Abstract
Previous studies have suggested that currently available brucellosis vaccines induce poor or no protection in elk (Cervus elaphus nelsoni). In this study, we characterized the immunologic responses of elk after initial or booster vaccination with Brucella abortus strains RB51 (SRB51) and 19 (S19). Elk were vaccinated with saline or 1010 CFU of SRB51 or S19 (n = seven animals/treatment) and booster vaccinated with a similar dosage of the autologous vaccine at 65 weeks. Compared to nonvaccinates, elk vaccinated with SRB51 or S19 had greater (P < 0.05) antibody responses to SRB51 or S19 after initial vaccination and after booster vaccination. Compared to nonvaccinated elk, greater (P < 0.05) proliferative responses to autologous antigen after initial vaccination occurred at only a few sample times in SRB51 (6, 14, and 22 weeks) and S19 (22 weeks) treatment groups. In general, proliferative responses of vaccinates to nonautologous antigens did not differ (P > 0.05) from the responses of nonvaccinated elk. Gamma interferon production in response to autologous or nonautologous Brucella antigens did not differ (P > 0.05) between controls and vaccinates after booster vaccination. Flow cytometric techniques suggested that proliferation occurred more frequently in immunoglobulin M-positive cells, with differences between vaccination and control treatments in CD4+ and CD8+ subset proliferation detected only at 22 weeks after initial vaccination. After booster vaccination, one technique ([3H]thymidine incorporation) suggested that proliferative responses to SRB51 antigen, but not S19 antigen, were greater (P < 0.05) in vaccinates compared to the responses of nonvaccinates. However, in general, flow cytometric and other techniques failed to detect significant anamnestic responses to autologous or nonautologous Brucella antigens in S19 or SRB51 vaccinates after booster vaccination. Although some cellular immune responses were detected after initial or booster vaccination of elk with SRB51 or S19, our data suggest that responses tend to be transient and much less robust than previously reported in SRB51-vaccinated cattle (Bos taurus) or bison (Bison bison). These data may explain why the vaccination of elk with S19 and SRB51 induces poor protection against brucellosis.
The regulatory programs for the elimination of brucellosis within the United States began in 1934 as a state-federal cooperative program to reduce the cattle population during severe drought conditions. As the Brucellosis Eradication Program for cattle nears completion in the United States after 70 years of regulatory efforts, the persistence of Brucella abortus in wildlife reservoirs remains a concern. The identification of Brucella-infected cattle herds in Wyoming in 2003 led to the loss of that state's brucellosis-free status. Epidemiologic and molecular data suggest that the likely reservoir for the Brucella abortus infections in Wyoming cattle were free-ranging elk (Cervus elaphus nelsoni).
The first public feedground in Wyoming, the National Elk Refuge, was established near Jackson in 1912 due to the decline in elk habitat caused by human intervention (21). In addition to the National Elk Refuge, there are currently 22 winter feedgrounds within Wyoming in the greater Yellowstone area (GYA). Brucellosis was first reported in the GYA in 1930 on the National Elk Refuge (24). Artificial winter feeding appears to be responsible for much of the maintenance and transmission of brucellosis in elk in the GYA (8). Through blood testing of adult female elk wintering on feedgrounds, the Wyoming Game and Fish Department estimates that 35% of those elk have been exposed to brucellosis compared to a 2 to 3% seropositive rate in elk that spend the winter on native range without supplemental feed (3).
B. abortus strain 19 (S19) has been used since 1985 for the ballistic vaccination of elk on Wyoming feedgrounds. Experimental data suggested that S19 prevents abortion in ca. 30% of vaccinates compared to paired controls (5, 23). Single or multiple vaccinations with a new brucellosis vaccine that is efficacious in bison (Bison bison) and cattle (Bos taurus) (2, 18), B. abortus strain RB51 (SRB51), did not protect elk against experimental Brucella challenge (6, 7). Some differences between the two vaccines may be in part due to the fact that S19 is a smooth Brucella that expresses the O side chain (perosamine residue) on its lipopolysaccharide, and SRB51 is rough, without O side chain expression. In a previous study using a small number of elk, strong antibody and transient proliferative responses were noted after SRB51 vaccination (16). In an effort to understand why current brucellosis vaccines have poor efficacy in elk, the study reported here expanded on previous work characterizing immune responses after vaccination and also evaluated immunologic responses to booster vaccination.
MATERIALS AND METHODS
B. abortus cultures.
A master seed stock of B. abortus strain RB51 (SRB51) was obtained (G. Schurig, Virginia Tech, Blacksburg, VA). The B. abortus strain 19 (S19) was obtained from the culture collection at the National Animal Disease Center. For experimental use, SRB51 and S19 bacteria were grown on tryptose agar for 48 h at 37°C. For antibody and proliferation assays, S19 and SRB51 suspensions (1012 CFU/ml) were inactivated by gamma irradiation (1.4 × 106 rads). After irradiation, suspensions were washed in 0.15 M sodium chloride (saline) and stored in 1-ml aliquots at −70°C.
For initial or booster vaccination of elk, S19 and SRB51 vaccines were diluted in saline to ∼1010 CFU by using spectrophotometric methods. After vaccination, the concentrations of viable bacteria within the inoculum were determined by standard plate counts.
Animals and inoculation.
