Abstract
The diagnostic utility of immunochromatographic (Leptotek) and enzyme-linked immunosorbent assay (ELISA; Panbio) tests for the detection of Leptospira immunoglobulin M antibodies was assessed in febrile adults admitted in Vientiane, Laos. Both tests demonstrated poor diagnostic accuracy using admission serum (Leptotek sensitivity of 47.3% and specificity of 75.5%: ELISA sensitivity of 60.9% and specificity of 65.6%) compared to the Leptospira “gold standard” microscopic agglutination test.
The laboratory diagnosis of acute Leptospira infection is usually dependent on serological methods. The “gold standard” microscopic agglutination test (MAT) requires paired specimens and considerable technical resources and training and is therefore not useful for acute patient management (5). Rapid methods, such as lateral flow immunochromatographic tests (ICT) and enzyme-linked immunosorbent assay (ELISA) formats that detect leptospiral immunoglobulin M (IgM) antibodies have demonstrated high diagnostic accuracy (1, 4, 8, 10, 11). However, a recent study in Viet Nam suggested a poor diagnostic utility of such tests there (9). Here we report the diagnostic accuracy of a commercial ELISA and an ICT for the detection of Leptospira IgM antibodies among adults with fever in the Lao People's Democratic Republic (Laos), where leptospirosis is endemic.
Human sera were collected after informed oral consent was obtained as part of a study to determine the causes of unexplained fever for patients presenting at Mahosot Hospital, Vientiane, Laos, between November 2001 and October 2003 (7). Paired admission and convalescent-phase serum specimens were available from 186 patients (total sample, n = 372) and stored at −85°C until tested. Unpaired sera were not included. Ethical approval was granted by the Ethical Review Committee of the Faculty of Medical Sciences, National University of Laos, Vientiane, Laos.
A commercial ELISA (Panbio Pty, Ltd., Australia) for the detection of IgM antibodies against Leptospira species was performed according to the manufacturer's instructions. The results were calculated as “Panbio units” with results of ≤9.0, 9.0 to 11.0, and ≥11.0 defined as negative, equivocal, and positive, respectively. Samples that initially returned an equivocal result were retested. An ICT (Leptotek; Organon-Teknika, The Netherlands) for the detection of Leptospira IgM antibodies was performed according to the manufacturer's instructions. All results were read by eye by the same operator and recorded as positive, equivocal, or negative for the presence of specific IgM antibody.
The MAT for Leptospira antibodies was performed by reference laboratories in The Netherlands and Australia. Samples 1 to 36 were assessed at WHO/FAO/OIE Collaborating Centre for Reference and Research on Leptospirosis, KIT Biomedical Research, Amsterdam, The Netherlands (2). Samples 37 to 186 were assessed at the WHO/FAO/OIE Collaborating Centre for Reference and Research on Leptospirosis in Australia. A patient was considered to have a current or recent Leptospira infection if a serum showed a titer of ≥1:400 or if paired sera demonstrated a fourfold rise over two specimens.
The diagnostic accuracy was calculated for ICT and ELISA by comparing results with the acute- and convalescent-phase MAT results for each patient as an individual case diagnosis. Only admission samples were tested by the ICT. For both ICT and ELISA, equivocal results were regarded as negative. Standard diagnostic accuracy indices of sensitivity, specificity, negative predictive values (NPVs) and positive predictive values (PPVs) with exact 95% confidence intervals (CI), positive and negative likelihood ratios, interquartile (IQR) ranges of days of fever and area under the receiver-operator characteristic curves (AUROCC) were calculated by using Stata/SE 8.0 (Stata Corp., College Station, Texas).
The percentage of patients with a true leptospirosis infection (as defined by MAT diagnostic criteria) was 12.4% (23 of 186). Of these, 78.2% (18 of 23) and 100% had admission and convalescent-phase sample titers of ≥1:400, respectively. The five patients with titers of <1:400 on admission demonstrated a ≥4-fold rise in titer in the convalescent-phase sample. On admission, patients had been ill for a median of 9 days (IQR 7 to 13), and the median interval between admission and convalescent-phase serum collection was 4.5 days (IQR 2 to 8). The diagnostic sensitivity of both assays was poor (Table 1). Overall, the sensitivity of the ELISA was 63.0%, with only a marginal increase in sensitivity when using convalescent-phase sera (65.2%) in comparison to admission sera (60.9%). The sensitivity of the ICT (47.8%) was lower than that of the ELISA. The specificities of the ELISA (55.5%) and ICT (75.5%) were also low, with a lower specificity for convalescent-phase sera than for the admission sera. In comparison to serum from all patients, samples from patients with only 1 to 7 days of fever had higher sensitivity (70.0%) and specificity (70 to 75%) in both assays but with very wide CIs (Table 1).
