep polyclonal antilactoferrin, anti-MG2, or control IgG antibodies for 1 h at 37°C in 5% CO2 prior to the addition of C8166 T cells. Samples were assessed for depletion in HIV inhibitory activity by quantification of p24 antigen (p24Ag) after 72 h using the 3-day microtiter assay. All samples were assayed in triplicate in three independent assays, and the means ± standard errors of the means are shown. V/C, virus control; WS, whole saliva; Control IgG, control antibody; hLf Ab, antilactoferrin antibody; SLPI Ab, SLPI antibody; MUC7 Ab, MG2 antibody. ♦, significantly different from the IgG control (P < 0.05 or less; see text).
Antibody blocking of HIV inhibitory activity in saliva fractions.
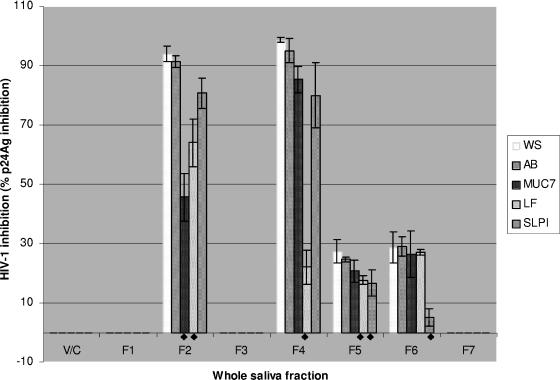
Whole-saliva FPLC fractions were subsequently assessed for reductions in HIV-1 inhibitory activity following preincubation with antibody (Fig. 5). Pretreatment with the anti-hLf antibody significantly reduced the inhibitory activities present in fraction 4 from 98% to 22% (P < 0.005), and there was also some reduction in activity in fractions 2 and 5 (P < 0.05). Preincubation of the fractions with anti-SLPI significantly reduced the anti-HIV-1 activity in fraction 6 (P < 0.005), with some reduction in fraction 5 (P < 0.05) (Fig. 5). In particular, fraction 6 showed the largest reduction (>5-fold) in anti-HIV-1 activity (28% to 5%). Preincubation of saliva fractions with anti-MG2 antibody resulted in a large decrease in the anti-HIV-1 activity of fraction 2 (95% to 48%; P < 0.01) while no significant reduction in activity was evident in fractions 4, 5, and 6 (Fig. 5).
FIG. 5.
FPLC gel filtration whole-saliva fractions (WS) incubated with anti-MG2 (MUC7), -Lf (LF), and -SLPI (SLPI) and control (IgG) antibodies (AB) to determine the reduction in HIV-1 inhibitory activity. Whole-saliva gel filtration fractions (1 to 7) were incubated with control antibody (IgG), anti-MG2, anti-hLf, or goat polyclonal anti-SLPI antibody for 1 h at 37°C in 5% CO2 prior to the addition of C8166 cells. Plates were then left for 72 h at 37°C in 5% CO2 and then assessed for HIV inhibition by quantification of p24 antigen (p24Ag). Samples were assessed in triplicate in three independent assays, and the means ± standard errors of the means are shown. V/C, virus control; ♦, significantly different from the IgG control (P < 0.05 or less; see text).
Thus, the activity in fraction 2 was strongly inhibited (55%) by anti-MUC7, with a smaller reduction with anti-hLF. Activity in fraction 4 was inhibited by >80% with anti-hLF but no other antisera, and activity in fraction 6 was inhibited only by anti-SLPI. The results are consistent with the presence in whole saliva of at least three active anti-HIV factors corresponding to mucin, lactoferrin, and SLPI.
DISCUSSION
Human mucosal fluids can inhibit HIV-1 replication in vitro (1, 11, 13, 15, 29, 39, 45, 52) and may have some role in preventing HIV-1 transmission in vivo. Direct comparisons between studies evaluating the inhibitory activity of mucosal secretions have been difficult due to the utilization of various host cells, HIV-1 strains, and techniques to assess antiviral activity (e.g., HeLa-CD4LTR β-galactosidase cell assay and assays to assess reverse transcriptase activity, p24 antigen content, and syncytium formation) (16, 43, 53). Therefore, we performed an evaluation that directly compared the anti-HIV-1 activities of seven mucosal fluids, utilizing a standardized infection assay to establish relative levels of HIV-1 inhibitory activity and to identify common components that inhibit HIV-1 activity.
