Abstract
The limitations of conventional microbiologic methods (CMM) for etiologic diagnosis of community pneumococcal pneumonia have made faster diagnostic techniques necessary. Our aim was to evaluate the usefulness of the immunochromatography (ICT) technique for detecting urinary Streptococcus pneumoniae antigen in the etiologic diagnosis of community-acquired pneumonias (CAP). This was a prospective study on in-patients with CAP in a tertiary hospital conducted from October 2000 to March 2004. Apart from using CMM to reach an etiologic diagnosis, we determined pneumococcal antigen in concentrated urine by ICT. We also determined the urinary pneumococcal antigen (UPA) content in patients from two control groups to calculate the specificity of the technique. One group was comprised of in-patients diagnosed with chronic obstructive pulmonary disease (COPD) or asthma, with respiratory infection, and without pneumonia; the other group included fractures. We studied 959 pneumonia patients and determined UPA content in 911 (95%) of them. We diagnosed the etiology of 253 cases (28%) using CMM; S. pneumoniae was the most common etiologic agent (57 cases). ICT analysis was positive for 279 patients (31%). Using this technique, the percentage of diagnoses of pneumococcal pneumonias increased by 26%, while the overall etiologic diagnosis increased from 28 to 49%. The technique sensitivity was 81%; the specificity oscillated between 80% in CAP with nonpneumococcal etiology and 99% for patients with fractures without infections. Determination of UPA is a rapid, simple analysis with good sensitivity and specificity, which increased the percentage of etiologic diagnoses. Positive UPA may persist in COPD patients with probable pneumococcal colonization or recent pneumococcal infections.
Community-acquired pneumonia (CAP) is a frequent infectious disease in adults that involves high morbidity and mortality rates (1, 3, 9, 8, 17). The incidence of CAP in Europe ranges from 5 to 11 cases/1,000 inhabitants/year (15), while the incidence in Spain ranges from 1.6 to 1.8 episodes/1,000 inhabitants/year (2). In the United States, CAP is the sixth most common cause of overall death, and the most common cause of death related to infection. CAP has an overall mortality rate of 14%, ranging from 37% of intensive care patients to 2% of outpatients (9).
Streptococcus pneumoniae is the most common cause of CAP (28). Moreover, it is probably the microorganism responsible for most pneumonias of unknown etiology. Failure to determine CAP etiology results from limitations in usual diagnosis methods, including (30) the following: (i) low blood culture yield and low gold standard sensitivity (which further decreases when antibiotics have previously been administered) (5), (ii) frequent pneumococcal colonization and infection of the lower airways in some at-risk subgroups, (iii) routine difficulties in obtaining good-quality sputum that can be evaluated to reach an etiologic diagnosis, and (iv) lag time in receiving conventional culture results which prevents providing an early and appropriate treatment.
The above-mentioned diagnostic difficulties, in addition to substantial increases in antibiotic resistance S. pneumoniae, have made it necessary to develop rapid diagnostic techniques to provide appropriate antibiotic treatment, especially in severe CAP cases (18). Consequently, counterimmunoelectrophoresis and latex agglutination techniques have been tested for efficacy in detecting pneumococcal antigens in diverse samples. However, the low sensitivity and specificity rates of these techniques, added to the complicated methodology involved, have made these tests difficult to establish in usual clinical practice (4, 29, 32).
Previous use of urinary antigen detection in other pulmonary infections, such as legionellosis (10, 16), have demonstrated possible diagnostic utility. The immunochromatography (ICT) membrane technique for detecting C polysaccharide antigen in urine, an antigen common to all S. pneumoniae serotypes (13), simplifies and standardizes obtaining a valid sample from most patients, unlike techniques based on sputum collection. The technique is quick and simple and has provided some promising initial results in the diagnosis of community-acquired pneumococcal pneumonias (CAPP) (6, 12, 21, 23). In practical application, it appears to have greater sensitivity if the urine is concentrated beforehand (21, 22, 23).
The aim of the present study was to evaluate the diagnostic usefulness of the ICT membrane for detecting S. pneumoniae antigen in concentrated urine by evaluating the sensitivity and specificity in a nonselected cohort of patients with CAP.
