Abstract
Iron is required by most organisms, but an excess of this metal is potentially toxic. Consequently, uptake and intracellular storage of iron are tightly controlled. The filamentous fungus A. nidulans lacks the iron storage compound ferritin but possesses an intracellular siderophore, which is accumulated in a highly regulated manner as iron-free desferri-ferricrocin or iron-containing ferricrocin via transcriptional regulation of the nonribosomal peptide synthetase SidC. Biosynthesis of desferri-ferricrocin was low during iron-replete conditions but up-regulated by both iron starvation and intracellular iron excess, the latter caused by either a shift from iron-depleted to high-iron conditions or deregulation of iron uptake. Consequently, ferricrocin constituted only about 5% of the total iron content under iron-replete conditions but up to 64% during conditions of intracellular excess. In contrast, during iron starvation, desferri-ferricrocin was accumulated, which appears to represent a proactive strategy to prevent iron toxicity. Accumulation of the intracellular siderophore was also up-regulated by oxidative stress, which underscores the intertwining of iron metabolism and oxidative stress. Lack of the intracellular siderophore causes pleiotropic effects, as SidC deficiency results in (i) less-efficient utilization of iron, indicated by reduced growth under iron-depleted conditions and a higher iron demand under iron-replete conditions, (ii) delayed germination under iron-depleted conditions, (iii) increased sensitivity of conidia to oxidative stress, and (iv) elimination of cleistothecia formation in homothallic conditions.
Iron is essential for all eukaryotes and most prokaryotes, as it serves as a cofactor in many enzymes and as a catalyst in electron transport systems. Excess or incorrect storage of iron, however, is toxic, as this metal has the capacity to reinforce the production of reactive oxygen species (14). Therefore, iron homeostasis is designed to maintain sufficient iron supply while, at the same time, preventing excess iron accumulation and minimizing cell-damaging effects.
In contrast to bacteria, plants, and animals (2, 3), most fungi lack ferritin-mediated iron storage and detoxification and, therefore, have developed alternative strategies. Most fungi excrete siderophores, which are low-molecular-mass, ferric-iron-specific chelators, to mobilize extracellular iron but also produce intracellular siderophores (11, 36). The function of the latter has not been studied in great detail so far due to the fact that Saccharomyces cerevisiae, which serves as a paradigm for fungal metabolism, can utilize iron bound to siderophores produced by other organisms but lacks the ability to synthesize siderophores by itself (11, 30, 35). Therefore, the vast knowledge of iron homeostasis in this yeast is of limited value for our understanding of iron uptake and storage in siderophore-producing fungi.
The ascomycetes Aspergillus nidulans and Aspergillus fumigatus are typical siderophore-producing fungi and are attractive for various aspects. A. fumigatus is the most common airborne fungal pathogen of humans, causing life-threatening invasive disease in immunocompromised patients (20); A. nidulans is a much rarer cause of disease but represents a model filamentous fungus because it possesses a sexual cycle and is therefore amenable to classical genetic analysis (6). Both Aspergillus species produce two major hydroxamate-type siderophores: desferri-triacetylfusarinine C (TAFC−Fe) to mobilize extracellular iron and desferri-ferricrocin (FC−Fe), which is involved in intracellular iron storage (26, 32). TAFC−Fe is a cyclic tripeptide consisting of three N2-acetyl-N5-cis-anhydromevalonyl-N5-hydroxyorhithine residues linked by ester bonds, and FC−Fe is a cyclic hexapeptide with the structure Gly-Ser-Gly-(N5-acetyl-N5-hydroxyornithine)3 (11). After uptake of iron-loaded TAFC−Fe, termed ferri-triacetylfusarinine C (TAFC+Fe), by specific transporters (12), the iron is transferred to metabolic pathways or stored by conversion of FC−Fe to ferricrocin (FC+Fe). TAFC−Fe is hydrolyzed by an esterase, and the hydrolysis products are excreted (26).
The first committed step of biosynthesis of both siderophores is the hydroxylation of ornithine catalyzed by N5-ornithine-monooxygenase, which is encoded by the sidA gene (9, 32). Deficiency in sidA causes the complete absence of siderophores in both fungal species. A. nidulans ΔsidA mutants are unable to grow unless supplemented with siderophores or high amounts of ferrous iron. In contrast, sidA-deficient A. fumigatus strains are viable due to the presence of a reductive iron assimilatory system, which is missing in A. nidulans. However, siderophore biosynthesis, but not reductive iron assimilation, has been found to be essential for A. fumigatus virulence in a murine model of invasive aspergillosis (32). In A. nidulans, two other components of the siderophore metabolism have been characterized: the nonribosomal FC−Fe synthetase SidC and the iron-regulatory GATA-type transcription factor SreA. SidC-deficient mutants still produce TAFC−Fe but lack synthesis of FC−Fe (9). SreA deficiency causes partial derepression of siderophore biosynthesis and iron uptake under iron-replete conditions (13, 26, 28).
Very little is known regarding the intracellular handling of iron in eukaryotes and, in particular, in ferritin-lacking fungi. We demonstrate that the accumulation of the intracellular siderophore is regulated by intra- and extracellular iron availability as well as oxidative stress in A. nidulans. The study revealed that FC+Fe is a major iron storage compound during intracellular iron excess but also suggested the presence of an alternative iron storage mechanism. Moreover, the absence of the intracellular siderophore was found to impair both sexual and asexual reproduction, to cause increased sensitivity of conidia to oxidative stress, and to result in delayed germination of conidia under iron-depleted conditions.
MATERIALS AND METHODS
Strains, media, and growth conditions.
The A. nidulans strains WGTRAN (argB2::pTran argB bgA0 biA1), SRKO1 (argB2 bgA0 biA1 sreA::argB), and SIC07 (argB2 bgA0 biA1 sidC::argB) are designated in the text as wt (wild type), ΔsreA, and ΔsidC, respectively (4, 9, 13). All three strains are derived from WG355 (4) and are isogenic, with the exception of the genomic localization of argB and the deficiencies in sreA and sidC.
Generally, fungal strains were grown at 37°C in minimal medium, according to the description of Pontecorvo et al. (31), containing 1% glucose as the carbon source, 20 mM glutamine as the nitrogen source, and 10 μM FeSO4 as the iron source supplemented with 20 μg liter−1 biotin but lacking the addition of iron for iron-depleted conditions. For iron-replete and high-iron conditions, TAFC+Fe was added to final concentrations of 10 μM and 100 μM, respectively. Shake flask cultures were obtained with 200 ml of medium in 1.0-liter Erlenmeyer flasks inoculated with 108 conidia. Conidia of wt, ΔsreA, and ΔsidC were derived from solid minimal medium containing either 1.5 mM FeSO4 or 10 μM FC+Fe after growth for 96 h. Importantly, ΔsidC conidia lack FC+Fe when derived from FeSO4 plates but have wt-like FC+Fe content when derived from FC+Fe plates.