Considerable differences in the mean levels of anti-HIV-1 activity were observed between the different mucosal fluid types, which may reflect the differences in relative transmission rates in vivo of HIV-1 by these fluids. We observed that whole saliva inhibited HIV-1 activity by approximately 75% (Fig. 1). The HIV-1 virus is rarely transmitted via the oral route (39), which could be explained partly by low titers of viable virus and partly by the presence of multiple secreted factors that inhibit HIV-1 replication (47). Shugars et al. (50) demonstrated that salivary HIV-1 RNA levels are approximately 10-fold lower than those of matched blood plasma, supporting the low-titer hypothesis. However, oral “hyper-excretors” with salivary HIV-1 viral loads that were at least fivefold higher than in matched blood plasma have also been identified (49). The latter finding corroborates the inhibitory factor hypothesis and indicates that these factors might act by reducing HIV-1 infectivity rather than viral load in saliva. Recently, Bolscher et al. (6) showed inhibition of HIV-1 infectivity by high-molecular-weight salivary components (possibly by entrapment of the virus particles) and strong HIV-1 neutralizing capacity in lower-molecular-weight components in both whole saliva and sm/sl saliva. Similarly, we observed that saliva possessed at least three components of different molecular sizes that appear to inhibit HIV-1 activity (Fig. 2). Thus, it is possible that saliva may affect different stages of the HIV-1 infection cycle through different factors, a hypothesis strongly supported by Bolscher et al. (6), who showed that salivary components acted both prior to and after HIV-1 replication after analyzing proviral DNA synthesis by reverse transcription.
Breast-feeding may account for 16% of vertical HIV-1 transmissions (60), a transmission rate much greater than that through saliva. We found that colostrum and breast milk possessed some of the highest levels of HIV-1 inhibitory activity, similar to whole saliva. However, the average amount of viable virus present in infected breast milk ranges from 240 to 8,100 copies/ml (60), compared with usually less than 1 copy/ml in saliva. This difference in viral titers might explain why the anti-HIV-1 factors present in breast milk have less-apparent protective effect in vivo: simply, there is too high a titer of viable virus for the endogenous protective factors to totally inhibit. It should be noted that some breast milk samples exhibited low antiviral activity (Fig. 1). Thus, one could hypothesize that these subjects may be particularly vulnerable to vertical HIV-1 transmission. Similarly, the low antiviral activity in nearly half of the seminal plasma samples and in some cervicovaginal secretion samples suggests that such subjects may be particularly susceptible to sexual transmission of HIV. In addition, in women, other sexually transmitted diseases often accompany HIV infection, and so, local inflammatory responses caused by sexually transmitted diseases such as Trichomonas vaginalis, which causes the release of host proteases, might further decrease function of HIV inhibitory factors, such as SLPI, in cervicovaginal secretions (7). In this study, it was required to dilute seminal plasma to a point where the cytotoxicity of the cell lines used was abrogated. Thus, the in vivo activity against HIV might be proportionately greater.
The intersubject variation in anti-HIV-1 activity shown with all mucosal fluid types in this study is consistent with intersubject variation observed in previous studies with saliva (6, 10, 24, 54) and seminal fluid (32). However, the finding that intersubject variation also occurs in fluids other than saliva and seminal fluid has not previously been demonstrated. Lower levels of certain anti-HIV-1 factors could give rise to increased susceptibility of an individual to HIV-1 infection via that mucosal route. A study by Pillay et al. (36) showed that rates of perinatal HIV-1 transmission were lower for women with levels of SLPI concentrations of >100 ng/ml and that concentrations did not correlate with local HIV-1 RNA levels. This supports the notion that levels of anti-HIV-1 inhibitory factors in vivo do vary between subjects. However, although we found intersubject variation in HIV-1 inhibitory activity in all fluids, particular fluid types tended to show a particular trend for high, medium, or low activity. Intrasubject variation from the donors that provided three types of saliva (whole, sm/sl, and parotid) was also observed, which confirms previous findings by Malamud et al. (24) and Nagashunmugam et al. (30). There was no apparent correlation between individual anti-HIV-1 activity in the three types of saliva; the majority of parotid saliva possessed low antiviral activity, the majority of sm/sl saliva possessed moderate antiviral activity, and the majority of whole saliva possessed high antiviral activity. In addition, in four subjects anti-HIV-1 activity was higher in parotid than in sm/sl saliva and in six subjects the reverse. This indicates that the antiviral activity of whole saliva is unlikely to be derived from parotid saliva and only partially derived from sm/sl saliva, leaving the possibility that minor salivary glands also contribute to the anti-HIV-1 activity. Fractionation of parotid saliva (data not shown) revealed HIV inhibitory activity at protein peaks corresponding to 80 and 40 kDa only, also suggesting that the majority of SLPI activity in whole saliva may not be derived from parotid saliva.