Twenty-one ∼7-month-old elk calves were obtained from a brucellosis-free herd. Upon arrival, the elk were vaccinated against Clostridium spp. (Ultrabac 7; Pfizer, New Groton, CT) and dewormed (Dectomax; Pfizer). Male elk were anesthetized with xylazine (Phoenix Pharmaceuticals, St. Joseph, MO) and ketamine (Fort Dodge Animal Health, Ft. Dodge, IA) intravenously and then castrated. After acclimation for 12 weeks, elk were randomly assigned to three groups (n = seven animals/group with three or four males/group) for subcutaneous (s.c.) vaccination with saline (control), SRB51, or S19. All inoculations were 2 ml in volume and were administered in the left cervical region drained by the superficial cervical (prescapular) lymph node. At 65 weeks after the initial vaccination, S19- and SRB51-vaccinated elk received s.c. booster vaccinations in a similar manner to that of the initial vaccinations.
Serologic evaluation.
Blood samples were collected by jugular venipuncture prior to vaccination, and at 2, 4, 6, 8, 10, 12, 18, 22, 26, and 29 weeks postinoculation. Blood samples were also obtained at the time of booster vaccination (week 65) and at 2, 4, 6, 8, and 12 weeks after booster vaccination. Blood was allowed to clot for 12 h at 4°C and then centrifuged. Serum was divided into 1-ml aliquots, frozen, and stored at −70°C.
Serological titers of animals to Brucella were determined by a previously described antibody dot blot assay in which gamma-irradiated SRB51 or S19 is used as antigen (15).
Blood cultures and monitoring of shedding by vaccinates.
To determine whether a prolonged SRB51 septicemia occurs in elk, 15 ml of blood was obtained from all elk at 1, 2, 3, 4, and 6 weeks after initial inoculation and mixed 1:1 with tryptose broth (Difco Laboratories, Detroit, MI) containing 1% sodium citrate. One milliliter of this mixture was directly plated onto tryptose agar containing 5% bovine sera. The remaining blood was held at −5°C for 24 h and then placed at 37°C in 5% CO2 with 1-ml volumes plated onto tryptose agar containing 5% bovine sera after 7, 14, 21, and 28 days of incubation. After incubation of the plates at 37°C and 5% CO2 for 72 h, SRB51 or S19 was identified on the basis of colony morphology and growth characteristics and confirmed by a PCR procedure using primers specific for the identification of B. abortus omp2a (9) or SRB51 (25).
Preparation of PBMC and lymph node cells for lymphocyte proliferation assays.
At 6, 8, 12, 14, 18, 22, 26, 29, and 31 weeks after vaccination, blood was obtained from the jugular vein of all elk and placed into an acid-citrate dextrose solution. Peripheral blood mononuclear cells (PBMC) were enriched by density centrifugation using a Ficoll-sodium diatrizoate gradient (Sigma Diagnostics, Inc., St. Louis, MO). PBMC were diluted in RPMI 1640 medium to 107 viable cells per ml as determined by trypan blue dye exclusion.
Portions (50 μl) of each cell suspension containing 5 × 105 cells were added to each of two separate flat-bottom wells of 96-well microtiter plates that contained 100 μl of RPMI 1640 medium only or 1640 medium containing gamma-irradiated SRB51 or S19 (109 to 105 bacteria per well). Wells containing 1 μg of pokeweed mitogen (Sigma Chemical)/ml were used as positive controls for proliferative responses. Microtiter plates were prepared prior to initiation of the study and maintained at −70°C until use. Cell cultures were incubated for 7 days at 37°C under 5% CO2. After 7 days of incubation, cell cultures were pulsed with 1.0 μCi of [3H]thymidine per well for 18 h. Cells were harvested onto glass filter mats and counted for radioactivity in a liquid scintillation counter. Cell proliferation results were converted to stimulation indices (counts per minute [cpm] of wells containing antigen/cpm in the absence of antigen) for statistical comparisons.
IFN-γ production.
Immediately prior to booster vaccination and at 2, 4, 6, and 8 weeks after, PBMC from each animal were adjusted to 107 viable cells per ml as described previously. Portions (50 μl) of each cell suspension, containing 5 × 105 cells, were added to flat-bottom wells of 96-well microtiter plates that contained 100 μl of RPMI 1640 medium only or 1640 medium containing gamma-irradiated SRB51 or S19 (2 × 108 bacteria per well). Cell cultures were incubated at 37°C under 5% CO2, and the supernatants were removed at 72 h after initiation of culture. Supernatants were held at −70°C until assayed for gamma interferon (IFN-γ) using a commercially available kit (Cervigam; CSL Veterinary, Victoria, Australia). Standard dilutions of a purified red deer IFN-γ of known quantity (108 to 0.211 ng/ml) were included on each microtiter plate. Optical density measurements at 450 nm were made by using an enzyme-linked immunosorbent assay plate reader (Molecular Devices, Sunnyvale, CA). Linear regression was used to prepare a standard curve from which the concentration of IFN-γ in each sample was determined. Antigen-specific net IFN-γ was determined for each individual by subtracting the concentration of IFN-γ in wells without antigen from IFN-γ concentrations in wells with antigen.
Flow cytometry.
