Abstract
Ferric citrate transport in Escherichia coli involves proteins encoded by the fec genes, including the transport and signaling protein FecA and the signal transducing protein FecR. Randomly isolated FecA point mutants showed a reduced interaction with FecR and a reduced transcription initiation of the ferric citrate transport genes. The mutations were localized on one side of the FecA signaling domain, which might form the interface to FecR. Some of the mutants showed strongly reduced iron transport rates, which suggests that the signaling domain affects the structure of the FecA transporter domain.
The outer membrane protein FecA displays two separate functions in ferric citrate transport in Escherichia coli: (i) it mediates transcription initiation of the fecABCDE transport genes and (ii) it transports ferric citrate from the cell surface into the periplasm (1). Mature FecA contains an N-proximal extension of 79 residues. This extension is characteristic of outer membrane transporters involved in transcriptional regulation. Deletion of the extension abolishes induction by ferric citrate but retains ferric citrate transport (7). The extension is located in the periplasm (7) and is not observed in the FecA crystal structures unloaded and loaded with diferric dicitrate (5, 14). This lack of a defined electron density is caused by the flexibility of the signaling domain, since nuclear magnetic resonance (NMR) spectroscopy of residues 1 to 79 yields a novel defined structure (6).
Previously, we demonstrated the interaction of the FecA signaling domain with the C-proximal region of the FecR protein (3, 4), residues 101 to 317 of which are located in the periplasm (9, 12). The interactions were indicated by binding of FecA to His10-FecR loaded on a Ni-nitrilotriacetic acid agarose column, by use of a bacterial two-hybrid system, and by isolation of randomly generated point mutants in FecR that were impaired in interaction and induction. In the two-hybrid system, FecA1-79 fused to mutated LexA1-87408 and FecR101-317 fused to wild-type LexA1-87 bind at two sites of the mutated sulA promoter and repress transcription of sulA-lacZ. In the present study, the latter approach was used to generate point mutants in the FecA signaling domain that display a reduced interaction with FecR and are affected in transcriptional activation.
fecA1-79 was randomly mutagenized by PCR (11). The fragments were fused to lexA1-87408 carried on plasmid pDP804 (2). The mutagenized plasmids were transformed (3) into E. coli SU202 sulA(op408/op+)::lacZ Δ(lacIPOZYA) lexA71::Tn5 sulA211 (2). The transformants were plated on MacConkey lactose agar. In the red colonies that formed, sulA-lacZ transcription was not completely repressed because the mutated FecA1-79 domains did not fully interact with the FecR101-317 domain and therefore the LexA1-87 DNA binding domains were impaired in dimer formation. Of 1,740 red colonies isolated, 10 colonies were randomly selected for further study.
β-Galactosidase activity of the mutants was determined. All FecA1-79 mutants showed a lower repression than wild-type FecA1-79 (Table 1), and five of these mutants displayed virtually no repression. The determined nucleotide sequences of the mutated fecA1-79 fragments revealed that seven mutants contained a single amino acid replacement, two mutants contained a double mutation, and one mutant carried a stop codon after residue 60 (Table 1) (mutants were designated according to the site and type of mutation, for example, N4Y means replacement of asparagine number 4 by tyrosine). The latter mutation somewhat repressed sulA-lacZ transcription, probably caused by some binding of the fragment to FecR101-317.
TABLE 1.
Interaction of mutated FecA1-79 with FecR101-317 and induction and transport activities of mutated FecA proteins
| FecA mutant | sulA-lacZ expression (U)a | Induction level(s) (%)b | Transport rate(s) (%)b |
|---|---|---|---|
| N4Y H41Rc | 155 | 66, 43 | 63, 6 |
| A18V | 204 | 35 | 95 |
| H20R | 157 | ND | 100 |
| F23S K62Rc | 169 | 39, 76 | 2, 100 |
| S37G | 160d | 100 | 100 |
| L40Q | 208 | 90 | 0 |
| D45G | 106 | 53 | 100 |
| S48G | 213 | 81 | 81 |
| Q52R | 190 | 56 | 0 |
| L54P | 217 | 58 | 0 |
| H41R Q52Re | NDf | 49 | 15 |
The β-galactosidase values of the selected interaction mutants are given (means of three experiments, with an average deviation of 7.5%). β-Galactosidase activity of the LexA1-87-FecA1-79 wild type was 65 U, and the activity of cells expressing no LexA1-87-FecA1-79 was 211 U.