We observed the mean highest levels of inhibitory activity against HIV-1 in whole saliva (∼75%), colostrum (∼75%), and breast milk (∼60%), confirming previous reports (11, 24, 33, 48, 60). FPLC gel filtration (size exclusion chromatography) and antibody blocking experiments demonstrated that SLPI, hLf, and MG2 (mucin) were the main identifiable inhibitory factors mediating anti-HIV-1 activity in whole saliva and that lactoferrin is a more powerful inhibitor than SLPI (Fig. 2, 3, and 5). It should be noted that we did observe some variance between the reactivity of saliva fractions with the antibodies (Fig. 3) and the inhibitory molecules present in the fractions (Fig. 5). For example, anti-Lf antibody pretreatment significantly reduced the inhibitory effect of the F2 fraction (though not as profoundly as F4, where Lf should be found based primarily on its molecular mass and chromatography with the hLf standard). One plausible explanation would be that hLf could be present in irreversible complexes with other salivary components (such as elastase and higher-molecular-mass proteins) and thus elute in earlier fractions. Similarly, although most SLPI activity was found in F6, where expected, some apparent SLPI activity was found in F5 and might also have been due to complexing. This may require further investigation.
SLPI (26, 47, 48, 62), mucins (24, 57), and hLf (13, 55, 56) have previously been identified in saliva and other mucosal fluids as factors that either independently or synergistically inhibit HIV-1 activity. SLPI might contribute to the reduced HIV-1 transmission through breast milk, as a study of 122 breast-fed infants born to HIV-1-infected mothers revealed that infants that were HIV-1 uninfected at 1 month had higher salivary SLPI levels than HIV-1-infected infants (8). Similarly, Wahl et al. (62) demonstrated that colostrum might contribute to reducing the rates of HIV-1 transmission, as it contains higher levels of SLPI and higher anti-HIV-1 activity than breast milk. Although we did not determine SLPI concentrations in our study, we also observed that on average, colostrum possessed greater anti-HIV-1 activity (∼75%) than breast milk (∼60%) (Fig. 1).
The exact mechanisms by which SLPI and Lf inhibit HIV-1 activity are still uncertain. SLPI appears to block HIV-1 entry by interacting with a non-CD4 cellular receptor protein (26), and recently, annexin II, which is a cofactor for macrophage HIV-1 infection, has been identified as a host ligand for SLPI and appears to be significant in mediating its anti-HIV-1 activity (23). Lf seems to act against HIV-1 on a number of levels. Studies using bovine Lf indicate that Lf can target HIV-1 reverse transcriptase (31) and HIV-1 entry (4, 5, 28), and Swart et al. (56) suggested that Lf might mediate its anti-HIV-1 effect by binding to the V3 domain of the HIV envelope protein gp120. Similarly, Groot et al. (12) recently proposed that Lf binds strongly to DC-SIGN, preventing virus capture and transmission.
