Abstract
Here we describe, for the first time, that budding yeast mitogen-activated protein kinase Hog1 and its upstream activators Pbs2 and Ssk1 are essential for the response to arsenite. Hog1 is rapidly phosphorylated in response to arsenite and triggers a transcriptional response that involves the upregulation of genes essential for arsenite detoxification.
Saccharomyces cerevisiae sets up a battery of mechanisms in response to arsenic that involve transcription factors (Yap1 and Arr1/Acr1/Yap8) (20), cell membrane transporters (Arr3/Acr3) (21), vacuole transporters that transfer arsenite-conjugated glutathione into the vacuole (Ycf1) (7), and an arsenate reductase (Arr2/Acr2) that reduces arsenate to arsenite before it can be a substrate of the transporters (15). However, it is still unknown if any of the mitogen-activated protein kinase (MAPK) pathways in Saccharomyces cerevisiae are involved in the response to arsenite.
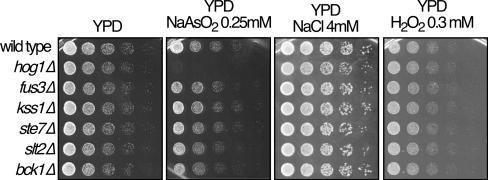
To study the function and importance of MAP kinase signaling in the response to arsenite in Saccharomyces cerevisiae, we monitored the sensitivity to arsenite of strains deficient in the MAPKs Hog1, Fus3, Kss1, and Slt2 as well as some of their upstream regulators, such as Ste7 and Bck1. As shown in Fig. 1, all the mutants and the wild type were able to grow at similar rates in rich medium (yeast extract-peptone-dextrose [YPD]). However, strains deficient in Hog1 (hog1Δ) did not grow in media containing sodium arsenite, indicating that Hog1 activity is necessary for the proper response of Saccharomyces cerevisiae to this metalloid.
FIG. 1.
Hog1 is essential for survival under arsenite stress. Serial dilutions (1:5) of the wild type and several mutants (hog1Δ, fus3Δ, kss1Δ, ste7Δ, slt2Δ, and bck1Δ) were plated on rich medium plates (YPD) in the presence of sodium arsenite, sodium chloride, and hydrogen peroxide. All of the strains used in this report were in the genetic background of the BY series, obtained from EUROSCARF. Pictures were taken after the plates were incubated for 2 to 3 days at 24°C.
This sensitivity could be explained by the toxicity caused by sodium in hog1Δ mutants (8). However, the presence of sodium in the media did not affect the growth of strains lacking Hog1 (Fig. 1), confirming that the toxicity of sodium arsenite in hog1Δ mutants was produced by As(III).
Some of the physiological effects caused by arsenite are thought to be produced by its capacity for producing reactive oxygen species (ROS). We monitored the sensitivity of the previously described mutants to hydrogen peroxide, a well-known ROS producer. As shown in Fig. 1, all the mutants and the wild type showed similar sensitivity to hydrogen peroxide, indicating that arsenite produced its toxic effects in the cell through a mechanism that cannot be exclusively explained by its ROS production capacity.
These results showed that Hog1 is required to respond to a new stimulus, namely sodium arsenite, distinct from hyperosmotic shock, hydrogen peroxide, or cold shock responses (16, 19).
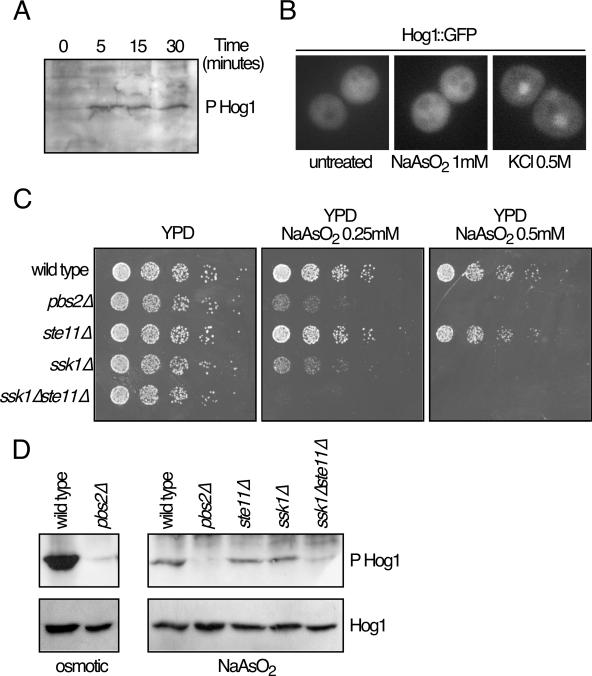
The sensitivity of hog1Δ cells to arsenite indicated that the presence of the MAPK Hog1 was required for the correct biochemical response to this insult. If signaling through Hog1 is essential for its function in these conditions, Hog1 should be activated by phosphorylation of residues Thr174 and Tyr176, which is required for Hog1 activation. Using commercial antibodies raised against phosphorylated human p38 (17852-R; Santa Cruz), we were able to detect phosphorylation of Hog1 protein in response to sodium arsenite (Fig. 2A) that appeared soon after arsenite treatment (5 min).