The values for induction and transport are given in percentages of values for wild-type FecA. The β-galactosidase values with wild-type FecA after induction were 386 U with 1 mM citrate, 12 U without citrate, and 4 U with no FecA. See footnote c for description of multiple values.
These derivatives were isolated as double mutants, and sulA-lacZ repression was determined. The mutations were separated for the determination of fecA-lacZ induction and ferric citrate transport. For example, FecA(N4Y) and FecA(H41R) transported iron with 63 and 6% of the wild-type rate, respectively.
Repression was determined with the mutant FecA(S37G) with a stop codon after Q60, whereas induction and transport were determined with FecA(S37G).
This double mutant was constructed to determine induction and transport.
ND, not determined.
To measure the induction of the fec transport genes by full-length FecA mutants, site-specific single mutations (Table 1) were introduced into the fecA gene. The fecA mutant genes were cloned in the low-copy-number plasmid pLCIRA (11) by replacement of the wild-type fecA gene. This plasmid also carries the fecI and fecR genes, which are required for induction. The pLCIRA derivatives were introduced by transformation into E. coli AA93 Δfec containing plasmid pMMO1034 fecA-lacZ (10). Transcription was determined by measuring β-galactosidase activity in response to 0.1 mM citrate, which forms ferric citrate with iron in the medium. The induction activities of the mutants ranged from 35 to 100% (Table 1). All mutants were induced by ferric citrate; none showed constitutive transcription in the absence of ferric citrate (transcription levels between 0 and 6%). Since none of the mutants showed only a baseline transcription level, the FecA(H41R Q52R) double mutant, in which two of the more strongly affected single-site induction mutations were combined, was constructed. This double mutant did not show a stronger transcription reduction than the single mutants (Table 1). Low sulA-lacZ repression levels, indicative of a low interaction of FecA1-79 with FecR101-317, were frequently but not consistently related to low induction levels. For example, low sulA-lacZ repression by FecA1-79(A18V)-FecR101-317 resulted in a reduced induction by FecA(A18V). In contrast, low repression by FecA1-79(L40Q) still resulted in high induction by FecA(L40Q). The comparison must take into account that interaction was studied with a FecA fragment located in the cytoplasm and that induction was studied with complete FecA located in the outer membrane and the periplasm. Some of the mutated signaling domains might assume a conformation in the cytoplasm that differs from the conformation assumed when the signaling domains are part of the complete FecA protein. In a previous study with mutations in the C-proximal region of FecR, interaction and induction levels matched more closely (3).
Mutations in the signaling sequence were not expected to affect transport since the signaling sequence can be removed without reducing the transport rate (6). Nevertheless, the transport rates of the mutants were determined as recently described (8). To ensure that these results were independent of the induction level, the wild-type and the mutated fecA1-79 genes were cloned downstream of the arabinose promoter in plasmid pBAD18, and transcription was induced by adding 0.2% arabinose to the growth medium. An example of the transport assays is given in Fig. 1. Surprisingly, transport of five mutants was virtually abolished: FecA(F23S), FecA(L40Q), FecA(H41R), FecA(Q52R), and FecA(L54P). The transport rates of FecA(N4Y), FecA(S48G), and FecA(H41R Q52R) were reduced (Table 1). The combination of mutations Q52R and H41R in the latter mutant resulted in a higher transport rate than the single mutation Q52R. The transport activity of the double mutant was determined with the mutated fecA gene cloned on plasmid pLCIRA; the genes were induced by addition of 0.1 mM ferric citrate.
FIG. 1.
Citrate-mediated 55Fe3+ transport into E. coli AA93 Δfec expressing the fecBCDE transport genes on plasmid pUP40 (8) and the indicated FecA wild-type (WT) and mutant proteins. The fecA genes were cloned on pBAD18; transcription was induced by addition of 0.2% arabinose.