Analysis of siderophores and iron.
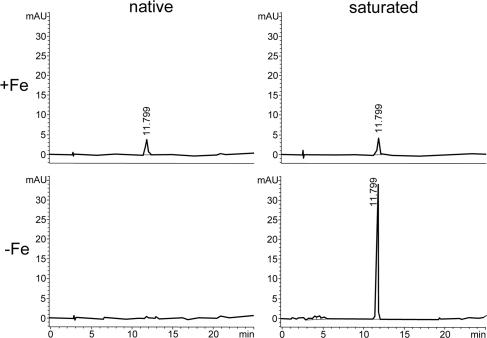
TAFC+Fe and FC+Fe were isolated from A. nidulans as described previously (26). Quantification of FC+Fe and FC−Fe was performed by reversed-phase high-performance liquid chromatography according to the method of Konetschny-Rapp et al. (18) as described previously (26). FC+Fe and FC−Fe were discriminated by analysis of the samples with and without the addition of ferric chloride to a final concentration of 1 mM, as photometric detection at 435 nm records only ferri-siderophores (Fig. 1). TAFC+Fe and TAFC−Fe can be discriminated just as well. TAFC+Fe and FC+Fe are distinguished by different retention times, as shown previously (26).
FIG. 1.
Reversed-phase high-performance liquid chromatography determination of cellular siderophore accumulation under iron-depleted (−Fe) and iron-replete (+Fe) conditions in A. nidulans wt with (saturated) and without (native) saturation with ferric chloride.
For determination of the total cellular iron content, 50 mg of freeze-dried mycelia was decomposed in closed polytetrafluorethylene vessels containing 2 ml of HNO3 and 0.5 ml of hydrogen peroxide using a high-performance microwave digestion unit (mls-1200 mega). Appropriate dilutions were made with distilled water, and the total iron content was determined by graphite furnace atomic absorption spectrometry (Hitachi Polarized Zeeman AAS Z8200) according to standard methods.
Analysis of fungal damage by exogenous H2O2.
The hydrogen peroxide diffusion assay was performed as described by Maerker et al. (23). Briefly, 108 conidia were plated on petri dishes containing iron-replete minimal medium. Holes of 1 cm in diameter were punched, and 100 μl of a 3% hydrogen peroxide solution was applied either immediately or after preincubation for 7 h at 37°C. Inhibition zones were measured after further incubation for 24 h at 37°C.
The sensitivity of conidia to killing by hydrogen peroxide was assayed essentially as described by Han et al. and Kawasaki et al. (15, 17). Briefly, 1 ml of conidial suspension containing approximately 105 spores was incubated for 30 min at 20°C with the indicated concentrations of hydrogen peroxide. To determine the number of surviving conidia, the spore suspensions were diluted 50-fold with sterile water and 0.1 ml was plated on iron-replete minimal medium. After incubation for 24 h at 37°C, colonies were counted and normalized to that without hydrogen peroxide treatment.
The sensitivity of hyphae to hydrogen peroxide was estimated by using a modification of the protocol of Kawasaki et al. (17). About 150 conidia were plated on iron-replete minimal medium and grown at 37°C for 19 h—at this time point, small colonies were countable. Subsequently, the plates were overlaid with 4.5 ml of the same medium as top agar but containing the indicated concentration of hydrogen peroxide. After further incubation for 24 h at 37°C, colonies able to resume growth were counted as survivors and normalized to the number before hydrogen peroxide treatment.
Analysis of sexual development.
To analyze homothalic sexual development, complete medium-agar plates were point inoculated with 106 conidia and incubated for 48 h at 37°C. Subsequently, small mycelial sections were transferred to minimal medium containing the iron source indicated (see Fig. 6) and further incubated for 24 h at 37°C. After sealing with Parafilm, incubation of the plates was continued for another 10 days under dark conditions. Sexual development was analyzed using a Leica MZ16 stereomicroscope. Huelle cells and ascospores were photographed using a Zeiss Axioplan light microscope.
FIG. 6.
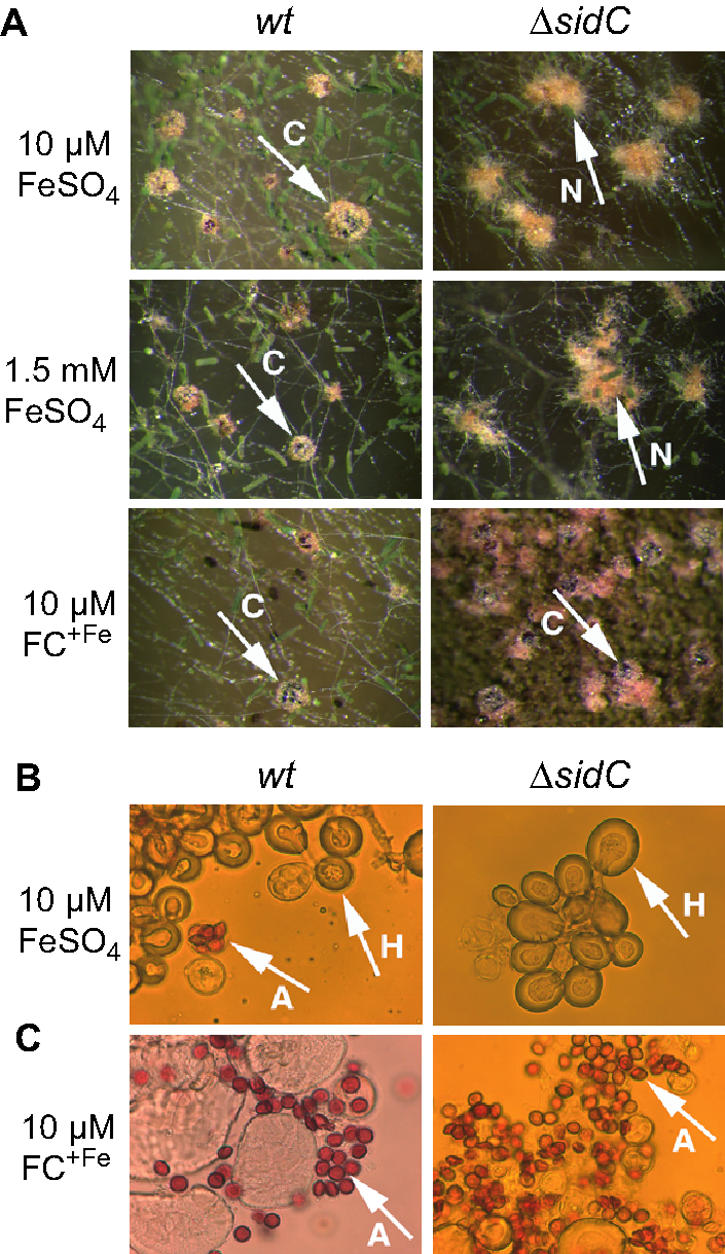
Ferricrocin deficiency causes self-sterility. (A) Cleistothecia (C) and nests (N) consisting of Huelle cells produced by A. nidulans wt and/or ΔsidC on minimal medium containing 10 μM FeSO4, 1.5 mM FeSO4, or 10 μM FC+Fe as the iron source. (B) Huelle cells (H) and ascospores (A) produced by wt and ΔsidC on 10 μM FeSO4. (C) Ascospores (A) produced by wt and ΔsidC on 10 μM FC+Fe. Magnification, ×50 (A) and ×1,000 (B and C).