FIG. 2.
Hog1 phosphorylation and localization. (A) Hog1 phosphorylation (P Hog1) in response to arsenite. Western blotting analysis of yeast extracts (13) showed that Hog1 is phosphorylated after 0.5 mM sodium arsenite treatment for the indicated times. Hog1 phosphorylation was detected using specific anti-phosphorylated Hog1 protein. (B) Hog1 localization. Hog1-deficient cells were transformed with plasmid pRS-Hog1-GFP (5). Hog1::GFP protein expressed from this plasmid was detected after treatment with 0.5 M KCl or 1 mM sodium arsenite for 10 min. (C) Mutants in the Hog1 pathway show different sensitivities to sodium arsenite. Serial dilutions of the wild type and pbs2Δ, ste11Δ, ssk1Δ, and ssk1Δste11Δ mutant strains were plated in rich media containing several concentrations of sodium arsenite. Pictures were taken after 2 to 3 days at 24°C. (D) Phosphorylation of Hog1 in different mutants. Strains described for panel C were treated with KCl (0.8 M) for 10 min (osmotic) or sodium arsenite (1 mM) for 10 min (NaAsO2). Phosphorylation and abundance of Hog1 were monitored by Western blotting using specific polyclonal antibodies (9079; Santa Cruz).
In response to high osmolarity, Hog1 phosphorylation is accompanied by translocation to the nucleus, increasing the abundance of the kinase in this cellular compartment (5). Using a HOG1::GFP allele, we monitored the localization of Hog1 in yeast cells after sodium arsenite treatment. As shown in Fig. 2B, Hog1 relocalization did not occur as much as observed in hyperosmotic stress-treated cells, indicating that the phosphorylation was not enough to trigger the accumulation of Hog1 protein in the nucleus. Nevertheless, it is possible that small amounts of Hog1 phosphorylation and Hog1 relocation to the nucleus are enough to trigger a complete response to arsenite.
Hog1 is activated through a series of phosphorylation events involving the MAPK kinase Pbs2 and two different MAPK kinase kinase (MAPKKK) branches involving, on the one hand, the MAPKKK Ste11, and on the other hand, the MAPKKKs Ssk2/Ssk22. To test whether any of those pathways was involved in the cellular response to arsenite, we monitored the sensitivity to sodium arsenite of several yeast strains carrying mutations in components of the Hog1 pathway. As shown in Fig. 2C, pbs2Δ mutants were highly sensitive to arsenite in a way similar to that of ssk1Δ and hog1Δ mutants (Fig. 1), while mutants in the MAPKKK Ste11 did not show any sensitivity to arsenite. Interestingly, the sensitivity of the double mutant ssk1Δste11Δ to sodium arsenite was higher than that of pbs2Δ or ssk1Δ.
We also tested the level of Hog1 activation after arsenite stress in several mutants (Fig. 2D). While the phosphorylation of Hog1 was undetectable in pbs2Δ and ssk1Δste11Δ mutants, ssk1Δ mutants showed a phosphorylation similar to that of the wild type and ste11Δ mutant, indicating that there is not a direct correlation between the amount of Hog1 phosphorylation and the sensitivity of S. cerevisiae to arsenite and that Ssk1 could have a role in arsenite response independent of its function on Hog1 phosphorylation.