MATERIALS AND METHODS
The present study was carried out at the tertiary Clínico Universitario Hospital in Valencia, which has 585 beds and caters to a population of 324,174 inhabitants. In a prospective study we analyzed a cohort of adult immunocompetent patients (≥15 years) consecutively diagnosed as having CAP and admitted between October 2000 and March 2004.
Pneumonia was initially diagnosed upon conformation of new infiltrate on a thorax X-ray alongside symptoms of respiratory infection. Patients from this population who were later diagnosed with tuberculosis, lung neoplasia, AIDS, serious neutropenia secondary to CAP, or any other diagnosis other than pneumonia were excluded from the study.
Our study included 959 patients with an initial diagnosis of pneumonia, with a mean age of 66.1 years (range, 15 to 97), 64% of whom were males. Patients first underwent an initial clinical and radiological assessment, and blood had been extracted to determine biochemical parameters; the following tests were also taken to reach a microbiologic diagnosis: two blood cultures in aerobe and anaerobe medium and paired serological determinations (on admission and at 4 to 6 weeks) for assessment by indirect immunofluorescence of antibodies against Legionella pneumophila and Chlamydophila pneumoniae. Furthermore, analysis by complement fixation for Mycoplasma pneumoniae, Chlamydia psittaci, Coxiella burnetii, respiratory syncytial virus, influenza viruses A and B, adenovirus, and parainfluenza viruses 1, 2, and 3 was performed. Urine was collected and concentrated for determination of the Legionella pneumophila serogroup 1 antigen. Where appropriate, fibrobronchoscopy was performed, and quantitative cultures of bronchoaspirate and/or bronchoalveolar lavage fluid were made. Pleural fluid was analyzed in all of the cases requiring a thoracentesis. If evaluative expectoration (more than 25 polymorphonuclear cells and fewer than 10 epithelial cells per field at ×100 magnification) was confirmed, Gram staining and sputum culture were carried out.
The conventional criteria for a definitive etiologic diagnosis included (i) isolation of microorganisms in uncontaminated samples (blood and pleural fluid), (ii) seroconversion of the pathogens analyzed in the serologic analysis, (iii) isolation of L. pneumophila in sputum or detection of its antigens using the urinary ICT test, and (iv) isolation of microorganisms in bronchoaspirate (≥105 CFU/ml) and bronchoalveolar lavage (104 CFU/ml) fluid. The patients who did not fulfill these criteria were diagnosed as having CAP of unknown etiology. A CAPP diagnosis was considered probable only when a concurrent presence of gram-positive diplococcus and positive sputum culture for S. pneumoniae was observed in the same sample.
Urine was collected and concentrated from CAP patients either on admission or during the first 24 h to determine the possible presence of the S. pneumoniae antigen. A membrane ICT assay, which can detect the soluble antigen of the pneumococcal polysaccharide C in urine (Binax-Now), was used. Analysis was performed 2 h after urine collection, once it was concentrated. Urine specimens were concentrated 25-fold by selective ultrafiltration (Minicom; Millipore Corp., Bedford, Mass.), boiled for 5 min, and centrifuged at 1,000 × g for 15 min before testing. The technique was performed according to the manufacturer's instructions. Urine is concentrated systematically at our hospital since there is a high incidence of Legionella in our region. The urine concentration step, therefore, did not increase the attendance cost of the process.
We calculated the test sensitivity of the ICT from samples in which the microbiologic diagnosis of CAPP was certain. We also determined the sensitivity in patients with Gram staining and with S. pneumoniae-positive sputum culture who were considered to have a probable CAPP diagnosis.
We used two groups to calculate the specificity of the technique: (i) nonpneumococcal patients with CAP of a known etiologic diagnosis and (ii) a control group of 235 in-patients who had a diagnosis other than CAP, chosen according to age, gender and comorbidities similar to those of CAP who tested antigen positive (Table 1).
TABLE 1.