This study compared the abilities of various mucosal secretions to inhibit the HIV-1 activity of cell-free virus and not cell-associated virus. We are aware that a significant proportion of HIV in these mucosal fluids occurs as infected leukocytes and that cell-associated virus may more effectively transmit HIV-1 than cell-free virus (14, 40, 42). However, our primary aim was to directly compare intrinsic anti-HIV-1 inhibitory activities in a variety of mucosal fluids and since mucosal secretions appear to inhibit cell-free virus more effectively than cell-associated virus (14, 40, 42), a cell-free assay system was utilized. Furthermore, we utilized the CXCR4-using, laboratory-adapted HIV-1 strain HIV-1RF and transformed T cells. Further studies using monocytotropic CCR5-using viral strains of HIV-1 and clinical isolates might be warranted to support the findings in this paper.
In summary, our study demonstrates the presence of endogenous factors in a number of human mucosal fluids that have the ability to exert an anti-HIV-1 effect in vitro. Whole saliva, breast milk, and colostrum exerted the highest levels of HIV-1 inhibitory activity relative to other human mucosal fluids. Lactoferrin and SLPI appear to be two of the most active anti-HIV-1 proteins in whole saliva. Our data suggest that the variation in HIV inhibitory activity between the fluids and between individuals may indicate major differences in susceptibility to HIV infection depending both on the individual and on the mucosal fluid involved. The data also suggest that further investigation of recombinant forms of active endogenous factors, such as lactoferrin, and their in vivo use would be merited.
Acknowledgments
This work was supported by a research scholarship from the Guy's Hospital Dental School Trust and by the Dunhill Medical Trust.
We would like to thank D. C. Shugars for her critical reading of the manuscript. We would also like to thank J. E. Mullen, K. Corbett, and C. F. Hodgson for their technical assistance and all of the volunteers who kindly participated in this study.
Footnotes
Published ahead of print on 23 August 2006.
REFERENCES
- 1.Atkinson, J. C., C. Yeh, F. G. Oppenheim, D. Bermudez, B. J. Baum, and P. C. Fox. 1990. Elevation of salivary antimicrobial proteins following HIV-1 infection. J. Acquir. Immune Defic. Syndr. 3:41-48. [PubMed] [Google Scholar]
- 2.Baron, S., J. Poast, and M. W. Cloyd. 1999. Why is HIV rarely transmitted by oral secretions? Saliva can disrupt orally shed, infected leukocytes. Arch. Intern. Med. 159:303-310. [DOI] [PubMed] [Google Scholar]
- 3.Baron, S., J. Poast, C. J. Richardson, D. Nguyen, and M. Cloyd. 2000. Oral transmission of human immunodeficiency virus by infected seminal fluid and milk: a novel mechanism. J. Infect. Dis. 181:498-504. [DOI] [PubMed] [Google Scholar]
- 4.Berkhout, B., R. Floris, I. Recio, and S. Visser. 2004. The antiviral activity of the milk protein lactoferrin against the human immunodeficiency virus type 1. Biometals 17:291-294. [DOI] [PubMed] [Google Scholar]
- 5.Berkhout, B., J. L. van Wamel, L. Beljaars, D. K. Meijer, S. Visser, and R. Floris. 2002. Characterization of the anti-HIV effects of native lactoferrin and other milk proteins and protein-derived peptides. Antivir. Res. 55:341-355. [DOI] [PubMed] [Google Scholar]