Alternatively, it was also possible that Hog1 response could be hampered by Skn7. Ssk1 and Skn7 are both regulated by the histidine phosphotransferase Ypd1. Skn7 has functions that counteract the Hog1 pathway (10-12). In fact, its deletion has a synthetic negative effect with the deletion of Ptc1, a phosphatase that negatively regulates Hog1 phosphorylation (10). As demonstrated by the kinetic studies performed by Janiak-Spens et al. (9), in regular conditions phosphotransfer from Ypd1 to Ssk1 is strongly favored over phosphotransfer to Skn7. Therefore, we hypothesized that elimination of Ssk1 could indirectly hyperactivate Skn7 and promote its counter-Hog1 effects.
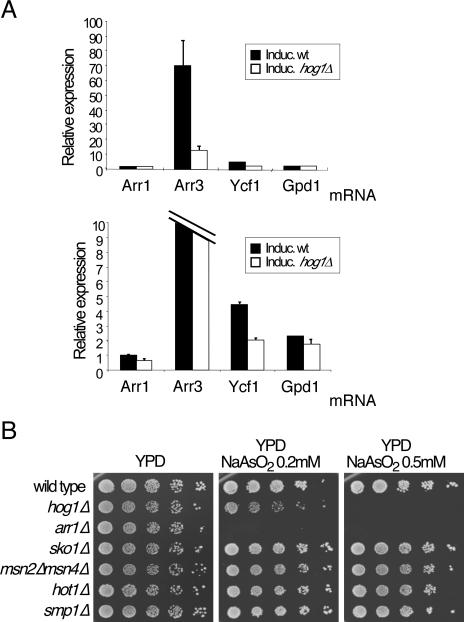
One of the main functions of MAPK pathways is the regulation of transcriptional events in response to specific stimuli. We studied the abundance of several mRNAs in the wild type and hog1Δ mutants in response to sodium arsenite by quantitative reverse transcription-PCR, as described previously (6). We monitored the expression of four mRNAs: the transcription factor Arr1, essential for the upregulation of several genes involved in the response to arsenite (2, 14); the plasma membrane transporter Arr3; the vacuole transporter Ycf1; and Gpd1, the product of a glycerol-3-phosphate dehydrogenase gene, essential in the response to hyperosmotic stress and regulated by Hog1 activity (1).
As shown in Fig. 3A, the Arr3 mRNA is highly induced in response to arsenite treatment, while Arr3 induction in hog1Δ mutants is much lower. A smaller but significant difference was observed in Ycf1 mRNA expression. This defective induction of Arr3 and Ycf1 expression is consistent with the high sensitivity of hog1Δ mutants to sodium arsenite.
FIG. 3.
Hog1-dependent gene expression in response to sodium arsenite. (A) Wild-type and hog1Δ strains were treated with 1 mM sodium arsenite for 1 h. After total RNA extraction, Arr1, Arr3, Ycf1, and Gpd1 mRNAs were quantified using quantitative reverse transcription-PCR (RT-PCRQ). Bars show the ratio of treated/untreated for each strain. Two graphs are shown for better observation of scale differences between experiments. Standard deviation of three experiments is shown. (B) Transcription factors and sensitivity to arsenite. Serial dilutions of the wild type and hog1Δ, arr1Δ, sko1Δ, msn2Δmsn4Δ, hot1Δ, and smp1Δ mutants were plated in rich media and media containing sodium arsenite. Pictures were taken after 2 to 3 days at 24°C. The double mutant msn2Δmsn4Δ was kindly provided by Raul García. wt, wild type.
The fact that the abundance of Arr3 and Ycf1 mRNAs depended on Hog1 led us to consider the possibility of transcriptional regulation of those genes by Hog1. To gain insight into the regulatory mechanism, we analyzed the sensitivity of strains deficient in the transcription factors Sko1, Msn2/4, Hot1, and Smp1, all regulated by Hog1. We also checked the arsenite resistance of a mutant lacking Arr1, which is responsible for the transcription of Arr3 and Ycf1 in response to arsenite.