Analysis of germination.
Approximately 104 conidia were incubated at 37°C in petri dishes containing 15 ml liquid minimal medium supplemented with different iron sources. Germination was scored microscopically at 0.5 h intervals. The time point when 45 out of 50 conidia developed a germ tube was defined as the time required for germination.
Northern analysis.
RNA was isolated using TRI reagent (Sigma-Aldrich). Generally, 10 μg of total RNA was electrophoresed on 1.2% agarose-2.2 M formaldehyde gels and blotted onto Hybond N membranes (Amersham). The hybridization probes used in this study were generated by PCR using oligonucleotides 5′-GCACCAGGTCATTGAGTACC-3′ and 5′-TCGTCAGCATGCTCTGGACG-3′ for sidC (9), 5′-GTATATCTTCGCGCAGGG-3′ and 5′-AACCCATCAACACCCGAG-3′ for mirB (12), and 5′-CGGTGATGAGGCACAGT-3′ and 5′-CGGACGTCGACATCAACA-3′ for γ-actin-encoding acnA (10).
RESULTS
FC+Fe is a major iron compound during intracellular iron excess.
As a first step toward the understanding of the function of the intracellular siderophore in iron metabolism of A. nidulans, we analyzed the respective amounts of FC−Fe, FC+Fe, and total cellular iron content during conditions of different iron availability. To this end, we compared A. nidulans wt and ΔsreA strains that were grown for 24 h under iron-depleted, iron-replete (10 μM TAFC+Fe), and high-iron (100 μM TAFC+Fe) conditions (Table 1). A. nidulans ΔsreA was analyzed because this mutant shows excess accumulation of iron under iron-replete conditions (13). TAFC+Fe was used as an iron source because it is soluble even in high concentrations and is the natural iron source of A. nidulans (9). FC−Fe binds iron stoichiometrically 1:1 (11), and therefore, the FC+Fe content directly corresponds to the amount of iron bound to intracellular siderophores. Under iron-depleted conditions, A. nidulans wt contained about 0.23 μmol iron and 1.78 μmol FC−Fe per g (dry weight) but did not contain detectable amounts of FC+Fe. In comparison, iron-replete and high-iron conditions increased the iron content 4.3-fold and 6.8-fold, respectively. Under both conditions, FC+Fe constituted about 5% of the total cellular iron content and FC−Fe was absent.
TABLE 1.
Steady-state cellular contents in FC−Fe, FC+Fe, and total iron of A. nidulans wt and ΔsreA after growth for 24 h under iron-depleted, iron-replete, and high-iron conditionsa
| Strain | Growth condition | Content (μmol g−1 [dry weight]) of:
|
FC+Fe/ total iron | ||
|---|---|---|---|---|---|
| FC−Fe | FC+Fe | Total iron | |||
| wt | −Fe | 1.78 ± 0.23 | 0.00 | 0.23 ± 0.03 | |
| 10 μM TAFC+Fe | 0.00 | 0.05 ± 0.01 | 0.99 ± 0.09 | 0.05 | |
| 100 μM TAFC+Fe | 0.00 | 0.08 ± 0.01 | 1.57 ± 0.33 | 0.04 | |
| ΔsreA | −Fe | 1.94 ± 0.21 | 0.00 | 0.23 ± 0.02 | |
| 10 μM TAFC+Fe | 0.00 | 1.72 ± 0.16 | 2.69 ± 0.22 | 0.64 | |
| 100 μM TAFC+Fe | 0.00 | 5.78 ± 0.29 | 18.24 ± 2.20 | 0.31 | |
The data represent the means ± standard deviations of results from three simultaneously harvested flasks.
A. nidulans ΔsreA did not show any significant difference from the wt under iron-depleted conditions with respect to FC−Fe and total iron content. However, under iron-replete conditions, the total iron content of ΔsreA, compared to the wt, was increased 2.7-fold with 64% bound to FC+Fe, which corresponds to a 34-fold increase in FC+Fe content. In other words, the surplus in FC+Fe made up for the entire surplus in iron excess in ΔsreA compared to the wt under iron-replete conditions. During high-iron conditions, the total iron content of ΔsreA was increased 11.6-fold with 31% consisting of FC+Fe—a 72-fold increase in FC+Fe compared to the wt under this condition. Remarkably, despite a 3.4-fold increase in FC+Fe content from iron-replete to high-iron conditions in ΔsreA, the ratio of FC+Fe to total iron decreased from 64% to 31%. The latter indicates that, under high-iron conditions, the iron is stored to an increasing degree independently of FC+Fe in ΔsreA, which might be explained by a limited capacity of FC+Fe-mediated iron storage. During high-iron conditions, the total iron content of ΔsreA was increased 79-fold compared to iron-depleted conditions, which indicates a wide range in the content of this potentially toxic metal. Taken together, these data revealed that SreA deficiency causes an increase of total iron content accompanied by an increase of FC+Fe content directly depending on ambient iron availability.
The high FC+Fe accumulation in ΔsreA might be a direct result of derepression of FC−Fe biosynthesis due to deficiency in SreA or caused indirectly by the resulting iron excess. To address this question, we analyzed the role of FC+Fe during iron excess in A. nidulans wt. The intracellular iron content cannot be raised significantly in A. nidulans wt by steady-state growth during high-iron conditions due to functional iron regulation. Therefore, the wt was grown for 22 h under iron-depleted conditions to fully activate iron uptake mechanisms and subsequently shifted into high-iron conditions by addition of TAFC+Fe to a final concentration of 100 μM to induce excessive uptake of iron. FC−Fe, FC+Fe, and total iron contents were measured after 0.5, 2.0, 5.0, and 8.0 h (Table 2). Within 0.5 h after the shift, the cellular iron content increased 25-fold and the complete FC−Fe pool was converted to FC+Fe, which constituted 44% of the total iron content. At 2 h after the shift, both the total iron and FC+Fe contents showed a maximum. Remarkably, the total iron content increased 86-fold compared to iron-depleted conditions and the FC+Fe content increased 2.9-fold compared to the initial FC−Fe pool. At this time point, the ratio of FC+Fe to total iron decreased to about 30%, indicating that the iron was stored to an increasing extent independently of FC+Fe. Within a further 3.0 h of incubation, both the total cellular iron and FC+Fe contents decreased about 0.6-fold. This appears to be due to distribution by cell division because the total biomass increased 4.6-fold during the time course experiment, and in contrast to the cellular level, the content of both FC+Fe and total iron of the entire culture increased. From the 5.0 h time point to the 8.0 h time point, the FC+Fe pool was maintained, indicating slow metabolization.