- 6.Bolscher, J. G., K. Nazmi, L. J. Ran, F. A. van Engelenburg, H. Schuitemaker, E. C. Veerman, and A. V. Nieuw Amerongen. 2002. Inhibition of HIV-1 IIIB and clinical isolates by human parotid, submandibular, sublingual and palatine saliva. Eur. J. Oral Sci. 110:149-156. [DOI] [PubMed] [Google Scholar]
- 7.Draper, D. L., D. V. Landers, M. A. Krohn, S. L. Hillier, H. C. Wiesenfeld, and R. P. Heine. 2000. Levels of vaginal secretory leukocyte protease inhibitor are decreased in women with lower reproductive tract infections. Am. J. Obstet. Gynecol. 183:1243-1248. [DOI] [PubMed] [Google Scholar]
- 8.Farquhar, C., T. C. VanCott, D. A. Mbori-Ngacha, L. Horani, R. K. Bosire, J. K. Kreiss, B. A. Richardson, and G. C. John-Stewart. 2002. Salivary secretory leukocyte protease inhibitor is associated with reduced transmission of human immunodeficiency virus type 1 through breast milk. J. Infect. Dis. 186:1173-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Florisa, R., I. Recio, B. Berkhout, and S. Visser. 2003. Antibacterial and antiviral effects of milk proteins and derivatives thereof. Curr. Pharm. Des. 9:1257-1275. [DOI] [PubMed] [Google Scholar]
- 10.Fox, P. C., A. Wolff, C. K. Yeh, J. C. Atkinson, and B. J. Baum. 1988. Saliva inhibits HIV-1 infectivity. J. Am. Dent. Assoc. 116:635-637. [DOI] [PubMed] [Google Scholar]
- 11.Fultz, P. N. 1986. Components of saliva inactivate human immunodeficiency virus. Lancet ii:1215. [DOI] [PubMed] [Google Scholar]
- 12.Groot, F., T. B. Geijtenbeek, R. W. Sanders, C. E. Baldwin, M. Sanchez-Hernandez, R. Floris, Y. van Kooyk, E. C. de Jong, and B. Berkhout. 2005. Lactoferrin prevents dendritic cell-mediated human immunodeficiency virus type 1 transmission by blocking the DC-SIGN-gp120 interaction. J. Virol. 79:3009-3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harmsen, M. C., P. J. Swart, M. P. de Bethune, R. Pauwels, E. De Clercq, T. H. The, and D. K. Meijer. 1995. Antiviral effects of plasma and milk proteins: lactoferrin shows potent activity against both human immunodeficiency virus and human cytomegalovirus replication in vitro. J. Infect. Dis. 172:380-388. [DOI] [PubMed] [Google Scholar]
- 14.Hocini, H., P. Becquart, H. Bouhlal, H. Adle-Biassette, M. D. Kazatchkine, and L. Belec. 2000. Secretory leukocyte protease inhibitor inhibits infection of monocytes and lymphocytes with human immunodeficiency virus type 1 but does not interfere with transcytosis of cell-associated virus across tight epithelial barriers. Clin. Diagn. Lab. Immunol. 7:515-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isaacs, C. E., K. S. Kim, and H. Thormar. 1994. Inactivation of enveloped viruses in human bodily fluids by purified lipids. Ann. N. Y. Acad. Sci. 724:457-464. [DOI] [PubMed] [Google Scholar]
- 16.Kimpton, J., and M. Emerman. 1992. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated β-galactosidase gene. J. Virol. 66:2232-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konopka, K., N. Shine, E. Pretzer, and N. Duzgunes. 1999. Secretory leukocyte protease inhibitor (SLPI): oxidation of SLPI does not explain its variable anti-HIV activity. J. Dent. Res. 78:1773-1776. [DOI] [PubMed] [Google Scholar]
- 18.Laemmli, U. K., E. Molbert, M. Showe, and E. Kellenberger. 1970. Form-determining function of the genes required for the assembly of the head of bacteriophage T4. J. Mol. Biol. 49:99-113. [DOI] [PubMed] [Google Scholar]
- 19.Lashley, K. S. 1916. Reflex secretion of the human parotid gland. J. Exp. Psychol. 1:461-474. [Google Scholar]