TABLE 1.
Sensitivities and specificities of the Panbio IgM ELISA and Leptotek IgM lateral flow test for the detection of IgM antibodies compared to the MATa
| Assay | Sample timing | Median days to fever onset (IQR) | Positive (+) or negative (−) | MAT result
|
% (95% CI)
|
||||
|---|---|---|---|---|---|---|---|---|---|
| + | − | Sensitivity | Specificity | PPV | NPV | ||||
| Panbio IgM | Overall | 10 (7-14.5) | + | 29 | 145 | 63.0 (47.6-76.8) | 55.5 (49.9-61.0) | 16.7 (11.5-23.1) | 91.4 (86.6-94.9) |
| ELISA | − | 17 | 181 | ||||||
| Admission | 9 (7-13) | + | 14 | 56 | 60.9 (38.5-80.3) | 65.6 (57.8-72.9) | 20.0 (11.4-31.3) | 92.2 (85.8-96.4) | |
| − | 9 | 107 | |||||||
| Convalescent-phase | 15 (10-20) | + | 15 | 89 | 65.2 (42.7-83.6) | 45.4 (37.6-53.4) | 14.4 (8.3-22.7) | 90.2 (81.7-95.7) | |
| − | 8 | 74 | |||||||
| Days 1 to 7 | 6 (5-7) | + | 7 | 18 | 70.0 (34.8-93.3) | 70.0 (56.8-81.2) | 28.0 (12.1-49.4) | 93.3 (81.7-98.6) | |
| of feverb | − | 3 | 42 | ||||||
| Leptotek lateral | Admission | 9 (7-14) | + | 11 | 40 | 47.3 (26.8-69.4) | 75.5 (68.1-81.9) | 21.6 (11.3-35.3) | 91.1 (85.0-95.3) |
| flow test | − | 12 | 123 | ||||||
| Days 1 to 7 | 6 (5-7) | + | 7 | 15 | 70.0 (34.8-93.3) | 75.0 (62.1-85.3) | 31.8 (13.9-54.9) | 93.8 (82.8-98.7) | |
| of fever | − | 3 | 45 | ||||||
The KIT panel of strains (with serovars in parentheses) included Jez Bratislava (Bratislava), Mus 127 (Ballum), Hond Utrecht IV (Canicola), Duyster (Grippotyphosa), Mandemakers (Grippotyphosa), Hebdomadis (Hebdomadis), Kantorowicz (Icterohaemorrhagiae), Wijnberg (Copenhageni), Poi (Poi), Pomona (Pomona), 1161 U (Proechimys), Hardjoprajitno (Hardjo), Lely 607 (Hardjo), Mus 24 (Saxkoebing), M 84 (Sejroe), Patoc I (Patoc), CH 11 (Andamana), Ballico (Australis), Rachmat (Rachmati), Swart (Bataviae), Celledoni (Celledoni), 3522 C (Cynopteri), Sari (Mini), CZ 214 K (Panama), Salinem (Pyrogenes), Veltat Semarang 173 (Semaranga), 1342 K (Shermani), and Perepelitsin (Tarassovi). The Australian panel of serovars included Pomona, Hardjo, Tarassovi, Grippotyphosa, Celledoni, Copenhageni, Australis, Pyrogenes, Canicola, Hebdomadis, Mini, Sarmin, Autumnalis, Cynopteri, Ballum, Bataviae, Djasiman, Javanica, Panama, Shermani, and Mwalok.
A combination of admission and convalescent-phase specimens.