TABLE 2.
Dry weight and content of FC−Fe, FC+Fe, and total iron in A. nidulans wt during a shift from iron-depleted to high-iron conditionsa
| Time (h) after shift | Content (μmol g−1 [dry weight]) of:
|
FC+Fe/total iron | Dry weight (g) | Dry weight × content (μmol) of:
|
|||
|---|---|---|---|---|---|---|---|
| FC−Fe | FC+Fe | Total iron | FC+Fe | Total iron | |||
| 0.0 | 2.1 ± 0.2 | 0.0 | 0.23 ± 0.04 | 0.10 ± 0.01 | 0.02 | 0.01 | |
| 0.5 | 0.0 | 2.5 ± 0.3 | 5.70 ± 0.59 | 0.44 | 0.16 ± 0.1 | 0.41 | 0.93 |
| 2.0 | 0.0 | 6.1 ± 0.5 | 19.81 ± 2.52 | 0.31 | 0.18 ± 0.01 | 1.11 | 3.61 |
| 5.0 | 0.0 | 3.4 ± 0.1 | 11.91 ± 2.11 | 0.28 | 0.35 ± 0.01 | 1.18 | 4.17 |
| 8.0 | 0.0 | 3.1 ± 0.4 | 11.19 ± 1.90 | 0.28 | 0.44 ± 0.02 | 1.37 | 4.94 |
A. nidulans wt was grown for 22 h in shake flask culture under iron-depleted conditions, and subsequently, TAFC+Fe was added to a final concentration of 100 μM. Measurements were performed at 0.0, 0.5, 1.0, 2.0, 5.0, and 8.0 h after the shift. The data represent the means ± standard deviations of results from three simultaneously harvested flasks.
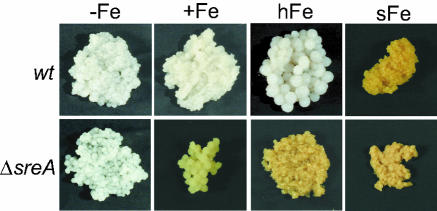
The analyses of the FC+Fe content of A. nidulans wt and ΔsreA indicated that the intracellular siderophore is a major iron storage compound in hyphae during conditions of intracellular iron excess. Remarkably, the mycelial mat of A. nidulans wt and ΔsreA displayed a brown coloration during conditions of high FC+Fe accumulation (Fig. 2), which is likely to be a direct effect, as FC+Fe has a brownish color.
FIG. 2.
Cellular accumulation of FC+Fe correlates with mycelial coloration. A. nidulans wt and ΔsreA were grown in shake flask culture for 24 h under iron-depleted (−Fe), iron-replete (+Fe, 10 μM TAFC+Fe), or high-iron (hFe, 100 μM TAFC+Fe) conditions or shifted for 2.0 h into high-iron conditions after growth for 22 h under iron-depleted conditions (sFe).
Oxidative stress causes increased siderophore accumulation.
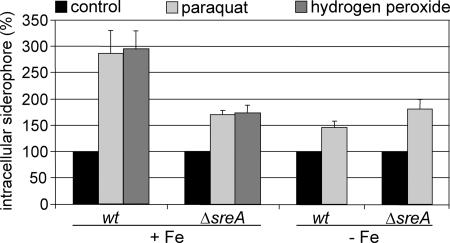
As shown above, in the shift of A. nidulans wt from iron-depleted to high-iron conditions, the FC+Fe content increased 2.9-fold within 2 h compared to the initial FC−Fe pool (Table 2). This might be caused directly by the intracellular iron excess or indirectly by the oxidative stress provoked by the iron excess. To investigate a possible effect of oxidative stress on the accumulation of the intracellular siderophore, wt and ΔsreA mycelia were exposed to hydrogen peroxide or the redox cycler paraquat (Fig. 3). Under iron-replete conditions, the wt strain responded to both modes of oxidative stress with a 2.9-fold increase in FC+Fe content. In ΔsreA, this treatment caused a 1.7-fold increase, and it has to be emphasized that this takes place at a 34-fold-higher level (compare FC+Fe content of wt and ΔsreA during growth in 10 μM TAFC+Fe in Table 1). Under iron-depleted conditions, the same hydrogen peroxide treatment caused killing of the wt and ΔsreA mycelia, which indicates higher sensitivity of iron-starved mycelia toward hydrogen peroxide. However, wt and ΔsreA responded to paraquat treatment by an increases in FC−Fe content of 1.5-fold and 1.8-fold, respectively. Remarkably, treatment with neither paraquat nor hydrogen peroxide affected the total iron content of wt and ΔsreA under iron-replete conditions.
FIG. 3.
Oxidative stress up-regulates accumulation of the intracellular siderophore. A. nidulans wt and ΔsreA were grown in shake flask culture for 16 h under iron-depleted (−Fe) or iron-replete conditions (+Fe). Subsequently, paraquat or hydrogen peroxide was added to a final concentration of 10 mM. For hydrogen peroxide, the addition of the same amount was repeated hourly. After further incubation for 8 h, mycelia were harvested and the FC+Fe and FC−Fe contents were measured and normalized to that of mycelia of the respective strain grown for 24 h without oxidative-stress treatment. The data represent the means ± standard deviations of results from three simultaneously harvested flasks. Columns represent the FC+Fe content under +Fe conditions and the FC−Fe content under −Fe conditions.
These data show that oxidative stress causes increased accumulation of the intracellular siderophore during both iron-replete and iron-depleted conditions independent of SreA and suggest that the increase in FC+Fe under iron-replete conditions is due to redistribution of intracellular iron rather than iron uptake.
Both intracellular iron excess and oxidative stress up-regulate expression of sidC.
To investigate at which level iron excess and oxidative stress regulate the synthesis of the intracellular siderophore, we performed expression analysis of sidC, which encodes the nonribosomal peptide synthetase involved in FC−Fe biosynthesis.
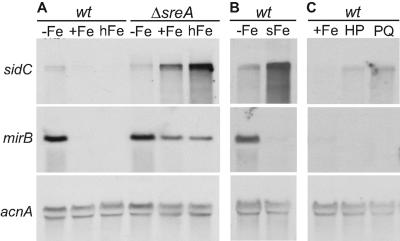
Northern analysis demonstrated (Fig. 4A), as shown previously (28), that expression of sidC is repressed under iron-replete conditions and derepressed under iron-depleted conditions in the wt. The mirB gene, which encodes an SreA-mediated iron-regulated transporter for uptake of TAFC+Fe (12), exhibited an expression pattern similar to that of sidC. In ΔsreA, however, the transcript level of sidC increased with elevating extracellular iron availability, which is directly related to the intracellular iron content in this mutant, whereas that of mirB decreased. Moreover, a shift from iron-depleted to high-iron conditions further increased the expression of sidC but repressed expression of mirB in the wt (Fig. 4B). Taken together, the expression analysis of sidC and mirB suggests that the increase of FC+Fe accumulation by intracellular iron excess in A. nidulans wt and ΔsreA is mediated by transcriptional up-regulation of sidC expression. Moreover, intracellular iron excess caused inverse regulation of siderophore-mediated iron storage and siderophore-mediated iron uptake, two processes which are coregulated under iron-depleted conditions.