- 20.Lin, A. L., D. A. Johnson, K. T. Stephan, and C. K. Yeh. 2004. Salivary secretory leukocyte protease inhibitor increases in HIV infection. J. Oral Pathol. Med. 33:410-416. [DOI] [PubMed] [Google Scholar]
- 21.Lonnerdal, B. 2004. Human milk proteins: key components for the biological activity of human milk. Adv. Exp. Med. Biol. 554:11-25. [PubMed] [Google Scholar]
- 22.Lu, L., G. Hangoc, A. Oliff, L. T. Chen, R. N. Shen, and H. E. Broxmeyer. 1987. Protective influence of lactoferrin on mice infected with the polycythemia-inducing strain of Friend virus complex. Cancer Res. 47:4184-4188. [PubMed] [Google Scholar]
- 23.Ma, G., T. Greenwell-Wild, K. Lei, W. Jin, J. Swisher, N. Hardegen, C. T. Wild, and S. M. Wahl. 2004. Secretory leukocyte protease inhibitor binds to annexin II, a cofactor for macrophage HIV-1 infection. J. Exp. Med. 200:1337-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malamud, D., T. Nagashunmugam, C. Davis, S. Kennedy, W. R. Abrams, R. Kream, and H. M. Friedman. 1997. Inhibition of HIV infectivity by human saliva. Oral Dis. 3(Suppl. 1):S58-S63. [DOI] [PubMed] [Google Scholar]
- 25.Mayer, K. H., and D. J. Anderson. 1995. Heterosexual HIV transmission. Infect. Agents Dis. 4:273-284. [PubMed] [Google Scholar]
- 26.McNeely, T. B., M. Dealy, D. J. Dripps, J. M. Orenstein, S. P. Eisenberg, and S. M. Wahl. 1995. Secretory leukocyte protease inhibitor: a human saliva protein exhibiting anti-human immunodeficiency virus 1 activity in vitro. J. Clin. Investig. 96:456-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNeely, T. B., D. C. Shugars, M. Rosendahl, C. Tucker, S. P. Eisenberg, and S. M. Wahl. 1997. Inhibition of human immunodeficiency virus type 1 infectivity by secretory leukocyte protease inhibitor occurs prior to viral reverse transcription. Blood 90:1141-1149. [PubMed] [Google Scholar]
- 28.Moriuchi, M., and H. Moriuchi. 2001. A milk protein lactoferrin enhances human T cell leukemia virus type I and suppresses HIV-1 infection. J. Immunol. 166:4231-4236. [DOI] [PubMed] [Google Scholar]
- 29.Nagashunmugam, T., H. M. Friedman, C. Davis, S. Kennedy, L. T. Goldstein, and D. Malamud. 1997. Human submandibular saliva specifically inhibits HIV type 1. AIDS Res. Hum. Retrovir. 13:371-376. [DOI] [PubMed] [Google Scholar]
- 30.Nagashunmugam, T., D. Malamud, C. Davis, W. R. Abrams, and H. M. Friedman. 1998. Human submandibular saliva inhibits human immunodeficiency virus type 1 infection by displacing envelope glycoprotein gp120 from the virus. J. Infect. Dis. 178:1635-1641. [DOI] [PubMed] [Google Scholar]
- 31.Ng, T. B., T. L. Lam, T. K. Au, X. Y. Ye, and C. C. Wan. 2001. Inhibition of human immunodeficiency virus type 1 reverse transcriptase, protease and integrase by bovine milk proteins. Life Sci. 69:2217-2223. [DOI] [PubMed] [Google Scholar]
- 32.O'Connor, T. J., D. Kinchington, H. O. Kangro, and D. J. Jeffries. 1995. The activity of candidate virucidal agents, low pH and genital secretions against HIV-1 in vitro. Int. J. STD AIDS 6:267-272. [DOI] [PubMed] [Google Scholar]
- 33.Orloff, S. L., J. C. Wallingford, and J. S. McDougal. 1993. Inactivation of human immunodeficiency virus type I in human milk: effects of intrinsic factors in human milk and of pasteurization. J. Hum. Lact. 9:13-17. [DOI] [PubMed] [Google Scholar]
- 34.Orsi, N. 2004. The antimicrobial activity of lactoferrin: current status and perspectives. Biometals 17:189-196. [DOI] [PubMed] [Google Scholar]