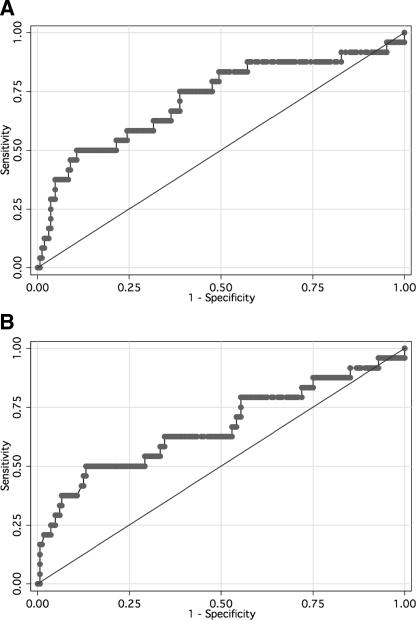
AUROCC analysis of ELISA accuracy (using the cutoff of ≥11.0 Panbio units as suggested by the manufacturer) versus MAT results gave AUROCC values for admission and convalescent-phase samples of 0.72 (95% CI = 0.59 to 0.84) and 0.67 (95% CI = 0.53 to 0.80) (Fig. 1), respectively, suggesting that the ELISA was diagnostically uninformative in both sample timing groups. Modeling of positivity cutoff values to improve the accuracy of the ELISA (Table 2) failed to find a compromise between sensitivity and specificity that provided sufficient accuracy for diagnostic utility. However, increasing the cutoff to ≥17.0 Panbio units made the assay at least as accurate as the ICT.
FIG. 1.
Receiver-operator characteristic curve of the Leptospira IgM ELISA versus the MAT for admission (A) and convalescent-phase (B) specimens.
TABLE 2.
Diagnostic accuracy indices at different positivity cutoff points for the Panbio Leptospira IgM ELISAa
| Cutoff point | %
|
Likelihood ratio
|
|||
|---|---|---|---|---|---|
| Sensitivity | Specificity | Correctly classified | Positive | Negative | |
| ≥9.1 | 75.6 | 53.4 | 56.6 | 1.62 | 0.46 |
| ≥10.1 | 73.2 | 59.1 | 61.1 | 1.79 | 0.45 |
| ≥11.0 | 68.3 | 64.0 | 64.6 | 1.90 | 0.50 |
| ≥13.0 | 61.0 | 71.7 | 70.1 | 2.15 | 0.54 |
| ≥15.0 | 53.7 | 77.3 | 74.0 | 2.37 | 0.60 |
| ≥17.0 | 46.3 | 83.0 | 77.8 | 2.73 | 0.65 |
| ≥19.1 | 43.9 | 88.3 | 81.9 | 3.74 | 0.63 |
| ≥21.0 | 39.0 | 91.5 | 84.0 | 4.59 | 0.67 |
| ≥23.3 | 39.0 | 91.9 | 84.4 | 4.82 | 0.66 |
| ≥25.8 | 34.2 | 94.3 | 85.8 | 6.02 | 0.70 |
| ≥27.0 | 26.8 | 95.1 | 85.4 | 5.52 | 0.77 |
True positivity values are as classified by MAT.
These data suggest that both assays have poor diagnostic accuracy on early serum specimens in Laos, making them unsuitable for the diagnosis of local acute leptospirosis. Similar poor performance of rapid assays has been reported in the adjacent region of Viet Nam where Leptospira is endemic and where a high proportion of clinically well individuals gave positive IgM results (9). The Viet Nam study differed from that presented here since the reference comparator was an in-house ELISA rather than the gold standard MAT. The persistence of leptospire IgM antibody for many months after recovery from leptospirosis (3, 6) and repeated exposure to nonpathogenic Leptospira during farming (9) may be significant factors in the poor specificities of the tests. The ICT and ELISA tests assessed in the present study are not sufficiently accurate for the diagnosis of acute leptospirosis infections in Laos. Antigen-based rapid tests or PCR may prove to be more accurate alternatives.