As shown in Fig. 3B, arr1Δ and hog1Δ are sensitive to different concentrations of arsenite. However, the rest of the mutants assayed (sko1Δ, msn2Δmsn4Δ, hot1Δ, and smp1Δ) did not show any growth defect in plates containing up to 1 mM sodium arsenite (Fig. 3B and data not shown). This result is consistent with a mechanism of Hog1 regulation of arsenite response that is independent of Sko1, Msn2/4, Hot1, and Smp1, which highlights a possible novel mechanism of Hog1-dependent transcriptional regulation.
The results described here have many similarities with those obtained in Schizosaccharomyces pombe and mammalian cells (3, 4, 17, 18). The fact that mammals, S. cerevisiae, and S. pombe use similar MAP kinase pathways to respond to arsenite indicates that the study of arsenite toxicity in those two yeasts can also render interesting results for the knowledge of the biochemical mechanisms of action of arsenite in humans.
Acknowledgments
We thank Rosa M. Perez for her help with the quantitative reverse transcription-PCR experiments. We also appreciate the kind gift of plasmids and strains from Raul Garcia, Javier Arroyo, and Francesc Posas. We thank Humberto Martín, Victoria Martín, and Peter Kaiser for critical reading of the manuscript and useful suggestions and María Molina for encouragement and support.
M.A.R.-G. is a Ramon y Cajal investigator. This work was partially funded by a grant awarded by Universidad Complutense de Madrid to M.A.R.-G.
Footnotes
Published ahead of print on 18 August 2006.
REFERENCES
- 1.Albertyn, J., S. Hohmann, J. M. Thevelein, and B. A. Prior. 1994. GPD1, which encodes glycerol-3-phosphate dehydrogenase, is essential for growth under osmotic stress in Saccharomyces cerevisiae, and its expression is regulated by the high-osmolarity glycerol response pathway. Mol. Cell. Biol. 14:4135-4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouganim, N., J. David, R. Wysocki, and D. Ramotar. 2001. Yap1 overproduction restores arsenite resistance to the ABC transporter deficient mutant ycf1 by activating ACR3 expression. Biochem. Cell Biol. 79:441-448. [PubMed] [Google Scholar]
- 3.Cavigelli, M., W. W. Li, A. Lin, B. Su, K. Yoshioka, and M. Karin. 1996. The tumor promoter arsenite stimulates AP-1 activity by inhibiting a JNK phosphatase. EMBO J. 15:6269-6279. [PMC free article] [PubMed] [Google Scholar]
- 4.Elbirt, K. K., and H. L. Bonkovsky. 1999. Heme oxygenase: recent advances in understanding its regulation and role. Proc. Assoc. Am. Phys. 111:438-447. [PubMed] [Google Scholar]
- 5.Ferrigno, P., F. Posas, D. Koepp, H. Saito, and P. A. Silver. 1998. Regulated nucleo/cytoplasmic exchange of HOG1 MAPK requires the importin beta homologs NMD5 and XPO1. EMBO J. 17:5606-5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia, R., C. Bermejo, C. Grau, R. Perez, J. M. Rodriguez-Pena, J. Francois, C. Nombela, and J. Arroyo. 2004. The global transcriptional response to transient cell wall damage in Saccharomyces cerevisiae and its regulation by the cell integrity signaling pathway. J. Biol. Chem. 279:15183-15195. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh, M., J. Shen, and B. P. Rosen. 1999. Pathways of As(III) detoxification in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 96:5001-5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giaever, G., A. M. Chu, L. Ni, C. Connelly, L. Riles, S. Veronneau, S. Dow, A. Lucau-Danila, K. Anderson, B. Andre, A. P. Arkin, A. Astromoff, M. El-Bakkoury, R. Bangham, R. Benito, S. Brachat, S. Campanaro, M. Curtiss, K. Davis, A. Deutschbauer, K. D. Entian, P. Flaherty, F. Foury, D. J. Garfinkel, M. Gerstein, D. Gotte, U. Guldener, J. H. Hegemann, S. Hempel, Z. Herman, D. F. Jaramillo, D. E. Kelly, S. L. Kelly, P. Kotter, D. LaBonte, D. C. Lamb, N. Lan, H. Liang, H. Liao, L. Liu, C. Luo, M. Lussier, R. Mao, P. Menard, S. L. Ooi, J. L. Revuelta, C. J. Roberts, M. Rose, P. Ross-Macdonald, B. Scherens, G. Schimmack, B. Shafer, D. D. Shoemaker, S. Sookhai-Mahadeo, R. K. Storms, J. N. Strathern, G. Valle, M. Voet, G. Volckaert, C. Y. Wang, T. R. Ward, J. Wilhelmy, E. A. Winzeler, Y. Yang, G. Yen, E. Youngman, K. Yu, H. Bussey, J. D. Boeke, M. Snyder, P. Philippsen, R. W. Davis, and M. Johnston. 