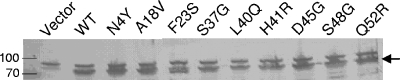
To examine whether the amounts of the FecA mutant proteins affected the induction and transport levels, cells were grown in the presence of 0.1 mM citrate and the amounts of FecA proteins were estimated by immunoblotting with anti-FecA antibodies. The blots showed small variations in the amounts of FecA derivatives, but the amounts were more than sufficient for full induction and transport (Fig. 2).
FIG. 2.
Immunoblot of wild-type (WT) FecA and mutant FecA proteins after sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The arrow indicates the position of FecA. The protein identities of the immunoreactive upper band in the sample lacking FecA and all of the other samples are unknown. Aliquots of cells corresponding to an identical absorbance were loaded on the gel. Numbers mark the positions of the 70- and 100-kDa standard proteins.
The mutation sites were localized on the known NMR structure of FecA1-79 (6). All but two mutations, F23S and K62R, were located in one half of the molecule in the α1 and α3 helices, in the β1 and β3 β-strands, and in the turns between α1 and β1, α2 and β3, and β3 and α3 (Fig. 3). Mutations N4Y, H20R, S37G, H41R, D45G, S48G, and Q52R are exposed at the surface of the signaling domain. In the wild type, these are polar residues, and their replacement by polar residues most likely did not alter the conformation of the signaling domain. These residues are probably involved in binding of FecR and are part of the interface to the C-proximal region of FecR. Since a FecA contact surface forms the region of interaction to FecR, single amino acid replacements contributed to but did not fully determine the level of interaction. The A18V substitution alters the interaction of helices α1 and α3 since the side chain of V is larger and more hydrophobic than the side chain of A. The L45P substitution might affect the α3 helix even though it is the second-to-last amino acid in the helix. The F23S substitution likely changes the conformation of the turn between α1 and β2, and it strongly reduced interaction, induction, and transport. In contrast, with the K62R substitution in β4, induction was slightly reduced, and transport remained at the wild-type level. The L40Q substitution, located in the turn between α2 and β3, hardly reduced interaction and induction but strongly reduced transport.
FIG. 3.
NMR structure of FecA1-74 (6). The sites of mutations isolated in this paper and the suppressor mutations (arrows with interrupted lines) (3) are approximately indicated.
Conformation of the signaling domain in the wild type is altered by binding of diferric dicitrate well above the cell surface and thus far away from signaling in the periplasm. This conformational change induces transcription of the fec transport genes via FecR and FecI. It is therefore conceivable that structural alterations in the signaling domain of FecA also affect the transporter domain. Indeed, transport was affected mostly by amino acid replacements in the core of the signaling domain that are predicted to alter its conformation. These alterations involve the TonB box, the cork domain, and probably also the β-barrel domain.
The randomly generated mutations were clustered mostly in the N-terminal half of the signaling domain, in which we previously have isolated two FecA mutations that suppressed mutations in FecR (3). The mutation FecA(G39R) restored the activity of the FecR(L269G) mutant to 37% of the wild-type level, and the mutation FecA(D43E) restored the activity of the FecR(F284L) mutant to 34% of the wild-type level. This finding further supports the conclusion that this FecA region forms the interface to FecR.
The mutations were at sites where signaling domains of other outer membrane proteins contained amino acid residues with similar properties (size, charge, and polarity). The sequences of the signaling domains are not highly conserved. Only 2 out of 79 sites contain in all signaling domains identical residues (listed in reference 6).
The results obtained with FecA in E. coli most likely also apply for transcription initiation in Shigella flexneri, various Bordetella strains, Serratia marcescens, Pseudomonas aeruginosa (10, 13), Pseudomonas putida, and Ralstonia solanacearum. In these species, a FecIRA type of transcription initiation has been demonstrated experimentally, and many predicted FecIRA-type regulatory devices have been predicted from sequenced genomes (listed in reference 1).