- 35.O'Shea, S., A. de Ruiter, J. Mullen, K. Corbett, I. Chrystie, M. L. Newell, and J. E. Banatvala. 1997. Quantification of HIV-1 RNA in cervicovaginal secretions: an improved method of sample collection. AIDS 11:1056-1058. [PubMed] [Google Scholar]
- 36.Pillay, K., A. Coutsoudis, A. K. Agadzi-Naqvi, L. Kuhn, H. M. Coovadia, and E. N. Janoff. 2001. Secretory leukocyte protease inhibitor in vaginal fluids and perinatal human immunodeficiency virus type 1 transmission. J. Infect. Dis. 183:653-656. [DOI] [PubMed] [Google Scholar]
- 37.Popovic, M., M. G. Sarngadharan, E. Read, and R. C. Gallo. 1984. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science 224:497-500. [DOI] [PubMed] [Google Scholar]
- 38.Robinovitch, M. R., R. L. Ashley, J. M. Iversen, E. M. Vigoren, F. G. Oppenheim, and M. Lamkin. 2001. Parotid salivary basic proline-rich proteins inhibit HIV-I infectivity. Oral Dis. 7:86-93. [PubMed] [Google Scholar]
- 39.Rothenberg, R. B., M. Scarlett, C. del Rio, D. Reznik, and C. O'Daniels. 1998. Oral transmission of HIV. AIDS 12:2095-2105. [DOI] [PubMed] [Google Scholar]
- 40.Rousseau, C. M., R. W. Nduati, B. A. Richardson, G. C. John-Stewart, D. A. Mbori-Ngacha, J. K. Kreiss, and J. Overbaugh. 2004. Association of levels of HIV-1-infected breast milk cells and risk of mother-to-child transmission. J. Infect. Dis. 190:1880-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salahuddin, S. Z., P. D. Markham, F. Wong-Staal, G. Franchini, V. S. Kalyanaraman, and R. C. Gallo. 1983. Restricted expression of human T-cell leukemia-lymphoma virus (HTLV) in transformed human umbilical cord blood lymphocytes. Virology 129:51-64. [DOI] [PubMed] [Google Scholar]
- 42.Satomi, M., M. Shimizu, E. Shinya, E. Watari, A. Owaki, C. Hidaka, M. Ichikawa, T. Takeshita, and H. Takahashi. 2005. Transmission of macrophage-tropic HIV-1 by breast-milk macrophages via DC-SIGN. J. Infect. Dis. 191:174-181. [DOI] [PubMed] [Google Scholar]
- 43.Schupbach, J., J. Boni, M. Flepp, Z. Tomasik, H. Joller, and M. Opravil. 2001. Antiretroviral treatment monitoring with an improved HIV-1 p24 antigen test: an inexpensive alternative to tests for viral RNA. J. Med. Virol. 65:225-232. [DOI] [PubMed] [Google Scholar]
- 44.Seganti, L., A. M. Di Biase, M. Marchetti, A. Pietrantoni, A. Tinari, and F. Superti. 2004. Antiviral activity of lactoferrin towards naked viruses. Biometals 17:295-299. [DOI] [PubMed] [Google Scholar]
- 45.Shine, N., K. Konopka, and N. Duzgunes. 1997. The anti-HIV-1 activity associated with saliva. J. Dent. Res. 76:634-640. [DOI] [PubMed] [Google Scholar]
- 46.Shine, N. R., S. C. Wang, K. Konopka, E. A. Burks, N. Duzgunes, and C. P. Whitman. 2002. Secretory leukocyte protease inhibitor: inhibition of human immunodeficiency virus-1 infection of monocytic THP-1 cells by a newly cloned protein. Bioorg. Chem. 30:249-263. [DOI] [PubMed] [Google Scholar]
- 47.Shugars, D. C. 1999. Endogenous mucosal antiviral factors of the oral cavity. J. Infect. Dis. 179(Suppl. 3):S431-S435. [DOI] [PubMed] [Google Scholar]
- 48.Shugars, D. C., A. L. Alexander, K. Fu, and S. A. Freel. 1999. Endogenous salivary inhibitors of human immunodeficiency virus. Arch. Oral Biol. 44:445-453. [DOI] [PubMed] [Google Scholar]
- 49.Shugars, D. C., L. L. Patton, S. A. Freel, L. R. Gray, R. T. Vollmer, J. J. Eron, Jr., and S. A. Fiscus. 2001. Hyper-excretion of human immunodeficiency virus type 1 RNA in saliva. J. Dent. Res. 80:414-420. [DOI] [PubMed] [Google Scholar]