Demographic and comorbidity characteristics of cases and controlsa
| Parameter | Data for:
|
|
|---|---|---|
| Cases (n = 911) | Controls (n = 235) | |
| Gender (male) (% of total) | 64 | 71 |
| Mean age (yr) ± SD | 66.1 ± 16 | 64.9 ± 14 |
| No. (%) of comorbidity cases | ||
| Diabetes | 268 (28) | 55 (23) |
| Arterial hypertension | 316 (33) | 75 (4) |
| Cardiopathy | 262 (27) | 67 (28) |
| Hepatopathy | 42 (4) | 40 (2) |
| Renal failure | 39 (4) | 11 (4) |
The descriptive statistical analysis was carried out by calculating frequencies in the case of qualitative variables and the mean, range and standard deviation for quantitative variables. We used the Student t test to compare the quantitative variables and the chi-square test for the qualitative variables, with P < 0.05 as the significance level. The statistics package used for data processing was the 11.5 version of SPSS for Windows. All case-versus-control comparisons were found to be not significant.
The descriptive statistical analysis was carried out by calculating frequencies in the case of qualitative variables and the mean, range, and standard deviation for quantitative variables. We used the Student t test to compare the quantitative variables and the chi-square test for the qualitative variables, using a P value of <0.05 as the significance level. The statistics package used for data processing was the 11.5 version of SPSS for Windows XP.
RESULTS
Urinary pneumococcal antigen (UPA) content was determined by the ICT technique in 911 of the 959 (95%) patients with CAP. The lack of ICT membrane kits during the first 24 h prevented antigen determination in 35 cases. Sample was unavailable for six patients due to anuria and for another seven patients due to early death.
The following techniques were used in an attempt to reach an etiologic diagnosis: blood cultures in 785 patients (86%), respiratory serology in 585 cases (64%), direct viewing and culture of pleural fluid in 59 patients (6%), endobronchial samples in 51 intubated patients (6%), and urinary Legionella antigen in 910 patients (95%). Sputum was obtained and evaluated in 654 cases (72%) in accordance with the criteria described by Murray and Washington (25).
A definitive etiologic diagnosis was reached by conventional microbiologic methods (CMM; not including Gram staining or sputum culture) for 253 (28%) of the 911 patients. S. pneumoniae proved to be the most common etiologic agent, being responsible for 57 cases (23% of the CAP with etiologic diagnoses): 51 cases were determined by blood cultures, 5 cases were determined by isolation and quantitative counts in bronchoaspirate, and 1 case was determined by pleural fluid culture (Table 2).
TABLE 2.
Etiologic diagnosis of CAP using conventional microbiologic methods and the rapid urinary antigen test
| Etiologic agent(s) | No. of patients overall (n = 253) | No. of patients positive by ICTa |
|---|---|---|
| Streptococcus pneumoniae | 50 | 40 |
| Legionella pneumophila | 35 | |
| Staphylococcus aureus | 13 | 3 |
| Escherichia coli | 7 | 2 |
| Enterobacter cloacae | 3 | |
| Pseudomonas aeruginosa | 10 | 3 |
| Haemophilus influenzae | 5 | 2 |
| Streptococcus spp. | 14 | 4 |
| Streptococcus pyogenes | 4 | 1 |
| Streptococcus agalactiae | 1 | 1 |
| Streptococcus constellatus | 1 | 1 |
| Streptococcus intermedius | 1 | |
| Streptococcus acidominimus | 1 | 1 |
| Streptococcus mitis | 1 | |
| Viridans group streptococci | 1 | |
| Bacteroides spp. | 1 | 1 |
| Chlamydophila pneumoniae | 14 | 4 |
| Mycoplasma pneumoniae | 8 | 2 |
| Chlamydophila psittaci | 4 | |
| Influenza A virus | 24 | 6 |
| Influenza B virus | 13 | 3 |
| Respiratory syncytial virus | 4 | 1 |
| Parainfluenza virus | 10 | 3 |
| Nocardia spp. | 2 | |
| Varicella-zoster virus | 5 | |
| Mixed etiology | ||
| Streptococcus pneumoniae and Mycoplasma pneumoniae | 2 | 2 |
| Streptococcus pneumoniae and parainfluenza virus | 3 | 2 |
| Streptococcus pneumoniae and influenza A virus | 1 | 1 |
| Streptococcus pneumoniae and Chlamydia pneumoniae | 1 | 1 |
| Mycoplasma pneumoniae and influenza A virus | 3 | |
| Varicella-zoster virus and influenza A virus | 2 | |
| Legionella pneumophila and influenza virus | 2 | |
| Haemophilus influenzae and Mycoplasma pneumoniae | 2 | |
| Staphylococcus aureus and Mycoplasma pneumoniae | 3 | 1 |
| Escherichia coli and respiratory syncytial virus | 2 | |
| Staphylococcus aureus and parainfluenza virus |
We calculated the sensitivity of the technique in probable CAPP cases. Gram staining and sputum culture were carried out in 654 patients (72%), which provided a probable CAPP diagnosis in 78 cases. ICT was positive for 46 cases, yielding a 59% sensitivity in these probable cases. There were no significant differences in age and gender between patients diagnosed by ICT or by conventional methods.