Acknowledgments
We are very grateful to the patients who participated in this study; the doctors, nurses, and staff of the Microbiology Laboratory, especially Mayfong Mayxay, Anisone Changthongthip, Sengmani Symanivong, Soulignasack Thongpaseuth, Kai-amporn Keopaseuth, Viengmala Siharath; and the Adult Infectious Disease Ward. We thank Chanpheng Thammavong and Bounkong Syhavong; the Minister of Health, Ponmek Dalaloy; and the Director of the Curative Department, Ministry of Health, Sommone Phounsavath, for their support for this study, which was part of the Wellcome Trust-Mahosot Hospital-Oxford Tropical Medicine Research Collaboration funded by the Wellcome Trust of Great Britain. We are very grateful to the British Embassy, Bangkok, Thailand, and to the British Ambassador to the Lao PDR for additional financial support under the Embassy Small Grants Scheme. We are also grateful to Sharon Peacock for helpful comments and suggestions during the preparation of the manuscript.
The authors have no conflict of interest in the conduct of this study. The ELISA tests were provided without charge by PanBio Pty, Ltd. PanBio Pty, Ltd., has had no role in the design, execution, analysis, writing, or submission of this study.
REFERENCES
- 1.Bajani, M. D., D. A. Ashford, S. L. Bragg, C. W. Woods, T. Aye, R. A. Spiegel, B. D. Plikaytis, B. A. Perkins, M. Phelan, P. N. Levett, and R. S. Weyant. 2003. Evaluation of four commercially available rapid serologic tests for diagnosis of leptospirosis. J. Clin. Microbiol. 41:803-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cole, J. R., C. R. Sulzer, and A. R. Pursell. 1973. Improved microtechnique for the leptospiral microscopic agglutination test. Appl. Microbiol. 25:976-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cumberland, P., C. O. Everard, J. G. Wheeler, and P. N. Levett. 2001. Persistence of anti-leptospiral IgM, IgG, and agglutinating antibodies in patients presenting with acute febrile illness in Barbados 1979-1989. Eur. J. Epidemiol. 17:601-608. [DOI] [PubMed] [Google Scholar]
- 4.Levett, P. N., S. L. Branch, C. U. Whittington, C. N. Edwards, and H. Paxton. 2001. Two methods for rapid serological diagnosis of acute leptospirosis. Clin. Diagn. Lab. Immunol. 8:349-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levett, P. N. 2001. Leptospirosis. Clin. Microbiol. Rev. 14:296-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lupidi, R., M. Cinco, D. Balanzin, E. Delprete, and P. E. Varaldo. 1991. Serological follow-up of patients involved in a localized outbreak of leptospirosis. J. Clin. Microbiol. 29:805-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phongmany, S., J. M. Rolain, R. Phetsouvanh, S. D. Blacksell, V. Soukkhaseum, B. Rasachack, K. Phiasakha, S. Soukkhaseum, K. Frichithavong, V. Chu, V. Keolouangkhot, B. Martinez-Aussel, K. Chang, C. Darasavath, O. Rattanavong, S. Sisouphone, M. Mayxay, S. Vidamaly, P. Parola, C. Thammavong, M. Heuangvongsy, B. Syhavong, D. Raoult, N. J. White, and P. N. Newton. 2006. Rickettsial infections and fever, Vientiane, Laos. Emerg. Infect. Dis. 12:256-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smits, H. L., C. K. Eapen, S. Sugathan, M. Kuriakose, M. H. Gasem, C. Yersin, D. Sasaki, B. Pujianto, M. Vestering, T. H. Abdoel, and G. C. Gussenhoven. 2001. Lateral-flow assay for rapid serodiagnosis of human leptospirosis. Clin. Diagn. Lab. Immunol. 8:166-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wagenaar, J. F., T. H. Falke, N. V. Nam, T. Q. Binh, H. L. Smits, F. G. Cobelens, and P. J. de Vries. 2004. Rapid serological assays for leptospirosis are of limited value in southern Vietnam. Ann. Trop. Med. Parasitol. 98:843-850. [DOI] [PubMed] [Google Scholar]
- 10.Winslow, W. E., D. J. Merry, M. L. Pirc, and P. L. Devine. 1997. Evaluation of a commercial enzyme-linked immunosorbent assay for detection of immunoglobulin M antibody in diagnosis of human leptospiral infection. J. Clin. Microbiol. 35:1938-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zochowski, W. J., M. F. Palmer, and T. J. Coleman. 2001. An evaluation of three commercial kits for use as screening methods for the detection of leptospiral antibodies in the UK. J. Clin. Pathol. 54:25-30. [DOI] [PMC free article] [PubMed] [Google Scholar]