FIG. 4.
Expression of sidC is up-regulated by intracellular iron excess and oxidative stress. Total RNA was isolated from mycelia harvested from shake flask cultures of strains and conditions described below and subjected to Northern analysis of expression of sidC and mirB. Hybridization with acnA served as a control for loading and quality of RNA. (A) A. nidulans wt and ΔsreA were grown for 24 h under iron-depleted (−Fe), iron-replete (+Fe, 10 μM TAFC+Fe), and high-iron (hFe, 100 μM TAFC+Fe) conditions. (B) A. nidulans wt was grown for 24 h under iron-depleted conditions (−Fe) or grown for 22 h under iron-depleted conditions and subsequently shifted for 2.0 h into high-iron conditions (sFe). (C) A. nidulans wt was grown for 24 h under iron-replete conditions (+Fe) or 20 h under iron-replete conditions and subsequently for 4 h in the presence of 10 mM hydrogen peroxide (HP) or paraquat (PQ), respectively.
Treatment with hydrogen peroxide and paraquat under iron-replete conditions up-regulated the transcript level of sidC, whereas no effect on the expression of mirB was found (Fig. 4C), suggesting that oxidative stress up-regulates expression of sidC but not mirB.
FC−Fe deficiency affects growth rate and iron content.
We have previously found no significant difference in radial growth rate on solid medium between A. nidulans wt and ΔsidC under iron-depleted and iron-replete conditions (9, 32). In three parallel shake flask cultures, the wt produced dry weights of 0.51 ± 0.08 g under iron-replete conditions and 0.47 ± 0.07 g under iron-depleted conditions after 24 h at 37°C. The dry weight of ΔsidC did not differ significantly under iron-replete conditions but was reduced by 27% under iron-depleted conditions. We have shown previously that TAFC−Fe production is mildly increased during iron-replete growth (9), which indicates an increased demand for iron. Consistently, under iron-replete conditions, the iron content of ΔsidC was found to be 1.88 ± 0.12 μmol g−1 (dry weight), which was 1.9-fold higher than that of the wt. Taken together, the decreased submerged growth rate under iron-depleted conditions and the increase of both TAFC−Fe production and iron content during iron-replete growth of ΔsidC suggest that the lack of the intracellular siderophore causes less-efficient utilization of intracellular iron.
The highest FC+Fe content in A. nidulans wt was found during conditions of iron excess caused by the shift from iron-depleted to high-iron conditions (Table 2), suggesting that the intracellular siderophore plays a crucial role during this transition. However, the growth curve of ΔsidC showed no significant difference to the wt in such an experiment (data not shown). At 5.0 h after the shift, the intracellular iron content of ΔsidC was 1.5-fold higher than that of the wt. These results indicate that the lack of effect of FC+Fe deficiency on growth rate is not due to reduced iron uptake and show that FC−Fe is dispensable in hyphae even during intracellular iron excess. In cellular extracts of both the wt and ΔsidC, TAFC+Fe was detected only in trace amounts during the shift (data not shown), which demonstrates that ΔsidC does not compensate FC+Fe deficiency by increased retention of TAFC+Fe.
Deficiency in SidC decreases the conidial iron content.
A. nidulans wt conidia contain FC+Fe, and FC−Fe-deficient strains are able to take up FC+Fe from the growth medium and store it in conidia (9). Therefore, conidia derived from ΔsidC supplemented with FC+Fe during sporulation have the same FC+Fe content as wt conidia despite their inability to synthesize the intracellular siderophore. Determination of the iron content revealed that wt conidia contain about 2.6 times less iron when harvested from mycelia grown under iron-depleted conditions than that grown under high-iron conditions or in FC+Fe-supplemented medium (Table 3). To our knowledge, these data indicate for the first time an influence of growth conditions on the characteristics of the formed conidia. The iron content of FC+Fe-lacking ΔsidC conidia was reduced by 34% compared to the wt, whereas FC+Fe-containing ΔsidC conidia showed a wt-like iron content.
TABLE 3.
Total iron content of A. nidulans wt and ΔsidC conidiaa
| Growth condition | Total conidial iron content in:
|
|
|---|---|---|
| wt | ΔsidC | |
| −Fe | 0.94 ± 0.05 | —b |
| hFe | 2.60 ± 0.22 | 1.72 ± 0.14 |
| FC | 2.51 ± 0.16 | 2.36 ± 0.32 |
Conidia were derived from iron-depleted solid minimal medium without the addition of iron (−Fe) or containing 100 μM FeSO4 (hFe) or 20 μM FC+Fe (FC). The data represent the means ± standard deviations of results from three independent measurements.
As shown previously (9), conidial production of ΔsidC is drastically reduced under iron-depleted conditions, impeding iron content determination (—).
FC+Fe-deficient conidia are sensitive to hydrogen peroxide.
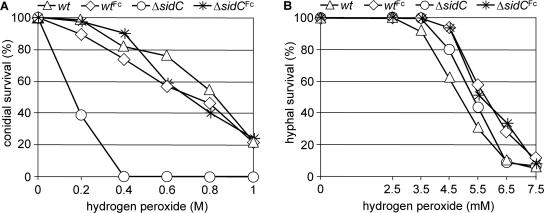
To analyze the role of FC+Fe in oxidative-stress resistance, wt and ΔsidC conidia derived from medium with or without supplementation with FC+Fe were exposed to hydrogen peroxide in a plate diffusion assay as described in Materials and Methods. FC+Fe-lacking ΔsidC conidia displayed increased hydrogen peroxide sensitivity (inhibition zone of 25 mm compared to 20 mm of the wt), which suggests a role of FC+Fe in oxidative-stress resistance. Preincubation for 7 h, to allow germination of the conidia before contact with hydrogen peroxide, decreased the sensitivity of both FC+Fe-lacking ΔsidC (inhibition zone of 19 mm) and the wt (inhibition zone of 17 mm). These data suggest that germination increases the hydrogen peroxide resistance and decreases the difference between FC+Fe-lacking ΔsidC and the wt. FC+Fe-containing ΔsidC conidia showed wt-like hydrogen peroxide resistance with and without preincubation, demonstrating that the hydrogen peroxide sensitivity of ΔsidC is indeed due to the absence of FC+Fe. To further analyze the impact of the intracellular siderophore on oxidative-stress resistance, the effect of hydrogen peroxide on conidial and hyphal survival was tested (Fig. 5). FC+Fe-lacking ΔsidC conidia were significantly more susceptible to killing by hydrogen peroxide, whereas FC+Fe-containing ΔsidC conidia were as resistant as wt conidia (Fig. 5A). In contrast, ΔsidC hyphae displayed wt-like resistance to killing by hydrogen peroxide (Fig. 5B). These data suggest that FC+Fe is more important for the oxidative-stress resistance of conidia than of hyphae.