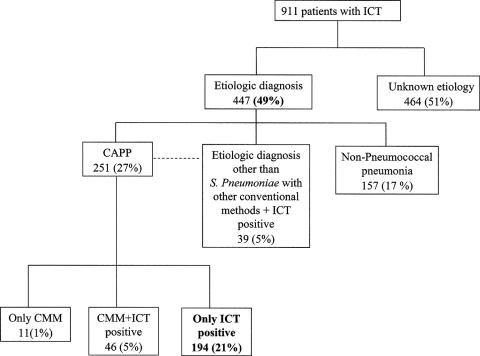
UPA content determined by the ICT method was positive in 279 cases (31%) (Table 3) of the 911 patients who were finally included in the study. Of these, 194 had no other etiologic diagnosis (Fig. 1). However, it was also positive for 39 of the 196 CAP cases with etiologies other than S. pneumoniae. As shown, the use of ICT increased the percentage of patients with a pneumococcal pneumonia diagnosis in the cohort regarding conventional methods by 26% (57 versus 290 cases), whereas the overall etiologic diagnosis increased from 28 to 49% (253 versus 447 cases).
TABLE 3.
ICT results of CAP with etiologic diagnoses made by conventional methods and in patients without CAP (COPD and orthopedic patients)
| ICT result | No. of cases
|
|||||
|---|---|---|---|---|---|---|
| Pneumococcal pneumonia
|
Nonpneumococcal pneumonia | Pneumonia of undetermined etiology | No pneumonia
|
|||
| Definite | Probable | COPD | Orthopedic | |||
| Positive | 46a | 46 | 39 | 148 | 12 | 1 |
| Negative | 11 | 32 | 157 | 432 | 143 | 79 |
| Total | 57 | 78 | 196b | 580 | 155c | 80d |
The sensitivity was 81% in patients with definitive pneumococcal pneumonia.
The specificity was 80% in patients with a definitive etiologic diagnosis of nonpneumococcal pneumonia.
The specificity was 92%.
The specificity was 99%.
FIG. 1.
Etiologic diagnosis of the cohort with conventional microbiology methods and with ICT.
UPA was detected in 46 of the 57 patients with a definitive CAPP diagnosis (obtained by blood culture, bronchoaspirate, and pleural fluid), yielding a test sensitivity of 81%. If only bacteremia cases are considered, the test sensitivity increases to 82% (42 of the 51 patients).
Likewise, we also calculated the sensitivity of the technique in probable CAPP cases. Gram staining and sputum culture were carried out in 654 patients (72%), which provided a probable CAPP diagnosis in 78 cases. ICT was positive for 46 cases, producing a 59% sensitivity in these probable cases. There were no significant differences in age and gender between patients diagnosed by ICT or by conventional methods.
Test specificity was calculated in diverse groups, including (i) patients with a definitive etiologic diagnosis of nonpneumococcal pneumonia (39 in 196 cases were antigen positive [80% specificity]), (ii) 155 patients with chronic obstructive pulmonary disease (COPD) and/or asthma without evidence of pneumonia, and (iii) 80 orthopedic patients without pneumonia. In group ii patients, ICT yielded positive results in 12 cases, which gave a specificity of 92%. In group iii patients, only one patient tested as pneumococcal antigen positive (99% specificity) (Table 3).
DISCUSSION
This study analyzes a large cohort of 959 patients admitted to our hospital for CAP over a period of several years. Our trial confirms the diagnostic usefulness of determining the UPA content in concentrated urine. In our experience, this noninvasive, rapid, and simple technique increases the diagnostic yield compared to conventional diagnostic methods. The test enables quicker initiation of therapy with the appropriate antibiotics. Consequently, this method has the potential to shorten patient hospital stays and reduce CAP mortality rates (11, 20).