At 14, 18, and 22 weeks after the initial vaccination and at 4, 8, and 12 weeks after the booster vaccination, aliquots of PBMC containing 2 × 107 cells were centrifuged, and the supernatant was discarded. The cells were stained with PKH-67 green fluorescent dye (Sigma Chemical) in accordance with the manufacturer's instructions. Cells were adjusted to 107 viable cells per ml as described above, and 50 μl of each cell suspension, containing 5 × 105 cells, was added to each of eight separate flat-bottom wells of 96-well microtiter plates that contained 100 μl of RPMI 1640 medium only or 1640 medium containing gamma-irradiated SRB51 (2 × 108 bacteria per well). Cell cultures were incubated for 7 days at 37°C under 5% CO2. Approximately 2 × 105 pooled cells in 200 μl of culture medium were added to individual wells of round-bottom microtiter plates, centrifuged (15 min, 400 × g), and resuspended in 100 μl of primary antibody(ies) (∼1 μg/well) in phosphate-buffered saline containing 1% fetal bovine sera and 0.1% sodium azide (fluorescence-activated cell sorting [FACS] buffer). Primary antibodies (VMRD, Pullman, WA) included anti-CD4 (17D1-immunoglobulin G [IgG]), anti-CD8 (ST8-IgM), anti-B cell (PIG45A-IgG2b), anti-γδ TCR (GB21A-IgG2b), and anti-WC1 (BAQ4A-IgG1). After a 15-min incubation at room temperature, cells were centrifuged (15 min, 400 × g) and resuspended in 100 μl each of peridinin chlorophyll protein (1 μg/ml)-conjugated rat anti-mouse IgG1 (Becton Dickinson, Franklin Lakes, NJ) and phycoerythrin (1 μg/well)-conjugated goat anti-mouse IgM or IgG2b (Southern Biotechnology Associates, Birmingham, AL). Cells in secondary antibody were incubated for 15 min at room temperature in the dark, washed with FACS buffer, resuspended in 200 μl of PBS containing 0.04% sodium azide, and analyzed on a flow cytometer (FacScan; Becton Dickinson). The data were analyzed by using commercially available software (CellQuest [Becton Dickinson] and Modfit [Verity Software House, Inc., Topsham, ME]).
Statistical analysis.
Serologic and proliferation data were analyzed as the logarithm of their value. Serologic data were compared over all times using a two-way analysis of variance model, whereas differences between treatments in flow cytometric, [3H]thymidine incorporation, and net IFN-γ data at each sampling time were compared by a general linear model procedure (SAS Institute, Inc., Cary, NC). Means for individual treatments were separated by use of a least-significant-difference procedure (P < 0.05).
RESULTS
Vaccine dosages.
Standard plate counts indicated that elk receiving the SRB51 and S19 treatments were initially vaccinated with 1.2 × 1010 and 0.8 × 1010 CFU, respectively. When booster vaccinated at 65 weeks after initial vaccination, SRB51 and S19 inocula contained 1.0 × 1010 and 1.2 × 1010 CFU, respectively.
Serologic evaluation. (i) SRB51 antigen.
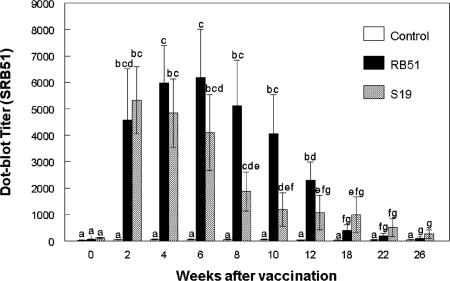
Prior to vaccination with S19 or SRB51, dot blot titers of vaccinated elk did not differ (P > 0.05) from nonvaccinates when SRB51 bacteria were used as the antigen (Fig. 1). Beginning at 2 weeks and continuing through 26 weeks postvaccination, S19- and SRB51-vaccinated elk had greater dot blot titers to SRB51 than nonvaccinated elk. Dot blot titers to SRB51 did not differ between SRB51- or S19-vaccinated elk at any sampling time.
FIG. 1.
Serologic responses of S19-vaccinated, SRB51-vaccinated, or control elk to gamma-irradiated SRB51 in a dot blot assay. Elk were vaccinated s.c. with saline, 1.2 × 1010 CFU of SRB51, or 0.8 × 1010 CFU of S19 (n = seven animals/treatment). The responses are presented as mean titers ± the standard error of the mean (SEM). Means with different superscripts are significantly different (P < 0.05).
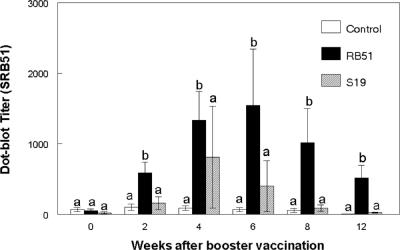
At the time of booster vaccination, the dot blot titers to SRB51 antigen did not differ (P > 0.05) between SRB51 vaccinates, S19 vaccinates, and nonvaccinated elk (Fig. 2). At between 2 and 12 weeks after booster vaccination, SRB51-vaccinated elk had greater (P < 0.05) dot blot titers to SRB51 than did nonvaccinated elk or elk booster vaccinated with S19. Dot blot titers to SRB51 antigen did not differ (P > 0.05) at any postbooster sampling time between nonvaccinated elk and elk booster vaccinated with S19.
FIG. 2.