FIG. 5.
The absence of FC+Fe causes increased sensitivity to hydrogen peroxide of conidia (A) but not hyphae (B). The conidia used were harvested from plates containing 1.5 mM FeSO4 or 10 μM FC+Fe (Fc). Analysis of hydrogen peroxide sensitivity of conidia (A) and hyphae (B) was carried out as described in Materials and Methods. Samples were prepared in triplicate, and the standard deviation did not exceed 15%.
FC+Fe deficiency causes delayed germination under iron-depleted conditions.
We analyzed the role of the conidial FC+Fe pool by comparison of the germination of wt, FC+Fe-containing ΔsidC, and FC+Fe-lacking ΔsidC conidia under iron-replete conditions as well as iron-depleted conditions with and without the iron-specific extracellular chelator bathophenanthroline disulfonate in a final concentration of 200 μM. Under all conditions, wt conidia formed germination tubes after 5.5 h of incubation at 37°C. However, the germination of FC+Fe-lacking ΔsidC conidia was delayed 1.5 h under iron-depleted conditions and 2.5 h in the presence of bathophenanthroline disulfonate. The time required for germination of FC+Fe-containing ΔsidC conidia was wt-like, which underlines that the delay in germination of FC+Fe-lacking ΔsidC conidia is a direct effect of deficiency in conidial FC+Fe. Under iron-replete conditions, FC+Fe-lacking ΔsidC conidia displayed no difference from the wt in the time required to germinate, indicating that the conidial FC+Fe pool serves as an iron source during germination but that its absence can be compensated for by uptake of extracellular iron.
FC+Fe is required for homothallic sexual development.
A. nidulans can form sexual fruiting bodies, termed cleistothecia, in both homothallic and heterothallic conditions (7). Mature black cleistothecia usually contain up to 10,000 asci in which eight red-pigmented ascospores (sexual spores) are present. Cleistothecia are surrounded by thick-walled globular cells, called Huelle cells, which are proposed to function in supporting fruiting body formation by providing necessary sources for energy and nutrients. During crossing experiments we found that ΔsidC could be successfully crossed with other common laboratory strains (data not shown) but was unable to develop homothallic cleistothecia in iron-replete conditions. However, it produced normal Huelle cells (Fig. 6). Cleistothecia formation and production of ascospores was restored by supplementation with FC+Fe but not high concentrations of iron, which demonstrates that self-sterility of ΔsidC is indeed due to lack of FC+Fe.
DISCUSSION
For a steady supply of cells with iron, not only uptake but also storage of this metal is of critical importance. Furthermore, the means of nontoxic iron storage is important in protection against iron-mediated oxidative stress. In organisms as diverse as bacteria, plants, and vertebrates, nontoxic storage of iron is conducted by ferritin and ferritin-like molecules (2, 3). With the exception of zygomycetes, fungi lack ferritin-like molecules. However, many fungal species, like A. nidulans, contain an intracellular siderophore—the focus of this study. In response to extracellular and intracellular iron concentrations, A. nidulans accumulates its intracellular siderophore as FC−Fe or FC+Fe. Under iron-depleted conditions, A. nidulans wt accumulated FC−Fe and did not contain FC+Fe. Under iron-replete conditions and high-iron conditions, the total cellular iron content increased 4.3-fold and 6.8-fold, respectively, and FC+Fe constituted about 5% of the total cellular iron content; FC−Fe was absent. Compared to iron-depleted conditions, the cellular iron content increased about 80-fold during a shift from iron-depleted to high-iron conditions or by derepression of iron uptake due to deficiency in the iron regulator SreA. This demonstrates the capacity of A. nidulans in coping with high amounts of this potentially toxic metal. Under these conditions of intracellular iron excess, FC+Fe became a major iron compound and represented up to 64% of the cellular iron content. In agreement, FC+Fe was also found to be the major iron compound under similar conditions in Neurospora crassa using Mössbauer spectroscopy (24). FC−Fe accumulation during iron depletion at first sight appears inconsistent with a role in iron storage but might represent a proactive strategy to cope with a potentially high incoming iron load due to the activation of iron transport systems. In this respect, the up-regulation of FC−Fe-dependent iron storage capacity under iron-depleted conditions might be comparable to induction of ZRC1 expression, which is essential for zinc tolerance by mediating vacuolar zinc storage, during zinc depletion in S. cerevisiae (22).
Accumulation of FC−Fe as well as FC+Fe requires synthesis of FC−Fe, which is subject to a remarkable regulation: FC−Fe synthesis is low under iron-replete conditions and up-regulated by both iron starvation and intracellular iron excess. Accumulation of the intracellular siderophore correlated with the transcript level of sidC, which encodes the nonribosomal FC−Fe synthetase, suggesting that accumulation of FC−Fe and FC+Fe is regulated at the level of expression of SidC. Comparison of expression of sidC and the TAFC+Fe transporter-encoding mirB indicated that siderophore-mediated iron uptake and siderophore-mediated iron storage are simultaneously up-regulated under iron-depleted conditions, which is at least partly a consequence of coregulation by the iron regulator SreA (26, 28). In contrast, intracellular iron excess caused up-regulation of siderophore-mediated iron storage but down-regulation of uptake. This inverse regulation seems appropriate to avoid iron-mediated oxidative stress and indicates an SreA-independent regulatory mechanism for up-regulation of FC−Fe synthesis during iron excess. This regulation might be part of the oxidative-stress response, as hydrogen peroxide or the redox cycler paraquat up-regulated expression of sidC and production of FC−Fe independent of SreA. Under iron-replete conditions, oxidative stress increased the FC+Fe pool, which appears to represent a redistribution of intracellular iron because the total cellular iron content was unaffected.