Given the diagnostic difficulties associated with CAP, blood culture results were viewed as the gold standard, providing 81% sensitivity (46 antigen-positive patients of the definitive 57 CAPP). These results are similar to results obtained in other studies with sensitivity rates between 77 and 82% (6, 7, 12, 23, 31); all listed studies calculate sensitivity with regard to the definitive diagnosis of CAPP. On the other hand, our study results differed from the sensitivity rate of 66% reported by Rosón et al.; this is probably explained by the fact that those authors considered CAPP cases not only those with positive blood culture and/or pleural fluid culture but also those with positive Gram staining and sputum culture (27).
In our study, only probable CAPP cases were considered and included in our sensitivity calculation. If our study had considered positive sputum culture as a CAPP diagnosis and the gold standard, in spite of the obvious limitations regarding collection difficulties and occasional poor validity (26, 33), our sensitivity rate would have fallen to 67%, similar to that of Rosón et al. (27). Furthermore, our study sensitivity rate clearly conflicts with the 100% reported by Marcos et al. (22). In our experience, the sensitivity of the ICT test differs for definite pneumococcal pneumonia versus probable pneumococcal pneumonia. This differential sensitivity might be due to the fact that, due to our definition for definite pneumococcal pneumonia, 89% of these patients were bacteremic, whereas none of the probable pneumococcal pneumonias were bacteremic. Bacteremic pneumococcal pneumonia might be more likely to produce positive urine antigen tests than nonbacteremic pneumonia. The specificity of the ICT varies depending on different studies. Domínguez et al. (6) recorded 97% specificity (two false positives in 71 patients with confirmed etiologic pneumonia were caused by a microorganism other than S. pneumoniae). This high specificity rate may be due to the fact that Domínguez et al. not only used blood culture as the gold standard for CAPP but also used the laborious contraimmunoelectrophoresis method for antigen detection of 84 urinary S. pneumoniae serotypes. Consequently, the percentage of CAPP diagnoses increased, and the number of false positives usually found in clinical practice decreased. Murdoch et al. studied 169 control in-patients without infectious pathology and found no positive cases, thus obtaining 100% specificity (23). On the other hand, Smith et al. (31) determined the UPA content in 106 patients with nonpneumonic bacteremia and found three positives, which gave a 97% specificity.
In view of these conflicting results (possibly due to different methodologies), we decided to calculate specificity in diverse groups. We assessed test specificity in cases with a definitive etiologic diagnosis other than S. pneumoniae, and we obtained 80% specificity, a specificity lower than the percentages reported in other studies (6, 12, 14). These results are not wholly reliable since some of the cases that were considered false positives may really have been hidden pneumococcal pneumonia, which cannot be diagnosed by other means. It has been calculated that one-third of the CAP cases that do not have an etiologic diagnosis are pneumococcal (7, 22) or that some CAP cases considered to have other etiologies in fact have a mixed nature with pneumococcal participation. It has been estimated that more than 10% of the CAP cases are of polymicrobial etiology. We must point out that there is a great debate regarding whether these patients have sequential (viral or atypical infection, followed by pneumococcal) or concomitant polymicrobial pneumonia. In the present study, the most common pathogens in the known etiology groups with positive UPA are listed in Table 2.
In view of this, and bearing in mind that it is nearly impossible to ascertain the exact number of CAPP cases due to diagnostic difficulties and the high frequency of mixed CAP, another more reliable possibility would be to calculate the specificity in subjects without CAP. To calculate this, we evaluated two different groups of patients. The first group consisted of patients with prior respiratory pathology (COPD and asthma); the second group consisted of patients in the hospital for diverse fractures awaiting surgery. The group of patients with COPD and asthma were considered because of the considerable frequency of pneumococcal carriers among these patients and the possibility of nonpneumonic infection exacerbation, which would make the specificity study more useful in these cases (24). UPA was detected in 12 of the 155 cases of COPD and asthmatic patients, yielding a specificity of 92%. The 12 false positives were found in patients with exacerbated COPD, which, in our experience, makes it advisable to evaluate a positive ICT result with caution due to the possibility of colonization of S. pneumoniae and the persistence of ICT in such patients. These conclusions have previously been reported in other studies (22, 24). Any patient in the COPD control group with a positive UPA had fever and/or purulent sputum. Using computed tomographic scans, posthidratation infiltrates were not observed in any case.