Serologic responses of S19-vaccinated, SRB51-vaccinated, or control elk to gamma-irradiated SRB51 in a dot blot assay after booster vaccination. Elk were vaccinated s.c. with saline, 1.2 × 1010 CFU of SRB51, or 0.8 × 1010 CFU of S19 (n = seven animals/treatment) and booster vaccinated 65 weeks later with saline, 1.0 × 1010 CFU SRB51, or 1.2 × 1010 CFU S19, respectively. The responses are presented as mean titers ± the SEM. Means with different superscripts are significantly different (P < 0.05).
(ii) S19 antigen.
Prior to vaccination with S19 or SRB51, dot blot titers of vaccinated elk did not differ (P > 0.05) from nonvaccinates when S19 bacteria were used as the antigen (data not shown). Strain 19-vaccinated elk had greater (P < 0.05) dot blot titers to S19 between 2 and 26 weeks after vaccination compared to SRB51-vaccinated or nonvaccinated elk. In a similar manner, SRB51-vaccinated elk had greater (P < 0.05) dot blot titers to S19 between 2 and 8 weeks after vaccination compared to nonvaccinated elk.
At the time of booster vaccination, dot blot titers to S19 bacteria did not differ (P > 0.05) between SRB51 vaccinates, S19 vaccinates, and nonvaccinated elk (data not shown). Dot blot titers to S19 antigen for nonvaccinated elk or elk booster vaccinated with SRB51 did not differ (P > 0.05) at any postbooster sampling time. Elk booster vaccinated with S19 had greater (P < 0.05) dot blot titers to S19 antigen between 2 and 12 weeks after booster vaccination than did nonvaccinated elk or elk booster vaccinated with SRB51.
Blood cultures.
SRB51 was recovered from the blood of SRB51-vaccinated elk at 1, 2, 3, and 6 weeks after vaccination (two, six, two, and one elk, respectively). In a similar manner, S19 was recovered from the blood of S19-vaccinated elk at 2 and 4 weeks after vaccination (two and one elk, respectively). Neither S19 nor SRB51 was recovered at any sampling time from the blood of cohoused, nonvaccinated elk.
Lymphocyte proliferation assays. (i) SRB51 antigen.
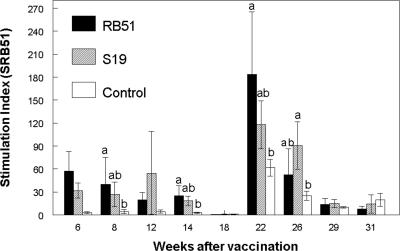
Compared to PBMC from nonvaccinated elk, proliferative responses (P < 0.05) to gamma-irradiated SRB51 were greater in PBMC from SRB51 vaccinates at 8, 14, and 22 weeks after vaccination (representative data are presented in Fig. 3). PBMC from S19-vaccinated elk had greater proliferative responses to γ-irradiated SRB51 at only the 26-week sampling time compared to nonvaccinated elk (P < 0.05). PBMC from elk vaccinated with SRB51 or S19 did not differ in proliferative responses to gamma-irradiated SRB51 at any sampling time (P > 0.05).
FIG. 3.
Proliferative responses to 108 CFU of gamma-irradiated SRB51 by PBMC from elk vaccinated with saline, 1.2 × 1010 CFU of SRB51, or 0.8 × 1010 CFU of S19 (n = seven animals/treatment). Cells were incubated at 37°C and 5% CO2 for 7 days and pulsed for 18 h with [3H]thymidine. The results are expressed as mean stimulation indexes. Means within a sampling time with different superscripts are significantly different (P < 0.05). The mean response of elk PBMC incubated in the absence of antigen was 1,991 ± 364 cpm. The mean response of elk PBMC incubated with 1 μg of pokeweed mitogen was 103,555 ± 7,550 cpm.
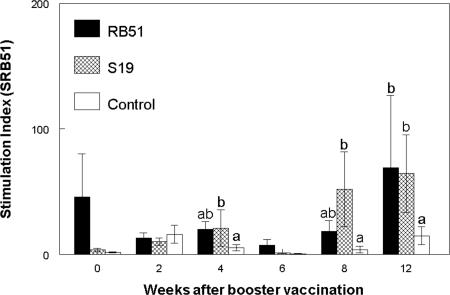
After booster vaccination with SRB51, proliferative responses to gamma-irradiated SRB51 by PBMC from SRB51-vaccinated elk were greater (P < 0.05) only at 12 weeks compared to PBMC from nonvaccinated elk (representative data are presented in Fig. 4). In comparison, PBMC from elk booster vaccinated with S19 had greater proliferative responses to gamma-irradiated SRB51 at 4, 8 and 12 weeks. However, the proliferative responses of PBMC from elk booster vaccinated with S19 or SRB51 did not differ (P > 0.05) at any sampling time after booster vaccination. Elk in all treatments demonstrated proliferative responses to pokeweed mitogen that were greater (P < 0.05) than proliferation in wells without antigen at all sampling times (data not shown).
FIG. 4.
Proliferative responses to 108 CFU of gamma-irradiated SRB51 by PBMC after booster vaccination of elk with saline, S19, or SRB51. Elk were initially vaccinated with saline, 1.2 × 1010 CFU of SRB51, or 0.8 × 1010 CFU of S19 (n = seven animals/treatment) and booster vaccinated 65 weeks later with saline, 1.0 × 1010 CFU SRB51, or 1.2 × 1010 CFU S19, respectively. Cells were incubated at 37°C and 5% CO2 for 7 days and then pulsed for 18 h with [3H]thymidine. The results are expressed as mean stimulation indexes. Means within a sampling time with different superscripts are significantly different (P < 0.05). The mean response of elk PBMC incubated in the absence of antigen was 2,278 ± 300 cpm. The mean response of elk PBMC incubated with 1 μg of pokeweed mitogen/ml was 70,626 ± 4,553 cpm.