A role of the intracellular siderophore in oxidative stress was suggested previously, as sidC deficiency results in an increased labile iron pool and slightly increased sensitivity to paraquat (9). In the present study, we found that FC+Fe deficiency causes significantly increased sensitivity of conidia but not of hyphae to hydrogen peroxide. As iron can potentiate oxidative stress, FC+Fe might be more important for the oxidative-stress resistance of conidia because FC+Fe is the major iron compound of conidia (25) but represents only about 5% of the total cellular iron content of hyphae under iron-replete conditions. On the other hand, oxidative-stress resistance depends on iron because catalases require heme as a cofactor. Consequently, the reduced conidial iron content of ΔsidC conidia might be responsible for the decreased hydrogen peroxide resistance. Compared to conidia, hyphal cells might be physiologically more versatile in the combat of oxidative stress by up-regulation of antioxidative enzymes. In support of the latter, deficiency in sidC results in up-regulation of the genes encoding hyphal catalase B and the Cu/Zn-superoxide dismutase (9). The lack of the siderophore system abrogates virulence of A. fumigatus (32), which might be due to the lack of production of TAFC−Fe and/or FC−Fe. The oxidative-stress sensitivity of ΔsidC conidia indicates a possible role of FC+Fe in virulence, as the killing of Aspergillus conidia by alveolar macrophages is mediated by reactive oxidant intermediates (29).
There are several indications that A. nidulans employs also FC−Fe-independent mechanisms for iron storage and detoxification: (i) deficiency in synthesis of FC−Fe is not lethal; (ii) during conditions of iron excess, a remarkably large FC+Fe-independent iron pool was detected; (iii) a shift from iron-depleted to high-iron conditions resulted in even higher iron accumulation in ΔsidC than in the wt but had no effect on the growth rate of ΔsidC. Iron might be stored in proteins requiring iron-containing cofactors, as indicated by up-regulation of the respective genes in ΔsreA during iron-replete growth (27) but which seems unlikely to account for buffering of vast overaccumulation of iron. A further possibility is vacuolar iron storage, as shown for S. cerevisiae (21). In S. cerevisiae, transport of iron into the vacuole is mediated by CCC1 and, in support of vacuolar iron storage in A. nidulans, a search of the A. nidulans genome (http://www.ncbi.nlm.nih.gov/BLAST/) revealed the presence of two putative CCC1 homologs, AN3681.2 and AN4990.2 (plastp E-values of 4e−32 and 3e−29). Nevertheless, the absence of the intracellular siderophore seems to cause a less-efficient utilization of iron, indicated by decreased growth in shake flask culturing under iron-depleted conditions as well as increased TAFC−Fe production and cellular iron content under iron-replete conditions of ΔsidC. The increased demand for iron might also be the reason for the reduced conidiation rate of ΔsidC found previously (9).
In A. nidulans, N. crassa, and Penicillium chrysogenum, the conidial siderophore storage was found to be an important germination factor, as germination of conidia, which have lost cellular siderophores by treatment with high salt concentrations, fails or is greatly delayed unless a suitable siderophore is supplied (8, 16). In agreement, germination of ΔsidC conidia was delayed under iron-depleted conditions—most likely due to the reduced iron store. Under iron-replete conditions, no difference from the wt was found, indicating that the lack of FC+Fe in ΔsidC can be compensated for by uptake of iron.
A. nidulans possesses both asexual and sexual reproduction cycles. We have previously shown that deficiency in the intracellular siderophore causes a reduction of asexual conidiospore production, which can be partly cured by supplementation with high concentrations of iron (9). Here we show that the lack of FC−Fe biosynthesis causes a defect in homothallic sexual development, which cannot be compensated by high iron supplementation. Consistent with the selection arena hypothesis, Bruggemann et al. (5) found that many auxotrophic mutants are self-sterile and produce only tiny cleistothecia lacking ascospores in homothallic conditions. SidC deficiency does not cause an auxotrophy and completely lacks cleistothecia formation, which might indicate a more specific role of FC+Fe in sexual development. The intracellular siderophore might be essential for sexual development due to its role in iron metabolism or oxidative-stress resistance. In this respect, it is important that reactive oxygen species are not only harmful molecules but also function as signaling molecules required, among others, for sexual development in A. nidulans (1). And here again, iron metabolism and regulatory production of reactive oxygen species are interconnected, as the A. nidulans NADPH-oxidase NoxA, which is proposed to induce sexual development by production of reactive oxygen species, requires heme iron as a cofactor (19). Remarkably, another heme-containing oxidoreductase, TmpA, is involved in regulation of asexual development in A. nidulans (33).
Taken together, our studies revealed that the intracellular siderophore is a central component of the fungal physiology, as it is involved in iron storage, oxidative-stress resistance, asexual and sexual reproduction, and germination. The regulation of FC−Fe biosynthesis by iron and oxidative stress resembles that of ferritin production in other organisms (34), emphasizing the similarity of these analogous mechanisms for storage and detoxification of iron.
Acknowledgments
This work was supported by the Austrian Science Foundation (FWF P-15959-B11 and FWF P-18606-B11).
REFERENCES
- 1.Aguirre, J., M. Rios-Momberg, D. Hewitt, and W. Hansberg. 2005. Reactive oxygen species and development in microbial eukaryotes. Trends Microbiol. 13:111-118. [DOI] [PubMed] [Google Scholar]
- 2.Andrews, S. C., A. K. Robinson, and F. Rodriguez-Quinones. 2003. Bacterial iron homeostasis. FEMS Microbiol. Rev. 27:215-237. [DOI] [PubMed] [Google Scholar]
- 3.Arosio, P., and S. Levi. 2002. Ferritin, iron homeostasis, and oxidative damage. Free Radic. Biol. Med. 33:457-463. [DOI] [PubMed] [Google Scholar]
- 4.Brakhage, A. A., and G. Turner. 1992. L-lysine repression of penicillin biosynthesis and the expression of penicillin biosynthesis genes acvA and ipnA in Aspergillus nidulans. FEMS Microbiol. Lett. 77:123-127. [DOI] [PubMed] [Google Scholar]
- 5.Bruggeman, J., A. J. Debets, and R. F. Hoekstra. 2004. Selection arena in Aspergillus nidulans. Fungal Genet. Biol. 41:181-188. [DOI] [PubMed] [Google Scholar]
- 6.Casselton, L., and M. Zolan. 2002. The art and design of genetic screens: filamentous fungi. Nat. Rev. Genet. 3:683-697. [DOI] [PubMed] [Google Scholar]