- 50.Shugars, D. C., G. D. Slade, L. L. Patton, and S. A. Fiscus. 2000. Oral and systemic factors associated with increased levels of human immunodeficiency virus type 1 RNA in saliva. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 89:432-440. [DOI] [PubMed] [Google Scholar]
- 51.Shugars, D. C., S. P. Sweet, D. Malamud, S. H. Kazmi, K. Page-Shafer, and S. J. Challacombe. 2002. Saliva and inhibition of HIV-1 infection: molecular mechanisms. Oral Dis. 8(Suppl. 2):169-175. [DOI] [PubMed] [Google Scholar]
- 52.Shugars, D. C., and S. M. Wahl. 1998. The role of the oral environment in HIV-1 transmission. J. Am. Dent. Assoc. 129:851-858. [DOI] [PubMed] [Google Scholar]
- 53.Si-Mohamed, A., L. Andreoletti, I. Colombet, M.-P. Carreno, G. Lopez, G. Chatelier, M. D. Kazatchkine, and L. Belec. 2001. Quantitation of human immunodeficiency virus type 1 (HIV-1) RNA in cell-free cervicovaginal secretions: comparison of reverse transcription-PCR amplification (AMPLICOR HIV-A MONITOR 1.5) with enhanced-sensitivity branched-DNA assay (Quantiplex 3.0). J. Clin. Microbiol. 39:2055-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skott, P., E. Lucht, M. Ehnlund, and E. Bjorling. 2002. Inhibitory function of secretory leukocyte proteinase inhibitor (SLPI) in human saliva is HIV-1 specific and varies with virus tropism. Oral Dis. 8:160-167. [DOI] [PubMed] [Google Scholar]
- 55.Superti, F., M. G. Ammendolia, P. Valenti, and L. Seganti. 1997. Antirotaviral activity of milk proteins: lactoferrin prevents rotavirus infection in the enterocyte-like cell line HT-29. Med. Microbiol. Immunol. (Berlin) 186:83-91. [DOI] [PubMed] [Google Scholar]
- 56.Swart, P. J., E. M. Kuipers, C. Smit, B. W. van der Strate, M. C. Harmsen, and D. K. Meijer. 1998. Lactoferrin. Antiviral activity of lactoferrin. Adv. Exp. Med. Biol. 443:205-213. [PubMed] [Google Scholar]
- 57.Tabak, L. A., M. J. Levine, I. D. Mandel, and S. A. Ellison. 1982. Role of salivary mucins in the protection of the oral cavity. J. Oral Pathol. 11:1-17. [DOI] [PubMed] [Google Scholar]
- 58.Teraguchi, S., H. Wakabayashi, H. Kuwata, K. Yamauchi, and Y. Tamura. 2004. Protection against infections by oral lactoferrin: evaluation in animal models. Biometals 17:231-234. [DOI] [PubMed] [Google Scholar]
- 59.Turpin, J. A., C. A. Schaeffer, M. Bu, L. Graham, R. W. Buckheit, Jr., D. Clanton, and W. G. Rice. 1996. Human immunodeficiency virus type-1 (HIV-1) replication is unaffected by human secretory leukocyte protease inhibitor. Antivir. Res. 29:269-277. [DOI] [PubMed] [Google Scholar]
- 60.Van de Perre, P. 2000. Breast milk transmission of HIV-1. Laboratory and clinical studies. Ann. N. Y. Acad. Sci. 918:122-127. [DOI] [PubMed] [Google Scholar]
- 61.van der Strate, B. W., L. Beljaars, G. Molema, M. C. Harmsen, and D. K. Meijer. 2001. Antiviral activities of lactoferrin. Antivir. Res. 52:225-239. [DOI] [PubMed] [Google Scholar]
- 62.Wahl, S. M., T. B. McNeely, E. N. Janoff, D. Shugars, P. Worley, C. Tucker, and J. M. Orenstein. 1997. Secretory leukocyte protease inhibitor (SLPI) in mucosal fluids inhibits HIV-I. Oral Dis. 3(Suppl. 1):S64-S69. [DOI] [PubMed] [Google Scholar]
- 63.Yamaguchi, H., S. Abe, and N. Takakura. 2004. Potential usefulness of bovine lactoferrrin for adjunctive immunotherapy for mucosal Candida infections. Biometals 17:245-248. [DOI] [PubMed] [Google Scholar]
- 64.Yeh, C. K., B. Handelman, P. C. Fox, and B. J. Baum. 1992. Further studies of salivary inhibition of HIV-1 infectivity. J. Acquir. Immune Defic. Syndr. 5:898-903. [PubMed] [Google Scholar]