(ii) S19 antigen.
Proliferative responses to S19 antigens were similar to responses to SRB51 antigens. With the exception of greater (P < 0.05) proliferative responses by PBMC from S19-vaccinated elk at 14 and 22 weeks, the proliferative responses of PBMC from SRB51-vaccinated, S19-vaccinated, and nonvaccinated elk to gamma-irradiated S19 did not differ (P > 0.05) in samples obtained up to 31 weeks after vaccination (data not shown). In a similar manner, PBMC from nonvaccinated elk or elk booster vaccinated with SRB51 or S19 did not differ (P > 0.05) in their proliferative responses to gamma-irradiated S19 when evaluated up to 12 weeks after booster vaccination (data not shown).
IFN-γ.
Production of IFN-γ after 72 h of incubation with gamma-irradiated SRB51 or S19 did not differ (P > 0.05) between PBMC isolated from control, SRB51-vaccinated, or S19-vaccinated elk at 0, 2, 4, 6, and 8 weeks after booster vaccination (data not shown).
Flow cytometry. (i) SRB51 antigen.
At 18 weeks, but not 14 weeks after initial vaccination, PBMC from SRB51- and S19-vaccinated elk had greater total cell proliferation to gamma-irradiated SRB51 compared to control elk (P < 0.05; data not shown). Proliferation responses in CD4+, CD8+, γδTCR+, or IgM+ subsets to gamma-irradiated SRB51 did not differ (P > 0.05) between treatments at any time after initial vaccination. Flow cytometry data at 22 weeks after vaccination measuring responses to gamma-irradiated SRB51 were eliminated due to the growth of contaminants within the microtiter plates at this sampling time.
At 8 weeks, but not at 4 or 12 weeks after booster vaccination, PBMC from SRB51- and S19-vaccinated elk had greater (P < 0.05) total proliferative responses to gamma-irradiated SRB51 compared to responses of PBMC from nonvaccinated elk (data not shown). PBMC from SRB51-vaccinated elk had greater (P < 0.05) CD4+ and CD8+ proliferative responses to gamma-irradiated SRB51 at 8 weeks at after booster vaccination compared to the responses of nonvaccinates. S19-vaccinated elk had greater (P < 0.05) CD8+ responses to gamma-irradiated SRB51 at 8 weeks and greater CD4+ responses at 12 weeks compared to the responses of control elk.
S19 antigen.
At 14, 18, and 22 weeks postvaccination, flow cytometric techniques demonstrated greater (P < 0.05) total proliferative responses to gamma-irradiated S19 in PBMC from SRB51- or S19-vaccinated elk than in PBMC from nonvaccinated elk (representative data are presented in Table 1). Compared to the responses of nonvaccinates, both S19 and SRB51 treatments had greater proliferation in IgM+ cells at 14 and 22 weeks, but only in S19 vaccinates at 18 weeks after vaccination. Greater (P < 0.05) proliferative CD4+ responses were detected in SRB51 and S19 vaccinates at 22 weeks after vaccination; however, only SRB51-vaccinated elk had greater proliferation (P < 0.05) in CD8+ cells at this sampling time than nonvaccinates.
TABLE 1.
Flow cytometric analysis of responses to B. abortus strain 19 after initial and booster vaccinationa
| Time postvaccination and animal group | Mean no. of proliferating cells/10,000 PBMC ± SEMb
|
||||
|---|---|---|---|---|---|
| Total | CD4+ | CD8+ | γδTCR+ | IgM+ | |
| 14 wk after initial vaccination | |||||
| Controls | 893 ± 407 | 126 ± 46 | 25 ± 11 | 22 ± 15 | 881 ± 174 |
| RB51 vaccinates | 1,833 ± 579* | 449 ± 351 | 19 ± 15 | 335 ± 311 | 1,927 ± 432* |
| S19 vaccinates | 1,262 ± 467 | 64 ± 42 | 64 ± 64 | 59 ± 59 | 1,942 ± 607* |
| 22 wk after initial vaccination | |||||
| Controls | 367 ± 367 | 353 ± 325 | 76 ± 42 | 38 ± 27 | 273 ± 184 |
| RB51 vaccinates | 2,161 ± 1,187* | 1,354 ± 384* | 381 ± 101* | 89 ± 89 | 1,277 ± 333* |
| S19 vaccinates | 2,063 ± 405* | 2,282 ± 245* | 529 ± 253 | 11 ± 93 | 1,873 ± 212* |
| 12 wk after booster vaccination | |||||
| Controls | 0 ± 0 | 404 ± 322 | 88 ± 34 | 28 ± 42 | 141 ± 30 |
| RB51 vaccinates | 0 ± 0 | 808 ± 488 | 232 ± 121 | 0 ± 0 | 467 ± 196 |
| S19 vaccinates | 1,000 ± 541 | 691 ± 303 | 306 ± 134 | 24 ± 41 | 637 ± 266 |
PKH-67-labeled PBMC from elk vaccinated s.c. with saline (control), SRB51, or S19 (n = seven animals/treatment) and booster vaccinated 65 weeks later were incubated with 108 CFU of gamma-irradiated B. abortus strain 19 at 37°C and 5% CO2 for 7 days, labeled with monoclonal antibodies, and analyzed in a flow cytometer.