Acknowledgments
We thank Klaus Hantke for advice, Christina Herrmann for performing the transport assays, and Karen A. Brune for critical reading of the manuscript.
This study was funded by the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie.
REFERENCES
- 1.Braun, V., and S. Mahren. 2005. Transmembrane transcriptional control (surface signalling) of the Escherichia coli Fec type. FEMS Microbiol. Rev. 29:673-684. [DOI] [PubMed] [Google Scholar]
- 2.Dmitrova, M., G. Younès-Cauet, P. Oertel-Buchheit, D. Porte, M. Schnarr, and M. Granger-Schnarr. 1998. A new LexA-based genetic system for monitoring and analyzing protein heterodimerization in Escherichia coli. Mol. Gen. Genet. 257:205-212. [DOI] [PubMed] [Google Scholar]
- 3.Enz, S., H. Brand, C. Orellana, S. Mahren, and V. Braun. 2003. Sites of interaction between the FecA and FecR signal transduction proteins of ferric citrate transport in Escherichia coli K-12. J. Bacteriol. 185:3745-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Enz, S., S. Mahren, U. Stroeher, and V. Braun. 2000. Surface signaling in ferric citrate transport gene induction: interaction of the FecA, FecR, and FecI regulatory proteins. J. Bacteriol. 182:637-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferguson, A., R. Chakraborty, B. Smith, L. Esser, D. van der Helm, and J. Deisenhofer. 2002. Structural basis of gating by the outer membrane transporter FecA. Science 295:1715-1719. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Herrero, A., and H. Vogel. 2005. Nuclear magnetic resonance solution structure of the periplasmic signalling domain of the TonB-dependent outer membrane transporter FecA from Escherichia coli. Mol. Microbiol. 58:1226-1237. [DOI] [PubMed] [Google Scholar]
- 7.Kim, I., A. Stiefel, S. Plantör, A. Angerer, and V. Braun. 1997. Transcription induction of the ferric citrate transport genes via the N-terminus of the FecA outer membrane protein, the Ton system and the electrochemical potential of the cytoplasmic membrane. Mol. Microbiol. 23:333-344. [DOI] [PubMed] [Google Scholar]
- 8.Mahren, S., H. Schnell, and V. Braun. 2005. Occurrence and regulation of the ferric citrate transport system in Escherichia coli B, Klebsiella pneumoniae, Enterobacter aerogenes, and Photorhabdus luminescens. Arch. Microbiol. 184:175-186. [DOI] [PubMed] [Google Scholar]
- 9.Ochs, M., S. Veitinger, I. Kim, D. Welz, A. Angerer, and V. Braun. 1995. Regulation of citrate-dependent iron transport of Escherichia coli: FecR is required for transcription activation by FecI. Mol. Microbiol. 15:119-132. [DOI] [PubMed] [Google Scholar]
- 10.Redly, G. A., and K. Poole. 2005. FpvIR control of fpv ferric pyoverdine receptor gene expression in Pseudomonas aeruginosa: demonstration of an interaction between FpvI and FpvR and identification of mutations in each compromising this interaction. J. Bacteriol. 187:5648-5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stiefel, A., S. Mahren, M. Ochs, P. T. Schindler, S. Enz, and V. Braun. 2001. Control of the ferric citrate transport system of Escherichia coli: mutations in region 2.1 of the FecI extracytoplasmic-function sigma factor suppress mutations in the FecR transmembrane regulatory protein. J. Bacteriol. 183:162-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Welz, D., and V. Braun. 1998. Ferric citrate transport of Escherichia coli: functional regions of the FecR transmembrane regulatory protein. J. Bacteriol. 180:2387-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson, M. J., and I. L. Lamont. 2006. Mutational analysis of an extracytoplasmic function-sigma factor to investigate its interactions with RNA polymerase and DNA. J. Bacteriol. 188:1935-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yue, W. W., S. Grizot, and S. K. Buchanan. 2003. Structural evidence for iron-free citrate and ferric citrate binding to the TonB-dependent outer membrane transporter FecA. J. Mol. Biol. 332:353-368. [DOI] [PubMed] [Google Scholar]