The specificity was 99% for the 80 patients hospitalized for diverse fractures and who did not have clinical infections. Only one case tested as antigen positive. This patient had manifestations of respiratory infection the month before, which had not required hospitalization and which could have conditioned a persistence in UPA. The data obtained from this group are similar to those in the study by Murdoch et al., who also used controls without infectious pathology (23). Therefore, the calculation of specificity presents difficulties because it involves CAP with nonpneumococcal etiology, which could in fact be of mixed etiology. As a consequence, when using COPD patients without CAP, there will probably be colonization and/or UPA persistence from previous respiratory infections.
In our cohort only a small percentage, 6%, was diagnosed with CAPP when CMM were used, whereas 26% were diagnosed with CAP without an etiologic diagnosis after testing antigen positive. Therefore, the use of ICT in urine should increase the diagnosis of CAPP from 6% (57 cases) to 32% (290 patients) compared to CMM. Clearly, the use of ICT should increase the diagnostic yield in CAPP and the percentage of overall etiologic diagnoses in the cohort from 28 to 49%. Given the absence of a gold standard with good sensitivity (blood culture is considered to be positive in only 15 to 40% of CAP cases [19]), the precise significance of these results cannot be assessed.
We believe the present study has some limiting factors, including the following: (i) previously discussed difficulties in calculating the specificity of the technique in the pneumonia group (the gold standard is of limited sensitivity and prevents research into the negative and positive predictive values of the technique); and (ii) possible repercussions of previous antibiotic treatment on the usefulness of the technique have not been studied in this article, nor have the possible connections between development time of CAP and test results. Both of these factors could affect the usefulness of the technique, and we will address them in a future study.
In our experience, this noninvasive technique is a useful test from the clinical viewpoint for diagnosing CAPP, especially in nonbacteremic manifestations (less frequently diagnosed). The test is rapid and simple and has good sensitivity and specificity. It is complemented by CMM, making possible an increased rate of etiologic diagnosis of CAPP and expediting the initiation of appropriate antibiotic treatment upon hospital admission. On the other hand, the interpretation of results requires greater caution with COPD patients. We believe new studies are needed to analyze the persistence of UPA in patients with recent CAP and in patients with probable pneumococcal colonization.
REFERENCES
- 1.Alfageme, I., J. Aspa, S. Bello, J. Blanquer, R. Blanquer, L. Borderías, J. Bravo, R. De Celis, X. De Gracia, J. Dorca, J. Gallardo, M. Gallego, R. Menéndez, L. Molinos, C. Paredes, O. Rajas, J. Rello, F. Rodríguez de Castro, J. Roig, F. Sánchez Gascón, A. Torres, R. Zalacaín, et al. 2005. Guidelines for the diagnosis and management of community-acquired pneumonia. Arch. Bronchopneumol. 41:272-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almirall, J., I. Bolíbar, J. Vidal, G. Sauca, P. Coll, B. Niklasson, M. Bartolomé, and X. Balanzo. 2000. Epidemiology of community-acquired pneumonia in adults: a population-based study. Eur. Respir. J. 15:757-763. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett, J. G., S. F. Dowell, L. A. Mandell, T. M. File, Jr., D. M. Musher, and M. J. Fine. 2000. Practice guidelines for the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 31:347-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boersma, W. G., A. Lowenberg, Y. Holloway, et al. 1993. Rapid detection of pneumococcal antigen in pleural fluid of patients with community acquired pneumonia. Thorax 48:160-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell, S. G., T. J. Marrie, R. Anstey, G. Dickinson, and S. Ackroyd-Stolarz. 2003. The contribution of blood cultures to the clinical management of adult patients admitted to the hospital with community-acquired pneumonia. Chest 123:1142-1150. [DOI] [PubMed] [Google Scholar]