Means within a sampling time with an asterisk are significantly different (P < 0.05) from means of controls labeled with the same monoclonal antibody.
Compared to other treatments, SRB51-vaccinated elk had greater (P > 0.05) total proliferation in response to S19 antigens at 8 weeks after the booster vaccination. With that exception, flow cytometric techniques did not detect any difference (P > 0.05) between treatments in all subsets at all sampling times after booster vaccination.
DISCUSSION
The results of the present study suggest that brucellosis vaccination of elk does not induce robust and persistent cellular immunologic responses even after booster vaccination. As with most facultative intracellular pathogens, cell-mediated immunity is believed to play a crucial role in long-term protection against Brucella (10) The lipopolysaccharide O side chain is an immunodominant antigen for B. abortus (28), and almost all brucellosis serologic tests are based on measuring antibody responses to this antigen. Since our study evaluated both smooth (S19) and rough (SRB51) strains of B. abortus, the failure of the vaccines to induce robust cell-mediated responses does not seem to be related to the presence or absence of the O side chain.
In the present study, both initial and booster vaccination failed to induce strong proliferative responses that persisted over time. After initial vaccination, [3H]thymidine incorporation suggested cellular immune responses to SRB51 antigen were present at four time points, but flow cytometric procedures suggested that the responses in most sampling times were confined to the IgM+ subset. Only at 22 weeks after initial vaccination, when peak proliferative responses were noted with [3H]thymidine incorporation, did flow cytometric data suggest responses in CD4+ or CD8+ subsets to S19 antigen. Although inconsistent and detected only in cells incubated with SRB51, booster vaccination did elicit some proliferation in the CD4+ or CD8+ cells of S19- or SRB51-vaccinated elk. However, the magnitudes of the anamnestic responses were less than we had anticipated. The observation of poor cellular immunity after brucellosis vaccination of elk is consistent with a previous study (16) in which SRB51-vaccinated elk had greater proliferative responses to SRB51 at 18 weeks after vaccination but not at 12 or 24 weeks after vaccination compared to nonvaccinated elk. Unpublished data from an additional study in our laboratory also demonstrated proliferation responses after strain 19 vaccination of elk that were similar to the responses reported here.
In comparison, in cattle and bison, where the SRB51 vaccine has been demonstrated to be protective (2, 18), increases in antigen-specific proliferative responses as measured by [3H]thymidine incorporation begin at 6 to 12 weeks and are consistently detected for as long as 24 weeks after parenteral vaccination with 1010 CFU of SRB51 (17, 22). Unpublished flow cytometric data from our laboratory indicates that SRB51-vaccinated cattle have strong proliferative responses in CD4+, CD8+, and IgM+ subsets between 12 and 16 weeks after vaccination versus nonvaccinated cattle. Although stimulation indices in the current elk study appear to be high compared to bison and cattle responses, background counts in wells without antigen were lower than previous studies in other species. The mean cpm of elk PBMC incubated in the absence of antigen over the course of the study was 1,991 ± 364. For a number of sampling times, including 22 weeks after the initial vaccination, the mean cpm for cells incubated in the absence of antigen was less than 1,000. In comparison, in a recent study in our laboratory, cattle PBMC incubated in the absence of antigen for a similar incubation time had a mean cpm of 5,317 ± 1,227. The relatively low background tended to amplify the magnitude of the mean stimulation indices in elk data such that indices are greater than those reported in other studies. However, it should be noted that failure to respond was not a function of incubation conditions or species-specific effects since elk PBMC demonstrated strong proliferative responses to 1 μg of pokeweed mitogen/ml, a finding comparable to similar data from our laboratory using cattle PBMC.
We have previously observed that SRB51 can persist for up to 34 weeks after vaccination in elk (16). One possibility for the failure to observe an anamnestic response after booster vaccination could be that the administration of live, proliferating bacteria, which persist for weeks in vivo, is sufficient to induce maximal primary and secondary immune responses such that additional antigenic stimulation does not induce immunologic responses of greater magnitude. The possibility that the regulation of immunologic memory in elk differs from other species also warrants consideration.
Our immunologic data suggested that both S19 and SRB51 did not induce robust cellular immune responses in elk, and there did not seem to be a difference between the two vaccines in their ability to induce cell-mediated immune responses after vaccination. Our data suggesting relatively poor cellular immunity in vaccinated elk may explain why previous studies have failed to demonstrate protection in elk that have received single or multiple dosages of 1010 CFU of SRB51 (6, 7). Although S19 vaccination gives relatively poor protection in elk, 109 CFU has been reported to prevent abortion in ca. 30% of vaccinated elk compared to unvaccinated controls (5, 7, 23). Since our data and others suggest that elk develop strong humoral responses and limited protection after S19 vaccination, it might be hypothesized that the limited protection induced by S19 vaccination of elk may be mediated through humoral responses. Antibodies induced by the smooth S19 strain may opsonize smooth field strains of B. abortus and induce phagocytosis, complement activation, and subsequent bacterial killing. In murine models of brucellosis, passive transfer with monoclonal antibodies against the O side chain induces protection for up to 4 weeks (1, 11). Since SRB51 does not express the O side chain, animals vaccinated with SRB51 do not develop antibodies against this antigen. Since lipopolysaccharides of smooth strains may make some surface antigens of B. abortus inaccessible to bovine antisera (20), antibodies developed against SRB51 may have limited abilities to opsonize smooth strains of B. abortus. Therefore, if opsonization is the mechanism for limited protection in vivo, the failure of SRB51 vaccination to induce comparable protection to S19 vaccination in elk may be because antibodies induced by SRB51 fail to bind and opsonize smooth strains of B. abortus.