2002. Functional profiling of the Saccharomyces cerevisiae genome. Nature 418:387-391. [DOI] [PubMed] [Google Scholar]
- 9.Janiak-Spens, F., P. F. Cook, and A. H. West. 2005. Kinetic analysis of YPD1-dependent phosphotransfer reactions in the yeast osmoregulatory phosphorelay system. Biochemistry 44:377-386. [DOI] [PubMed] [Google Scholar]
- 10.Ketela, T., J. L. Brown, R. C. Stewart, and H. Bussey. 1998. Yeast Skn7p activity is modulated by the Sln1p-Ypd1p osmosensor and contributes to regulation of the HOG pathway. Mol. Gen. Genet. 259:372-378. [DOI] [PubMed] [Google Scholar]
- 11.Li, S., A. Ault, C. L. Malone, D. Raitt, S. Dean, L. H. Johnston, R. J. Deschenes, and J. S. Fassler. 1998. The yeast histidine protein kinase, Sln1p, mediates phosphotransfer to two response regulators, Ssk1p and Skn7p. EMBO J. 17:6952-6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu, J. M., R. J. Deschenes, and J. S. Fassler. 2003. Saccharomyces cerevisiae histidine phosphotransferase Ypd1p shuttles between the nucleus and cytoplasm for SLN1-dependent phosphorylation of Ssk1p and Skn7p. Eukaryot. Cell 2:1304-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin, H., J. M. Rodriguez-Pachon, C. Ruiz, C. Nombela, and M. Molina. 2000. Regulatory mechanisms for modulation of signaling through the cell integrity Slt2-mediated pathway in Saccharomyces cerevisiae. J. Biol. Chem. 275:1511-1519. [DOI] [PubMed] [Google Scholar]
- 14.Menezes, R. A., C. Amaral, A. Delaunay, M. Toledano, and C. Rodrigues-Pousada. 2004. Yap8p activation in Saccharomyces cerevisiae under arsenic conditions. FEBS Lett. 566:141-146. [DOI] [PubMed] [Google Scholar]
- 15.Mukhopadhyay, R., J. Shi, and B. P. Rosen. 2000. Purification and characterization of ACR2p, the Saccharomyces cerevisiae arsenate reductase. J. Biol. Chem. 275:21149-21157. [DOI] [PubMed] [Google Scholar]
- 16.Panadero, J., C. Pallotti, S. Rodriguez-Vargas, F. Randez-Gil, and J. A. Prieto. 2006. A downshift in temperature activates the high osmolarity glycerol (HOG) pathway, which determines freeze tolerance in Saccharomyces cerevisiae. J. Biol. Chem. 281:4638-4645. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Gabriel, M. A., and P. Russell. 2005. Distinct signaling pathways respond to arsenite and reactive oxygen species in Schizosaccharomyces pombe. Eukaryot. Cell 4:1396-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rouse, J., P. Cohen, S. Trigon, M. Morange, A. Alonso-Llamazares, D. Zamanillo, T. Hunt, and A. R. Nebreda. 1994. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell 78:1027-1037. [DOI] [PubMed] [Google Scholar]
- 19.Staleva, L., A. Hall, and S. J. Orlow. 2004. Oxidative stress activates FUS1 and RLM1 transcription in the yeast Saccharomyces cerevisiae in an oxidant-dependent manner. Mol. Biol. Cell 15:5574-5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vincent, A. C., and K. Struhl. 1992. ACR1, a yeast ATF/CREB repressor. Mol. Cell. Biol. 12:5394-5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wysocki, R., P. Bobrowicz, and S. Ulaszewski. 1997. The Saccharomyces cerevisiae ACR3 gene encodes a putative membrane protein involved in arsenite transport. J. Biol. Chem. 272:30061-30066. [DOI] [PubMed] [Google Scholar]