- 6.Domínguez, J., N. Galí, S. Blanco, P. Pedroso, C. Prat, L. Matas, and V. Ausina. 2001. Detection of Streptococcus pneumoniae antigen by a rapid immunochromatographic assay in urine samples. Chest 119:243-249. [DOI] [PubMed] [Google Scholar]
- 7.Farina, C., M. Arosio, F. Vailati, F. Moioli, and A. Goglio. 2002. Urinary detection of Streptococcus pneumoniae antigen for diagnosis of pneumonia. New Microbiol. 25:259-263. [PubMed] [Google Scholar]
- 8.File, T. M. 2003. Community-acquired pneumonia. Lancet 362:1991-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fine, M. J., M. A. Smith, C. A. Carson, S. S. Sankey, L. A. Weissfeld, and W. N. Kapoor. 1996. Prognosis and outcomes of patients with community-acquired pneumonia: a meta-analysis. JAMA 275:134-141. [PubMed] [Google Scholar]
- 10.Formica, N., M. Yates, M. Beers, J. Carnie, G. Hogg, N. Ryan, and G. Tallis. 2001. The impact of diagnosis by legionella urinary antigen test on the epidemiology and outcomes of Legionnaires' disease. Epidemiol. Infect. 127:275-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guchev, I. A., V. L. Yu, A. Sinopalnikov, O. I. Klochkov, R. S. Kozlov, and L. S. Stratchounski. 2005. Management of nonsevere pneumonia in military trainees with the urinary antigen test for Streptococcus pneumoniae: an innovative approach to targeted therapy. Clin. Infect. Dis. 40:1608-1616. [DOI] [PubMed] [Google Scholar]
- 12.Gutierrez, F., M. Masia, J. C. Rodríguez, A. Ayelo, B. Soldán, L. Cebrián, C. Mirete, G. Royo, and A. M. Hidalgo. 2003. Evaluation of the immunochromatographic Binax NOW assay for detection of Streptococcus pneumoniae urinary antigen in a prospective study of community-acquired pneumonia in Spain. Clin. Infect. Dis. 36:286-292. [DOI] [PubMed] [Google Scholar]
- 13.Henney, J. E. 1999. Quick test for pneumonia. JAMA 282:1218.10517411 [Google Scholar]
- 14.Ishida, T., T. Hashimoto, M. Arita, Y. Tojo, H. Tachibana, and M. Jinnai. 2004. A 3-year prospective study of a urinary antigen-detection test for Streptococcus pneumoniae in community-acquired pneumonia: utility and clinical impact on the reported etiology. J. Infect. Chemother. 10:359-363. [DOI] [PubMed] [Google Scholar]
- 15.Jokinen, C., L. Heiskanen, H. Juvonen, S. Kallinen, K. Karkola, M. Korppi, S. Kurki, P. R. Ronnberg, A. Seppa, and S. Soimakallio. 1993. Incidence of community-acquired pneumonia in the population of four municipalities in eastern Finland. Am. J. Epidemiol. 137:977-988. [DOI] [PubMed] [Google Scholar]
- 16.Lim, W. S., J. T. Macfarlane, T. C. Boswell, T. G. Harrison, D. Rose, M. Leinonen, and P. Saikku. 2001. Study of community-acquired pneumonia etiology (SCAPA) in adults admitted to hospital: implications for management guidelines. Thorax 56:296-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macfarlane, J. T., et al. 2001. BTS Guidelines for the management of community-acquired pneumonia in adults. Thorax 56(Suppl. IV):iv1-iv64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macfarlane, J. T., and D. Boldy. 2004. Update of BTS pneumonia guidelines: what's new? Thorax 59:364-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandell, L. A. 1995. Community-acquired pneumonia. Etiology, epidemiology, and treatment. Chest 108:35S-42S. [DOI] [PubMed] [Google Scholar]