Protection against Brucella, as an intracellular pathogen, is believed to be associated with the secretion of interleukin-12 (29) and IFN-γ (13) by Th1-type helper T cells and the subsequent activation of cytotoxic T cells, NK cells, and macrophages. In comparison, cytokines associated with a Th2-type response can stimulate antibody responses but can also have negative regulatory effects on Th1-type responses (4, 12) and associated reductions in protection against intracellular pathogens (19). Since elk develop robust antibody responses to Brucella, the Th1-Th2 paradigm is an attractive mechanism for explaining the lack of cellular immune responses.
After vaccination of elk with Mycobacterium bovis bacillus Calmette-Guérin (BCG), a vaccine for another intracellular bacterial pathogen, antigen-specific proliferative responses are primarily associated with IgM+ cells (26). In comparison, cattle and white-tailed deer (Odocoileus virginianus) develop strong cellular immune responses after BCG vaccination (14, 27). The failure of elk to develop robust cellular immune responses to brucellosis vaccines is therefore not unique to Brucella. Rather, data currently available would suggest that the failure to develop strong cell-mediated responses is intrinsic to the host (elk) rather than the pathogen (B. abortus). Development of an efficacious brucellosis vaccine for elk will most likely require finding adjuvants or other mechanisms to modulate elk immune responses toward protective Th1-type responses.
Acknowledgments
We thank Aileen Duit, Allen Jensen, Terry Krausman, Katie Lies, Darl Pringle, Deb Buffington, Donnie Robinson, Larry Wright, and Dennis Weuve for technical assistance. We also thank the Oregon Department of Fish and Wildlife for the donation of the elk used in this research.
Product names are necessary to report factually on the available data; however, the U.S. Department of Agriculture neither guarantees nor warrants the standard of the product, and the use of the name by the U.S. Department of Agriculture implies no approval of the product to the exclusion of others that may also be suitable.
REFERENCES
- 1.Araya, L. N., P. H. Elzer., G. E. Rowe, F. M. Enright, and A. J. Winter. 1989. Temporal development of protective cell-mediated and humoral immunity in BALB/c mice infected with Brucella abortus. J. Immunol. 143:3330-3337. [PubMed] [Google Scholar]
- 2.Cheville, N. F., S. C. Olsen, A. E. Jensen, M. G. Stevens, and M. V. Palmer. 1996. Effects of age at vaccination on efficacy of Brucella abortus strain RB51 to protect cattle against brucellosis. Am. J. Vet. Res. 57:1153-1156. [PubMed] [Google Scholar]
- 3.Claus, D., S. Kilpatrick, R. Dean, and B. Smith. 2002. Brucellosis-feedground-habitat program: an integrated management approach to brucellosis in elk in Wyoming, p. 80-96. In T. J. Kreeger (ed.), Proceedings of Brucellosis in Elk and Bison in the Greater Yellowstone Area. Wyoming Game and Fish Department, Cheyenne, Wyo.
- 4.Fernandes, D., and C. Baldwin. 1995. Interleukin-10 downregulates protective immunity to Brucella abortus. Infect. Immun. 63:1130-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herriges, J. D., E. T. Thorne, S. L. Anderson, and H. A. Dawson. 1989. Vaccination of elk in Wyoming with reduced dose strain 19 Brucella: controlled studies and ballistic implant field trials. Proc. U. S. Anim. Health Assoc. 93:640-655. [Google Scholar]
- 6.Kreeger, T. J., M. W. Miller, M. Wild, P. H. Elzer, and S. C. Olsen. 2000. Safety and efficacy of Brucella abortus strain RB51 vaccine in captive pregnant elk. J. Wildl. Dis. 36:477-483. [DOI] [PubMed] [Google Scholar]
- 7.Kreeger, T. J., W. E. Cook, W. H. Edwards, P. H. Elzer, and S. C. Olsen. 2002. Brucella abortus strain RB51 vaccination in elk II. Failure of high dosage to prevent abortion. J. Wildl. Dis. 38:27-31. [DOI] [PubMed] [Google Scholar]
- 8.Kreeger, T. J., and S. C. Olsen. 2002. Brucellosis vaccination in elk, p. 43-50. In T. J. Kreeger (ed.), Proceedings of Brucellosis in Elk and Bison in the Greater Yellowstone Area. Wyoming Game and Fish Department, Cheyenne, Wyo.