- 7.Champe, S. P., D. L. Nagle, and L. N. Yager. 1994. Sexual sporulation. Prog. Ind. Microbiol. 29:429-454. [PubMed] [Google Scholar]
- 8.Charlang, G., B. Ng, N. H. Horowitz, and R. M. Horowitz. 1981. Cellular and extracellular siderophores of Aspergillus nidulans and Penicillium chrysogenum. Mol. Cell. Biol. 1:94-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisendle, M., H. Oberegger, I. Zadra, and H. Haas. 2003. The siderophore system is essential for viability of Aspergillus nidulans: functional analysis of two genes encoding l-ornithine N 5-monooxygenase (sidA) and a non-ribosomal peptide synthetase (sidC). Mol. Microbiol. 49:359-375. [DOI] [PubMed] [Google Scholar]
- 10.Fidel, S., J. H. Doonan, and N. R. Morris. 1988. Aspergillus nidulans contains a single actin gene which has unique intron locations and encodes a gamma-actin. Gene. 70:283-293. [DOI] [PubMed] [Google Scholar]
- 11.Haas, H. 2003. Molecular genetics of fungal siderophore biosynthesis and uptake: the role of siderophores in iron uptake and storage. Appl. Microbiol. Biotechnol. 62:316-330. [DOI] [PubMed] [Google Scholar]
- 12.Haas, H., M. Schoeser, E. Lesuisse, J. F. Ernst, W. Parson, B. Abt, G. Winkelmann, and H. Oberegger. 2003. Characterization of the Aspergillus nidulans transporters for the siderophores enterobactin and triacetylfusarinine C. Biochem. J. 371:505-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haas, H., I. Zadra, G. Stoffler, and K. Angermayr. 1999. The Aspergillus nidulans GATA factor SREA is involved in regulation of siderophore biosynthesis and control of iron uptake. J. Biol. Chem. 274:4613-4619. [DOI] [PubMed] [Google Scholar]
- 14.Halliwell, B., and J. M. Gutteridge. 1984. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 219:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han, K. H., J. A. Seo, and J. H. Yu. 2004. Regulators of G-protein signalling in Aspergillus nidulans: RgsA downregulates stress response and stimulates asexual sporulation through attenuation of GanB (Galpha) signalling. Mol. Microbiol. 53:529-540. [DOI] [PubMed] [Google Scholar]
- 16.Horowitz, N. H., G. Charlang, G. Horn, and N. P. Williams. 1976. Isolation and identification of the conidial germination factor of Neurospora crassa. J. Bacteriol. 127:135-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawasaki, L., D. Wysong, R. Diamond, and J. Aguirre. 1997. Two divergent catalase genes are differentially regulated during Aspergillus nidulans development and oxidative stress. J. Bacteriol. 179:3284-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konetschny-Rapp, S., H. G. Huschka, G. Winkelmann, and G. Jung. 1988. High-performance liquid chromatography of siderophores from fungi. Biol. Met. 1:9-17. [DOI] [PubMed] [Google Scholar]
- 19.Lara-Ortiz, T., H. Riveros-Rosas, and J. Aguirre. 2003. Reactive oxygen species generated by microbial NADPH oxidase NoxA regulate sexual development in Aspergillus nidulans. Mol. Microbiol. 50:1241-1255. [DOI] [PubMed] [Google Scholar]
- 20.Latge, J. P. 1999. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12:310-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li, L., O. S. Chen, D. McVey Ward, and J. Kaplan. 2001. CCC1 is a transporter that mediates vacuolar iron storage in yeast. J. Biol. Chem. 276:29515-29519. [DOI] [PubMed] [Google Scholar]
- 22.MacDiarmid, C. W., M. A. Milanick, and D. J. Eide. 2003. Induction of the ZRC1 metal tolerance gene in zinc-limited yeast confers resistance to zinc shock. J. Biol. Chem. 278:15065-15072. [DOI] [PubMed] [Google Scholar]
- 23.Maerker, C., M. Rohde, A. A. Brakhage, and M. Brock. 2005. Methylcitrate synthase from Aspergillus fumigatus. Propionyl-CoA affects polyketide synthesis, growth and morphology of conidia. FEBS J. 272:3615-3630. [DOI] [PubMed] [Google Scholar]
- 24.Matzanke, B. F., E. Bill, G. I. Muller, A. X. Trautwein, and G. Winkelmann. 1987. Metabolic utilization of 57Fe-labeled coprogen in Neurospora crassa. An in vivo Mossbauer study. Eur. J. Biochem. 162:643-650. [DOI] [PubMed] [Google Scholar]
- 25.Matzanke, B. F., E. Bill, A. X. Trautwein, and G. Winkelmann. 1987. Role of siderophores in iron storage in spores of Neurospora crassa and Aspergillus ochraceus. J. Bacteriol. 169:5873-5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oberegger, H., M. Schoeser, I. Zadra, B. Abt, and H. Haas. 2001. SREA is involved in regulation of siderophore biosynthesis, utilization and uptake in Aspergillus nidulans. Mol. Microbiol. 41:1077-1089. [DOI] [PubMed] [Google Scholar]
- 27.Oberegger, H., M. Schoeser, I. Zadra, M. Schrettl, W. Parson, and H. Haas. 2002. Regulation of freA, acoA, lysF, and cycA expression by iron availability in Aspergillus nidulans. Appl. Environ Microbiol. 68:5769-5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oberegger, H., I. Zadra, M. Schoeser, B. Abt, W. Parson, and H. Haas. 2002. Identification of members of the Aspergillus nidulans SREA regulon: genes involved in siderophore biosynthesis and utilization. Biochem. Soc. Trans. 30:781-783. [DOI] [PubMed] [Google Scholar]
- 29.Philippe, B., O. Ibrahim-Granet, M. C. Prevost, M. A. Gougerot-Pocidalo, M. Sanchez Perez, A. Van der Meeren, and J. P. Latge. 2003. Killing of Aspergillus fumigatus by alveolar macrophages is mediated by reactive oxidant intermediates. Infect. Immun. 71:3034-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Philpott, C. C., O. Protchenko, Y. W. Kim, Y. Boretsky, and M. Shakoury-Elizeh. 2002. The response to iron deprivation in Saccharomyces cerevisiae: expression of siderophore-based systems of iron uptake. Biochem. Soc. Trans. 30:698-702. [DOI] [PubMed] [Google Scholar]
- 31.Pontecorvo, G., J. A. Roper, L. M. Hemmons, K. D. MacDonald, and A. W. J. Bufton. 1953. The genetics of Aspergillus nidulans. Adv. Genet. 5:141-238. [DOI] [PubMed] [Google Scholar]
- 32.Schrettl, M., E. Bignell, C. Kragl, C. Joechl, T. Rogers, H. N. Arst, Jr., K. Haynes, and H. Haas. 2004. Siderophore biosynthesis but not reductive iron assimilation is essential for Aspergillus fumigatus virulence. J. Exp. Med. 200:1213-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soid-Raggi, G., O. Sanchez, and J. Aguirre. 2006. TmpA, a member of a novel family of putative membrane flavoproteins, regulates asexual development in Aspergillus nidulans. Mol. Microbiol. 59:854-869. [DOI] [PubMed] [Google Scholar]
- 34.Torti, F. M., and S. V. Torti. 2002. Regulation of ferritin genes and protein. Blood 99:3505-3516. [DOI] [PubMed] [Google Scholar]
- 35.Van Ho, A., D. M. Ward, and J. Kaplan. 2002. Transition metal transport in yeast. Annu. Rev. Microbiol. 56:237-261. [DOI] [PubMed] [Google Scholar]
- 36.Winkelmann, G. 1993. Kinetics, energetics, and mechanisms of siderophore iron transport in fungi, p. 219-239. In L. L. Barton and B. C. Hemmings (ed.), Iron chelation in plants and soil microorganisms. Academic Press, Inc., New York, N.Y.