- 20.Mandell, L. A., J. G. Bartlett, S. F. Dowell, T. M. File, Jr., and D. M. C. Whitney. 2003. Update of practice guidelines for the management of community-acquired pneumonia in immunocompetent adults. Clin. Infect. Dis. 37:1405-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marcos, M. A., J. Gonzalez, J. Angrill, F. Sánchez, A. Raño, T. T. Bauer, J. Mensa, A. Torres, and M. T. Jimenez de Anta. 2000. The Streptococcus pneumoniae urinary antigen test for improvement of the diagnosis of community-acquired pneumonia. Am. J. Respir. Crit. Care Med. 161:A307. [Google Scholar]
- 22.Marcos, M. A., M. T. Jimenez de Anta, J. P. de la Bellacasa, J. González, E. Martínez, E. García, J. Mensa, A. de Roux, and A. Torres. 2003. Rapid urinary antigen test for diagnosis of pneumococcal community-acquired pneumonia in adults. Eur. Respir. J. 21:209-214. [DOI] [PubMed] [Google Scholar]
- 23.Murdoch, D. R., R. T. Laing, G. D. Mills, N. C. Karalus, G. I. Town, S Mirrett, and L. B. Reller. 2001. Evaluation of a rapid immunochromatographic test for detection of Streptococcus pneumoniae antigen in urine samples from adults with community-acquired pneumonia. J. Clin. Microbiol. 39:3495-3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murdoch, D. R., R. T. R. Laing, and J. M. Cook. 2003. The Now S. pneumoniae urinary antigen test positivity rate 6 weeks after pneumonia onset and among patients with COPD. Clin. Infect. Dis. 37:153-154. [DOI] [PubMed] [Google Scholar]
- 25.Murray, P. R., and J. A. Washington. 1975. Microscopic and bacteriologic analysis of expectorated sputum. Mayo Clin. Proc. 50:339-344. [PubMed] [Google Scholar]
- 26.Pesola, G. R. 2001. The urinary antigen test for the diagnosis of pneumococcal pneumonia. Chest 119:9-11. [DOI] [PubMed] [Google Scholar]
- 27.Rosón, B., N. Fernández, J. Carratalá, R. Verdaguer, J.Dorca, F. Manresa, and F. Gudiol. 2004. Contribution of a urinary antigen assay (Binax Now) to the early diagnosis of pneumococcal pneumonia. Clin. Infect. Dis. 38:222-226. [DOI] [PubMed] [Google Scholar]
- 28.Ruiz, M., S. Ewig, M. A. Marcos, J. A. Martínez, F. Arancibia, J. Mensa, and A. Torres. 1999. Etiology of community-acquired pneumonia: impact of age, comorbidity, and severity. Am. J. Respir. Crit. Care Med. 160:397-405. [DOI] [PubMed] [Google Scholar]
- 29.Ruiz-Gonzalez, A., A. Nogues, M. Falguera, J. M. Porcel, E. Huelin, and M. Rubio-Caballero. 1997. Rapid detection of pneumococcal antigen in aspirates: comparison with culture and PCR technique. Respir. Med. 91:201-206. [DOI] [PubMed] [Google Scholar]
- 30.Ruiz-Gonzalez, A., M. Falguera, A. Nogués, and M. Rubio-Caballero. 1999. Is Streptococcus pneumoniae the leading cause of pneumonia of unknown etiology? A microbiologic study of lung aspirates in consecutive patients with community-acquired pneumonia. Am. J. Med. 106:385-390. [DOI] [PubMed] [Google Scholar]
- 31.Smith, M. D., P. Derrington, R. Evans, M. Creek, R. Morris, D. A. B. Dance, and K. Cartwright. 2003. Rapid diagnosis of bacteremic pneumococcal infections in adults by using the Binax NOW Streptococcus pneumoniae urinary antigen test: a prospective, controlled clinical evaluation. J. Clin. Microbiol. 47:2810-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van der Auwera, P., A. Andre, G. Bulliard, J. C. Legrand, B. Gordts, H. Van Landuyt, and F. Schuyteneer. 1983. Comparison of latex agglutination and counterimmunoelectrophoresis in the diagnosis of acute Streptococcus pneumoniae infections. Eur. J. Clin. Microbiol. 2:534-540. [DOI] [PubMed] [Google Scholar]
- 33.Van der Eerden, M. M., F. Vlaspolder, C. S. de Graaff, T. Groot, H. M. Jansen, and W. G. Boersma. 2005. Value of intensive diagnostic microbiological investigation in low- and high-risk patients with community-acquired pneumonia. Eur. J. Clin. Microbiol. Infect. Dis. 24:241-249. [DOI] [PubMed] [Google Scholar]