- 9.Lee, I.-K., S. C. Olsen, and C. A. Bolin. 2001. Effects of exogenous recombinant interleukin-12 on immune responses and protection against Brucella abortus in a murine model. Can. J. Vet. Res. 65:223-228. [PMC free article] [PubMed] [Google Scholar]
- 10.Montaraz, J. A., and A. J. Winter. 1986. Comparison of living and nonliving vaccines for Brucella abortus in BALB/c mice. Infect. Immun. 53:245-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montaraz, J. A., A. J. Winter, D. M. Hunter, B. A. Sowa, A. M. Wu, and L. G. Adams. 1986. Protection against Brucella abortus in mice with O-polysaccharide-specific monoclonal antibodies. Infect. Immun. 51:961-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morel, P. A., and T. B. Oriss. 1998. Crossregulation between Th1 and Th2 cells. Crit. Rev. Immunol. 18:275-303. [DOI] [PubMed] [Google Scholar]
- 13.Murphy, E. A., J. Sathiyaseelan, M. A. Parent, B. Zou, and C. L. Baldwin. 2001. Interferon-gamma is crucial for surviving a Brucella abortus infection in both resistant C57BL/6 and susceptible BALB/c mice. Immunology 103:511-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nonnecke, B. J., W. R. Waters, M. R. Foote, M. V. Palmer, B. L. Miller, T. E. Johnson, H. B. Perry, and M. A. Fowler. 2005. Development of an adult-like cell-mediated immune response in calves after early vaccination with Mycobacterium bovis bacillus Calmette-Guérin. J. Dairy Sci. 88:195-210. [DOI] [PubMed] [Google Scholar]
- 15.Olsen, S. C., M. G. Stevens, N. F. Cheville, and G. G. Schurig. 1997. Experimental use of a dot blot assay to measure serologic responses of cattle vaccinated with Brucella abortus strain RB51. J. Vet. Diagn. Investig. 9:363-367. [DOI] [PubMed] [Google Scholar]
- 16.Olsen, S. C., T. J. Kreeger, and M. V. Palmer. 2002. Immune responses of elk to vaccination with Brucella abortus strain RB51. J. Wildl. Dis. 38:746-751. [DOI] [PubMed] [Google Scholar]
- 17.Olsen, S. C., T. J. Kreeger, and W. Schultz. 2002. Immune responses of bison to ballistic or hand vaccination with Brucella abortus strain RB51. J. Wildl. Dis. 34:738-745. [DOI] [PubMed] [Google Scholar]
- 18.Olsen, S. C., A. E. Jensen, W. C. Stoffregen, and M. V. Palmer. 2003. Efficacy of calfhood vaccination with Brucella abortus strain RB51 in protecting bison against brucellosis. Res. Vet. Sci. 74:17-22. [DOI] [PubMed] [Google Scholar]
- 19.Rook, G. A. W., K. Dheda, and A. Zumla. 2006. Immune systems in developed and developing countries; implications for the design of vaccines that will work where BCG does not. Tuberculosis 86:152-162. [DOI] [PubMed] [Google Scholar]
- 20.Schurig, G. G., A. T. Pringle, and S. S. Breese. 1981. Localization of Brucella antigens that elicit a humoral immune response in Brucella abortus-infected cattle. Infect. Immun. 34:1000-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith, B. L. 2001. Winter feeding of elk in Western North America. J. Wildl. Meet. 65:173-190. [Google Scholar]
- 22.Stevens, M. G., S. C. Olsen, and N. F. Cheville. 1995. Comparative analysis of immune responses in cattle vaccinated with Brucella abortus strain 19 or strain RB51. Vet. Immunol. Immunopathol. 44:223-235. [DOI] [PubMed] [Google Scholar]
- 23.Thorne, E. T., T. J. Walthall, and H. A. Dawson. 1981. Vaccination of elk with strain 19 Brucella abortus. Proc. U. S. Anim. Health Assoc. 85:359-374. [Google Scholar]
- 24.Tunnicliff, E. A., and H. Marsh. 1935. Bang's disease in bison and elk in the Yellowstone National Park and on the National Bison Range. J. Am. Vet. Med. Assoc. 86:745-752. [Google Scholar]
- 25.Vemulappalli, R., J. R. Mcquiston, G. G. Schurig, N. Sriranganathan, S. M. Halling, and S. M. Boyle. 1999. Identification of an IS711 element interrupting the wboA gene of Brucella abortus vaccine strain RB51 and a PCR assay to distinguish strain RB51 from other Brucella species and strains. Clin. Diagn. Lab. Immunol. 6:760-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waters, W. R., M. V. Palmer, S. C. Olsen, R. E. Sacco, and D. L. Whipple. 2003. Immune responses of elk to Mycobacterium bovis bacillus Calmette Guerin vaccination. Vaccine 21:1518-1526. [DOI] [PubMed] [Google Scholar]
- 27.Waters, W. R., M. V. Palmer, D. L. Whipple, R. E. Slaughter, and S. L. Jones. 2004. Immune responses of white-tailed deer (Odocoileus virginianus) to Mycobacterium bovis BCG vaccination. J. Wildl. Dis. 40:66-78. [DOI] [PubMed] [Google Scholar]
- 28.Winter, A. J., G. E. Rowe., J. R. Duncan, M. J. Eise, J. Widom, B. Ganem, and B. Morein. 1988. Effectiveness of natural and synthetic complexes of porin and O polysaccharide as vaccines against Brucella abortus in mice. Infect. Immun. 56:2808-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhan, Y., and C. Cheers. 1995. Endogenous interleukin-12 is involved in resistance to Brucella abortus infections. Infect. Immun. 63:1387-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]